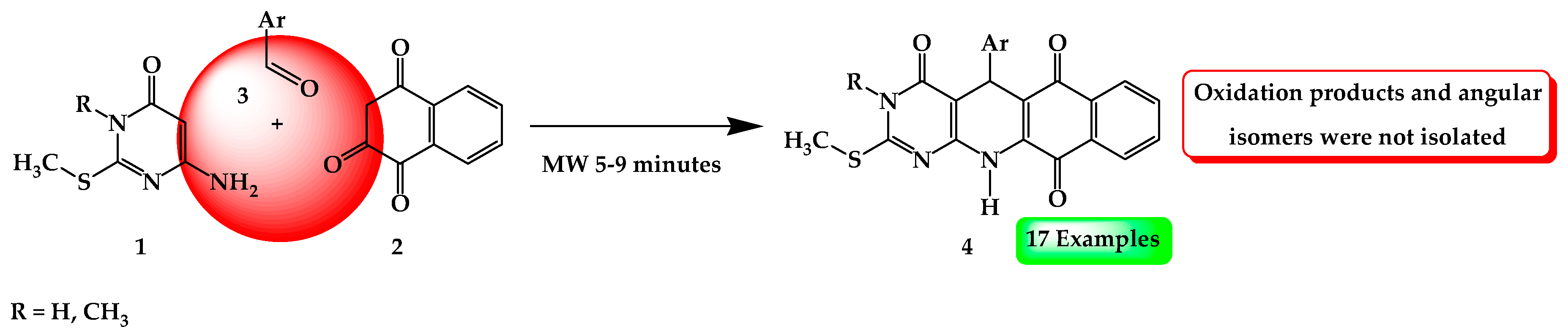
General Procedure for the MCR of Compounds 4a–q
The mixture of 6-aminopyrimidin-4-one 1 (1 mmol), naphthalene-1,2,4(3H)-trione 2 (1 mmol) and aldehyde 3 (1 mmol), were irradiated for 5–9 min and 200 °C under solvent-free conditions. Upon completion, monitored by thin-layer chromatography (TLC), the reaction mixture was cooled to room temperature. The solid was further purified by recrystallization from EtOH (95%).
2-methylthio-5-phenyl-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4a). 80%. m.p. > 300 °C. IR (KBr, υ cm−1), 3398 (N-H st), 2689 (CH3 st), 1652, 1630 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.55 (s, 3H, SCH3), 5.25 (s, 1H, CH), 7.11 (t, J = 6.78 Hz, 1H, Hp), 7.21 (t, J = 7.28 Hz, 2H, Hm), 7.30 (d, J = 7.78 Hz, 2H, Ho), 7.76–7.84 (m, 2H, H8, H9), 7.90 (d, J = 7.28 Hz, 1H, H7), 8.03 (d, J = 7.03 Hz, 1H, H10), 9.60 (s, 1H, NH), 12.49 (s, 1H, NH). 13C NMR δ (ppm): 12.7 (SCH3), 54.3 (C5), 117.4 (C4a), 124.5 (Cp), 125.3 (Co), 125.6 (C10), 127.3 (Cm), 127.9 (C7), 130.3, 131.8 (C10a), 133.2 (C8), 134.6 (C9), 139.3 (C5a), 145.0 (Ci), 179.1 (C=O), 181.6 (C=O). MS: (70 eV) m/z = 401 (16, M+), 325 (19), 324 (100), 276 (13). Anal. Calcd. for C22H15N3O3S C, 65.82; H, 3.77; N, 10.47; found C, 65.85; H, 3.80; N, 10.45.
2-methylthio-5-(4-methylphenyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4b). 80%. m.p. > 300 °C (dec). IR (KBr, υ cm−1), 3395 (N-H st), 2929 (CH3 st), 1651 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.18 (s, 3H, CH3), 2.55 (s, 3H, SCH3), 5.21 (s, 1H, CH), 7.00 (d, J = 8.03 Hz, 2H, Hm), 7.16 (d, J = 8.03 Hz, 2H, Ho), 7.77–7.84 (m, 2H, H8, H9), 7.90 (d, J = 7.53 Hz, 1H, H7), 8.02 (d, J = 7.03 Hz, 1H, H10), 9.58 (s, 1H, NH), 12.51 (s, 1H, NH). 13C NMR δ (ppm): 12.7 (p-CH3), 20.5 (SCH3), 34.1 (C5), 117.7 (C4a), 125.6 (C10), 125.9 (C7), 127.7 (Co), 128.7 (Cm), 130.3 (C6a), 131.9 (C10a), 133.3 (C8), 134.8 (C9), 135.6 (C2), 139.2 (C5a), 142.2 (Ci), 179.2 (C=O), 181.7 (C=O). MS: (70 eV) m/z (%) = 415 (30, M+), 324 (100), 276 (14). Anal. Calcd. for C23H17N3O3S C, 66.49; H, 4.12; N, 10.11; found C, 66.48; H, 4.13; N, 10.14.
5-(4-methoxyphenyl)-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4c). Yellow crystalline solid, 80%. m.p. > 300 °C (dec). IR (KBr, υ cm−1), 3272 (N-H st), 2841 (CH3 st), 1674, 1650 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.55 (s, 3H, SCH3), 3.65 (s, 3H, OCH3), 5.18 (s, 1H, H5), 6.76 (d, J = 8.79 Hz, 2H, Ho), 7.18 (d, J = 8.79 Hz, 2H, Hm), 7.77–7.85 (m, 2H, H8, H9), 7.90 (d, J = 7.53 Hz,1H, H7), 8.02 (d, J =7.03 Hz, 1H, H10), 9.60 (s, 1H, H12), 12.52 (s, 1H, H3). 13C NMR δ (ppm): 12.7 (SCH3), 34.0 (OCH3), 55.0 (C5), 114.0 (Co), 129.0 (Cp), 158.0 (C=O). MS: (70 eV) m/z = 431 (61, M+), 325 (19), 324 (100), 276 (18), 248 (12). Anal. Calcd. for C23H17N3O4S C, 64.03; H, 3.97; N, 9.74; found C, 64.02; H, 4.01; N, 9.78.
2-methylthio-5-(3,4,5-trimethoxyphenyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4d). 80%. m.p. 265 °C. IR (KBr, υ cm−1), 3264 (N-H st), 2933 (CH3 st), 1656 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.55 (s, 3H, SCH3), 3.58 (s, 3H, OCH3), 3.67 (s, 6H, OCH3), 5.22 (s, 1H, CH), 6.57 (s, 2H), 7.77–7.85 (m, 2H, H9, H8), 7.93 (d, J = 8.53 Hz, 1H, H7), 8.03 (d, J = 8.78 Hz, 1H, H10), 9.49 (s, 1H, NH), 12.48 (s, 1H, NH). 13C NMR δ (ppm): 12.5 (SCH3), 34.5 (C5), 55.7 (OCH3), 59.6 (OCH3), 105.2 (Co), 125.4 (C7), 125.7 (C10), 133.0 (C8), 134.5 (C9), 135.6, 136.3 (C5a), 139.2, 140.3 (Ci), 152.4 (C11a), 178.9 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 491 (48, M+), 460 (17), 325 (19), 324 (100), 276 (18). Anal. Calcd. for C25H21N3O6S C, 61.09; H, 4.31; N, 8.55; found C, 61.12; H, 4.30; N, 8.58.
2-methylthio-5-(2-thienyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4e). 70%. m.p. > 300 °C. IR (KBr, υ cm−1), 3384 (N-H st), 2969 (CH3 st), 1655 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.55 (s, 3H, SCH3), 5.53 (s, 1H, CH), 6.81–6.85 (m, 2H, Hetaryl), 7.25 (d, J = 6.28 Hz, 1H, Hetaryl) 7.77–7.86 (m, 2H, H9, H8), 7.97 (d, J = 8.53 Hz, 1H, H7), 8.04 (d, J = 8.53 Hz, 1H, H10), 9.86 (s, 1H, NH), 12.64 (s, 1H, NH). 13C NMR δ (ppm): 12.7 (SCH3), 29.1 (CH), 116.6 (C4a), 124.3 (CH, Hetaryl), 124.5 (CH, Hetaryl), 125.7 (C10), 126.0 (CH, Hetaryl), 126.7 (C7), 130.3 (C6a), 131.7 (C10a), 133.3 (C8), 134.8 (C9), 138.9 (C5a), 148.0 (C2), 179.1 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 407 (100, M+), 392 (11), 346 (18), 324 (59), 276 (18). Anal. Calcd. for C20H13N3O3S2 C, 58.95; H, 3.22; N, 10.31; found C, 58.99; H, 3.25; N, 10.34.
5-(4-fluorophenyl)-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4f). 70%. m.p. > 300 °C. IR (KBr, υ cm−1), 3337 (NH st), 1656 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.56 (s, 3H, SCH3), 5.25 (s, 1H, H5), 7.03 (t, J = 8.79 Hz, 2H, Ho), 7.34 (d, J = 7.03 Hz, 2H, Hm), 7.79–7.83 (m, 2H, H8, H9), 7.90 (d, J = 6.78 Hz, 1H, H7), 8.03 (d, J = 7.28 Hz, 1H, H10), 9.64 (s, 1H, NH), 12.53 (s, 1H, NH). 13C NMR δ (ppm): 12.8 (SCH3), 35.7 (C5), 114.8 (C4a), 124.8 (C10), 128.6 (C7), 129.4 (Co), 129.5 (Cm), 131.1 (C8), 134.6 (C9), 141.2 (Ci), 145.5, 162.3, 178.6 (C=O). MS: (70 eV) m/z (%) = 419 (84, M+), 417, (25), 474 (50), 324 (100). Anal. Calcd. for C22H14FN3O3S C, 63.00; H, 3.36; N, 10.02; found C, 63.04; H, 3.34; N, 10.05.
5-(4-chlorophenyl)-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4g). 80%. m.p. > 300 °C. IR (KBr, υ cm−1), 3367 (NH st), 2684 (CH3 st), 1652, 1626 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.55 (s, 3H, SCH3), 5.23 (s, 1H, H5), 7.26 (d, J = 8.54 Hz, 2H, Ho), 7.32 (d, J = 8.54 Hz, 2H, Hm), 7.79–7.82 (m, 2H, H8, H9), 7.90 (d, J = 7.03 Hz, 1H, H7), 8.03 (d, J = 7.03 Hz, 1H, H10), 9.66 (s, 1H, NH), 12.53 (s, 1H, NH). 13C NMR δ (ppm): 12.7 (SCH3), 34.3 (C5), 125.6 (C10), 125.9 (C7), 128.1 (Co), 129.7 (Cm), 130.4 (C6a), 131.8 (C10a), 133.3, 134.7 (C9), 139.4 (C5a), 143.9 (Ci), 178.5 (C4), 179.2 (C=O), 181.6 (C=O). MS: (70 eV) m/z (%) = 437 (6, M+2), 436 (5.7, M+1), 435 (15, M+), 326 (6), 325 (18), 324 (100), 276 (13). Anal. Calcd. for C22H14ClN3O3S C, 60.62; H, 3.24; N, 9.64; found C, 60.64; H, 3.22; N, 9.68.
5-(4-bromophenyl)-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4h). 80%. m.p. > 300 °C. IR (KBr, υ cm−1), 3369 (NH st), 1658 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.56 (s, 3H, SCH3), 5.33 (s, 1H, H5), 7.54 (d, J = 8.28 Hz, 2H, Ho), 7.58 (d, J = 8.28 Hz, 2H, Hm), 7.77–7.82 (m, 2H, H8, H9), 7.89 (d, 1H, J = 7.27 Hz, H7), 8.03 (d, 1H, J = 7.03 Hz, H10), 9.72 (s, 1H, NH), 12.55 (s, 1H, NH). 13C NMR δ (ppm): 12.7 (SCH3), 35.0 (C5), 116.5 (C5a), 125.0, 125.6 (C10), 125.9 (C7), 127.8 (Co), 129.5 (Cm), 131.7 (C10a), 133.2 (C8), 134.7 (C9), 139.7 (Ci), 149.3 (C2), 162.2, 178.9 (C4), 179.0 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 469 (17), 467 (8), 325 (19), 324 (100), 276 (14). Anal. Calcd. for C22H14BrN3O3S C, 55.01; H, 2.94; N, 8.75; found C, 55.04; H, 2.98 N, 8.72.
3-methyl-2-(methylthio)-5-phenyl-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4i). Red solid. 81%. m.p. 277 °C. IR (KBr, υ cm−1), 3227 (N-H st), 1647 (C=O, st), 1522 (C=C, st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62 (s, 3H, SCH3), 3.31 (s, 3H, NCH3), 5.24 (s, 1H. CH) 7.10 (t, J = 7.03 Hz, 1H, Hp), 7.20 (t, J = 7.53 Hz, 2H, Hm), 7.29 (d, J = 7.28 Hz, 2H, Ho), 7.70–7.81 (m, 2H, H9, H8), 7.87 (d, J = 7.53 Hz, 1H, H7), 8.01 (d, J = 7.28 Hz, 1H, H10), 9.68 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 30.0 (NCH3), 35.3 (C5), 117.4 (C4a), 125.6 (C10), 125.8 (C7), 126.4 (Cp), 128.0 (Co), 128.6 (Cm), 130.3 (C6a), 131.8 (C10a), 133.2 (C8), 134.7 (C9), 139.2 (C5a), 145.0 (Ci), 149.7 (C2), 160.2 (C4, C=O), 161.6 (C12a), 179.1 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 414 (11, M+), 337 (100). Anal. Calcd. for C23H17N3O3S C, 66.49; H, 4.12; N, 10.11; found C, 66.47; H, 4.15; N, 10.12.
3-methyl-2-(methylthio)-5-(4-methylphenyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4j). Red solid. 75 %. m.p. 280 °C. IR (KBr, υ cm−1), 3234 (NH st), 1650 (C=0 st), (1521 C=C st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.18 (s, 3H, p-CH3), 2.63 (s, 3H, SCH3), 2.75 (s, 3H, NCH3), 5.22 (s, 1H, H5), 7.02 (d, J = 8.03 Hz, 2H, Hm), 7.17 (d, J = 8.03, 2H, Ho), 7.79–7.83 (m, 2H, H8, H9), 7.90 (d, J = 7.28 Hz, 1H, H7), 8.03 (d, J = 7.28 Hz, 1H, H-10), 9.72 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (p-CH3), 20.5 (SCH3), 29.8 (NCH3), 34.9 (C5), 117.6 (C4a), 125.6 (C10), 125.8 (C7), 127.8 (Co), 128.6 (Cm), 130.3 (C6a), 131.8 (C10a), 133.2 (C8), 134.7 (C9), 135.5 (C11a), 139.1 (C5a), 142.1 (Ci), 149.7 (C2), 160.2 (C=O), 161.4 (C12a), 179.2 (C=O), 181.6 (C=O). MS: (70 eV) m/z (%) = 429 (22, M+), 337 (100). Anal. Calcd. for C24H19N3O3S C, 67.12; H, 4.46; N, 9.78; found C, 67.15; H, 4.45; N, 9.76.
3-methyl-5-(4-methoxyphenyl)-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4k). Red solid. 70%. m.p. 282 °C. IR (KBr, υ cm−1), 3222 (NH st), 1647 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.63 (s, 3H, SCH3), 3.32 (s, 3H, NCH3), 3.84 (s, 3H, OCH3), 5.21 (s, 1H, H5), 6.77 (d, J = 8.79 Hz, 2H, Ho), 7.21 (d, J = 8.53 Hz, 2H, Hm), 7.78–7.82 (m, 2H, H8, H9), 7.89 (d, J = 8.54 Hz, 1H, H7), 8.03 (d, J = 8.53 Hz, 1H, H10), 9.66 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 29.9 (NCH3), 34.4 (C5), 54.9 (OCH3), 113.5 (Co), 117.7 (C4a), 125.6 (C10), 128.8 (Cm), 130.3 (C6a), 131.8 (Ci), 133.1 (C7), 134.7 (C9), 137.3 (C10a), 138.9 (C5a), 149.6 (C2), 157.8 (C2), 160.2 (C=O), 161.4 (C12a). MS: (70 eV) m/z (%) = 445 (45, M+), 337 (100). Anal. Calcd. for C24H19N3O4S C, 64.71; H, 4.30; N, 9.43; found C, 64.75; H, 4.27; N, 9.45.
3-methyl-2-(methylthio)-5-(3,4,5-trimethoxyphenyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4l). Brown solid. 80%. m.p. 263 °C. IR (KBr, υ cm−1), 3243 (NH st), 1648 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62 (s, 3H, SCH3), 3.33 (s, 3H, NCH3), 3.56 (s, 3H, OCH3), 3.67 (s, 6H, OCH3), 5.21 (s, 1H, H5), 6.57 (s, 2H, Ho) 7.78–7.82 (m, 2H, H8, H9), 7.91 (d, J = 7.28 Hz, 1H, H7), 8.03 (d, J = 7.28 Hz, 1H, H10), 9.60 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 29.9 (NCH3), 35.6 (C5), 55.8 (OCH3), 59.7 (OCH3), 105.6 (Co), 117.1 (C4a), 125.6 (C7), 125.8 (C10), 130.4 (C6a), 131.8 (C10a), 133.1 (C8), 134.6 (C9), 136.5 (C5a), 140.6 (Ci), 149.7 (C2), 152.5 (C11a), 161.5 (C12a), 179.1 (C=O), 181.6 (C=O). MS: (70 eV) m/z (%) = 505 (76, M+), 474 (15), 337 (100). Anal. Calcd. for C26H23N3O6S C, 61.77; H, 4.59; N, 8.31; found C, 61.79; H, 4.62; N, 8.34.
3-methyl-2-(methylthio)-5-(4-trifluoromethylphenyl)-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4m). Red solid. 75%. m.p. >300 °C. IR (KBr, υ cm−1), 3447 (NH st), 1687 (C=O st), 1519 (C=C st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.74 (s, 3H, SCH3), 2.88 (s, 3H, NCH3), 5.33 (s, 1H, H5), 7.39 (d, J = 7.03 Hz, 1H, H7), 7.56 (d, J = 7.06 Hz, 2H, Ho), 7.72–7.74 (d, J = 7.03 Hz, 2H, Hm), 7.80 (t, 1H, H8), 7.89 (t, 1H, H9), 8.03 (d, J = 7.03 Hz, 1H, H10), 9.88 (s, 1H, NH). 13C NMR δ (ppm): 14.5 (SCH3), 29.9 (NCH3), 35.7 (C5), 99.5 (C4a), 111.6 (C5a), 127.0 (C10), 127.3 (Co), 128.0 (C7), 128.9 (Cm), 133.2 (C8), 135.4 (C9), 139.7 (Ci), 149.8 (C2), 157.3, 159.2 (C6a), 160.2 (C=O), 166.1 (C12a), 178.3 (C=O), 198.50 (C6). MS: (70 eV) m/z (%) = 480 (18, M+), 337 (100), 437 (40). Anal. Calcd. for C24H16F3N3O3S C, 59.62; H, 3.34; N, 8.69; found C, 59.65; H, 3.30; N, 8.73.
5-(benzo[d][1,3]dioxol-6-yl)-3-methyl-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4n). Red solid. 75%. m.p. 254 °C. IR (KBr, υ cm−1), 3225 (NH st), 1647 (C=O st), 1519 (C=C st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.63 (s, 3H, SCH3), 3.34 (s, 3H, NCH3), 5.19 (s, 1H, H5), 5.91 (s, 2H, CH2), 6.74 (d, J = 6.79 Hz, 2H, aryl), 6.86 (s, 1H, aryl), 7.78–7.84 (m, 2H, H8, H9), 7.91 (d, J = 7.89 Hz, 1H, H7), 8.03 (d, J = 8.02 Hz, 1H, H10), 9.72 (s, 1H, NH). 13C NMR δ (ppm): 14.45 (SCH3), 29.9 (NCH3), 35.0 (C5), 100.7 (CH2), 107.8, 108.6 (C2′-C6′), 117.3 (C4a), 121.0 (C5′), 125.6 (C10), 125.9 (C7), 130.4 (C6a), 131.8 (C10a), 133.2 (C8), 134.7 (C9), 139.1 (C5a), 145.8 (Ci), 146.9 (C11a), 149.6 (C2), 160.3 (C=O), 161.6 (C12a), 179.2 (C=O), 181.6 (C6). MS: (70 eV) m/z (%) = 458 (38, M+), 337 (100). Anal. Calcd for C24H17N3O5S C, 62.74; H, 3.73; N, 9.15; found C, 62.71; H, 3.71; N, 9.18.
5-(4-fluorophenyl)-3-methyl-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4o). Red solid. 74%. m.p. 276 °C. IR (KBr, υ cm−1), 3237 (NH st), 1646 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.61 (s, 3H, SCH3), 3.30 (s, 3H, NCH3), 5.22 (s, 1H, H5), 7.01 (d, J = 7.30 Hz, 2H, Ho), 7.32 (t, J = 7.30 Hz, 2H, Hm), 7.77–7.82 (m, 2H, H8, H9), 7.87 (d, J = 7.98 Hz, 1H, H7), 8.01 (d, J = 7.03 Hz, 1H, H10), 9.73 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 29.9 (NCH3), 34.8 (C5), 114.6 (C4a), 114.8 (C6a), 117.1 (C10a), 125.6 (C10), 125.9 (C7), 129.8 (Co), 130.3 (Cm), 131.7 (C11a), 133.2 (C8), 134.7 (C9), 139.3 (Ci), 149.8 (C2), 179.1 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 441 (7, M+), 432 (22), 430 (42), 387 (75), 337 (100). Anal. Calcd. for C23H16FN3O3S C, 63.73; H, 3.72; N, 9.69; found C, 63.77; H, 3.76; N, 9.70.
5-(4-chlorophenyl)-3-methyl-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4p). Red solid. 71%. m.p. 283 °C. IR (KBr, υ cm−1), 3233 (NH st), 1648 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62 (s, 3H, SCH3), 3.31 (s, 3H, NCH3), 5.24 (s, 1H, H5), 7.24 (d, J =8.28 Hz, 2H, Ho), 7.32 (d, J = 8.28 Hz, 2H, Hm), 7.78–7.82 (m, 2H, H8, H9), 7.88 (d, J = 7.28 Hz, 1H, H7), 8.02 (d, J = 7.28 Hz, 1H, H10), 9.75 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 29.8 (NCH3), 35.1 (C5), 116.8 (C4a), 125.6 (C10), 125.9 (C7) 127.9 (Co), 129.8 (Cm), 133.2 (C8), 134.7 (C9), 139.5 (C5a), 143.9 (Ci), 149.7 (C2), 160.2 (C4), 161.8 (C12a), 179.1 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) 448 (12, M+), 337 (100). Anal. Calcd. for C23H16ClN3O3S C, 61.40; H, 3.58; N, 9.34; found C, 61.37; H, 3.57; N, 9.33.
5-(4-bromophenyl)-3-methyl-2-methylthio-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-4,6,11(3H)-trione (4q). Red solid. 76%. m.p. 264 °C. IR (KBr, υ cm−1), 3228 (NH st), 1650 (C=O st). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.62 (s, 3H, SCH3), 3.30 (s, 3H, NCH3), 5.20 (s, 1H, H5), 7.25 (d, J = 8.29 Hz, 2H, Ho), 7.37 (d, J = 8.53 Hz, 2H, Hm), 7.77–7.81 (m, 2H, H8, H9), 7.88 (d, J = 7.27 Hz, 1H, H7), 8.01 (d, J = 8.03 Hz, 1H, H10), 9.73 (s, 1H, NH). 13C NMR δ (ppm): 14.4 (SCH3), 29.8 (NCH3), 35.2 (C5), 99.4 (C4a), 116.7 (C5a), 119.5 (Cp) 125.5 (C10), 125.8 (C7), 130.2 (Co), 130.3 (C6a), 130.9 (Cm), 131.7 (C10a), 133.2 (C8), 134.7 (C9), 139.4 (Ci), 144.3 (C11a), 149.7 (C2), 160.1 (C4), 161.8 (C12a), 179.0 (C=O), 181.5 (C=O). MS: (70 eV) m/z (%) = 492 (9, M+), 337 (100). Anal. Calcd. for C23H16BrN3O3S C, 55.88; H, 3.26; N, 8.50; found C, 55.91; H, 3.29; N, 8.49.
1H NMR spectra and spectroscopic data for all compounds are included in the supplementary materials.