Intrant ELISA: A Novel Approach to Fabrication of Electrospun Fiber Mat-Assisted Biosensor Platforms and Their Integration within Standard Analytical Well Plates
Abstract
:1. Introduction
2. Materials and Methods
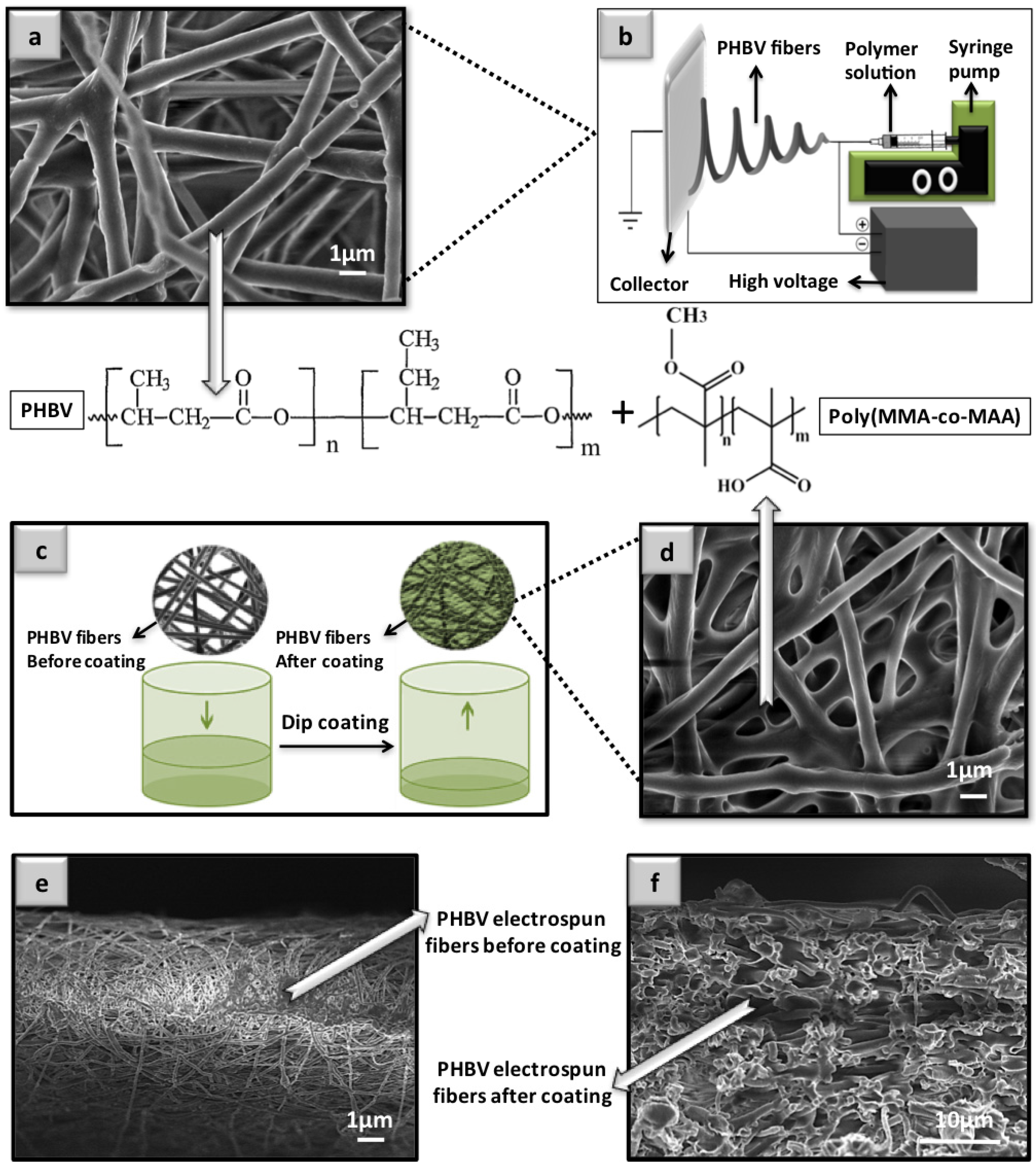
2.1. Fabrication of the Polymer-Coated Electrospun Fibers
2.2. Fabrication of i-ELISA
2.3. Morphology Analysis by Scanning Electron Microscopy (SEM) and Water-in-Air Contact (WCA) Angle Analysis
2.4. Colorimetric Sandwich ELISA for DENV Detection
3. Results and Discussion
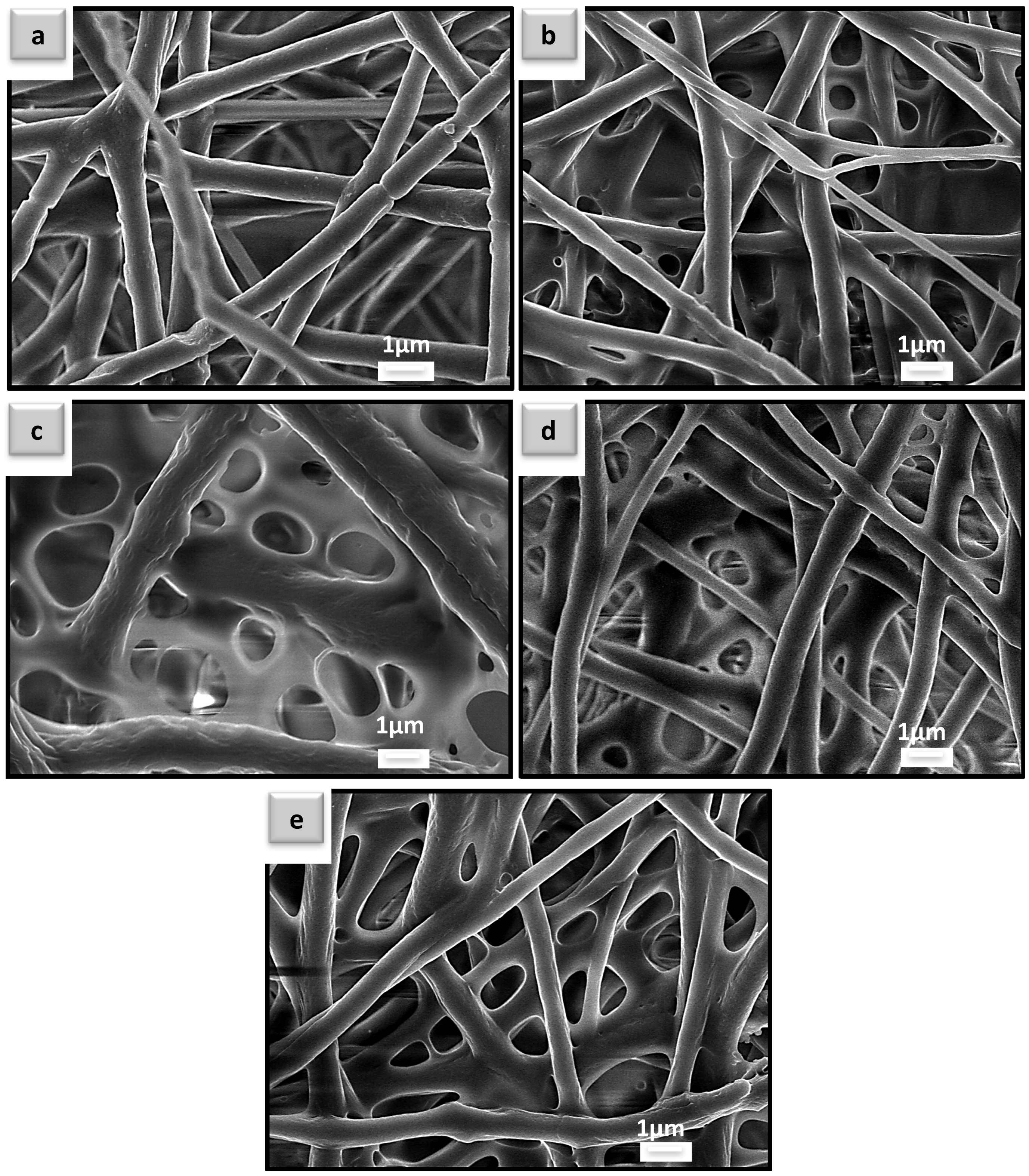
3.1. Morphology Analysis of the Coated and Uncoated Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Electrospun Fiber Mats
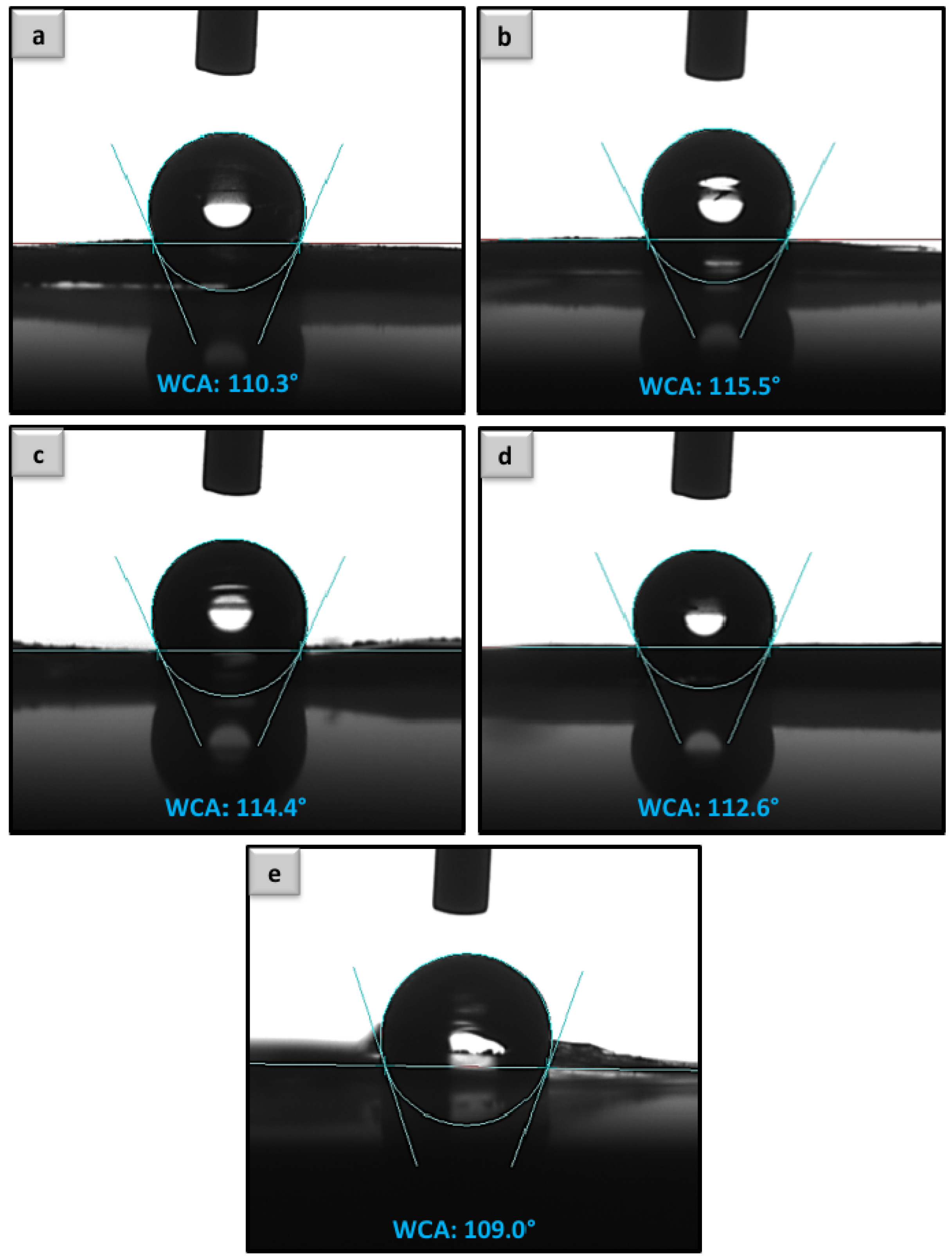
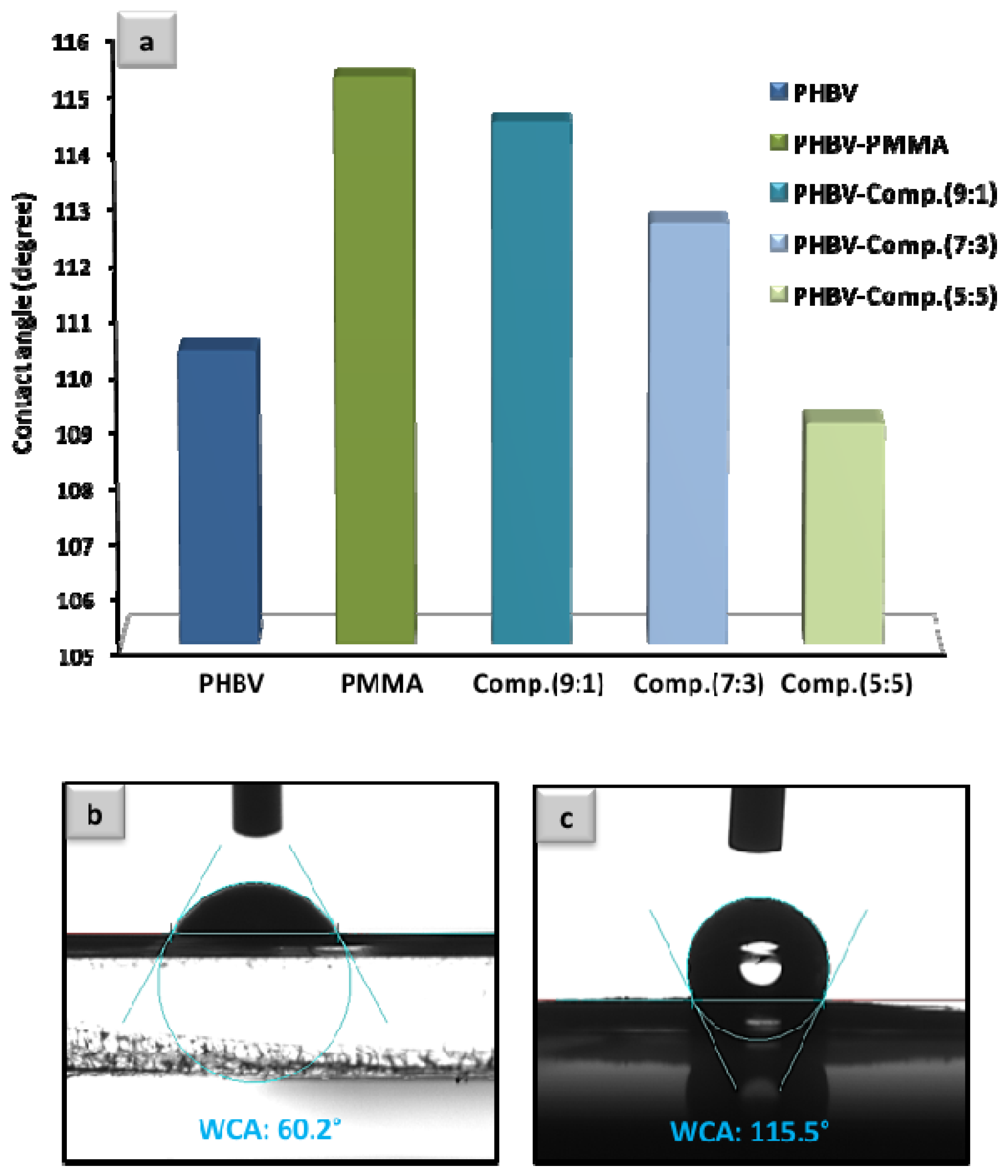
3.2. Contact Angle Analysis and the Fundamental Aspect of Physical Interaction between Protein Molecules and Poly Methyl Methacrylate-Co-Methacrylic (poly(MMA-co-MAA))-Coated Fibers
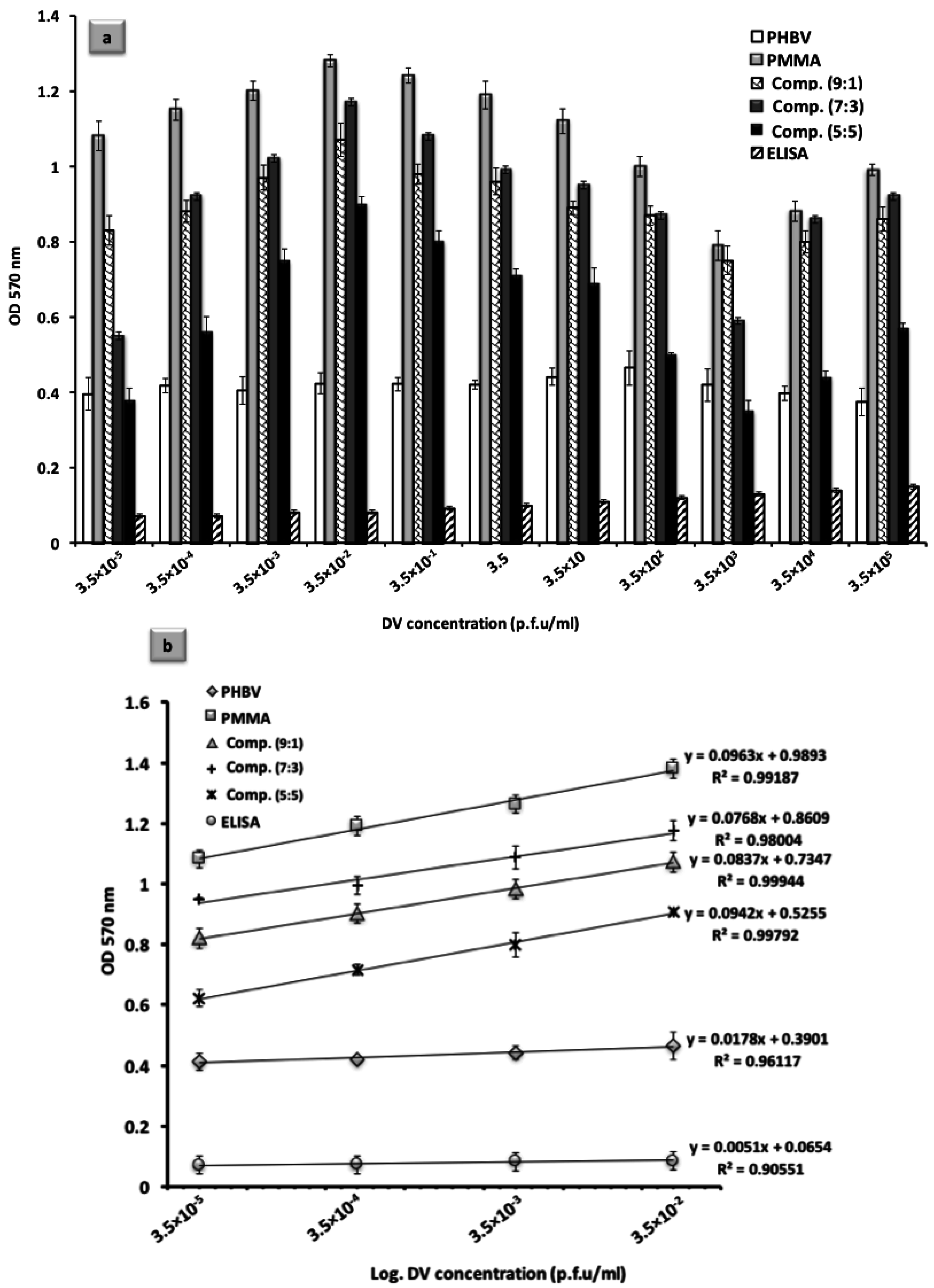
3.3. Analytical Method Optimization: Calibration and Evaluation of the Assay
3.4. Detection Performance as a Function of Poly(MMA-co-MAA) Polymer Coating Composition
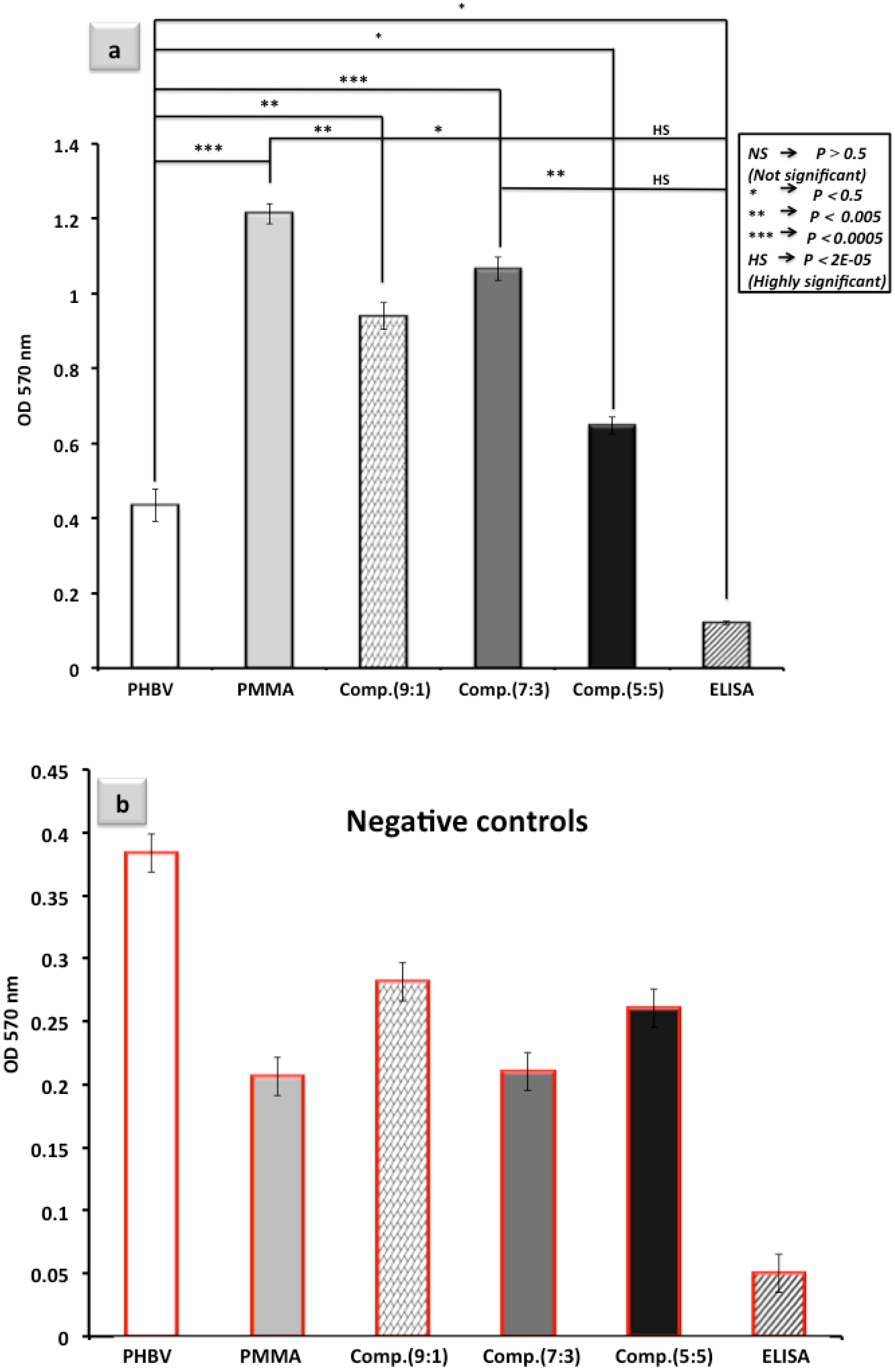
3.5. Advantages of i-ELISA in Comparison to the Competing Technologies
- I-ELISA is the first developed electrospun fiber mat-assisted analytical well plate that strictly follows the principles of the standard ELISA method.
- I-ELISA well plates can be fabricated by a slight modification in the current well plate by the injection molding process. Therefore, our platform can be highly cost-effective in comparison to the vying products.
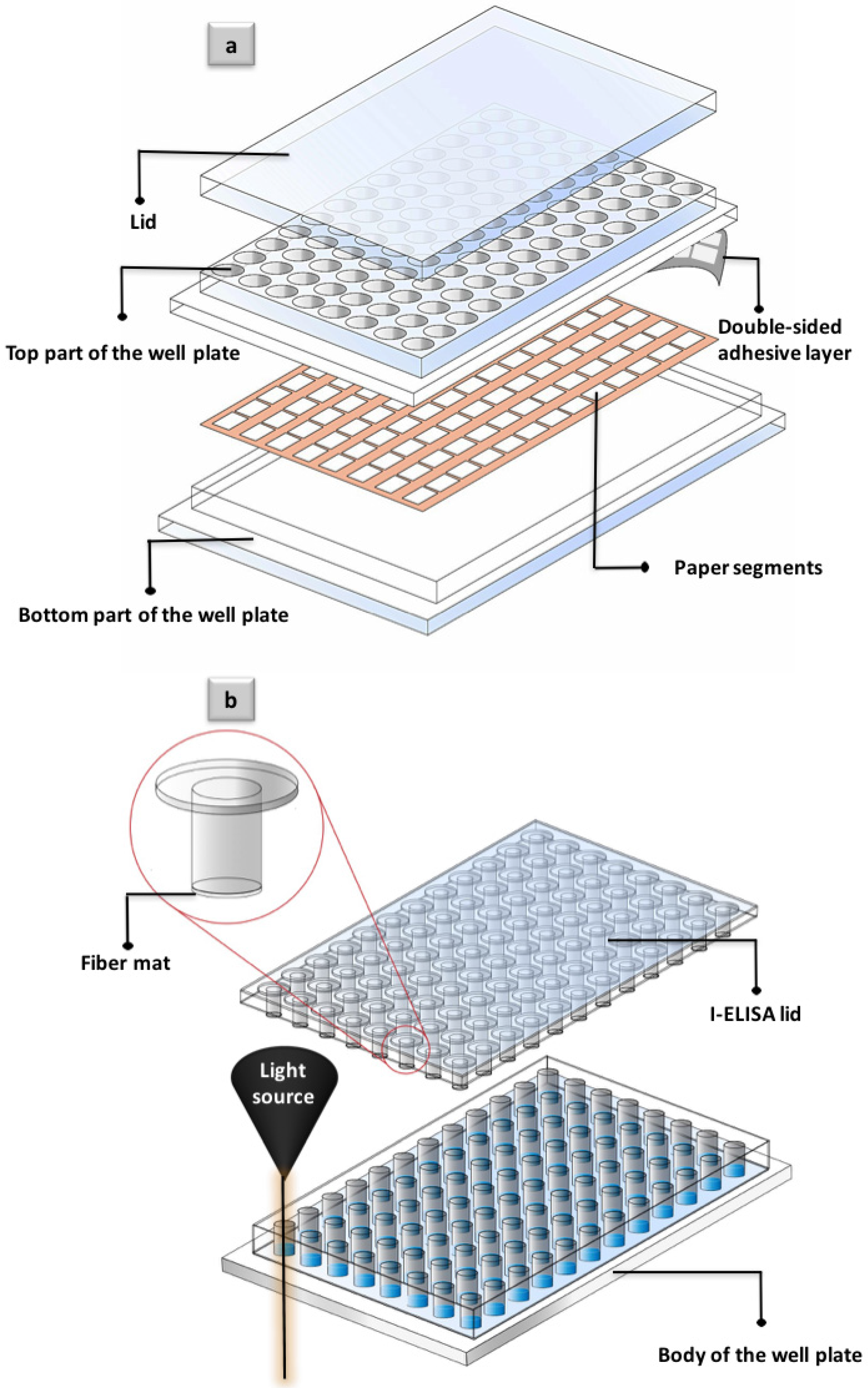
- Unlike previously developed paper-based well plates, which are often composed of several different layers that need to be assembled before the application (Figure 7a), i-ELISA consists of only two parts (lid and body of the well plate, Figure 7b), in the same fashion as commercially available analytical well plates. This fact to a considerable extent makes i-ELISA practical as the proper installation of the multilayer platforms is often beyond expertise of the lab technicians.
- I-ELISA can be used as a universal approach through which a wide variety of fiber/paper products with their special properties and functionalities can be introduced to the assay.
- As a unique platform, i-ELISA can offer the visual detection judgment as the white color of the paper makes the perfect background for colorimetric detection. More importantly, i-ELISA enables routine quantification of the exact signal intensity, as the bottom part of the well plate stays intact for a regular colorimetric readout (Figure 7b).
- I-ELISA is privileged with a great stability of the fiber parts as well as the well plate itself. In most of the paper-based well plates, the paper segments are printed or sprayed at the bottom of the well plate [2]. Printed sections are highly sensitive and are normally damaged through the numerous pipetting steps that are performed in a typical assay. In the present prototype, however, the paper part is barely touched throughout the pipetting procedure and thus remains whole (Figure 7b).
- Unlike previous nitrocellulose NC-based analytical platforms, polymer-coated electrospun PHBV is a customized fiber material that can possess tailored surface properties, tuned wettability and specific morphological characteristics. Fiber segments of i-ELISA not only offer desirable surface characteristics but also are benefited with inherent –COOH functional groups that make the application of surface modification techniques needless.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Supplementary Section
Fabrication of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Fibers
- 20 mg of PHBV was dissolved (10 wt %) in chloroform/ dimethylformamide (DMF) (9:1) and ejected from a 20 G needle (inner diameter = 0.9 mm) at the speed of 3 mL/h.
- A voltage of 10 kV was loaded between the needle and an aluminum plate (40 cm × 40 cm), which was the target for fiber deposition. The distance between the needle and the aluminum plate was set to be 18 cm.
- The polymer solutions were drawn into the PHBV fibers and were deposited on the aluminum plate. The sheet of PHBV electrospun fiber was then peeled off from the aluminum plate.
Synthesis and Processing of Poly Methyl Methacrylate-Co-Methacrylic (poly(MMA-co-MAA)) Compositions
Dip-Coating Procedure
Dengue Virus Propagation in Mosquito Cells and Titration
Sandwich ELISA Procedure
Evaluation of the Assay
- True positive (TP)
- True negative (TN)
- False positive (FP)
- False negative (FN)
Scanning Electron Microscopy (SEM) Analysis of the Uncoated and Coated PHBV Electrospun Fibers
Water-in-Air Contact Angle Analysis of the Uncoated and Coated PHBV Electrospun Fibers
References
- Carvalhal, R.F.; Simão Kfouri, M.; De Oliveira Piazetta, M.H.; Gobbi, A.L.; Kubota, L.T. Electrochemical detection in a paper-based separation device. Anal. Chem. 2010, 82, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Bui, M.-P.N.; Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens. Bioelectron. 2016, 77, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Yeow, N.; McLiesh, H.; Guan, L.; Shen, W.; Garnier, G. Paper-based assay for red blood cell antigen typing by the indirect antiglobulin test. Anal. Bioanal. Chem. 2016, 408, 5231–5238. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, E.; Ibrahim, F.; Hosseini, S.; Rothan, H.A.; Yusof, R.; Koole, L.H.; Djordjevic, I. A novel approach for application of nylon membranes in the biosensing domain. Appl. Surf. Sci. 2015, 353, 1310–1319. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R. Proteolytic assays on quantum-dot-modified paper substrates using simple optical readout platforms. Anal. Chem. 2013, 85, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, P.; Ge, S.; Li, N.; Yu, J.; Yan, M.; Huang, J. Photoelectrochemical Lab-on-Paper Device Based on an Integrated Paper Supercapacitor and Internal Light Source. Anal. Chem. 2013, 85, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Karra-Châabouni, M.; Bouaziz, I.; Boufi, S.; do Rego, A.M.B.; Gargouri, Y. Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: activity and stability studies. Colloids Surf. B Biointerfaces 2008, 66, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Koschella, A.; Kadry, G.; Heinze, T. Evaluation of cellulose and carboxymethyl cellulose/poly (vinyl alcohol) membranes. Carbohydr. Polym. 2013, 95, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, C.; Tomar, L.K.; Singh, H. Surface modification of cellulose filter paper by glycidyl methacrylate grafting for biomolecule immobilization: Influence of grafting parameters and urease immobilization. J. Appl. Polym. Sci. 2009, 111, 1381–1390. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Colorimetric sensing of cyanide anions in aqueous media based on functional surface modification of natural cellulose materials. Tetrahedron 2011, 67, 4939–4947. [Google Scholar] [CrossRef]
- Bessoff, K.; Delorey, M.; Sun, W.; Hunsperger, E. Comparison of two commercially available Dengue Virus (DENV) NS1 capture enzyme-linked immunosorbent assays using a single clinical sample for diagnosis of acute DENV infection. Clin. Vaccine Immunol. 2008, 15, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Shu, P.-Y.; Chen, L.-K.; Chang, S.-F.; Yueh, Y.-Y.; Chow, L.; Chien, L.-J.; Lin, T.-H.; Huang, J.-H. Comparison of Capture Immunoglobulin M (IgM) and IgG Enzyme-Linked Immunosorbent Assay (ELISA) and Nonstructural Protein NS1 Serotype-Specific IgG ELISA for Differentiation of Primary and Secondary Dengue Virus Infections. Clin. Diagn. Lab. Immunol. 2003, 10, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-Linked Immunosorbent Assay Specific to Dengue Virus Type 1 Nonstructural Protein NS1 Reveals Circulation of the Antigen in the Blood during the Acute Phase of Disease in Patients Experiencing Primary or Secondary Infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Gahan, M.E.; Mahalingam, S.; Keller, P.A. The medicinal chemistry of dengue fever. J. Med. Chem. 2009, 52, 7911–7926. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Di, B.; Pan, Y.-X.; Qiu, L.-W.; Wang, Y.-D.; Hao, W.; He, L.-J.; Yuen, K.-Y.; Che, X.-Y. Serotype 1-Specific Monoclonal Antibody-Based Antigen Capture Immunoassay for Detection of Circulating Nonstructural Protein NS1: Implications for Early Diagnosis and Serotyping of Dengue Virus Infections. J. Clin. Microbiol. 2006, 44, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Tan, J.L.; Smith, S.A.; de Alwis, R.; Ng, T.-S.; Kostyuchenko, V.A.; Jadi, R.S.; Kukkaro, P.; de Silva, A.M.; Crowe, J.E.; et al. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Gurugama, P.; Garg, P.; Perera, J.; Wijewickrama, A.; Seneviratne, S. Dengue viral infections. Indian J. Dermatology 2010, 55, 68–78. [Google Scholar]
- Allwinn, R. Significant increase in travel-associated dengue fever in Germany. Med. Microbiol. Immunol. 2011, 200, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lapphra, K.; Sangcharaswichai, A.; Chokephaibulkit, K.; Tiengrim, S.; Piriyakarnsakul, W.; Chakorn, T.; Yoksan, S.; Wattanamongkolsil, L.; Thamlikitkul, V. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn. Microbiol. Infect. Dis. 2008, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Linares, E.M.; Pannuti, C.S.; Kubota, L.T.; Thalhammer, S. Immunospot assay based on fluorescent nanoparticles for Dengue fever detection. Biosens. Bioelectron. 2013, 41, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Ibrahim, F.; Djordjevic, I.; Rothan, H.A.; Yusof, R.; van der Mareld, C.; Koole, L.H. Synthesis and Processing of ELISA Polymer Substitute: The Influence of Surface Chemistry and Morphology on Detection Sensitivity. Appl. Surf. Sci. 2014, 317, 630–638. [Google Scholar] [CrossRef]
- Hosseini, S.; Azari, P.; Farahmand, E.; Gan, S.N.; Rothan, H.A.; Yusof, R.; Koole, L.H.; Djordjevic, I.; Ibrahim, F. Polymethacrylate coated electrospun PHB fibers: An exquisite outlook for fabrication of paper-based biosensors. Biosens. Bioelectron. 2015, 69, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Ibrahim, F.; Djordjevic, I.; Koole, L.H. Polymethyl methacrylate-co-methacrylic acid coatings with controllable concentration of surface carboxyl groups: A novel approach in fabrication of polymeric platforms for potential bio-diagnostic devices. Appl. Surf. Sci. 2014, 300, 43–50. [Google Scholar] [CrossRef]
- Reinholt, S.J.; Sonnenfeldt, A.; Naik, A.; Frey, M.W.; Baeumner, A.J. Developing new materials for paper-based diagnostics using electrospun nanofibers. Anal. Bioanal. Chem. 2014, 406, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Ibrahim, F. Current Optical Biosensors in Clinical Practice. Novel Polymeric Biochips for Enhanced Detection of Infectious Diseases; Springer: Berlin, Germany, 2016; pp. 1–12. [Google Scholar]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Swamy, N.; Prashanth, K.N.; Basavaiah, K. Titrimetric and spectrophotometric assay of diethylcarbamazine citrate in formulations using iodate and iodide mixture as reagents. Braz. J. Pharm. Sci. 2015, 51, 43–52. [Google Scholar] [CrossRef]
- Gad, M.; Zaazaa, H.; Amer, S.; Korany, M. Static headspace gas chromatographic method for the determination of residual solvents in cephalosporins. RSC Adv. 2015, 5, 17150–17159. [Google Scholar] [CrossRef]
- Bauer, S.M.; Gehringer, M.; Laufer, S.A. A direct enzyme-linked immunosorbent assay (ELISA) for the quantitative evaluation of Janus Kinase 3 (JAK3) inhibitors. Anal. Methods 2014, 6, 8817–8822. [Google Scholar] [CrossRef]
- Pereira, A.G.; D’Avila, F.B.; Ferreira, P.C.L.; Holler, M.G.; Limberger, R.P.; Froehlich, P.E. Determination of cocaine, its metabolites and pyrolytic products by LC-MS using a chemometric approach. Anal. Methods 2014, 6, 456–462. [Google Scholar] [CrossRef]
- Zhu, M.; Zuo, W.; Yu, H.; Yang, W.; Chen, Y. Superhydrophobic surface directly created by electrospinning based on hydrophilic material. J. Mater. Sci. 2006, 41, 3793–3797. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin, Germany, 2013; pp. 3–34. [Google Scholar]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Surface modified nonwoven polysulphone (PSU) fiber mesh by electrospinning: A novel affinity membrane. J. Membr. Sci. 2006, 272, 179–187. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Park, H.-Y.; Kim, J.-H.; Kim, W.-S. Adsorption of BSA on highly carboxylated microspheres—Quantitative effects of surface functional groups and interaction forces. J. Colloid Interface Sci. 1996, 177, 613–620. [Google Scholar] [CrossRef]
- Hosseini, S.; Ibrahim, F.; Rothan, H.A.; Yusof, R.; Marel, C.; Djordjevic, I.; Koole, LH. Aging effect and antibody immobilization on –COOH exposed surfaces designed for dengue virus detection. Biochem. Eng. J. 2015, 99, 183–192. [Google Scholar] [CrossRef]
- Hosseini, S.; Ibrahim, F. Application of Biochips in Dengue Virus Detection. In Novel Polymeric Biochips for Enhanced Detection of Infectious Diseases; Springer: Berlin, Germany, 2016; pp. 39–47. [Google Scholar]
- Bai, Y.; Koh, C.G.; Boreman, M.; Juang, Y.-J.; Tang, I.C.; Lee, L.J.; Yang, S.-T. Surface Modification for Enhancing Antibody Binding on Polymer-Based Microfluidic Device for Enzyme-Linked Immunosorbent Assay. Langmuir 2006, 22, 9458–9467. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Sam, S.; Touahir, L.; Salvador Andresa, J.; Allongue, P.; Chazalviel, J.N.; Gouget-Laemmel, A.C.; Henry de Villeneuve, C.; Moraillon, A.; Ozanam, F.; Gabouze, N.; et al. Semiquantitative Study of the EDC/NHS Activation of Acid Terminal Groups at Modified Porous Silicon Surfaces. Langmuir 2009, 26, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Federica Rusmini, Z.Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, Q.; Liu, H.-B.; Zhou, X.-H.; Xiao, S.-J. Different EDC/NHS Activation Mechanisms between PAA and PMAA Brushes and the Following Amidation Reactions. Langmuir 2011, 27, 12058–12068. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Jasieniak, M.; Griesser, S.S.; Griesser, H.J. Controlled covalent surface immobilisation of proteins and peptides using plasma methods. Surf. Coat Technol. 2013, 233, 169–177. [Google Scholar] [CrossRef]
- Hosseini, S.; Ibrahim, F.; Djordjevic, I.; Rothan, H.A.; Yusof, R.; Marel, C.; Benzina, A.; Koole, LH. Synthesis and characterization of methacrylic microspheres for biomolecular recognition: Ultrasensitive biosensor for dengue virus detection. Eur. Polym. J. 2014, 60, 14–21. [Google Scholar] [CrossRef]
- Peissker, T.; Deschaume, O.; Rand, D.R.; Boyen, H.-G.; Conard, T.; Van Bael, M.J.; Bartic, C. Selective Protein Immobilization onto Gold Nanoparticles Deposited under Vacuum on a Protein-Repellent Self-Assembled Monolayer. Langmuir 2013, 29, 15328–15335. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Najioullah, F.; Verlaeten, O.; Martial, J.; Brichler, S.; Kaidomar, S.; Moravie, V.; Cabié, A.; Césaire, R. Relationship between Nonstructural Protein 1 Detection and Plasma Virus Load in Dengue Patients. Am. J. Trop. Med. Hyg. 2010, 83, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Aeinehvand, M.M.; Uddin, S.M.; Benzina, A.; Rothan, H.A.; Yusof, R.; Koole, L.H.; Madou, M.J.; Djordjevic, I.; Ibrahim, F. Microsphere Integrated Microfluidic Disk: Synergy of Two Techniques for Rapid and Ultrasensitive Dengue Detection; NCBI: Bethesda, MD, USA, May 2015.
- Pan, Y.-J.; Lin, J.-H.; Chiang, K.-C. Biomedical Applications of Antibacterial Nanofiber Mats Made of Electrospinning with Wire Electrodes. Appl. Sci. 2016, 6. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Dalavoy, T.S.; Wernette, D.P.; Gong, M.; Sweedler, J.V.; Lu, Y.; Flachsbart, B.R.; Shannon, M.A.; Bohn, P.W.; Cropek, D.M. Immobilization of DNAzyme catalytic beacons on PMMA for Pb2+ detection. Lab Chip 2008, 8, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pham, J.Q.; Johnston, K.P.; Green, P.F. Contact Angle of Water on Polystyrene Thin Films: Effects of CO2 Environment and Film Thickness. Langmuir 2007, 23, 9785–9793. [Google Scholar] [CrossRef] [PubMed]


| DENV Status | PHBV | PMMA | Comp.(9:1) | Comp.(7:3) | Comp.(5:5) | ELISA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | + | − | + | − | |
| Positive (TP, FP) | 70 | 4 | 71 | 1 | 70 | 3 | 71 | 1 | 69 | 2 | 55 | 5 |
| Negative (FN, TN) | 2 | 20 | 1 | 23 | 2 | 21 | 1 | 23 | 3 | 22 | 17 | 19 |
| Total | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 | 72 | 24 |
| Sensitivity (%) | 97.22 | 98.61 | 97.22 | 98.61 | 95.83 | 76.39 | ||||||
| Specificity (%) | 83.33 | 95.83 | 87.50 | 95.83 | 91.67 | 79.17 | ||||||
| Accuracy (%) | 93.75 | 97.91 | 94.79 | 97.91 | 94.79 | 77 | ||||||
| LoD (p.f.u/mL) | 413.99 | 9.9 | 14.1 | 16.2 | 10.41 | 5 × 103 | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, S.; Azari, P.; Aeinehvand, M.M.; Rothan, H.A.; Djordjevic, I.; Martinez-Chapa, S.O.; Madou, M.J. Intrant ELISA: A Novel Approach to Fabrication of Electrospun Fiber Mat-Assisted Biosensor Platforms and Their Integration within Standard Analytical Well Plates. Appl. Sci. 2016, 6, 336. https://doi.org/10.3390/app6110336
Hosseini S, Azari P, Aeinehvand MM, Rothan HA, Djordjevic I, Martinez-Chapa SO, Madou MJ. Intrant ELISA: A Novel Approach to Fabrication of Electrospun Fiber Mat-Assisted Biosensor Platforms and Their Integration within Standard Analytical Well Plates. Applied Sciences. 2016; 6(11):336. https://doi.org/10.3390/app6110336
Chicago/Turabian StyleHosseini, Samira, Pedram Azari, Mohammad M. Aeinehvand, Hussin A. Rothan, Ivan Djordjevic, Sergio O. Martinez-Chapa, and Marc J. Madou. 2016. "Intrant ELISA: A Novel Approach to Fabrication of Electrospun Fiber Mat-Assisted Biosensor Platforms and Their Integration within Standard Analytical Well Plates" Applied Sciences 6, no. 11: 336. https://doi.org/10.3390/app6110336
APA StyleHosseini, S., Azari, P., Aeinehvand, M. M., Rothan, H. A., Djordjevic, I., Martinez-Chapa, S. O., & Madou, M. J. (2016). Intrant ELISA: A Novel Approach to Fabrication of Electrospun Fiber Mat-Assisted Biosensor Platforms and Their Integration within Standard Analytical Well Plates. Applied Sciences, 6(11), 336. https://doi.org/10.3390/app6110336







