Modeling Coloration of a Radiochromic Film with Molecular Dynamics-Coupled Finite Element Method
Abstract
:1. Introduction
2. Materials and Methods
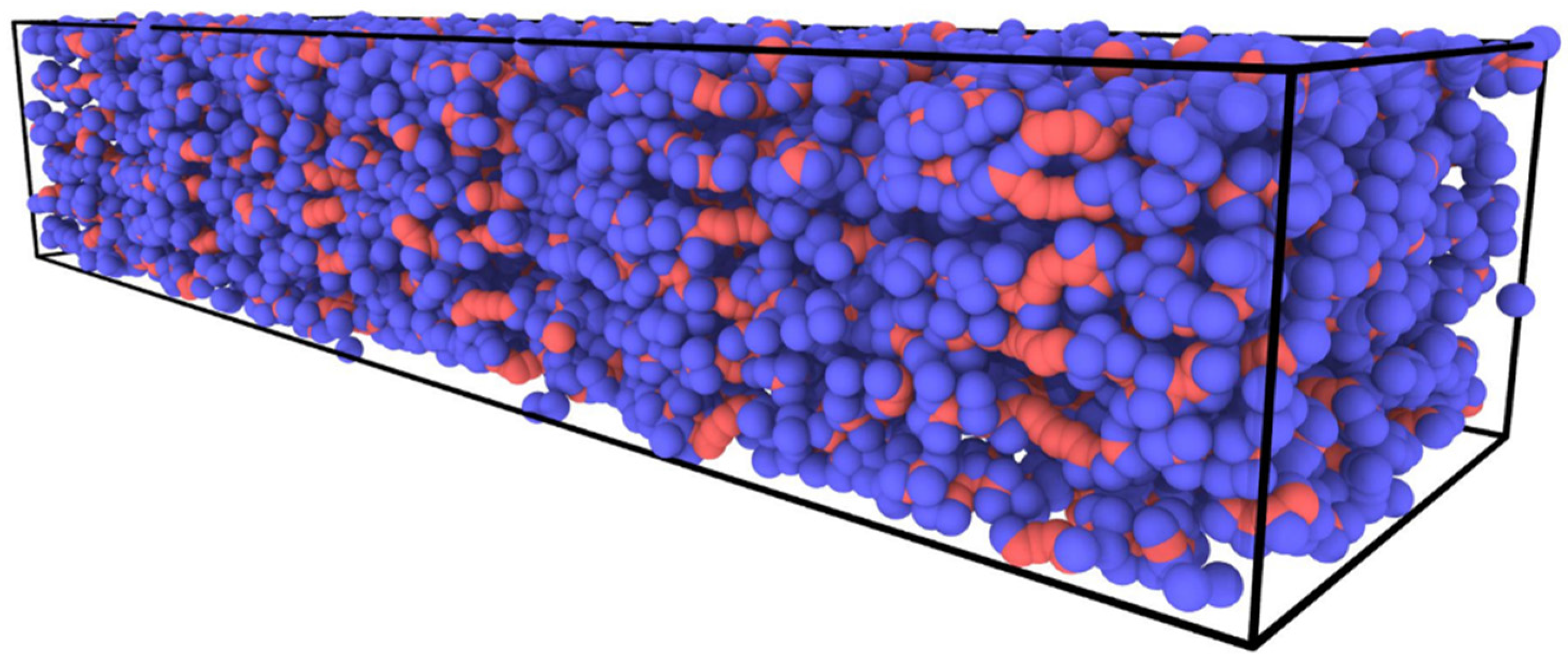
2.1. Atomic Scale Model
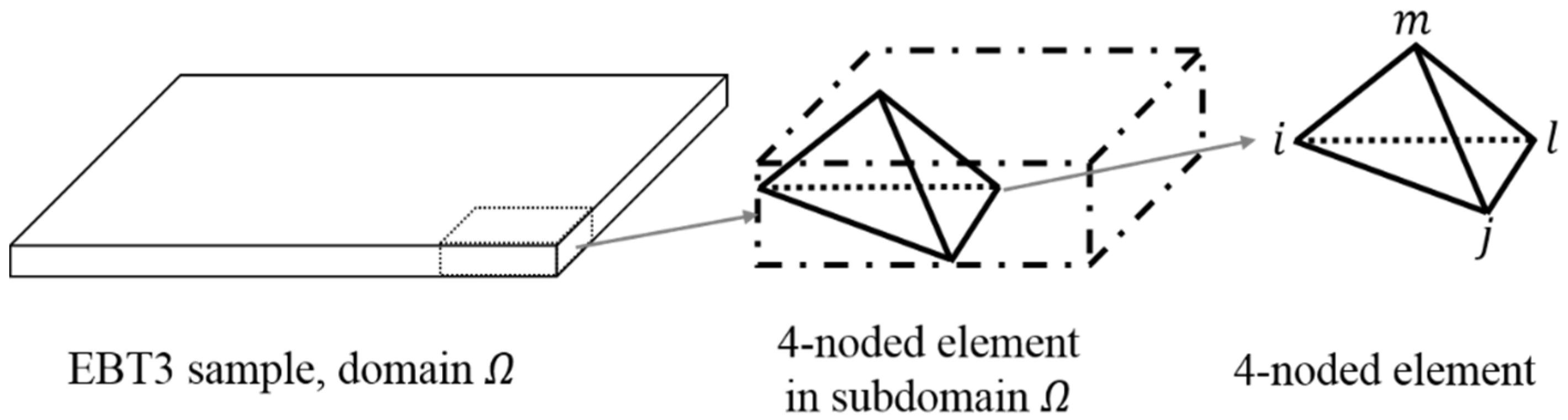
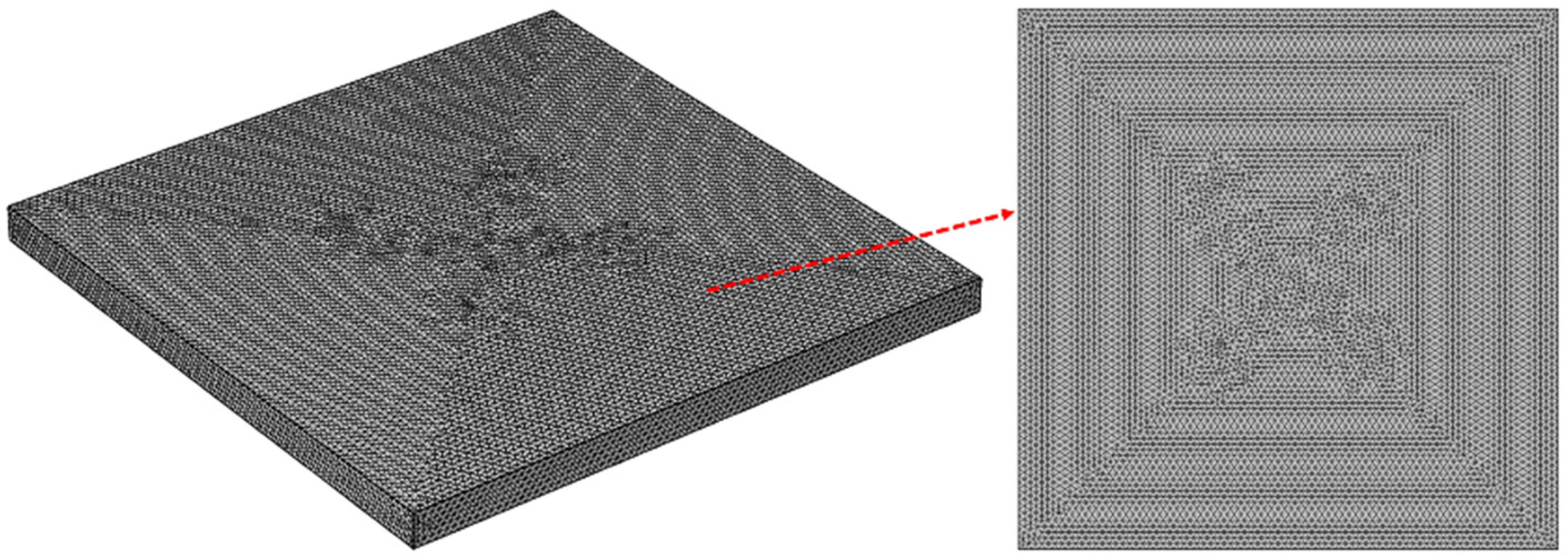
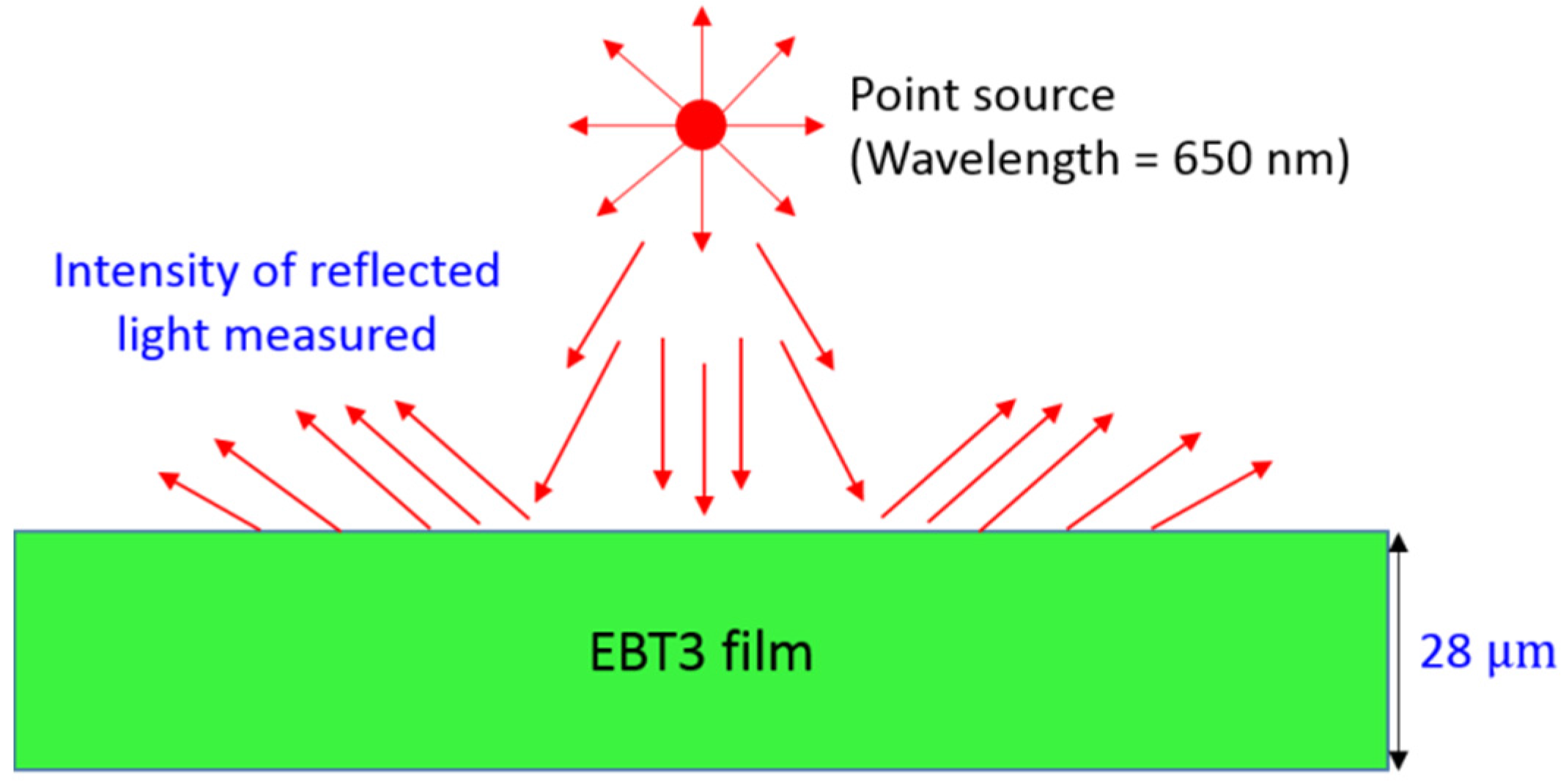
2.2. Macroscopic Scale Model
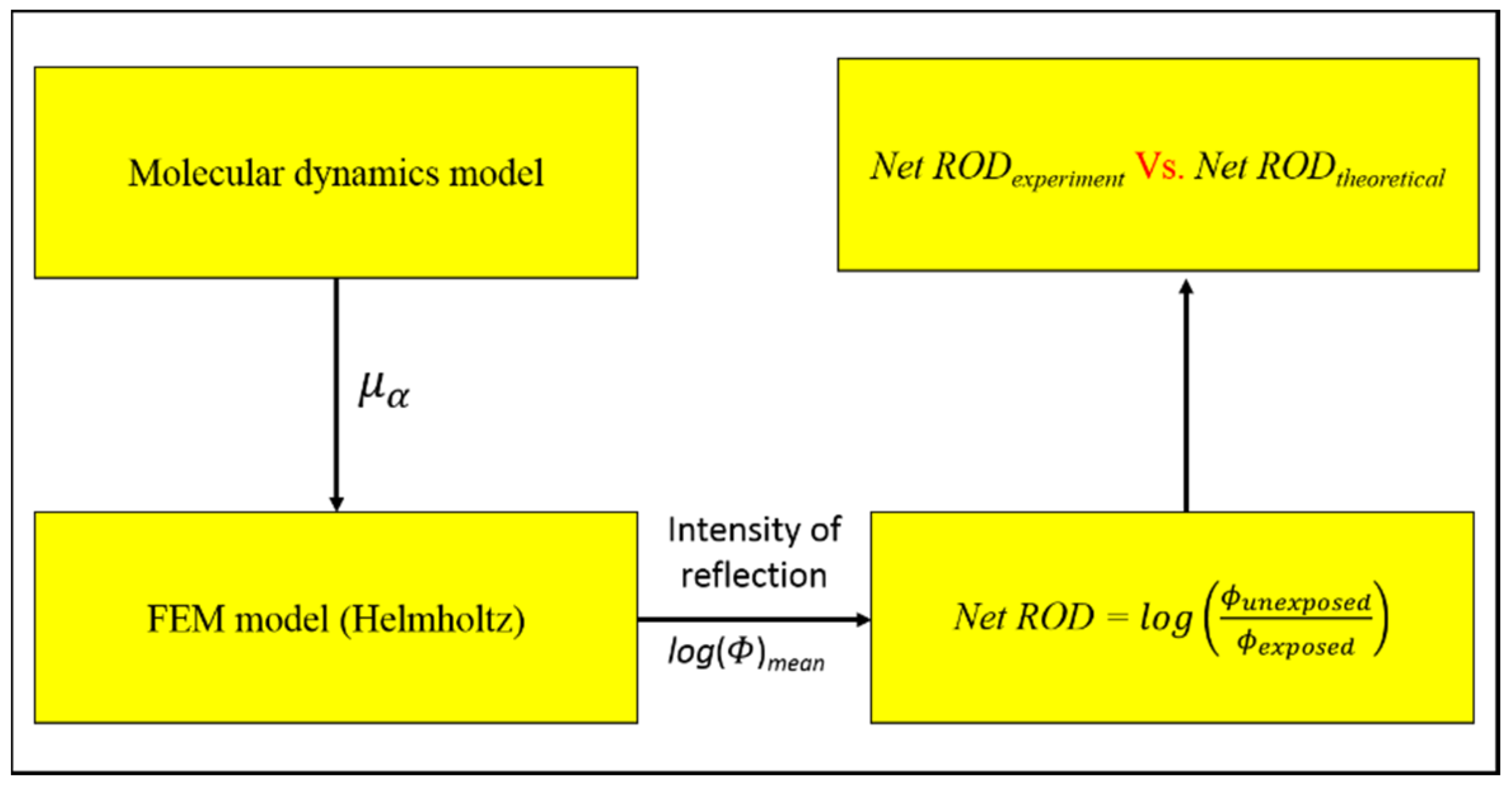
2.3. Computation Scheme
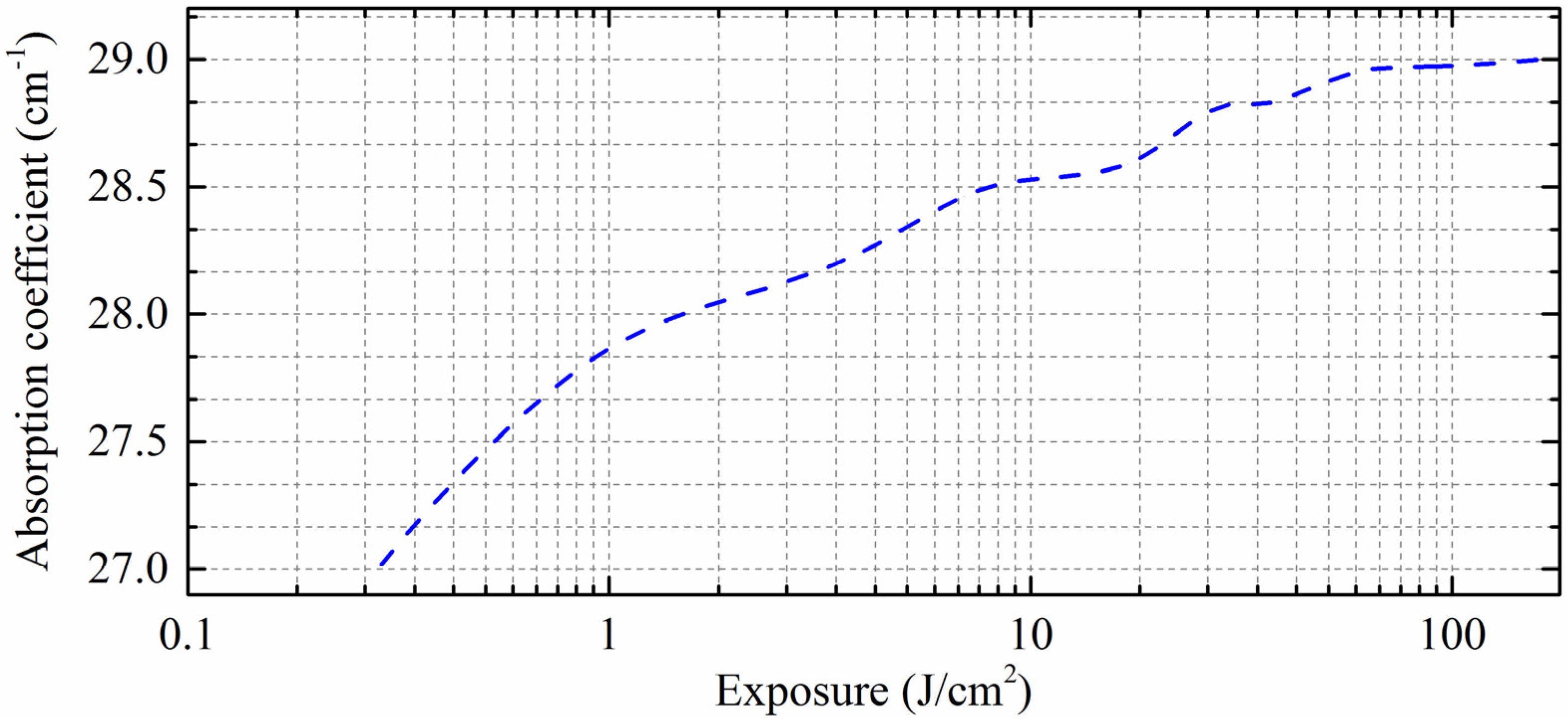
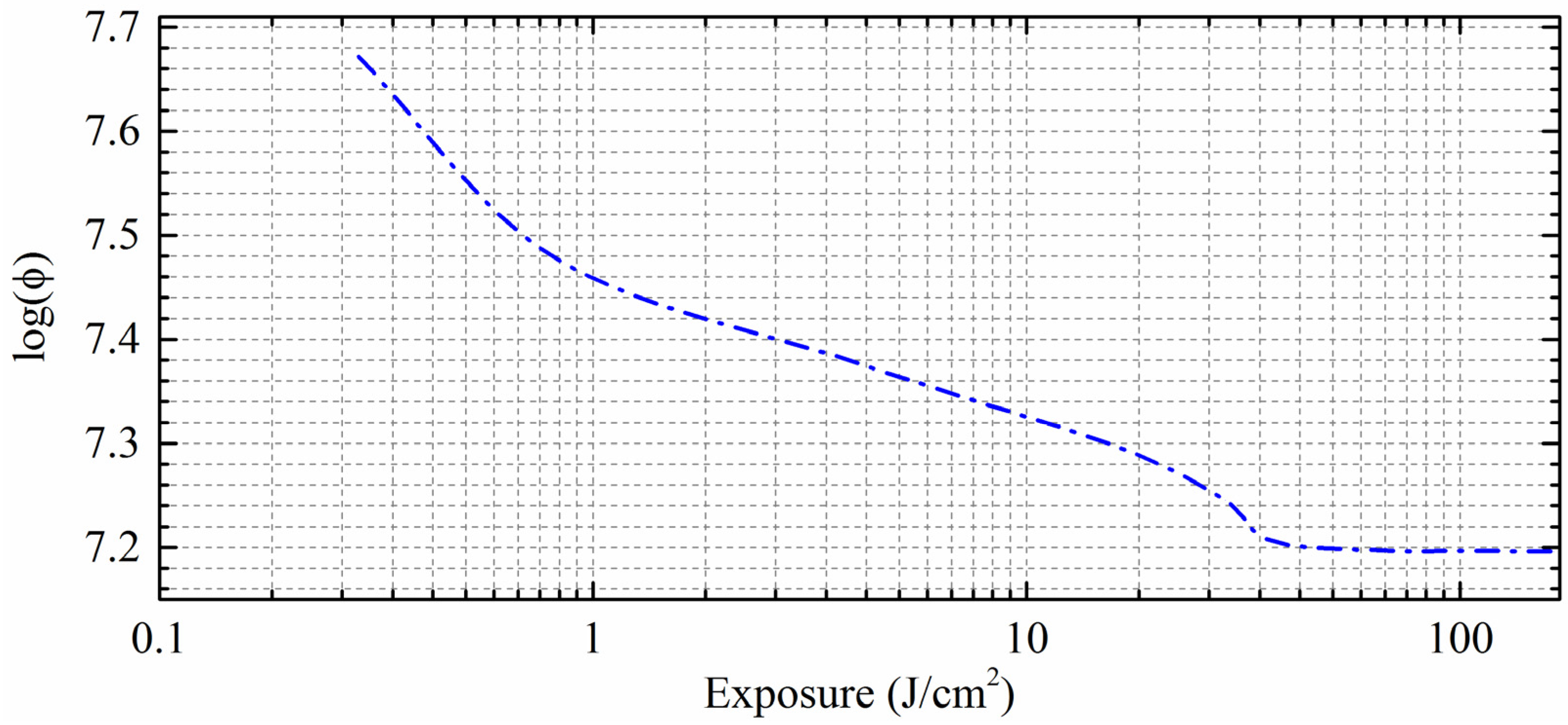
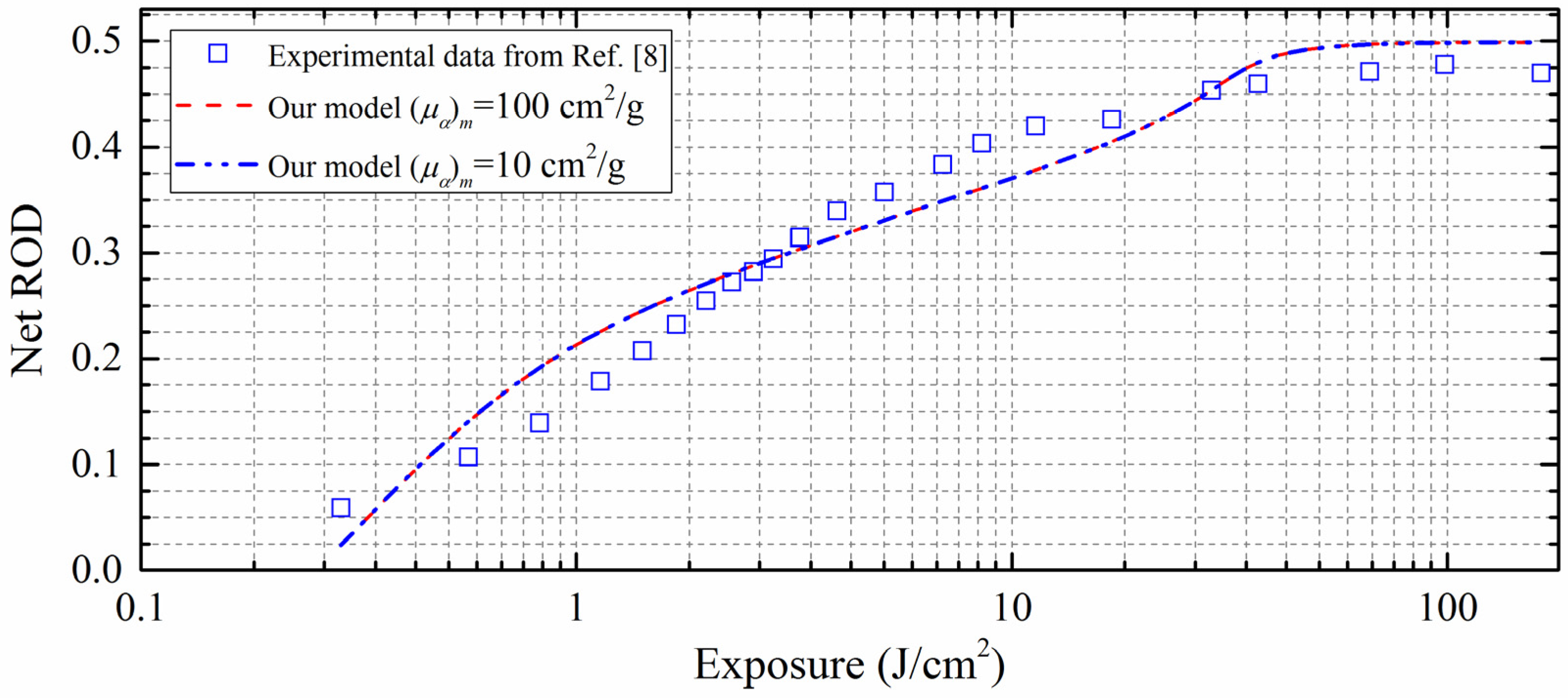
3. Results and Discussions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- McLaughlin, W.L.; Al-Sheikhly, M.; Lewis, D.F.; Kovács, A.; Wojnárovits, L. Radiochromic Solid-State Polymerization Reaction. In Irradiation of Polymers, Chapter 11; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 620, pp. 152–166. Available online: http://pubs.acs.org/doi/abs/10.1021/bk-1996–0620.ch011 (accessed on 10 September 2017).
- Butson, M.J.; Yu, P.K.N.; Cheung, T.; Metcalfe, P. Radiochromic film for medical radiation dosimetry. Mater. Sci. Eng. R 2003, 41, 61–120. Available online: http://www.sciencedirect.com/science/article/pii/S0927796X03000342 (accessed on 10 September 2017). [CrossRef]
- Cheung, T.; Butson, M.J.; Yu, P.K.N. Evaluation of a fluorescent light densitometer for radiochromic film analysis. Radiat. Meas. 2002, 35, 13–16. Available online: http://www.sciencedirect.com/science/article/pii/S1350448701002517 (accessed on 10 September 2017). [CrossRef]
- Butson, M.J.; Cheung, T.; Yu, P.K.N. Fluorescent light effects on FWT-60 radiochromic film. Phys. Med. Biol. 2005, 50, N209–N213. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/50/16/N02/meta (accessed on 10 September 2017). [CrossRef] [PubMed]
- Butson, M.J.; Cheung, T.; Yu, P.K.N.; Abatti, D.; Greenoak, G. Ultraviolet radiation dosimetry with radiochromic film. Phys. Med. Biol. 2000, 45, 1863–1868. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/45/7/311/meta (accessed on 10 September 2017). [CrossRef]
- Butson, E.T.; Cheung, T.; Yu, P.K.N.; Butson, M.J. Measuring solar UV radiation with EBT radiochromic film. Phys. Med. Biol. 2010, 55, N487–N493. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/55/20/N01/meta (accessed on 10 September 2017). [CrossRef] [PubMed]
- Butson, E.T.; Yu, P.K.N.; Butson, M.J. Solar ultraviolet radiation response of EBT2 Gafchromic, radiochromic film. Phys. Med. Biol. 2013, 58, N287–N294. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/58/21/N287/meta (accessed on 10 September 2017). [CrossRef] [PubMed]
- Chun, S.L.; Yu, P.K.N. Note: Calibration of EBT3 radiochromic film for measuring solar ultraviolet radiation. Rev. Sci. Instrum. 2014, 85, 106103. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25362462 (accessed on 10 September 2017). [CrossRef] [PubMed]
- Cheung, T.; Butson, M.J.; Yu, P.K.N. Experimental energy response verification of XR type T radiochromic film. Phys. Med. Biol. 2004, 49, N371–N376. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/49/21/N02/meta (accessed on 10 September 2017). [CrossRef] [PubMed]
- Cheung, T.; Butson, M.J.; Yu, P.K.N. Weak energy dependence of EBT Gafchromic film dose response in the 50 kVp—10 MVp X-ray range. Appl. Radiat. Isot. 2006, 64, 60–62. Available online: http://www.sciencedirect.com/science/article/pii/S0969804305002332 (accessed on 10 September 2017).
- Butson, M.J.; Cheung, T.; Yu, P.K.N. Measurement of energy dependence for XRCT Radiochromic film. Med. Phys. 2006, 33, 2923–2925. Available online: http://onlinelibrary.wiley.com/doi/10.1118/1.2219330/full (accessed on 10 September 2017). [CrossRef] [PubMed]
- Butson, M.J.; Cheung, T.; Yu, P.K.N. Measuring energy response for RTQA Radiochromic film to improve quality assurance procedures. Australas. Phys. Eng. Sci. 2008, 31, 203–206. Available online: https://link.springer.com/article/10.1007/BF03179345 (accessed on 10 September 2017). [CrossRef]
- Butson, M.J.; Yu, P.K.N.; Cheung, T.; Alnawaf, H. Energy response of the new EBT2 radiochromic film to x-ray radiation. Radiat. Meas. 2010, 45, 836–839. Available online: http://www.sciencedirect.com/science/article/pii/S1350448710000739 (accessed on 10 September 2017). [CrossRef]
- Butson, M.J.; Yu, P.K.N.; Cheung, T.; Inwood, D. Polarization effects on a high sensitivity radiochromic film. Phys. Med. Biol. 2003, 48, N207–N211. Available online: http://iopscience.iop.org/article/10.1088/0031–9155/48/15/401/meta (accessed on 10 September 2017). [CrossRef] [PubMed]
- Butson, M.J.; Cheung, T.; Yu, P.K.N. Scanning orientation effects on EBT Gafchromic film dosimetry. Australas. Phys. Eng. Sci. 2006, 29, 281–284. Available online: https://link.springer.com/article/10.1007/BF03178579 (accessed on 10 September 2017). [CrossRef]
- Alnawaf, H.; Butson, M.J.; Cheung, T.; Yu, P.K.N. Scanning orientation and polarization effects for XRQA Radiochromic film. Phys. Med. 2010, 26, 216–219. Available online: http://www.sciencedirect.com/science/article/pii/S1120179710000050 (accessed on 10 September 2017). [CrossRef] [PubMed]
- Yu, P.K.N.; Butson, M.J.; Cheung, T. Does mechanical pressure on radiochromic film affect optical absorption and dosimetry? Australas. Phys. Eng. Sci. 2006, 29, 285–287. Available online: https://link.springer.com/article/10.1007/BF03178580 (accessed on 10 September 2017). [CrossRef]
- Chan, P.M.; Ng, C.Y.P.; Shahmohammadi Beni, M.; Law, S.S.; Yu, K.N. Auto-development issue in quality assurance of biological X-ray irradiator using Gafchromic EBT3 film. Results Phys. 2017, 7, 444–445. Available online: http://www.sciencedirect.com/science/article/pii/S2211379716307471 (accessed on 10 September 2017). [CrossRef]
- Shahmohammadi Beni, M.; Ng, C.Y.P.; Krstic, D.; Nikezic, D.; Yu, K.N. Conversion coefficients for determination of dispersed photon dose during radiotherapy: NRUrad input code for MCNP. PLoS ONE 2017, 12, e0174836. Available online: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0174836 (accessed on 10 September 2017). [CrossRef] [PubMed]
- Girard, F.; Bouchard, H.; Lacroix, F. Reference dosimetry using radiochromic film. J. Appl. Clin. Med. Phys. 2012, 13, 339–353. Available online: http://onlinelibrary.wiley.com/doi/10.1120/jacmp.v13i6.3994/full (accessed on 10 September 2017). [CrossRef] [PubMed]
- MUMPS Solver. Available online: http://mumps.enseeiht.fr/ (accessed on 10 September 2017).
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. Available online: http://aip.scitation.org/doi/abs/10.1063/1.481208 (accessed on 10 September 2017). [CrossRef]
- Arlett, P.L.; Bahrani, A.K.; Zienkiewicz, O.C. Application of finite elements to the solution of Helmholtz’s equation. Proc. Inst. Electr. Eng. 1968, 115, 1762–1766. Available online: http://ieeexplore.ieee.org/document/5249030/ (accessed on 10 September 2017). [CrossRef]
- Shahmohammadi Beni, M.; Yu, K.N. Computational Fluid Dynamics Analysis of Cold Plasma Plume Mixing with Blood Using Level Set Method Coupled with Heat Transfer. Appl. Sci. 2017, 7, 578. Available online: https://www.mdpi.com/2076–3417/7/6/578/htm (accessed on 10 September 2017). [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beni, M.S.; Krstic, D.; Nikezic, D.; Yu, K.N. Modeling Coloration of a Radiochromic Film with Molecular Dynamics-Coupled Finite Element Method. Appl. Sci. 2017, 7, 1031. https://doi.org/10.3390/app7101031
Beni MS, Krstic D, Nikezic D, Yu KN. Modeling Coloration of a Radiochromic Film with Molecular Dynamics-Coupled Finite Element Method. Applied Sciences. 2017; 7(10):1031. https://doi.org/10.3390/app7101031
Chicago/Turabian StyleBeni, Mehrdad Shahmohammadi, D. Krstic, D. Nikezic, and K.N. Yu. 2017. "Modeling Coloration of a Radiochromic Film with Molecular Dynamics-Coupled Finite Element Method" Applied Sciences 7, no. 10: 1031. https://doi.org/10.3390/app7101031
APA StyleBeni, M. S., Krstic, D., Nikezic, D., & Yu, K. N. (2017). Modeling Coloration of a Radiochromic Film with Molecular Dynamics-Coupled Finite Element Method. Applied Sciences, 7(10), 1031. https://doi.org/10.3390/app7101031



_Kwan_Ngok_Yu.png)
