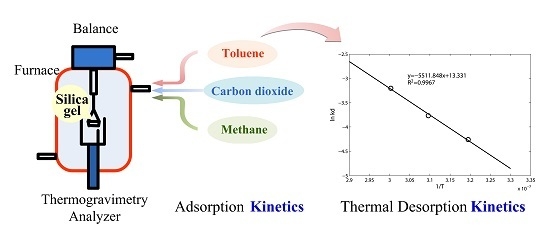
Kinetics Study of Gas Pollutant Adsorption and Thermal Desorption on Silica Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent and Adsorbates
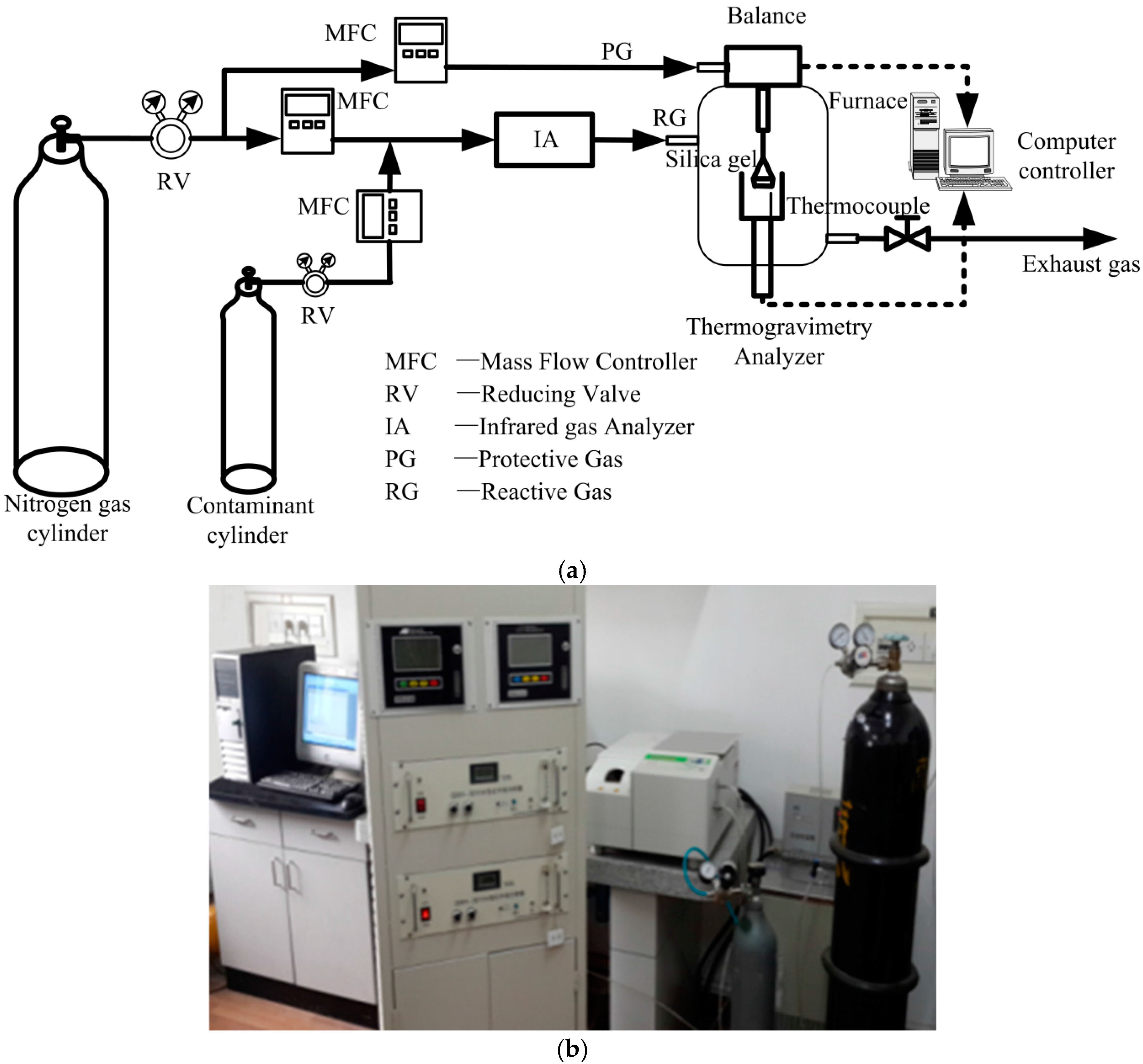
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
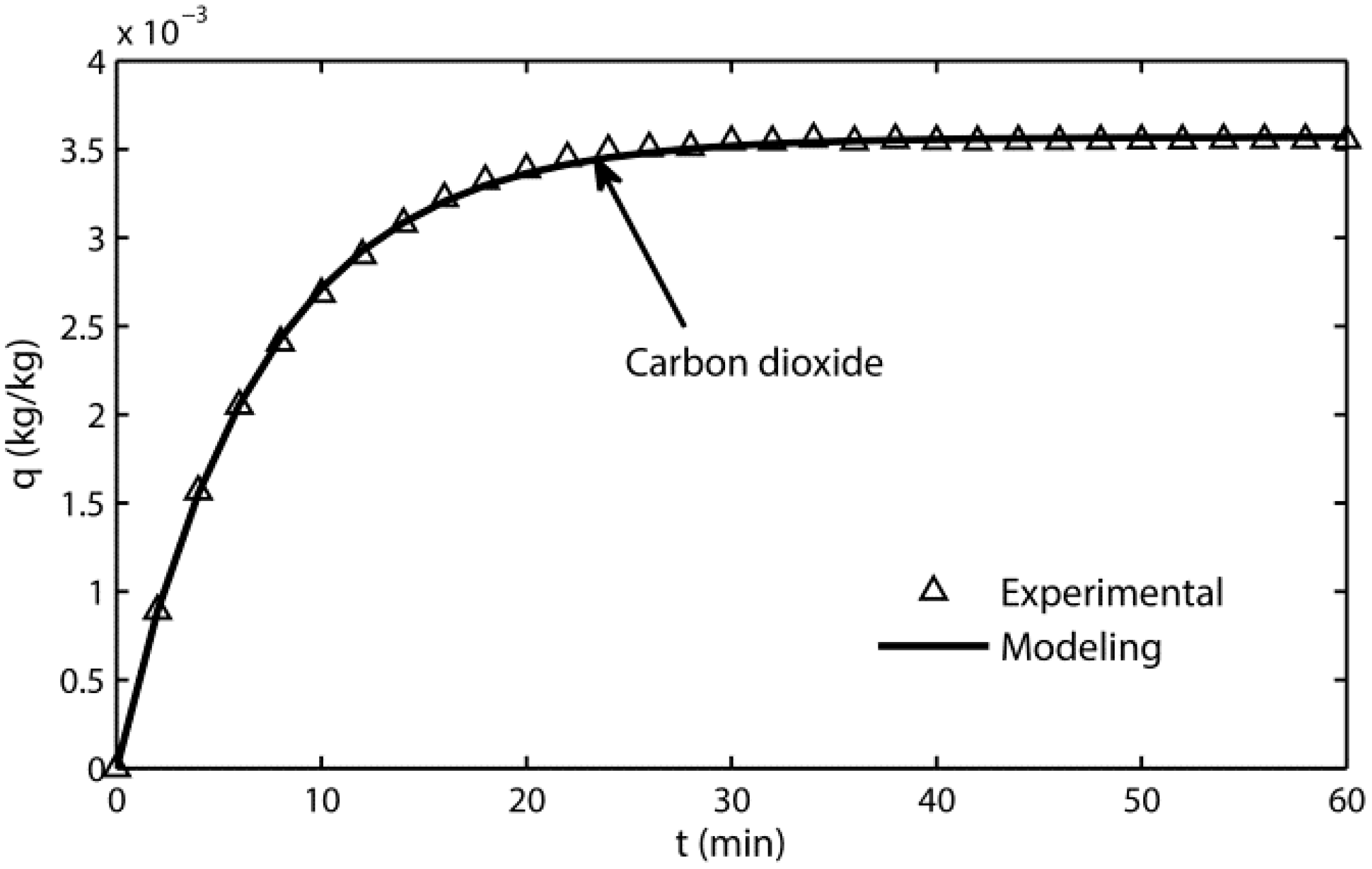
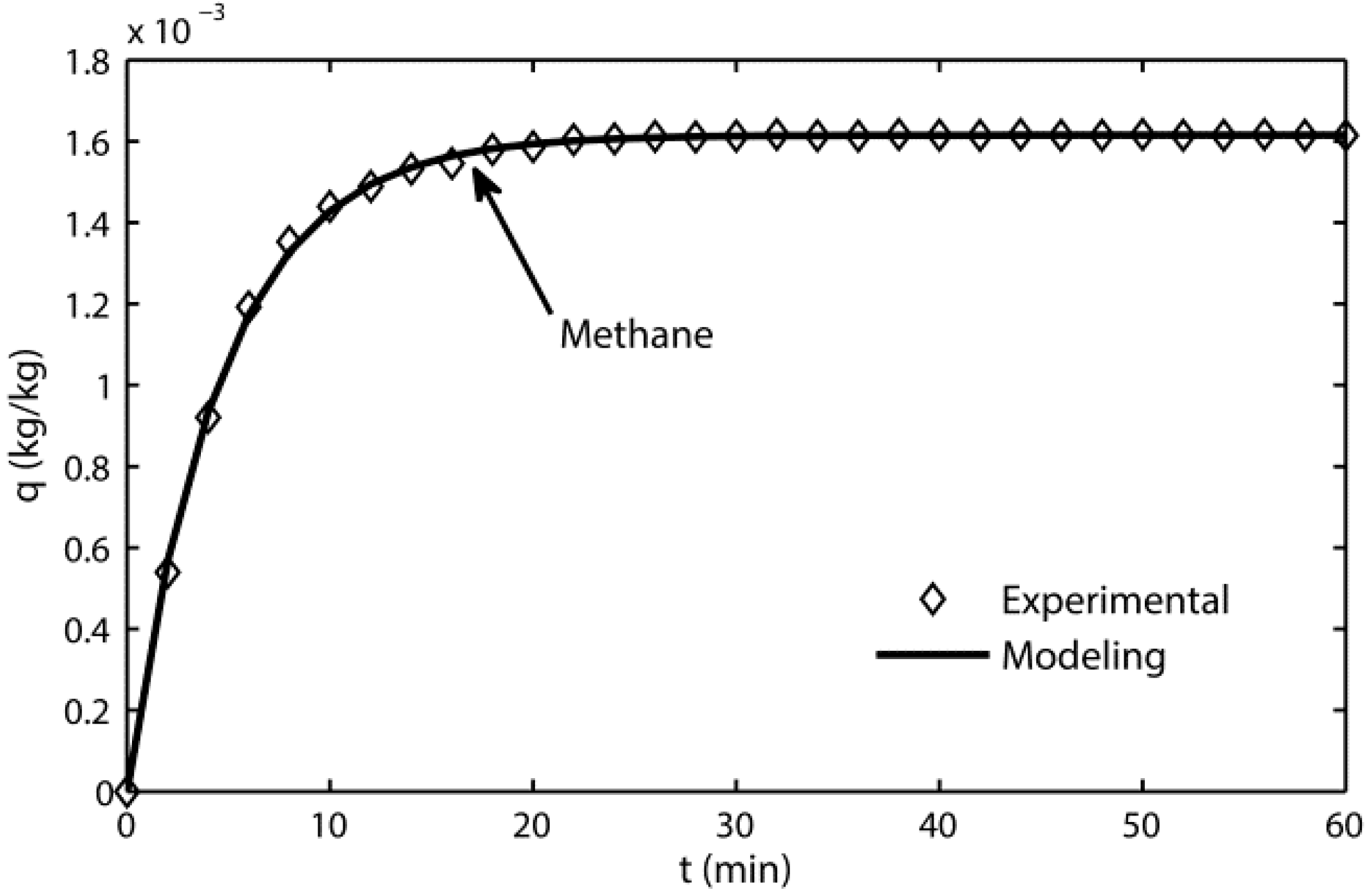
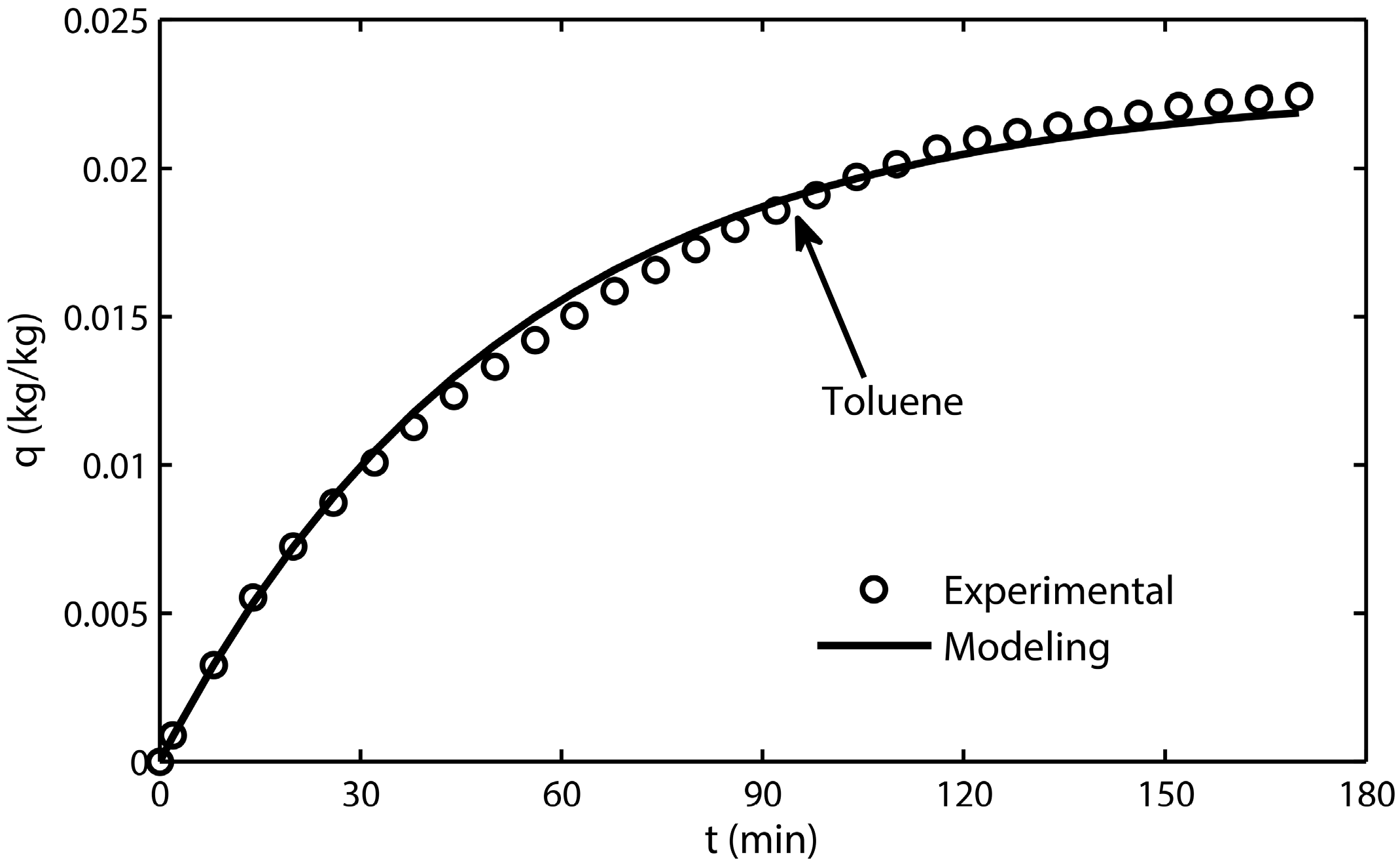
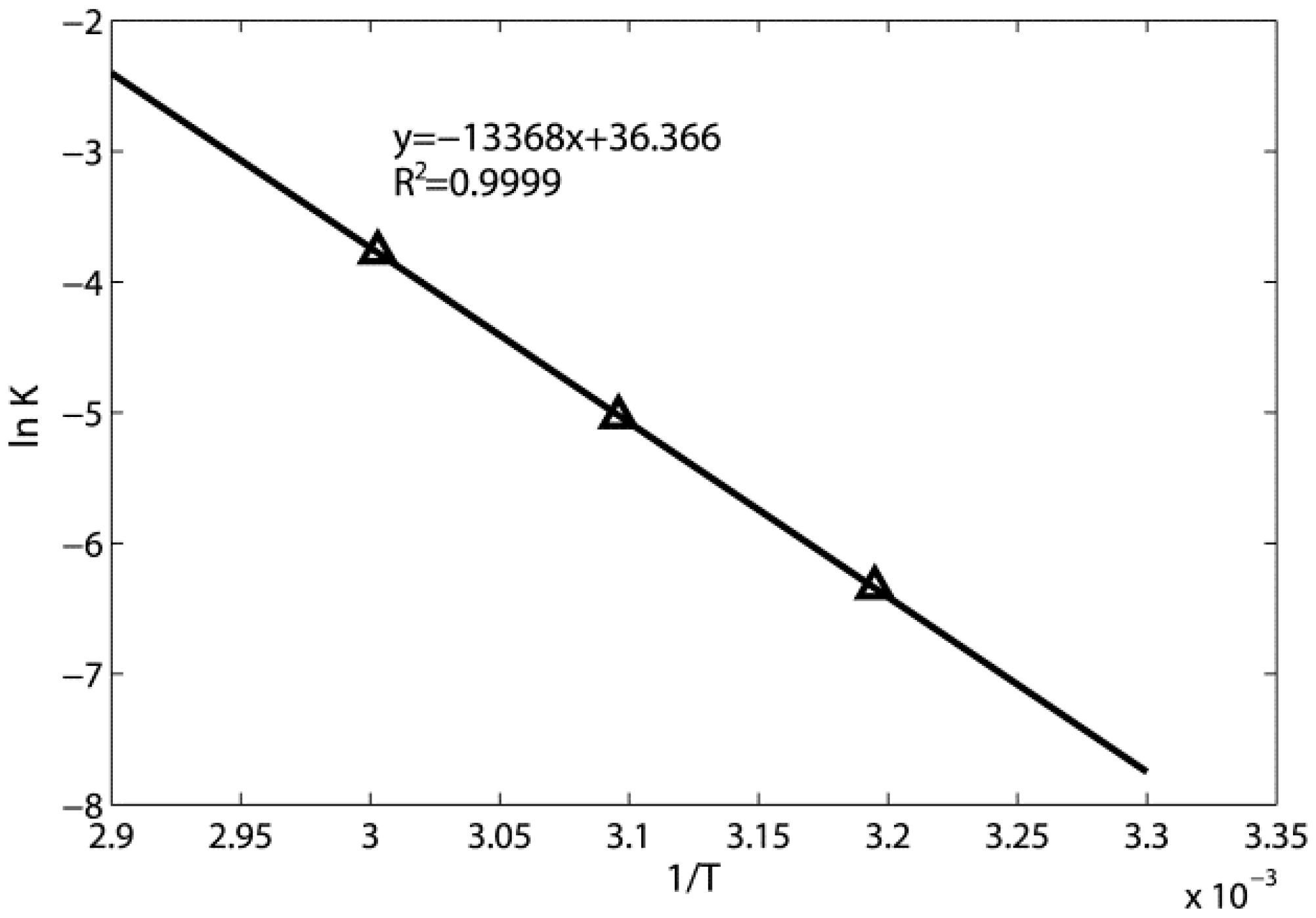
3.1. Adsorption Kinetic Model
- The adsorption-terms are the only dominant mechanism for silica gel adsorption processes at 298 K.
- The kinetic model reflects the adsorption mechanism for silica gel because of its high fitting accuracy.
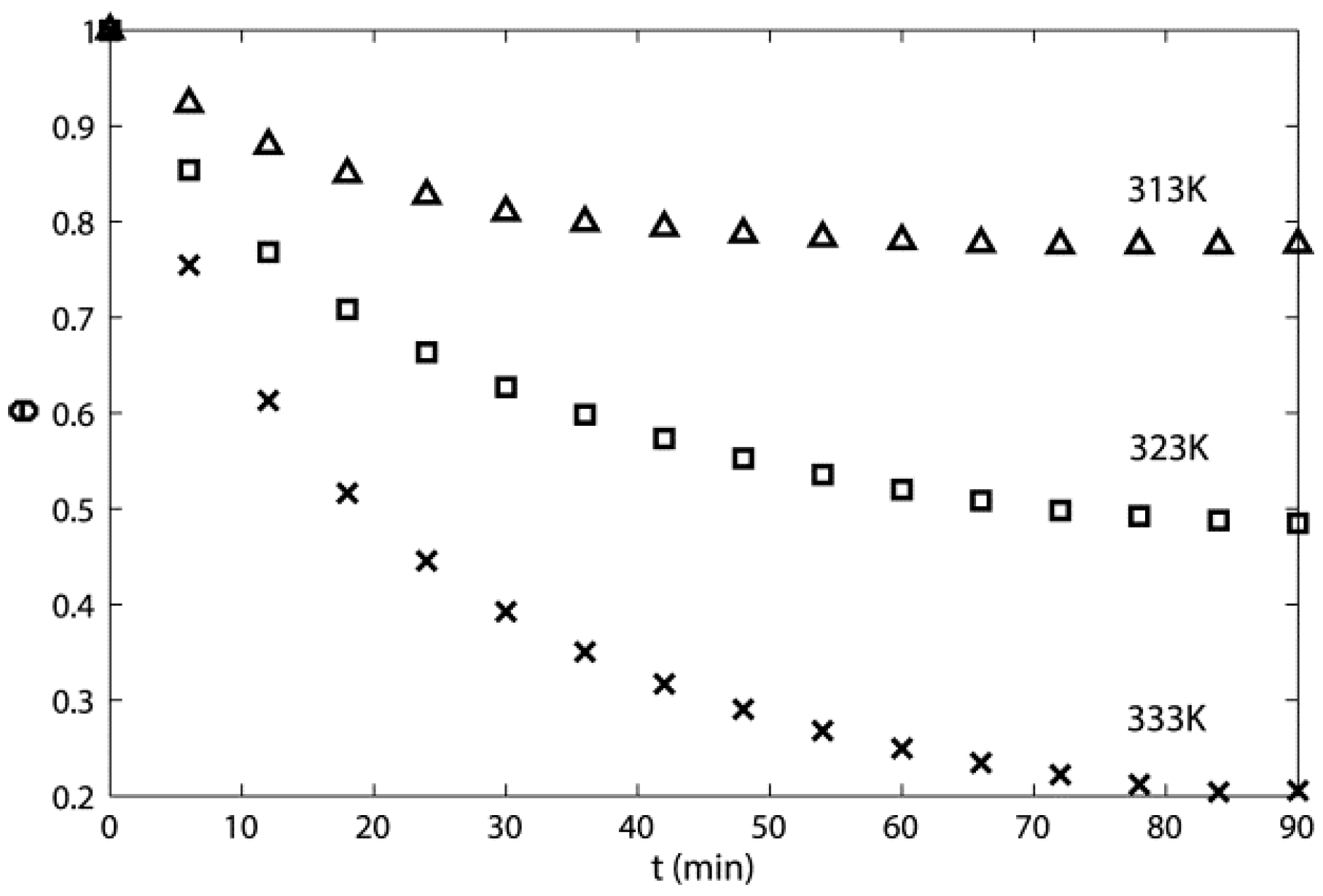
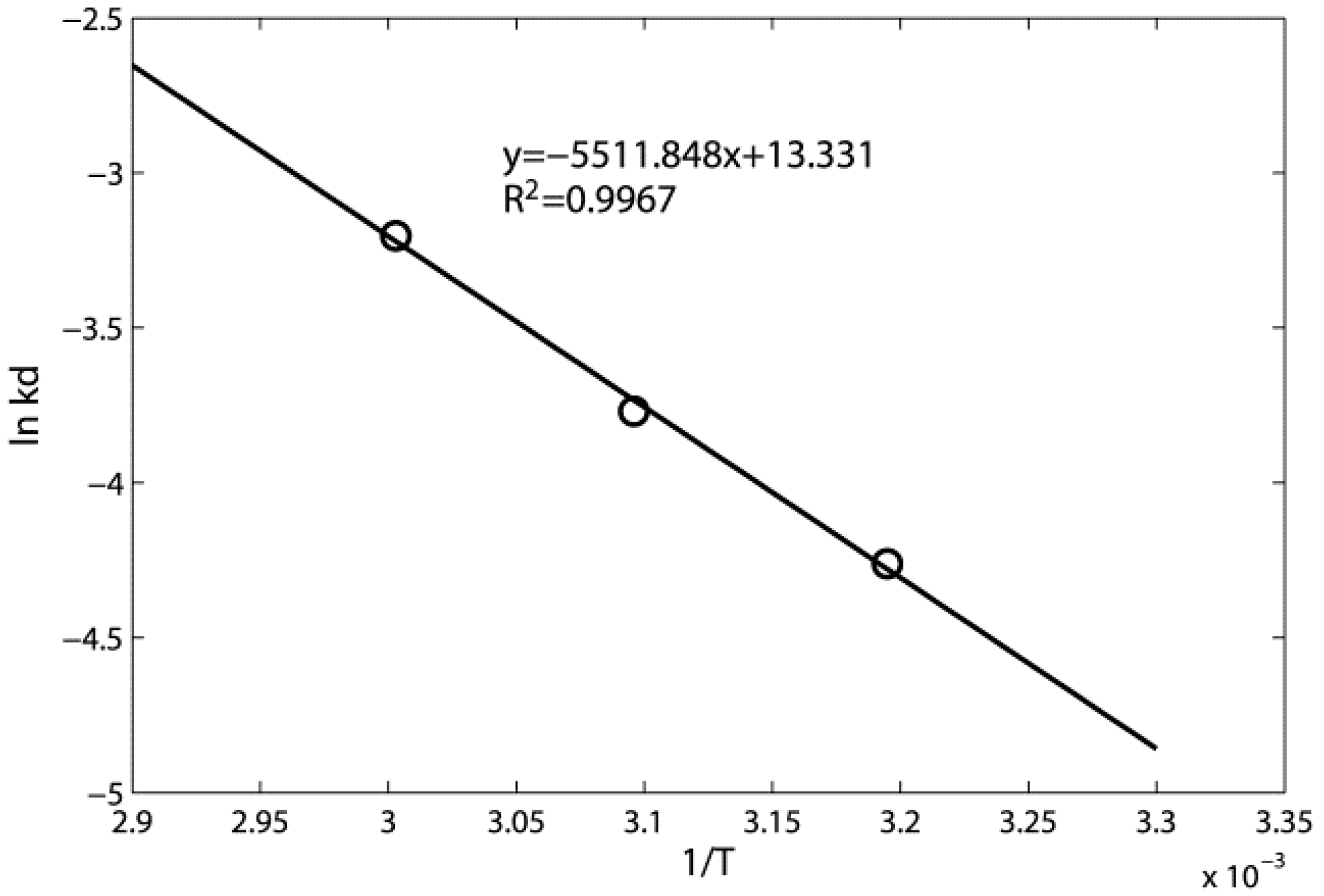
3.2. Toluene Thermal Desorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Research Council (U.S.). Health Surveillance. In The Airliner Cabin Environment and the Health of Passengers and Crew; National Academies Press: Washington, DC, USA, 2002; pp. 223–236. ISBN 0-309-56770-X. [Google Scholar]
- Tao, H.; Meng, L.; Liping, P.; Jun, W. Studies on new air purification and air quality control system of airliner cabin. Procedia Eng. 2011, 17, 343–353. [Google Scholar] [CrossRef]
- Bull, K. Cabin air filtration: Helping to protect occupants from infectious diseases. Travel Med. Infect. Dis. 2008, 6, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Boggia, S.; Rüd, K. Intercooled recuperated gas turbine engine concept. In Proceedings of the 41st Aiaa/Asme/Sae/Asee Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2005. [Google Scholar]
- Shim, W.G.; Kim, S.C. Heterogeneous adsorption and catalytic oxidation of benzene, toluene and xylene over spent and chemically regenerated platinum catalyst supported on activated carbon. Appl. Surf. Sci. 2010, 256, 5566–5571. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Cohn, S.; Fisk, W.J. Energy efficient indoor voc air cleaning with activated carbon fiber (acf) filters. Build. Environ. 2012, 47, 357–367. [Google Scholar] [CrossRef]
- Liu, M.; Yang, D.S.; Pang, L.P.; Yu, Q.N.; Huang, Y. Experimental and computational investigation of adsorption performance of TC-5A and PSA-5A for manned spacecraft. Chin. J. Aeronaut. 2015, 28, 1583–1592. [Google Scholar] [CrossRef]
- Li, G.; Pang, L.; Liu, M.; Yang, D.; Yu, Q.; Rong, A. Multiobjective optimal method for carbon dioxide removal assembly in manned spacecraft. J. Aerosp. Eng. 2016, 29, 04016052. [Google Scholar]
- Ginestet, A.; Pugnet, D.; Rowley, J.; Bull, K.; Yeomans, H. Development of a new photocatalytic oxidation air filter for aircraft cabin. Indoor Air 2005, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, L.; Wyon, D.P.; Wisthaler, A.; Lagercrantz, L.; Strøm-Tejsen, P. Experimental research on photocatalytic oxidation air purification technology applied to aircraft cabins. Build. Environ. 2008, 43, 258–268. [Google Scholar] [CrossRef]
- Wisthaler, A.; Strøm-Tejsen, P.; Fang, L.; Arnaud, T.J.; Hansel, A.; MÄrk, T.D.; Wyon, D.P. PTR-MS assessment of photocatalytic and sorption-based purification of recirculated cabin air during simulated 7-h flights with high passenger density. Environ. Sci. Technol. 2007, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bull, K.; Roux, P. Cabin air filtration systems—Novel technological solutions for commercial aircraft. In Proceedings of the 40th International Conference on Environmental Systems, Barcelona, Spain, 11–15 July 2010; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2010. [Google Scholar]
- Metts, T.A. Heterogeneous reactions of ozone and d-limonene on activated carbon. Indoor Air 2007, 17, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, G.; Wisthaler, A. Desiccant wheels as gas-phase absorption (gpa) air cleaners: Evaluation by ptr-ms and sensory assessment. Indoor Air 2008, 18, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Fang, L.; Zhang, G.; Sheng, Y.; Kong, X.; Zhang, Y.; Olesen, B.W. Theoretical study on volatile organic compound removal and energy performance of a novel heat pump assisted solid desiccant cooling system. Build. Environ. 2015, 85, 233–242. [Google Scholar] [CrossRef]
- Zhang, G.; He, W.; Fang, L.; Ma, F. Numerical study on the contribution of convective mass transfer inside high-porosity adsorbents in the voc adsorption process. Indoor Built. Environ. 2013, 22, 551–558. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.F.; Fang, L. Theoretical study of simultaneous water and vocs adsorption and desorption in a silica gel rotor. Indoor Air 2008, 18, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Strom-Tejsen, P.; Zukowska, D.; Fang, L.; Space, D.R.; Wyon, D.P. Advantages for passengers and cabin crew of operating a gas-phase adsorption air purifier in 11-h simulated flights. Indoor Air 2008, 18, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Fathieh, F.; Nezakat, M.; Evitts, R.W.; Simonson, C.J. Effects of physical and sorption properties of desiccant coating on performance of energy wheels. J. Heat Transf. 2017, 139, 062601. [Google Scholar] [CrossRef]
- Naghash, M.; Fathieh, F.; Besant, R.W.; Evitts, R.W.; Simonson, C.J. Measurement of convective heat transfer coefficients in a randomly packed bed of silica gel particles using ihtp analysis. Appl. Therm. Eng. 2016, 106, 361–370. [Google Scholar] [CrossRef]
- Kafui, K.D. Transient heat and moisture transfer in thin silica gel beds. J. Heat Transf. 1994, 116, 946–953. [Google Scholar] [CrossRef]
- Michaelis, S. Aviation Contaminated Air Reference Manual; Susan Michaelis: Horsham, UK, 2007; ISBN 9780955567209. [Google Scholar]
- Nagda, N.L.; Rector, H.E. A critical review of reported air concentrations of organic compounds in aircraft cabins. Indoor Air 2003, 13, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ncube, T.; Kumar Reddy, K.S.; Al Shoaibi, A.; Srinivasakannan, C. Benzene, toluene, m-xylene adsorption on silica-based adsorbents. Energy Fuels 2017, 31, 1882–1888. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Xu, Z.; Zhang, T.; Liang, D.; Xin, Q.; Ying, P. Interaction of methane with surfaces of silica, aluminas and HZSM-5 zeolite. A comparative FT-IR study. Catal. Lett. 1995, 35, 245–258. [Google Scholar] [CrossRef]
- Monazam, E.R.; Spenik, J.; Shadle, L.J. CO2 desorption kinetics for immobilized polyethylenimine (pei). Energy Fuels 2014, 28, 650–656. [Google Scholar] [CrossRef]
- Villa, A.C.; Gutierrez, J.S.; Gomez, C.J.N.; De los Rios, G.S.A.; Villalobos, M.R.; Palacios, L.C.; Ortiz, A.L.; Collins-Martinez, V. Kinetic study of the CO2 desorption process by carbonated Na2ZrO3 solid absorbent. Int. J. Hydrog. Energy 2015, 40, 17338–17343. [Google Scholar] [CrossRef]
| Parameters | Unit | Value |
|---|---|---|
| BET specific surface area | m2/g | 610 |
| Total pore volume | cm3/g | 0.36 |
| Average pore size | nm | 2.4 |
| Mean particle diameter | mm | 2.10 |
| Gas Pollutant | C (ppm) | ka (s−1) | kaC (s−1) | R2 |
|---|---|---|---|---|
| Carbon dioxide | 1000 | 0.240 | 0.0024 | 0.9999 |
| Methane | 1000 | 0.360 | 0.0036 | 0.9999 |
| Toluene | 609 | 0.053 | 0.00032 | 0.9903 |
| Temperature (K) | kaC (s−1) | ka (s−1) | kd (s−1) | K | R2 |
|---|---|---|---|---|---|
| 313 | 0.0486 | 7.9803 | 0.0141 | 0.00177 | 0.9989 |
| 323 | 0.0216 | 3.5468 | 0.0231 | 0.00651 | 0.9952 |
| 333 | 0.0108 | 1.7734 | 0.0406 | 0.02289 | 0.9955 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A, R.; Liu, M.; Pang, L.; Yang, D.; Wang, J.; Zhou, Y. Kinetics Study of Gas Pollutant Adsorption and Thermal Desorption on Silica Gel. Appl. Sci. 2017, 7, 609. https://doi.org/10.3390/app7060609
A R, Liu M, Pang L, Yang D, Wang J, Zhou Y. Kinetics Study of Gas Pollutant Adsorption and Thermal Desorption on Silica Gel. Applied Sciences. 2017; 7(6):609. https://doi.org/10.3390/app7060609
Chicago/Turabian StyleA, Rong, Meng Liu, Liping Pang, Dongsheng Yang, Jun Wang, and Yue Zhou. 2017. "Kinetics Study of Gas Pollutant Adsorption and Thermal Desorption on Silica Gel" Applied Sciences 7, no. 6: 609. https://doi.org/10.3390/app7060609
APA StyleA, R., Liu, M., Pang, L., Yang, D., Wang, J., & Zhou, Y. (2017). Kinetics Study of Gas Pollutant Adsorption and Thermal Desorption on Silica Gel. Applied Sciences, 7(6), 609. https://doi.org/10.3390/app7060609