Stabilization of Iron (Micro)Particles with Polyhydroxybutyrate for In Situ Remediation Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
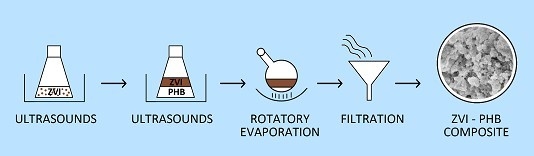
2.2. Preparation of ZVI–PHB Complexes
2.3. Scanning Electron Microscopy
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR–FTIR) Analysis
2.5. Textural Analysis
2.6. Thermogravimetric Analysis (TGA) Measurements
2.7. Disc Centrifuge Analysis
2.8. Zeta Potential Measurements
3. Results and Discussion
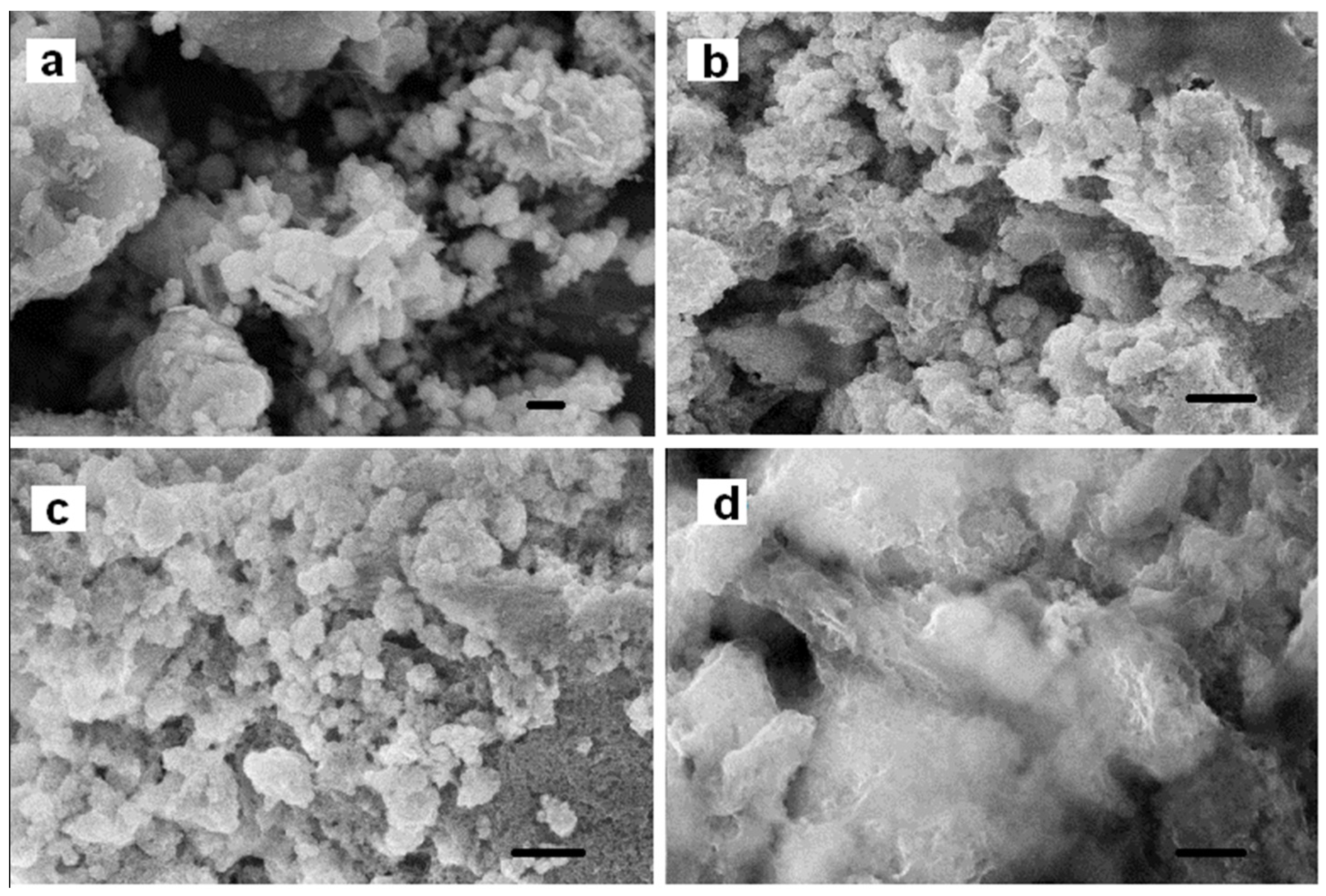
3.1. Preparation and Morphological Characterization of ZVI–PHB Complexes
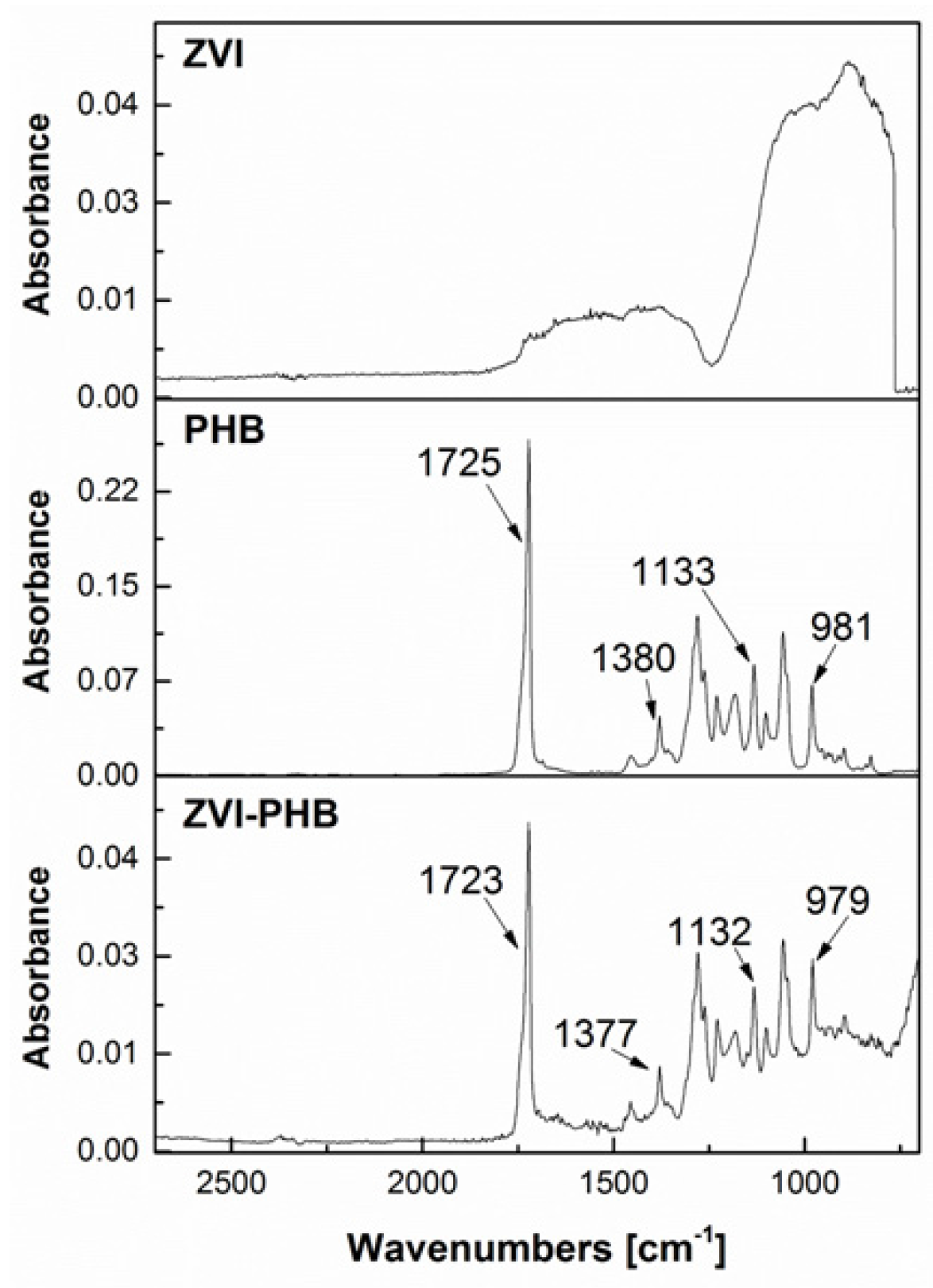
3.2. FTIR Measurements
3.3. Textural Analysis
3.4. TGA Measurements
3.5. Disc Centrifuge Analysis
3.6. Zeta Potential Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.C. Organic Contaminants in the Environment: Environmental Pathways & Effects; Elsevier Science Publishers Ltd.: Barking, UK, 2013. [Google Scholar]
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2014.182.01.0052.01.ENG (accessed on 28 September 2016).
- Mackay, D.M.; Cherry, J.A. Groundwater contamination: Pump-and-treat remediation. Environ. Sci. Technol. 1989, 23, 630–636. [Google Scholar] [CrossRef]
- Park, D.K.; Ko, N.; Lee, K. Optimal groundwater remediation design considering effects of natural attenuation processes: Pumping strategy with enhanced-natural-attenuation. Geosci. J. 2007, 11, 377–385. [Google Scholar] [CrossRef]
- Park, Y.; Jeong, J.; Eom, S.; Jeong, U.P. Optimal management design of a pump and treat system at the industrial complex in Wonju, Korea. Geosci. J. 2011, 15, 207–223. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J.J. Chemistry and Microbiology of Permeable Reactive Barriers for In Situ Groundwater Clean up. Crit. Rev. Microbiol. 2000, 26, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumfor, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.I.; Kim, S.K.; Lee, Y.S.; Yu, J. Permeable reactive barrier using atomized slag material for treatment of contaminants from landfills. Geosci. J. 2007, 11, 137–145. [Google Scholar] [CrossRef]
- Moraci, N.; Calabrò, P.S. Heavy metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. J. Environ. Manag. 2010, 91, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Matheson, L.J.; Tratnyek, P.G. Reductive Dehalogenation of Chlorinated Methanes by Iron Metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.; Totten, L.A.; Arnold, W.A.; Burris, D.R.; Campbell, T.J. Reductive Elimination of Chlorinated Ethylenes by Zero-Valent Metals. Environ. Sci. Technol. 1996, 30, 2654–2659. [Google Scholar] [CrossRef]
- Reardon, E.J. Zerovalent Irons: Styles of Corrosion and Inorganic Control on Hydrogen Pressure Buildup. Environ. Sci. Technol. 2005, 39, 7311–7317. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Heal. A 2014, 49, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Btatkeu, B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215, 959–968. [Google Scholar] [CrossRef]
- Lojkasek-Lima, P.; Aravena, R.; Shouakar-Stash, O.; Frape, S.K.; Marchesi, M.; Fiorenza, S.; Vogan, J. Evaluating TCE abiotic and biotic degradation pathways in a permeable reactive barrier using compound specific isotope analysis. Ground Water Monit. R. 2012, 32, 53–62. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jin, S.; Fallgren, P.H.; Swoboda-Colberg, N.G.; Liu, F.; Colberg, P.J. Electrochemical depassivation of zero-valent iron for trichloroethene reduction. J. Hazard. Mater. 2012, 239, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Carraway, E.R. Reduction of chlorinated ethanes by nanosized zero-valent iron: Kinetics, pathways, and effects of reaction conditions. Environ. Sci. Technol. 2005, 39, 6237–6245. [Google Scholar] [CrossRef] [PubMed]
- Arjoon, A.; Olaniran, A.O.; Pillay, B. Enhanced 1,2-dichloroethane degradation in heavy metal co-contaminated wastewater undergoing biostimulation and bioaugmentation. Chemosphere 2013, 93, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, D. Preparation and Characterization of a New Class of Starch-Stabilized Bimetallic Nanoparticles for Degradation of Chlorinated Hydrocarbons in Water. Environ. Sci. Technol. 2005, 39, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.M.; Oleniuk, A.J.; Kocur, C.M.; Sleep, B.E.; Bennett, P.; Xiong, Z.; O’Carroll, D.M. A Field-Validated Model for In Situ Transport of Polymer-Stabilized nZVI and Implications for Subsurface Injection. Environ. Sci. Technol. 2013, 47, 7332–7340. [Google Scholar] [CrossRef] [PubMed]
- Gastone, F.; Tosco, T.; Sethi, R. Green stabilization of microscale iron particles using guar gum: Bulk rheology, sedimentation rate and enzymatic degradation. J. Colloid Interface Sci. 2014, 421, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Velimirovic, M.; Simons, Q.; Bastiaens, L. Guar gum coupled microscale ZVI for in situ treatment of CAHs: Continuous-flow column study. J. Hazard. Mater. 2014, 265, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Valentino, F.; Barbetta, A.; Martino, L.; Scandola, M.; Majone, M. Polyhydroxyalkanoates production with mixed microbial cultures: From culture selection to polymer recovery in a high-rate continuous process. N Biotechnol. 2014, 31, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Chronopoulou, L.; Petrangeli Papini, M.; Vinod, V.T.P.; Palocci, C.; Kupčík, J.; Černík, M. Enhancement of stability and reactivity of nanosized zero-valent iron with polyhydroxybutyrate. Desalin. Water Treat. 2016. [Google Scholar] [CrossRef]
- Baric, M.; Majone, M.; Beccari, M.; Petrangeli Papini, M. Coupling of polyhydroxybutyrate (PHB) and zero valent iron (ZVI) for enhanced treatment of chlorinated ethanes in permeable reactive barriers (PRBs). Chem. Eng. J. 2012, 195–196, 22–30. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: London, UK, 1982. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M. Self-Assembled Monolayers of Mercaptoacetic Acid on Ag Powder: Characterization by FT-IR Diffuse Reflection Spectroscopy. Bull. Korean Chem. Soc. 2004, 25, 1461–1462. [Google Scholar]
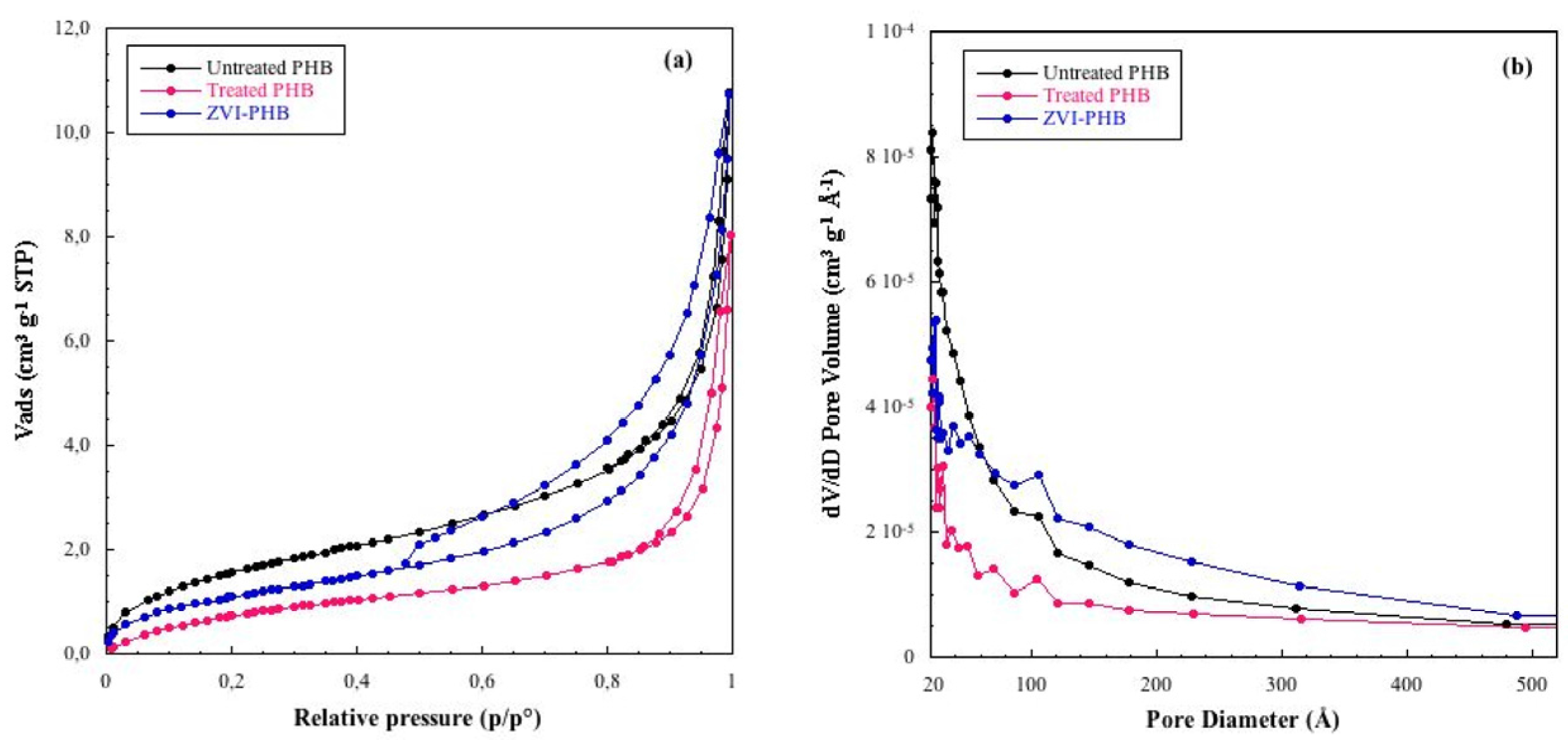
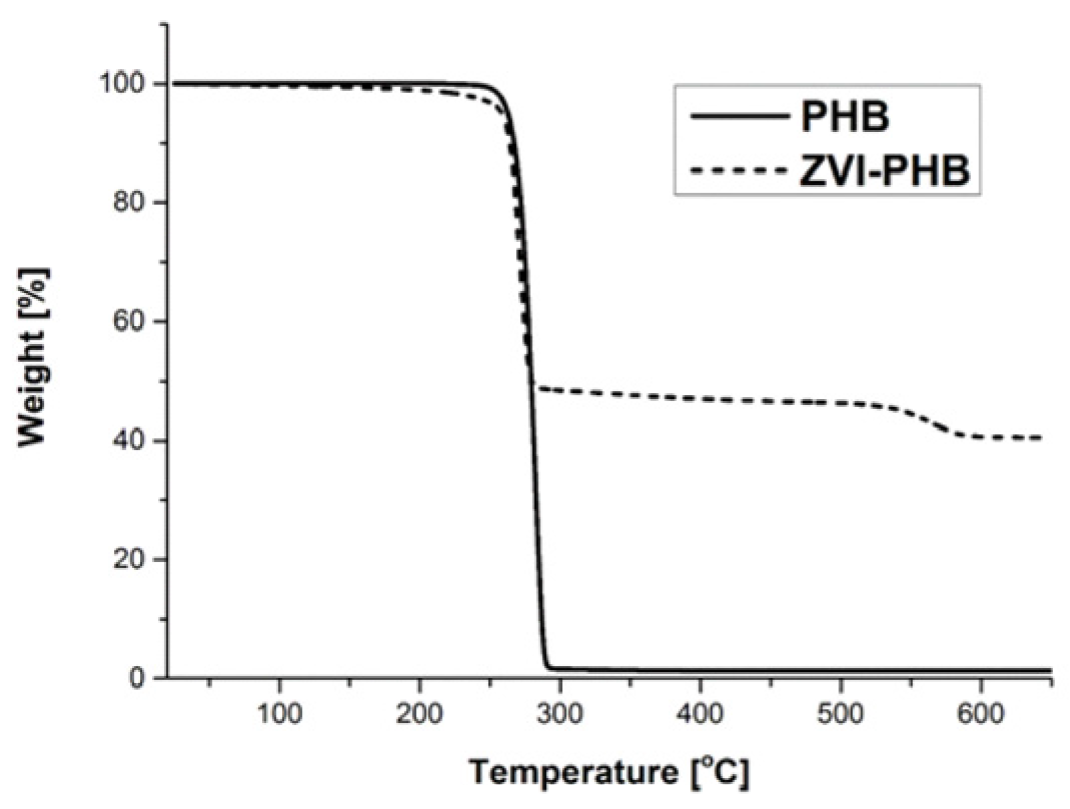
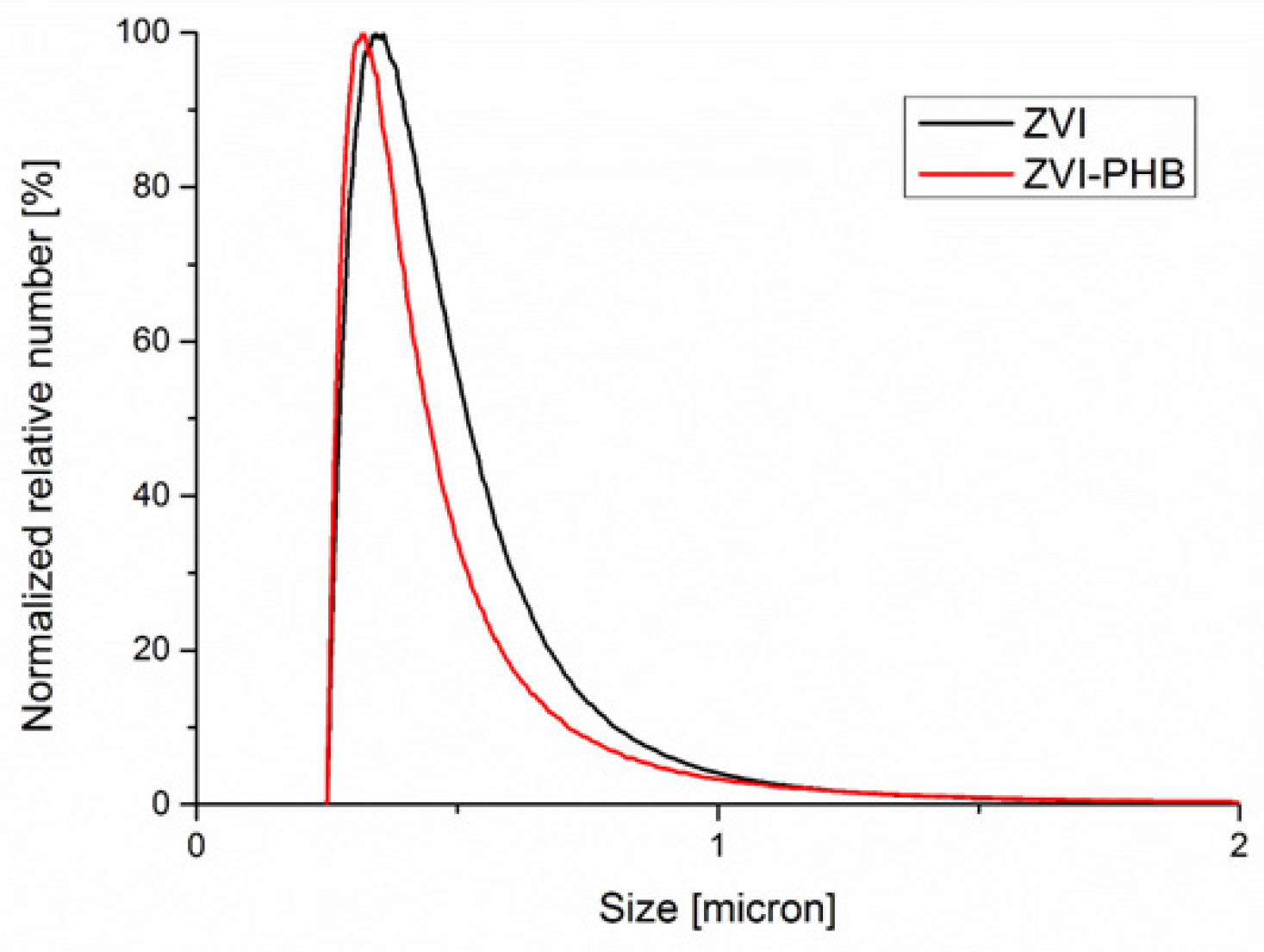
| Sample | Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| Untreated PHB | 6.1 ± 0.5 | 0.0160 |
| Treated PHB | 3.5 ± 0.5 | 0.0095 |
| ZVI–PHB Composite | 4.3 ± 0.5 | 0.0162 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chronopoulou, L.; Palocci, C.; Valentino, F.; Pettiti, I.; Wacławek, S.; Černík, M.; Petrangeli Papini, M. Stabilization of Iron (Micro)Particles with Polyhydroxybutyrate for In Situ Remediation Applications. Appl. Sci. 2016, 6, 417. https://doi.org/10.3390/app6120417
Chronopoulou L, Palocci C, Valentino F, Pettiti I, Wacławek S, Černík M, Petrangeli Papini M. Stabilization of Iron (Micro)Particles with Polyhydroxybutyrate for In Situ Remediation Applications. Applied Sciences. 2016; 6(12):417. https://doi.org/10.3390/app6120417
Chicago/Turabian StyleChronopoulou, Laura, Cleofe Palocci, Francesco Valentino, Ida Pettiti, Stanisław Wacławek, Miroslav Černík, and Marco Petrangeli Papini. 2016. "Stabilization of Iron (Micro)Particles with Polyhydroxybutyrate for In Situ Remediation Applications" Applied Sciences 6, no. 12: 417. https://doi.org/10.3390/app6120417
APA StyleChronopoulou, L., Palocci, C., Valentino, F., Pettiti, I., Wacławek, S., Černík, M., & Petrangeli Papini, M. (2016). Stabilization of Iron (Micro)Particles with Polyhydroxybutyrate for In Situ Remediation Applications. Applied Sciences, 6(12), 417. https://doi.org/10.3390/app6120417