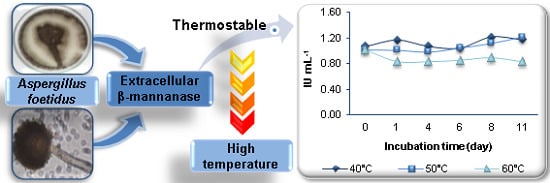
Partial Purification and Characterization of a Thermostable β-Mannanase from Aspergillus foetidus
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Residue Pretreatment
2.3. Bromatological Analysis
2.4. Organism and Enzyme Production
2.5. Enzyme Assay
2.6. Partial Purification of Man 58
2.7. Electrophoresis and Zymogram
2.8. Enzyme Characterization
2.9. Effect of Autohydrolysis Liquor
3. Results and Discussion
3.1. Enzyme Production
3.2. Partial Purification of Man 58
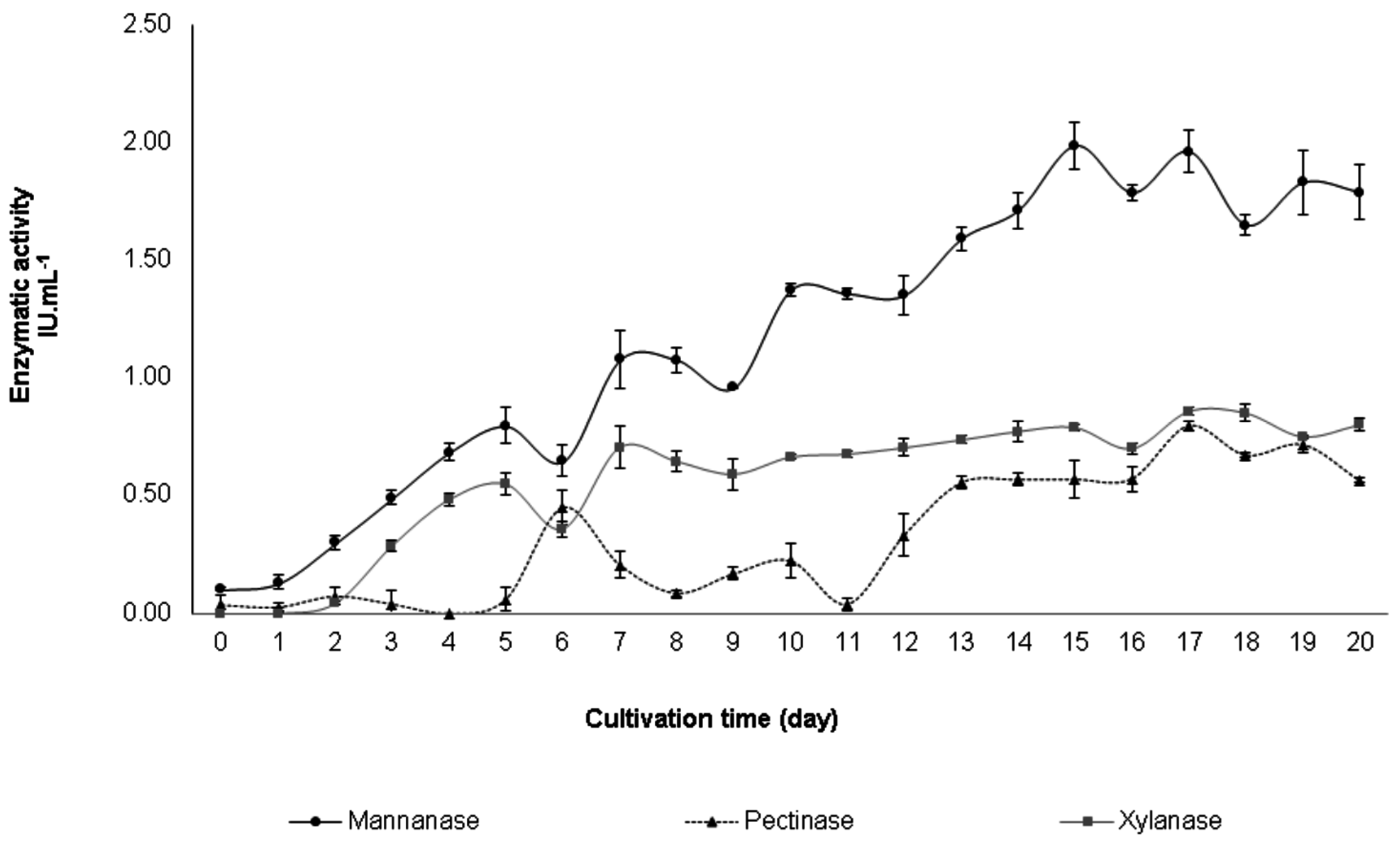
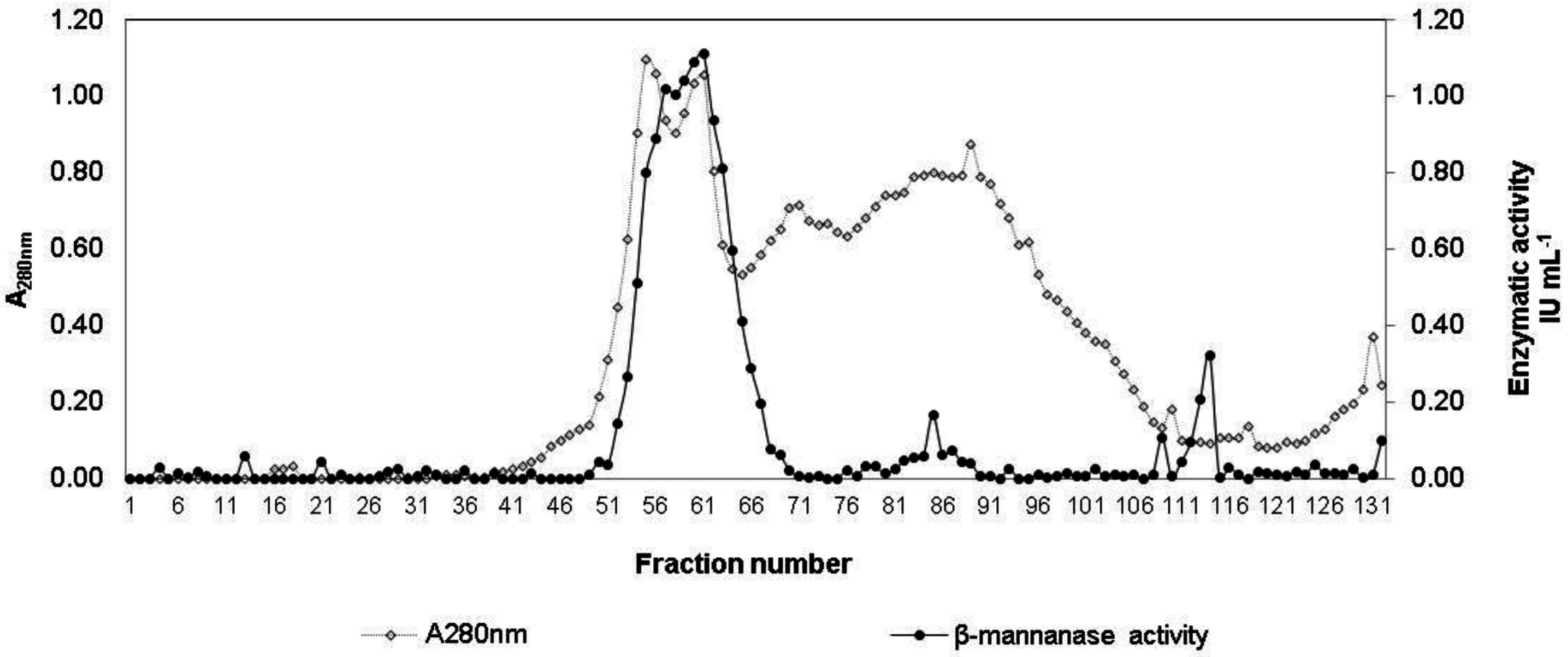
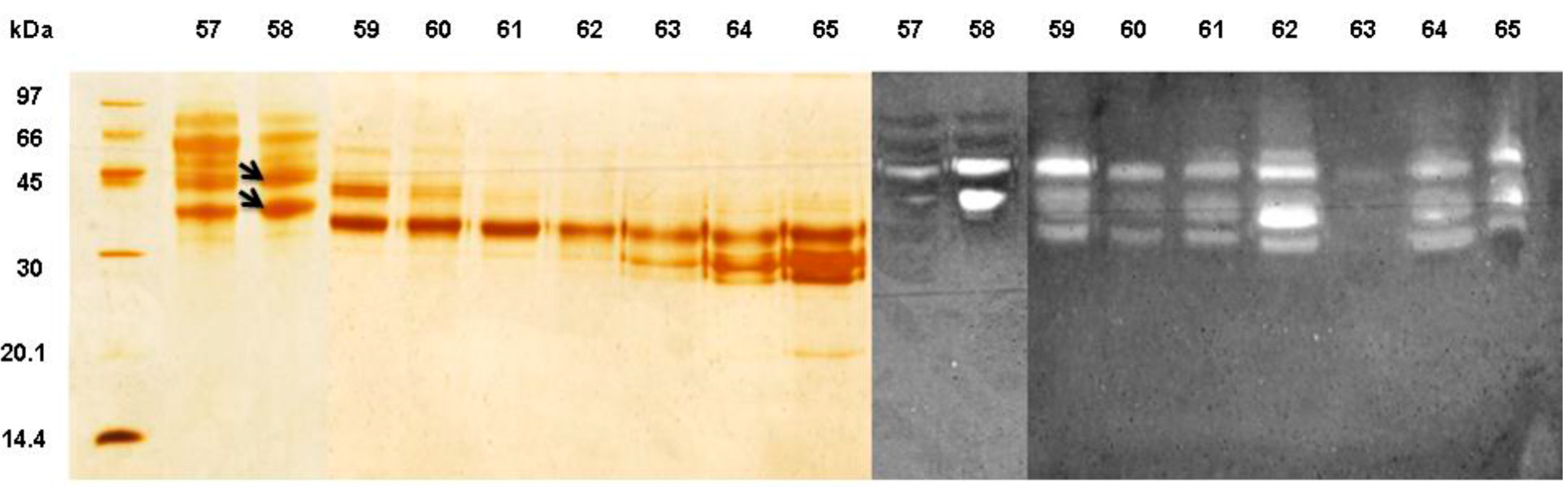
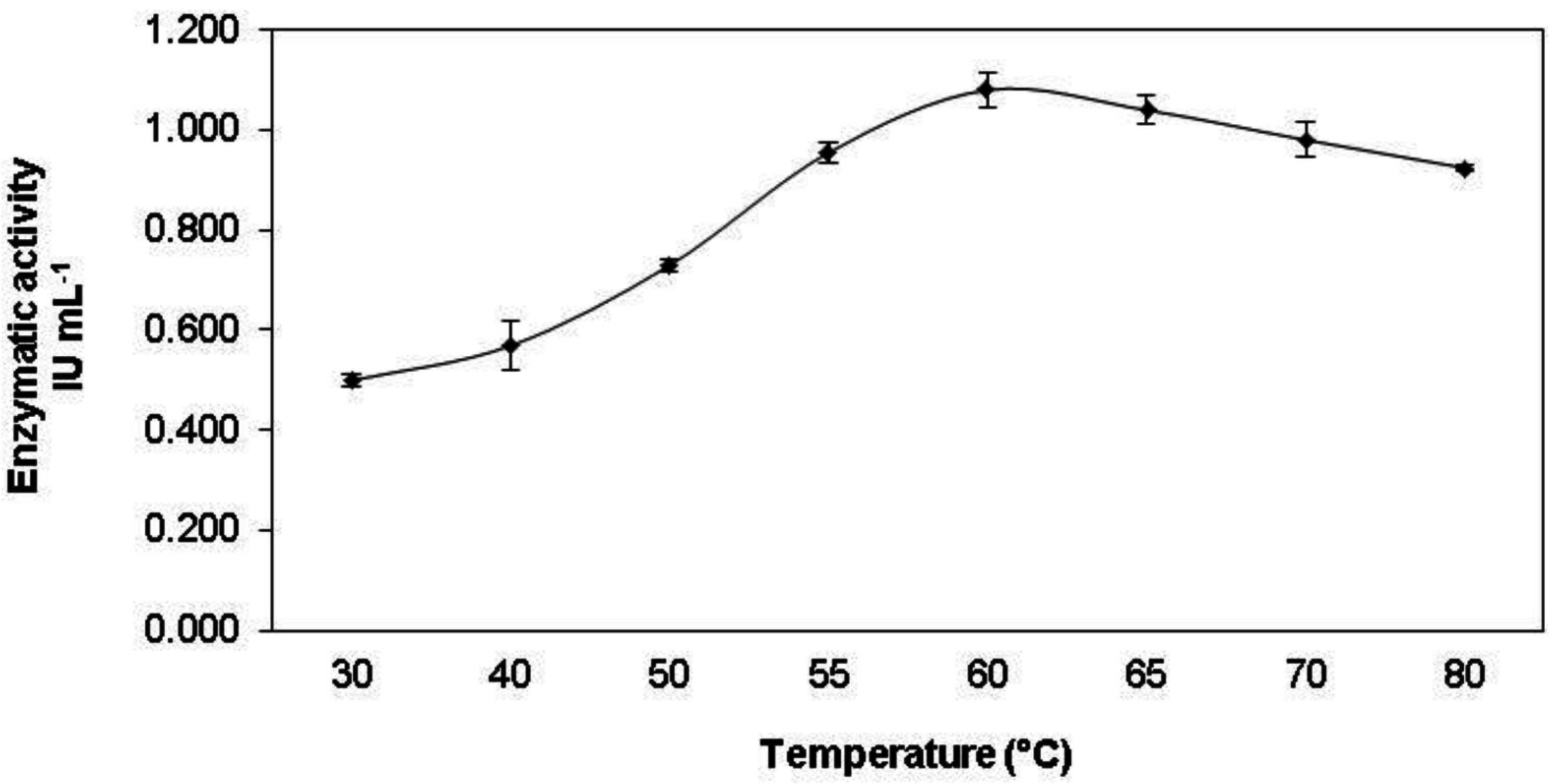
3.3. Enzyme Characterization

| Man 58 | ||
|---|---|---|
| Relative Activity % | ||
| Metal Ions and EDTA | 1 mM | 10 mM |
| Control | 100.00 ± 0.057 | 100.00 ± 0.075 |
| MgSO4 | 80.24 ± 0.039 | 91.90 ± 0.017 |
| FeSO4 | 105.66 ± 0.034 | 121.99 ± 0.046 |
| CoCl2 | 92.68 ± 0.086 | 110.86 ± 0.022 |
| FeCl3 | 79.21 ± 0.036 | 62.42 ± 0.030 |
| CuSO4 | 81.50 ± 0.098 | 65.00 ± 0.028 |
| CaCl2 | 101.50 ± 0.024 | 87.53 ± 0.023 |
| MgCl2 | 86.06 ± 0.029 | 84.50 ± 0.035 |
| ZnCl2 | 95.90 ± 0.030 | 87.25 ± 0.057 |
| ZnSO4 | 94.09 ± 0.043 | 89.76 ± 0.047 |
| CuCl2 | 90.39 ± 0.089 | 64.29 ± 0.068 |
| KCl | 81.65 ± 0.133 | 94.12 ± 0.047 |
| NaCl | 106.93 ± 0.077 | 92.52 ± 0.010 |
| EDTA | 73.30 ± 0.028 | 89.05 ± 0.023 |
| Man 58 | |
|---|---|
| Relative Activity (%) | |
| Control | 100.00 ± 0.024 |
| Ferulic acid | 151.06 ± 0.008 |
| Cinnamic acid | 93.37 ± 0.010 |
| P-coumaric acid | 103.24 ± 0.006 |
| 4-hydroxybenzoic acid | 107.89 ± 0.026 |
| Tannic acid | 98.16 ± 0.014 |
| Vanillin | 98.02 ± 0.038 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akinyele, J.B.; Olaniyi, O.O.; Adetunji, C.O. Screening and optimization of nutritional conditions for mannanase production by Penicillium italicum LAD-A5 in solid state cultivation. J. Biotechnol. Pharm. Res. 2013, 4, 103–109. [Google Scholar]
- Ferreira, H.M.; Filho, E.X.F. Purification and characterization of a β-mannanase from Trichoderma harzianum strain T4. Carbohydr. Polym. 2004, 57, 23–29. [Google Scholar] [CrossRef]
- Moreira, L.R.S.; Filho, E.X.F. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Puls, J.; Schuseil, J. Chemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. In Hemicellulose and Hemicellulases; Coughlan, M.P., Hazlewood, G.P., Eds.; Portland Press: London, UK, 1993; pp. 1–27. [Google Scholar]
- Singh, S.; Madlala, A.M.; Prior, B.A. Thermomyces lanuginosus: Properties of strains and their hemicellulases. FEMS Microbiol. Rev. 2003, 27, 3–16. [Google Scholar] [CrossRef]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. β-Mannanase (Man26A) and α-galactosidase (Aga27A) synergism—A key factor for the hydrolysis of galactomannan substrates. Enzym. Microb. Technol. 2015, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, P.; Lu, H.; Wang, H.; Luo, H.; Huang, H.; Yang, P.; Yao, B. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem. 2015, 173, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, A.; Nadaroglu, H.; Adiguzel, G. Purification and characterization of β-mannanase from Bacillus pumilus (M27) and its applications in some fruit juices. J. Food Sci. Technol. 2015, 52, 5292–5298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sang, Q. Production and extraction optimization of xylanase and β-mannanase by Penicillium chrysogenum QML-2 and primary application in saccharification of corn cob. Biochem. Eng. J. 2015, 97, 101–110. [Google Scholar] [CrossRef]
- Chiyanzu, I.; Brienzo, M.; García-Aparicio, M.P.; Görgens, J.F. Application of Endo-β-1,4,d-mannanase and cellulase for the release of mannooligosaccharides from steam-pretreated spent coffee ground. Appl. Biochem. Biotechnol. 2014, 172, 3538–3557. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Bailey, C.A.; Cartwright, A.L. β-Mannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult. Sci. 2003, 82, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, F.G.; Filho, E.X.F. Plant Cell Wall as a substrate for the production of enzymes with industrial applications. Mini-Rev. Org. Chem. 2010, 7, 54–60. [Google Scholar]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yan, R.; Liu, Y.; Jiang, W. In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose and lignin. Bioresour. Technol. 2010, 101, 8217–8223. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Sunna, A.; Puls, J.; Antranikian, G. Characterization of the xylanolytic enzyme system of the extreme thermophilic anaerobic bacteria Thermotoga maritima, T-neapolitana, and T-thermarum. Comp. Biochem. Physiol. 1997, 118, 453–461. [Google Scholar] [CrossRef]
- Leatherbarrow, R.J. Enzfitter Manual, a Non-Linear Curve Fitting Program for Windows; Biosoft: London, UK, 1999; pp. 1–104. [Google Scholar]
- Michelin, M.; Polizeli, M.L.T.M.; Ruzene, D.S.; Silva, D.P.; Ruiz, H.A.; Vicente, A.A.; Jorge, J.A.; Terenzi, H.F.; Teixeira, J.A. Production of xylanase and b-xylosidase from autohydrolysis liquor of corncob using two fungal strains. Bioprocess Biosyst. Eng. 2012, 35, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Naganagouda, K.; Salimath, P.; Mulimani, V.H. Purification and Characterization of Endo-β-1,4 Mannanase from Aspergillus niger gr for Application in Food Processing Industry. J. Microbiol. Biotechnol. 2009, 19, 1184–1190. [Google Scholar] [PubMed]
- Benech, R.O.; Li, X.; Patton, D.; Powlowski, J.; Storms, R.; Bourbonnais, R.; Paice, M.; Tsang, A. Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzym. Microb. Technol. 2007, 41, 740–747. [Google Scholar] [CrossRef]
- Kote, N.V.; Patil, A.G.G.; Mulimani, V.H. Optimization of the Production of Thermostable endo-β-1,4 Mannanases from a Newly Isolated Aspergillus niger gr and Aspergillus flavus gr. Appl. Biochem. Biotechnol. 2009, 152, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, Z.; Hong, Y.; Li, C.; Fu, X.; Liu, Z. A novel β-mannanase from Pantoea agglomerans A021: Gene cloning, expression, purification and characterization. World J. Microbiol. Biotechnol. 2010, 26, 1777–1784. [Google Scholar] [CrossRef]
- Xu, B.; Sellos, D.; Janson, J.C. Cloning and expression in Pichia pastoris of a blue mussel (Mytilus edulis) β-mannanase gene. Eur. J. Biochem. 2002, 269, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, Y.; Araki, T.; Amagoi, H.; Mori, H.; Morishita, T. Purification and characterization of an extracellular β-1,4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl. Environ. Microbiol. 1995, 61, 4454–4458. [Google Scholar] [PubMed]
- Moreira, L.R.S.; Campos, M.C.; Siqueira, P.H.V.M.; Silva, L.P.; Ricart, C.A.O.; Martins, P.A.; Queiroz, R.M.L.; Filho, E.X.F. Two β-xylanases from Aspergillus terreus: Characterization and influence of phenolic compounds on xylanase activity. Fungal Gen. Biol. 2013, 60, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Boukari, I.; O’donohue, M.; Rémond, C.; Chabbert, B. Probing a family GH11 endo-β-1,4-xylanase inhibition mechanism by phenolic compounds: Role of functional phenolic groups. J. Mol. Catal. B 2011, 72, 130–138. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.A.; Monsier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, J.D.C.I.; Neto, G.P.d.S.; Castro, C.F.d.S.; Michelin, M.; Polizeli, M.D.L.T.M.; Filho, E.X.F. Partial Purification and Characterization of a Thermostable β-Mannanase from Aspergillus foetidus. Appl. Sci. 2015, 5, 881-893. https://doi.org/10.3390/app5040881
De Marco JDCI, Neto GPdS, Castro CFdS, Michelin M, Polizeli MDLTM, Filho EXF. Partial Purification and Characterization of a Thermostable β-Mannanase from Aspergillus foetidus. Applied Sciences. 2015; 5(4):881-893. https://doi.org/10.3390/app5040881
Chicago/Turabian StyleDe Marco, Juliana Da Conceição Infante, Geraldo Pereira de Souza Neto, Carlos Frederico de Souza Castro, Michele Michelin, Maria De Lourdes T. M. Polizeli, and Edivaldo Ximenes Ferreira Filho. 2015. "Partial Purification and Characterization of a Thermostable β-Mannanase from Aspergillus foetidus" Applied Sciences 5, no. 4: 881-893. https://doi.org/10.3390/app5040881
APA StyleDe Marco, J. D. C. I., Neto, G. P. d. S., Castro, C. F. d. S., Michelin, M., Polizeli, M. D. L. T. M., & Filho, E. X. F. (2015). Partial Purification and Characterization of a Thermostable β-Mannanase from Aspergillus foetidus. Applied Sciences, 5(4), 881-893. https://doi.org/10.3390/app5040881