1. Introduction
Helicobacter pylori (
H. pylori) is a common bacterium that was first identified in 1983 [
1].
H. pylori cells are adapted to live in the harsh, acidic environment of the stomach and their spiral shape allows them to penetrate the stomach lining.
H. pylori colonizes the stomachs of approximately 50% of the human population and contributes to overt gastric disease, peptic inflammation, and ulceration.
H. pylori also have an etiological relationship with gastric cancer [
2]; there is a gut microbe-host relationship that exists between
H. pylori and the human or animal gut. The ability of
H. pylori to interact with cells within the gastric epithelium is greatly influenced by its motility [
3,
4].
In practice, first-line therapies fail 10%–20% of the time [
5,
6], in part due to antibiotic resistance and poor compliance with complicated dosing schedules [
7]. Motility in
H. plyori is essential for initiating and maintaining infections in humans and animal models [
8]. Therefore, motility is a major virulence factor, and studies have reported that non-motile
H. pylori cells are less virulent [
9]. Therefore, chemicals that modulate motility play an important role in virulence and are able to affect the severity of symptoms during
H. pylori infection [
9].
H. pylori cells utilize multiple flagella to move through the gastric environment, a process which is governed by chemotaxis (typically towards areas of lower pH) [
10,
11].
Chemotaxis is defined as the preferential movement towards more favorable chemical environments. In the growth cycle of
H. pylori within the gastric mucosal layer, chemotaxis-directed motility is a remarkable characteristic [
9]. Chemotaxis helps
H. pylori to establish and maintain infection in animal models [
10]. Although a significant amount of work has been done to measure chemotaxis and general motility in
H. pylori, there is no consensus on how these processes work during infection. For example, Abdollahi and Tadjrobehkar [
9] and Worku
et al. [
12] used very similar experimental methods, but obtained opposite findings in regards to the response of
H. pylori to aspartate. Using
in vivo models, Williams
et al. [
13] demonstrated that
H. pylori uses chemotaxis to guide itself to the stomach epithelium. Many other studies have assessed the response of
H. pylori to gradients of physiologically relevant compounds
in vitro [
14,
15]. However, the experimental conditions in those studies do not accurately mimic the conditions present
in vivo. In particular, these models do not incorporate a viscoelastic medium or pH gradient, which are important environmental conditions relevant to infection [
16].
It has been demonstrated that the pathogenic mechanisms of
H. pylori are influenced by urea. Urease activity allows
H. pylori cells to survive in an acidic gastric environment by creating a “cloud” of ammonia around the bacterium that helps to neutralize the pH of the surrounding environment, thus playing an important role in virulence and pathogenesis of gastric and peptic ulcers; ammonia generation leads to the destruction of the gastric epithelium [
17,
18]. Furthermore, studies show that cytoplasmic urease plays a critical role in the chemotactic behaviors of
H. pylori [
19,
20].
Traditionally, microbes have been studied in the laboratory as cultures of isolated species using conventional microbiological techniques, in part due to the complexities involved in designing and building physiologically relevant environments. Microfluidics overcomes the limitations of traditional methods, and offers techniques for cultivating and investigating microbes in a more clinically realistic and physiologically relevant environment. Microfluidic devices have been become powerful tools used in cell biology applications, including cell culture [
21] and handling and analysis [
22,
23]. These devices allow researchers to better mimic
in vivo physiological conditions due to their unique properties of the microenvironment control, customizable microstructure, and perfusion controllability [
24]. Microfluidics provides a large surface area to volume ratio compared to standard cell culture plates due to their micrometer dimensions. The small dimensions of the microchannels facilitate the formation of laminar flow, such that the liquid–liquid interface mass transport is realized by diffusion. This feature also establishes microfluidic systems as tools for mimicking the
in vivo environment.
In addition, single cell
H. pylori chemotaxis has not been studied mainly due to the challenges associated in culturing the cells under anaerobic conditions. Although preferring microaerophilic conditions,
H. pylori has the ability to grow equally well
in vitro under microaerobic or aerobic conditions, if cultured at high bacterial concentrations [
25]. With the advent of microfluidic technology and with instrumentation, it is possible to create a conducive microenvironment to study the chemotaxis of cells in their natural state. Microfluidics is reportedly much more sensitive when measuring chemotaxis in
Escherichia coli than the capillary and soft agar assays traditionally used with
H. pylori [
26]. The same order of the size of the cells and microchannel enables to observe the cells and the subtle movements of single cells at high resolution. Currently there are no reports where microfluidics has been used to study
H. pylori. In this study, the single cell swimming dynamics of
H. pylori were investigated using a combination of imaging and microfluidic systems. Results generated with our methodology work towards improving the understanding of the behavior of this pathogenic bacterium and also provide a promising way to investigate urease inhibition with regards to the treatment of
H. pylori infection.
2. Materials and Methods
2.1. Bacterial Strains and Cell Culturing
H. pylori strain SS1 (provided as a gift from The Hospital for Sick Children, Toronto, ON, Canada) was streaked on 5% horse blood agar plates (Fisher Scientific, Burlington, ON, Canada). Plates were then incubated in a jar filled with premixed gas (5% O2, 10% CO2, balance N2) at 37 °C for four days. A single colony was inoculated into a test tube with Brucella broth and incubated in the premixed gas filled jar at 37 °C for one to four days. Before an assay, the bacteria culture was washed with chemotaxis buffer and pelleted by centrifugation (SciLogex D3024, Berlin, CT, USA) at 3000 rpm for 3 min, a total of twice. The pellet was then gently resuspended in chemotaxis buffer for further use. Potassium phosphate buffer of 10 mM (pH 7.0) served as the chemotaxis buffer and washing solution.
All of the chemicals (urea, sodium and potassium bicarbonate) and Brucella culture medium used for bacterial growth were purchased from Sigma Aldrich (Oakville, ON, Canada).
2.2. Microfluidic Platform and Device Fabrication
The details for the design of the microfluidic device used in this study have been described in our previous study [
27]. Briefly, the microfluidic chip consists of one central (chemotaxis) channel (415 μm in width) with a viewing area (200 μm in width) in the middle and two side channels (280 μm in width) for feeding solution. The side channels have narrow neck of 40 μm in the middle. The “neck” is connected with the viewing area by hundreds of nanoporous structure membranes (800 nm in diameter) that are used for solution diffusion. The depth of the microfluidic chip is 10 μm.
The silicon and polymer master was fabricated using a combination of electron beam lithography, anisotropic silicon etching, and cross linking of the polymer [
26]. The PDMS microfluidic chip was made by using a master mold and standard soft lithography. Briefly, a degassed mixture of PDMS prepolymer and curing agent (10:1
w/
w, Sylgard, Dow Corning, Burlington, ON, Canada) was poured over the top of the silicon mold and baked for 4 h at 75 °C to harden the PDMS. The PDMS replica was then peeled off the master mold, punched to form the inlets and outlets, and bonded onto a glass slide (25 × 75 × 1 mm, VWR International, Suwanee, GA, USA) after plasma cleaning (Harrick Plasma, Ithaca, NY, USA) for 40 s. Finally, the bonded chip was baked for 30 min at 60 °C to solidify the bond.
The gradient generation test of the microfluidic device was also carried out as described previously [
27]. Two fluorescent dyes (5 μm Fluorescein and 5 μm Texas Red) were used in the test to determine the diffusion rate. A rapid concentration gradient was clearly observed using the liquid–liquid interface.
2.3. Experimental Setup
In each assay, a blocking step was performed to avoid the nonspecific binding of bacteria to the surfaces by filling the microchannel with 0.1% bovine serum albumin (BSA) for 10 min and washing with distilled water.
To begin, we determined the baseline (control) motility of
H. pylori. After blocking, bacterial solution was manually dispensed into the central channel with a 1 mL syringe. Chemotaxis buffer was dispensed into the side channels using a syringe pump (Chemyx Fusion Touch, Stafford, TX, USA) to maintain a constant flow rate of 30 μL per hour. After the viewing area filled with bacterial solution, the chip stood for a couple of minutes to stop mechanical flow so that only bacterial self-propelled motion would be present in the viewing chamber. The inlet and outlet of the central channel was then plugged with PDMS stoppers. A video of the viewing area was recorded. Similarly, for the chemotaxis assay, after filling the chamber with bacterial solution, chemotaxis buffer and urea solution were dispensed into the side channels using a syringe pump (Chemyx Fusion Touch) to maintain a constant flow rate of 30 μL per hour. A video of the viewing chamber was recorded
(Supplementary Video 1 online).
2.4. Imaging Processing and Analysis
Videos of the viewing chamber were taken with a Nikon Eclipse Ti inverted microscope mounted with a Nikon DS-QiMc microscope camera and a Nikon NIS Elements BR version 4.13 software (Nikon Instruments Inc., Melville, NY, USA). The video was captured at 640 × 512 resolution with a 10 ms exposure, 2× analog gain, and 15 fps in 40 s-long sections.
Image processing and data analysis was carried out using the public domain program ImageJ (NIH, Bethesda, MD, USA) (The taken video was first divided into 10 s clips that were processed individually. Three independent tests were conducted and 20–30 bacteria in each were selected for tracking in each test. Tracking was performed on single bacteria, frame by frame, using the Manual Tracking Plugin (Fabrice Cordelires, Institut Curie, Orsay, France). Data obtained were then exported to the Chemotaxis and Migration Tool (Ibidi Software, Munich, Germany) for further analysis. The cell movement was characterized by microscopic imaging, cellular velocity analysis, and directness analysis, which refers to the linearity of cell trajectories calculated by comparing the length of the line segment connecting the starting and ending points of the cell's trajectory to the total length travelled by the cell.
3. Results and Discussion
Chemotaxis aids
H. pylori in nutrient acquisition, allowing it to persist within the mammalian host [
15]. Urea is synthesized in the liver, circulated in the bloodstream, and diffuses into the gastric lumen via capillaries, which generates concentration gradients of urea across the mucous layer [
19]. In this study, we conducted two types of chemotaxis assays: spatial assays observing bacterial migration to a particular area in response to a urea gradient and temporal assays monitoring the flagellar switching of a single cell. The most popular assay to investigate the migration of
H. pylori is the soft-agar method [
28], which requires rich media and serum to support growth and does not evaluate the effect of a single chemotactic agent; this method assesses only general chemotactic ability [
15]. Using our microfluidic device, a concentration gradient of urea was generated in the microchannel [
27], allowing us to examine the response of
H. pylori to a single chemoeffector. Another advantage of our microfluidic device is that the small scale of the observation area allows for the surveillance of the subtle movements of a single cell at high resolution. We thus investigated the chemotactic activities of a single bacterium, including its complex bacterial swimming behavior and the frequency and type of directional changes or tumbles.
In the viewing chamber, it is required to have no overall flow other than the self-propelled motion of the cells. In our study, we evaluated that the cells were in such an environment by analyzing the forward migration index of the swimming cells in the chamber.
Table S1 shows an example of the forward migration index of
H. pylori cells in an individual test for baseline, 10 mM and 100 mM urea. The low values indicated that no movement caused by the environment and any movement recorded was caused by the cells’ own propulsion.
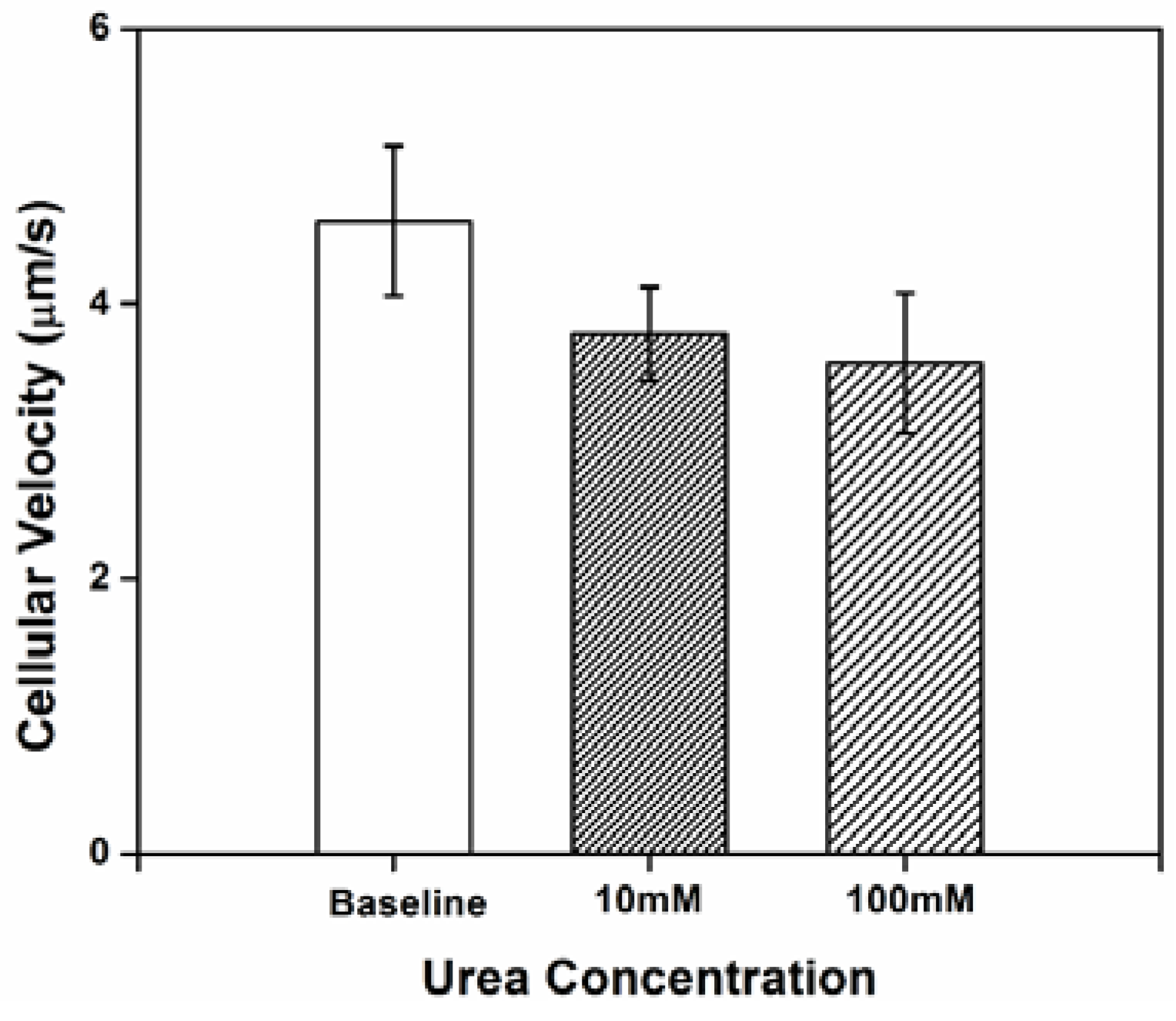
3.1. Impact of Urea on H. pylori Cellular Velocity
Three independent tests were conducted and 20–30 cells in each test were randomly selected and tracked for at least 10 s to analyze individual bacterial swimming behavior. Cellular velocity is shown in
Figure 1. At baseline (no urea added), the cells exhibited a low velocity. The relative limited motility of
H. pylori in chemotaxis buffer may be attributed to the lack of an adequate internal energy store to power the flagella [
28]; chemotaxis buffer is nutrient poor. The relatively large standard deviation for cellular velocity reflects the instability in
H. pylori motility, which might be partially the result of a frameshift mutation-prone repetitive sequence in fliP, the flagellar biosynthetic gene [
29]. A decrease in the cellular velocity was observed (compared to baseline) when concentration gradients of urea were generated in the microenvironment. However, there were no significant differences in velocity between cells exposed to 10
vs. 100 mM urea.
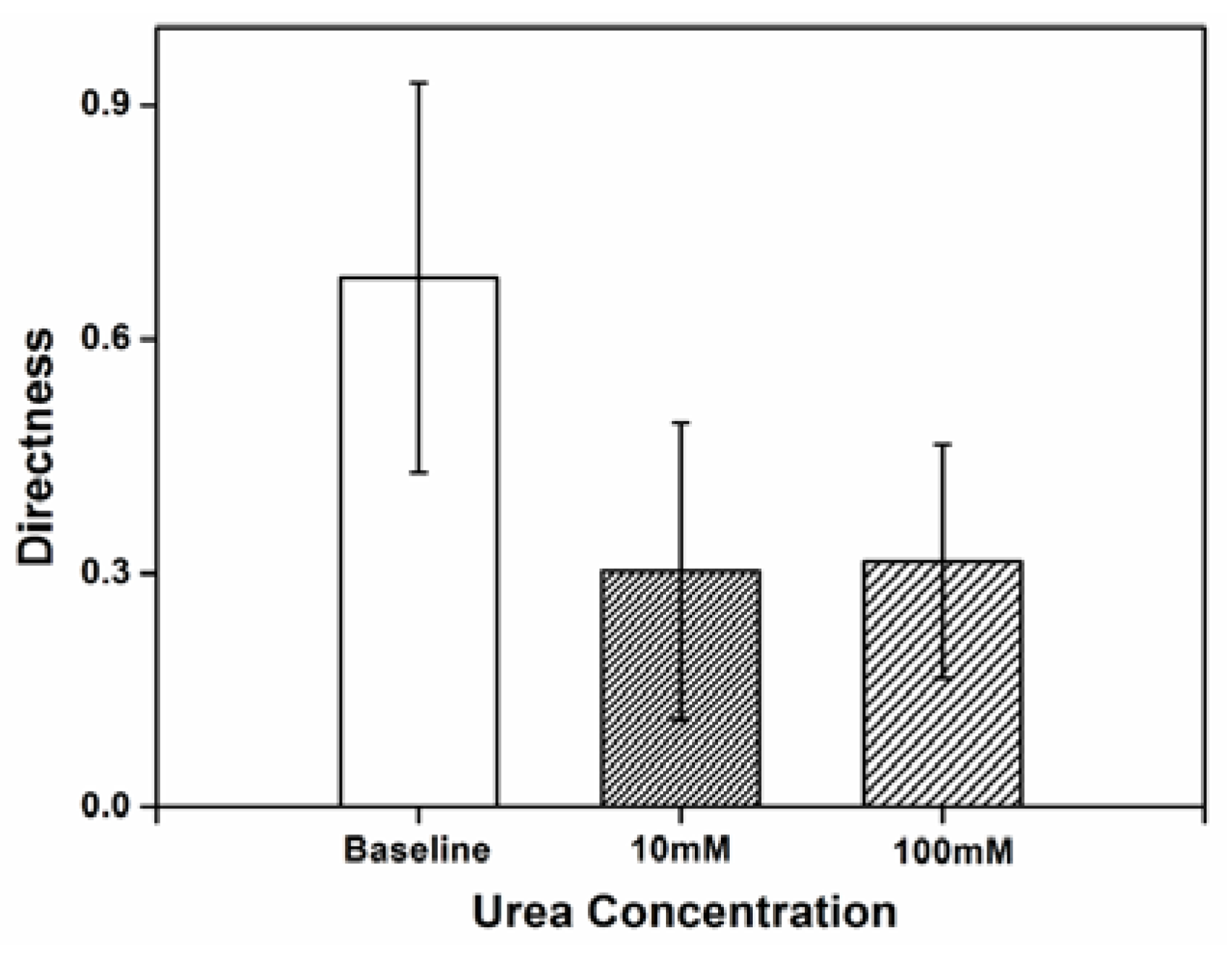
3.2. Impact of Urea on H. pylori Directness
Directness was used as a measure of how often the cell changed its traveling path and the randomness of movement, comparing the actual distance covered by the cell to the straight line distance between the starting point and the end point of the cell’s movement. The high directness value obtained reflects the inherently random movement of the
H. pylori cells in chemotaxis buffer.
Figure 2 shows the directness of
H. pylori movement when exposed to concentration of 10 and 100 mM urea within the microenvironment. Compared to baseline, the randomness of
H. pylori cellular movement decreases with exposure to urea. The twirling and tumbling behaviors, which refers to the rotating on cell’s center of mass and rolling towards any directions, exhibited in chemotaxis buffer without urea are practically absent when urea is present. Sparse tumbling was observed with urea, but this was only in the direction of the urea source.
3.3. Impact of Urea on the Type and Frequency of H. pylori Movements
The cell dynamics and mechanics of
H. pylori during motility in chemotaxis buffer were also characterized by analyzing the video recorded by the microscope at slower acquisition recording rates. Most bacterial cells exhibited twirling and tumbling behavior in chemotaxis buffer.
Figure 3 gives an example of the typical motility behavior of a single cell. Three dimensional rotations were observed, as indicated by arrows shown in the images.
The chemotactic behavior of
H. pylori, dictated by flagellar locomotion, is a crucial factor in the bacterial colonization process [
19].
H. pylori cells normally have 2–6 sheathed flagella that the bacterium uses to move to and within its ecological niche. The
H. pylori flagella consist of three structural components: a basal body, an external helically shaped filament, and a hook. These components contain the proteins required for flagellar rotation and chemotaxis, which function as a propeller when rotated at its base [
30]. Nakamura
et al. [
19] have suggested that
H. pylori utilize a proton motive force for flagellar motility. It has also been reported that the velocity of
H. pylori cells decreases in response to increasing concentrations of urea, likely due to lack of protons that would be required to neutralize excess ammonia when urea is present in a more neutral (pH 4.5–7) environment [
31].
In vitro tests show that
H. pylori is acid-sensitive, but is protected from acid (pH < 4.0) by the ammonia released during urea hydrolysis, which is associated with its inherent urease activity [
32]. Therefore,
H. pylori can survive in the presence of urea when exposed to acid
in vitro. In keeping with these observations, we found that
H. pylori exhibits restricted motility in a near neutral environment.
H. pylori is generally thought to have four chemoreceptors: TlpA, TlpB, TlpC, and T1pD Proteins TlpA, TlpB, and TlpC span the inner membrane, which means that part of the protein is located within the periplasm, which is the space between the two membranes of Gram-negative organisms, while another part of the protein is located in the cytoplasm; TlpD is localized completely within the cytoplasm [
15]. In
H. pylori, four different regulatory proteins, CheA, CheY, CheW, and CheZ, are responsible for the transduction of the signal that initiates flagellar motor. When compounds, such as urea bind to the Tlp receptor, allosteric changes in the receptor propagate to a CheY kinase, called CheA. CheA then catalyzes the transfer of a phosphate from adenosine triphosphate (ATP; cellular energy source) to a cytoplasmic protein, CheY; the phosphorylated form of CheY is called CheY-P. CheY-P is able to interact with the motor at the base of the flagellum. The flagellar motor essentially operates as a sensor for CheY-P, whose output is a tendency toward rotation of the flagellum leading to cell tumbling [
30]. The interaction of physically proximal receptors may be an important factor in deciding how cells react to even small changes in chemoattractant concentrations.
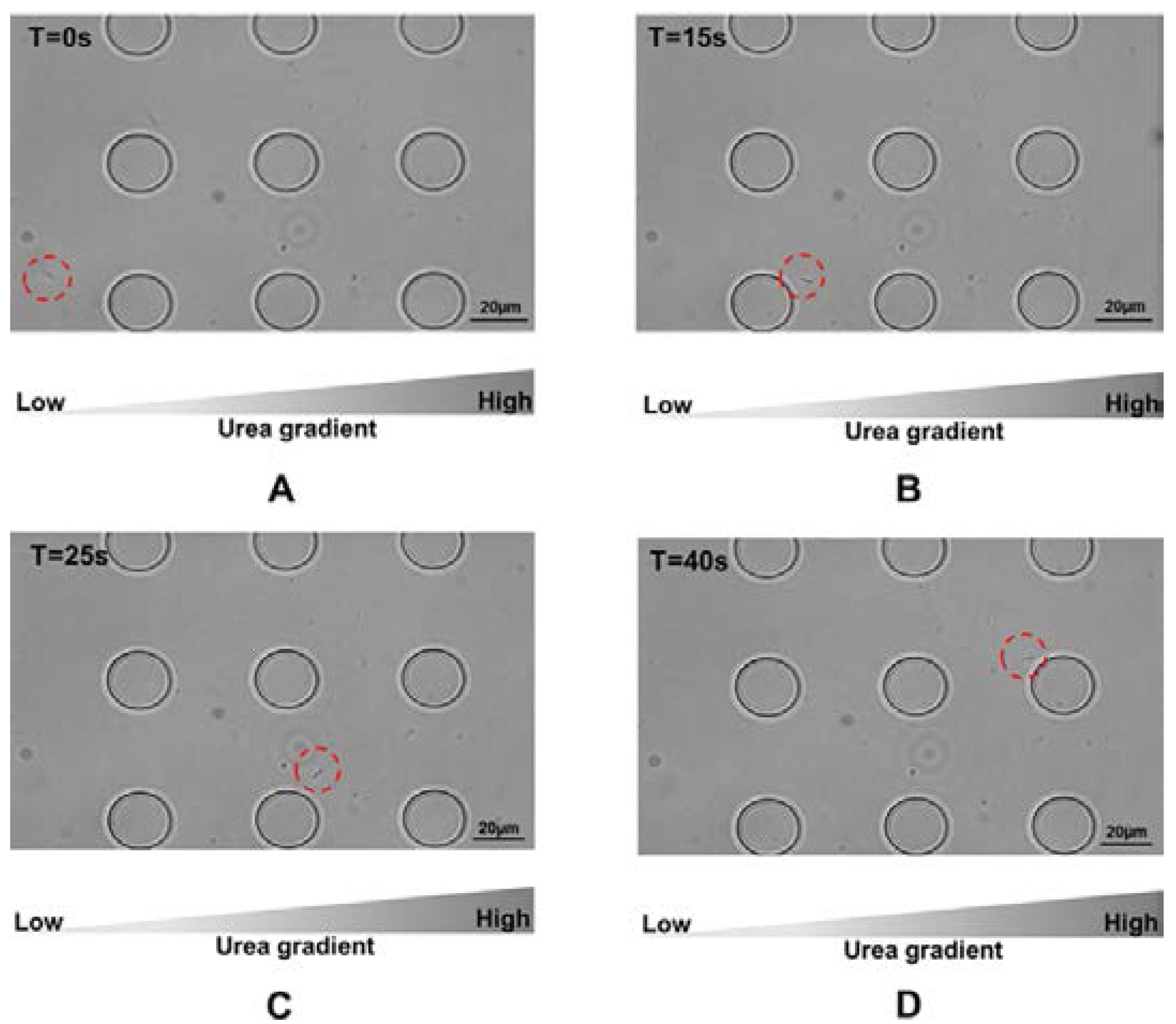
In our study,
H. pylori cells were attracted to urea.
Figure 4 gives an example how the cells moved towards the side of the microfluidic device where the concentration of urea was greatest. A video was provided in the
supplementary to give an example that cells were attracted by urea at a concentration of 100 mM. The chemotactic activities exhibited by
H. pylori presented in response to urea suggest that the proton motive force generated in the process is adequate for swimming in chemotaxis buffer. As discussed, adaptive mechanisms are responsible for the chemotactic movement toward attractants [
30,
33]. In
H. pylori, chemoattractant sensing is mediated by methyl-accepting chemotaxis proteins (MCP), which contain a large cytoplasmic signaling and adaptation domain. When an attractant binds to an MCP, the activity of CheA is inhibited, resulting in increased methylation of the Tlp receptor by CheR. Consequently, adaptation of the receptor returns signaling to a state similar to that prior to stimulus challenge, which generates periodical “running” induced by dephosphorylated CheA and CheY [
30]. Attractant-bound MCP may suppress CheA activity, reducing the pools of CheY-P, which may explain why the
H. pylori exhibited less frequent tumbling when exposed higher concentrations of urea. Ultimately, this may help to explain how
H. pylori senses concentration gradients of urea to move towards higher concentrations. In combination with the restricted motility discussed earlier, this helps to explain the lower directness (reflecting less random movement).