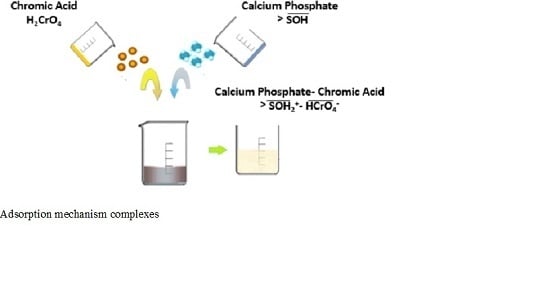
Adsorption of Chromium (VI) on Calcium Phosphate: Mechanisms and Stability Constants of Surface Complexes
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Methods
2.2. Synthesis and Characterization of OCP
2.3. Surface Properties and Adsorption Experiments
3. Results and Discussion
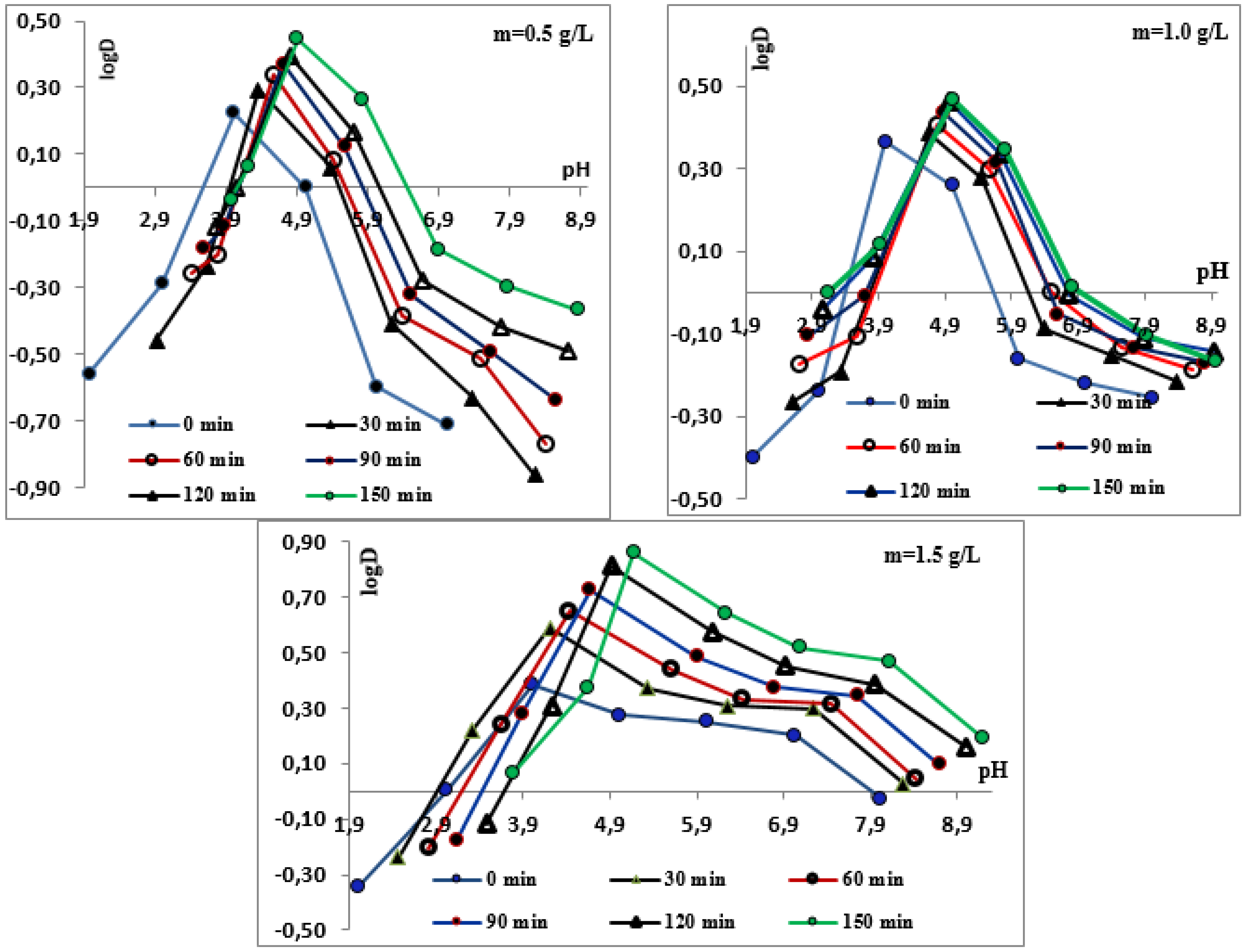
3.1. Effect of pH on Chromium Adsorption
3.2. Effect of Contact Time
3.3. Chromium (VI) Adsorption Reactions and Stability Constants
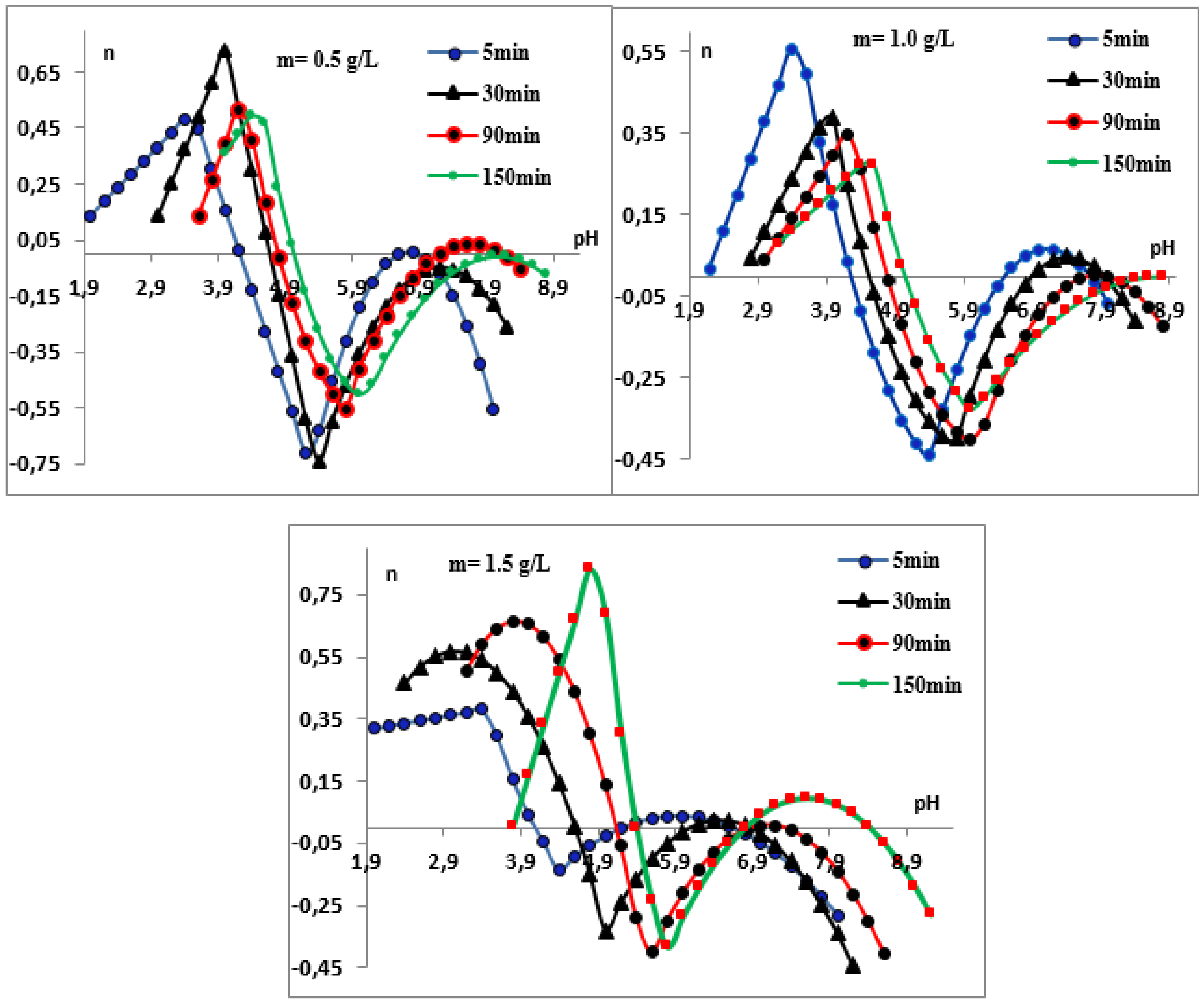
3.3.1. Surface Complexes and Effect of pH and Contact Time on H3O+/OH− Exchange
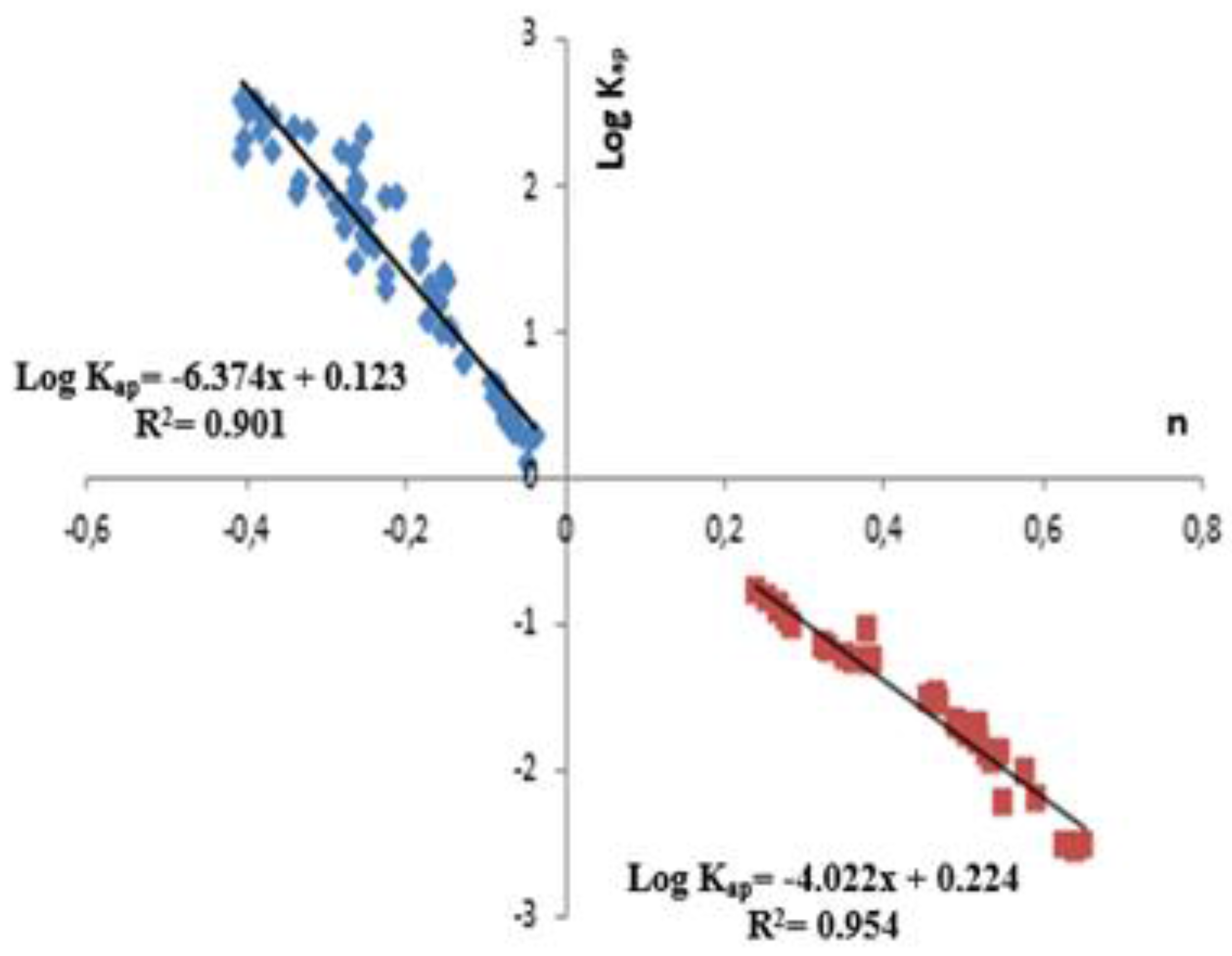
3.3.2. Equilibrium Constants
4. Conclusions
Abbreviations
| OCP | Octacalcium Phosphate (Ca8H2(PO4)6.5H2O) |
| XRD | X-ray Diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| D | distribution coefficient (the ratio of concentration of adsorbed Cr (VI) to its concentration in aqueous phase) |
| m | OCP sorbent amounts in g/L |
| t | contact time. |
| K+, K | surface stability of active site > SOH |
| l | number of functional surface group involved in adsorption reaction |
| H n | hydrogen atoms (n < 0) or OH groups (n > 0) |
| Kln | adsorption constant |
| Ψ0 | surface potential |
| R | universal gas constant (8.31 J/mol·K) |
| T | temperature (K) |
References
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. Hazard J. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Zachara, J.M.; Ainsworth, C.; Brown, G.E., Jr.; Catalano, J.G.; McKinley, J.P.; Qafoku, O.; Smith, S.C.; Szecsody, J.E.; Traina, S.J.; Warner, J.A. Chromium speciation and mobility in a high level nuclear waste vadose zone plume. Geochim. Cosmochim. Acta 2004, 68, 13–30. [Google Scholar] [CrossRef]
- Zhou, X.; Korenaga, T.; Takahashi, T.; Moriwake, T.; Shinoda, S. A process monitoring/controlling system for the treatment of wastewater containing Chromium (VI). Water Res. 1993, 27, 1049–1054. [Google Scholar] [CrossRef]
- Tiravanti, G.; Petruzzelli, D.; Passino, R. Pretreatment of Industrial Wastewaters II, Selected Proceedings of the Second IAWQ International Conference on Pretreatment of Industrial Wastewaters. Water Sci. Technol. 1997, 36, 197–207. [Google Scholar] [CrossRef]
- Pagilla, K.R.; Canter, L.W. Biosorption of Hexavalent Chromium from Aqueous Medium with Opuntia BiomassAthens, Greece. Environ. J. Eng. 1999, 125, 243–248. [Google Scholar] [CrossRef]
- Bruce Manning, A.; Goldberg, S. Modeling Competitive Adsorption of Arsenate with Phosphate and Molybdate on Oxide Minerals. Soil. Sci. Soc. Am. J. 1996, 60, 121–131. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Removal of Heavy Metals from Wastewater by Adsorption and Membrane Processes: A Comparative Study. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Dahbi, S.; Azzi, M.; de la Guardia, M. Removal of hexavalent chromium from wastewaters by bone charcoal. Fresenius J. Anal. Chem. 1999, 363, 404–407. [Google Scholar] [CrossRef]
- Dahbi, S.; Azzi, M.; Saib, N.; de la Guardia, M.; Faure, R.; Durand, R. Removal of trivalent chromium from tannery waste waters using bone charcoal. Anal. Bioanal. Chem. 2002, 374, 540–546. [Google Scholar] [PubMed]
- Hyder, A.H.M.G.; Shamim, A.B.; Nosa, O.E. Adsorption isotherm and kinetic studies of hexavalent chromium removal from aqueous solution onto bone char. J. Environ. Chem. Eng. 2015, 3, 1329–1336. [Google Scholar] [CrossRef]
- Yoon, J. Phosohate-Induced Lead Immobilization in Contaminated Soil. Master’s Thesis, University of Florida, Gainesville, FL, USA, May 2005. [Google Scholar]
- Chowdhury, S.R.; Ernest Yanful, K. Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Elzinga, E.J.; Reeder, R. Lead and zinc removal with storage period in porous asphalt pavement. J. Environ. J. Sci. Technol. 2005, 39, 4042–4048. [Google Scholar] [CrossRef]
- Qureshi, M.; Varshney, K.G. Inorganic Ion Exchangers; Chemical Analysis CRC Press: Boca Raton, FL, USA, 2002; p. 282. [Google Scholar]
- Correa, F.G.; Martínez, J.B.; Gómez, J.S. Synthesis and characterization of calcium phosphate and its relation to Cr (VI) adsorption properties. Rev. Int. Contam. Ambient. 2010, 26, 129–134. [Google Scholar]
- Conca, J.L.; Lu, N.; Parker, G.; Moore, B.; Adams, A.; Wright, J.V.; Heller, P. Remediation of Chlorinated and Recalcitrant Compounds. J. Environ. Qual. 2000, 7, 319–326. [Google Scholar]
- Saxena, S.; D’Souza, S.F. Heavy metal pollution abatement using rock phosphate mineral. Environ. Int. 2006, 32, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Aklil, A.; Mouflih, M.; Sebti, S. Removal of Cadmium from Water Using Natural Phosphate as Adsorbent. Hazard J. Mater. A 2004, 112, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Corami, A.; D’Acapito, F.; Mignardia, S.; Ferrini, V. Removal of Cu from Aqueous Solutions by Synthetic Hydroxyapatite: EXAFS Investigation. Mater. Sci. Eng. B 2008, 149, 209–213. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhu, Y.-J.; Zhao, J.; Lu, B.-Q.; Chen, F.; Qi, C.; Wu, J. Hydroxyapatite nanosheet-assembled microspheres: Hemoglobin-templated synthesis and adsorption for heavy metal ions. J. Colloid Interface Sci. 2014, 416, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gómez, J.; Ramírez-Sandoval, J.; Bonifacio-Martínez, J.; Granados-Correa, F.; Badillo-Almaraz, V.E. Uptake of CrO42-Ions by Fe-Treated Tri-Calcium Phosphate. J. Mex. Chem. Soc. 2010, 54, 34–39. [Google Scholar]
- Razzouki, B.; El Hajjaji, S.; ElYahyaoui, A.; Lamhamdi, A.; Jaafar, A.; Azzaoui, K.; Boussaoud, A.; Zarrouk, A. Kinetic investigation on arsenic (III) adsorption onto iron hydroxide (III). Pharm. Lett. 2015, 7, 53–59. [Google Scholar]
- Soundarrajan, M.; Gomathi, T.; Sudha, P.N. Understanding the Adsorption Efficiency of Chitosan Coated Carbon on Heavy Metal Removal. Int. J. Sci. Res. Publ. 2013, 3, 2250–3153. [Google Scholar]
- Sakai, S.; Anada, T.; Tsuchiya, K.; Ymazaki, H.; Margouli, H.C.; Suzuki, O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent. Mater. J. 2016, 35, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Osamu, S. Octacalcium phosphate (OCP)-based bone substitute materials. Jpn. Dent. Sci. Rev. 2013, 49, 58–71. [Google Scholar]
- Samake, D. Treatment of Tannery Wastewater Using Clay Materials. Ph.D. Thesis, Joseph Fourier-Grenoble University, Bamako, Mali, December 2008. [Google Scholar]
- Zachara, J.M.; Cowan, C.E.; Schmidt, R.L.; Ainsworth, C.C. Chromate Adsorption by Kaolinite. Clays Clay Miner. 1988, 36, 317–326. [Google Scholar] [CrossRef]
- Grossl, V.; Eick, M.; Sparks, D.; Goldberg, S.; Ainsworth, C.C. Arsenate and Chromate Retention Mechanisms on Goethite. 2. Kinetic Evaluation Using a Pressure-Jump Relaxation Technique. Environ. Sci. Technol. 1997, 31, 321–326. [Google Scholar] [CrossRef]
- Lorphensri, O.; Intravijit, J.; Sabatini, D.A.; Kibbey, T.C.G.; Osathaphan, K.; Saiwan, C. Characterization of Commercial Ceramic Adsorbents and its Application on Naphthenic Acids Removal of Petroleum Distillates. Water Res. 2006, 40, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Harding, I.S.; Rashid, N.; Hing, K.A. urface charge and the effect of excess calcium ions on the hydroxyapatite surface. Biomaterials 2005, 26, 6818–6826. [Google Scholar] [CrossRef] [PubMed]
- Zachara, J.M.; Girvin, D.C.; Scmidt, R.L.; Resch, C.T. Chromate adsorption on amorphous iron oxyhydroxide in presence of major ground water ion. Environ. Sci. Technol. 1987, 21, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, A.; Pashalidis, I. Spectophotometric studies on the competitive adsorption of boric acid (B(iii)) and chromate (Cr(Vi)) onto iron (oxy) hydroxide (Fe(O)OH). Glob. Nest J. 2012, 14, 32–39. [Google Scholar]
- Hsia, T.H.; Lo, S.L.; Lin, C.F.; Lee, D.Y. Chemica and spectroscopie evidence for specifie adsorption of chromate on hydrous iron oxide. Chemosphere 1993, 26, 1897–1904. [Google Scholar] [CrossRef]
- Aoyama, M.; Tsuda, M. Removal of Cr(VI) from aqueous solutions by larch bark. Wood Sci. Technol. 2001, 35, 425–434. [Google Scholar] [CrossRef]
- Odeh, L.; Odeh, I.; Khamis, M.; Khatib, M.; Qurie, M.; Shakhsher, Z.; Qutob, M. Hexavalent Chromium Removal and Reduction to Cr (III) by Polystyrene Tris(2-aminoethyl)amine. Sci. Res. Acad. Publ. 2016, 6, 26–37. [Google Scholar] [CrossRef]
- Chang, L. Metal Ions Removal from polluted Waters by Sorption onto Exhausted Coffee Waste. Application to Metal Finishing Industries Wastewater Treatment. Ph.D. Thesis, University of Girona, Girona, Spain, September 2014. [Google Scholar]
- Stankiewicz, A.I.; Moulijn, J.A. Process Intensification: Transforming Chemical Engineering. Chem. Eng. Prog. 2000, 96, 22–34. [Google Scholar] [CrossRef]
- Oldham, D.J.; Mohsen, E.A. A technique for predicting the performance of self-protecting buildings with respect to traffic noise. Noise Control Eng. J. 1980, 15, 11–19. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Swain, S.K.; Sengupta, D.K.; Singh, B.P. Effect of heat treatment of hydroxyapatite on its dispersibility in aqueous medium. J. Colloids Surf. 2006, 277, 164–170. [Google Scholar] [CrossRef]
- Wu, X.W.; Ma, H.W.; Zhang, Y.R. Adsorption of chromium (VI) from aqueous solution by a mesoporous aluminosilicate synthesized from microcline. Appl. Clay Sci. 2010, 48, 538–541. [Google Scholar] [CrossRef]
- Pandey, P.K.; Sharma, S.K.; Sambi, S.S. Kinetics and equilibrium study of chromium adsorption on zeoliteNaX. Int. J. Environ. Sci. Technol. 2010, 7, 395–404. [Google Scholar] [CrossRef]
- Schmuhl, R.; Krieg, H.M.; Keizer, K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies. Water SA 2001, 27, 1–8. [Google Scholar] [CrossRef]
- Asgari, G.; Rahmani, A.R. Preparattion of an Adsorbent from Pumice Stone and Its Adsorption Potential for Removal of Toxic Recalcitrant Contaminants. J. Res. Health Sci. 2013, 13, 53–57. [Google Scholar] [PubMed]
- Dzombak, D.A.; Morel, F.M.M. Adsorption of Inorganic Contaminants in Ponded Effluents from Coal-Fired Power Plants; Massachusetts Institute of Technology Cambridge: Cambridge, MA, USA, 1985. [Google Scholar]
- Farley, K.J.; Dzombak, D.A.; Morel, F.M.M. Distinguishing Adsorption from Surface Precipitation. J. Colloid Surf. Sci. 1985, 106, 226–242. [Google Scholar] [CrossRef]
- Correa, F.G.; Gómez, J.S.; Martínez, J.B. Synthesis and Characterization of Inorganic Materials to Be Used as Adsorbents of Toxic Metals and of Nuclear Interest. In Contributions of the National Institute of Nuclear Research to the Advance of Science and Technology in Mexico. Commemorative Edition 2010; Instituto Nacional de Investigaciones Nucleares: Ocoyoacac, Mexico, 2010; pp. 195–210. [Google Scholar]
- Müller, A.; Duffek, A. Similar Adsorption Parameters for Trace Metals with Different Aquatic Particles. J. Aquat. Geochem. 2001, 7, 107–126. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D. Arsenate and Chromate Retention Mechanisms on Goethite. 1. Surface Structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Fuller, C.C.; Davis, J.A. Surface complexation and precipitate geometry for aqueous Zn (II) sorption on ferrihydrite. Geochim. Cosmochim. Acta 2002, 66, 1119–1137. [Google Scholar] [CrossRef]
- Mario, V.; Maya, A.T.; James, O.L. Surface Complexation Modeling of Carbonate Effects on the Adsorption of Cr(VI), Pb(II), and U(VI) on Goethite. Environ. Sci. Technol. 2001, 35, 3849–3856. [Google Scholar]
- Raven, K.P.; Jain, A.; Loeppert, R.H. rsenite and Arsenate Adsorption on Ferrihydrite: Kinetics, Equilibrium, and Adsorption Envelopes. Environ. Sci. Technol. 1998, 32, 344–349. [Google Scholar] [CrossRef]
- Perrone, J.; Fourest, B.; Giffaut, E. Surface and Physicochemical Characterization of Phosphates Vivianite, Fe2(PO4)3 and Hydroxyapatite, Ca5(PO4)3OH. J. Colloid Interface Sci. 2002, 249, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Smičiklas, I.; Dimović, S.; Plećaš, I.; Mitrić, M. Adsorption and removal of strontium in aqueous solution by synthetic hydroxyapatite. Water Res. 2006, 40, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs. anion-exchange. J. Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, M. Surface Complexation at the Iron Oxide/Water Interface Experimental Investigations and Theoretical Developments; Institution för kemi Göteborgs University: Göteborgs, Germany, 2002. [Google Scholar]
- Jain, A.; Raven, K.P.; Loeppert, R.H. Understanding Arsenate Reaction Kinetics with Ferric Hydroxides. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Álvarez-Ayusço, E.; García-Sánchez, A.; Querol, X. Adsorption of Heavy Metals from Aqueous Solutions on Synthetic Zeolite. Hazard J. Mater. 2007, 142, 191–198. [Google Scholar]
- Ellouzi, K.; Elyahyaoui, A.; Bouhlassa, S. Octacalcium phosphate: Microwave-assisted hydrothermal synthesisand potentiometric determination of the Point of Zero Charge (PZC) and isoelectric point (IEP). Pharm. Lett. 2015, 7, 152–159. [Google Scholar]
- Jin-Wook, K. The Modeling of Arsenic Removal from Contaminated Water Using Coagulation and Sorption; Texas A&M University: College Station, TX, USA, 2005. [Google Scholar]
- Singh, S.P.N.; Mattigod, S.V. Modelling boron adsorption on kaolinite. Clays Clay Miner. 1992, 40, 192–205. [Google Scholar] [CrossRef]
- Eick, M.J.; Peak, J.D.; Brady, W.D. The effect of oxyanions on the oxalatepromoted dissolution of goethite. Soil. Sci. Soc. Am. J. 1999, 63, 1133–1141. [Google Scholar] [CrossRef]
- Tanuma, Y.; Anada, T.; Honda, Y.; Kawai, T.; Kamakura, S.; Echigo, S.; Suzuki, O. Granule Size–Dependent Bone Regenerative Capacity of Octacalcium Phosphate in Collagen Matrix. Tissue Eng. A 2011, 18, 546–557. [Google Scholar] [CrossRef] [PubMed]
| Species | n | Adsorption Reaction | logK1n | logKcol |
|---|---|---|---|---|
| Acidic medium (pH < 5) | ||||
| 0 | 0.1 | |||
| 0 | 0.1 | |||
| +1 | −4.0 | 4.1 | ||
| 0.5 | 3.0 | 2.4 | ||
| Lower acidic to alkaline medium (pH > 5) | ||||
| 0 | 0.2 | |||
| −1 | −6.7 | 6.1 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyahyaoui, A.; Ellouzi, K.; Al Zabadi, H.; Razzouki, B.; Bouhlassa, S.; Azzaoui, K.; Mejdoubi, E.M.; Hamed, O.; Jodeh, S.; Lamhamdi, A. Adsorption of Chromium (VI) on Calcium Phosphate: Mechanisms and Stability Constants of Surface Complexes. Appl. Sci. 2017, 7, 222. https://doi.org/10.3390/app7030222
Elyahyaoui A, Ellouzi K, Al Zabadi H, Razzouki B, Bouhlassa S, Azzaoui K, Mejdoubi EM, Hamed O, Jodeh S, Lamhamdi A. Adsorption of Chromium (VI) on Calcium Phosphate: Mechanisms and Stability Constants of Surface Complexes. Applied Sciences. 2017; 7(3):222. https://doi.org/10.3390/app7030222
Chicago/Turabian StyleElyahyaoui, Ahmed, Kawtar Ellouzi, Hamzeh Al Zabadi, Brahim Razzouki, Saidati Bouhlassa, Khalil Azzaoui, El Miloud Mejdoubi, Othman Hamed, Shehdeh Jodeh, and Abdellatif Lamhamdi. 2017. "Adsorption of Chromium (VI) on Calcium Phosphate: Mechanisms and Stability Constants of Surface Complexes" Applied Sciences 7, no. 3: 222. https://doi.org/10.3390/app7030222
APA StyleElyahyaoui, A., Ellouzi, K., Al Zabadi, H., Razzouki, B., Bouhlassa, S., Azzaoui, K., Mejdoubi, E. M., Hamed, O., Jodeh, S., & Lamhamdi, A. (2017). Adsorption of Chromium (VI) on Calcium Phosphate: Mechanisms and Stability Constants of Surface Complexes. Applied Sciences, 7(3), 222. https://doi.org/10.3390/app7030222