Fluid-Rock Interaction Signature in Palomares Fault Zone—New Mineralogical and Geochemical Insights into the Tectono-Magmatic Águilas Arc Geothermal System (SE Spain)
Featured Application
Abstract
1. Introduction
2. Geological Setting
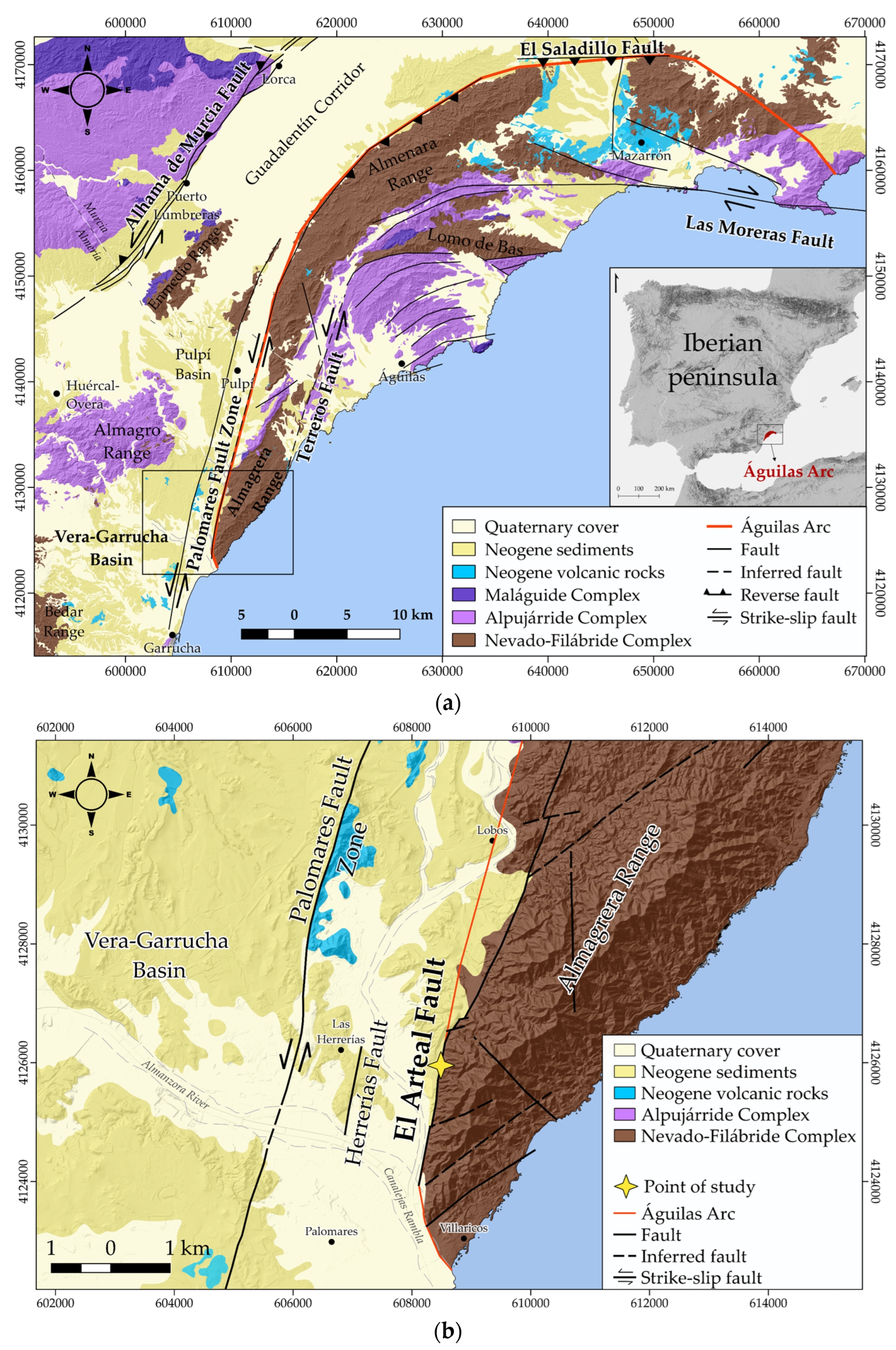
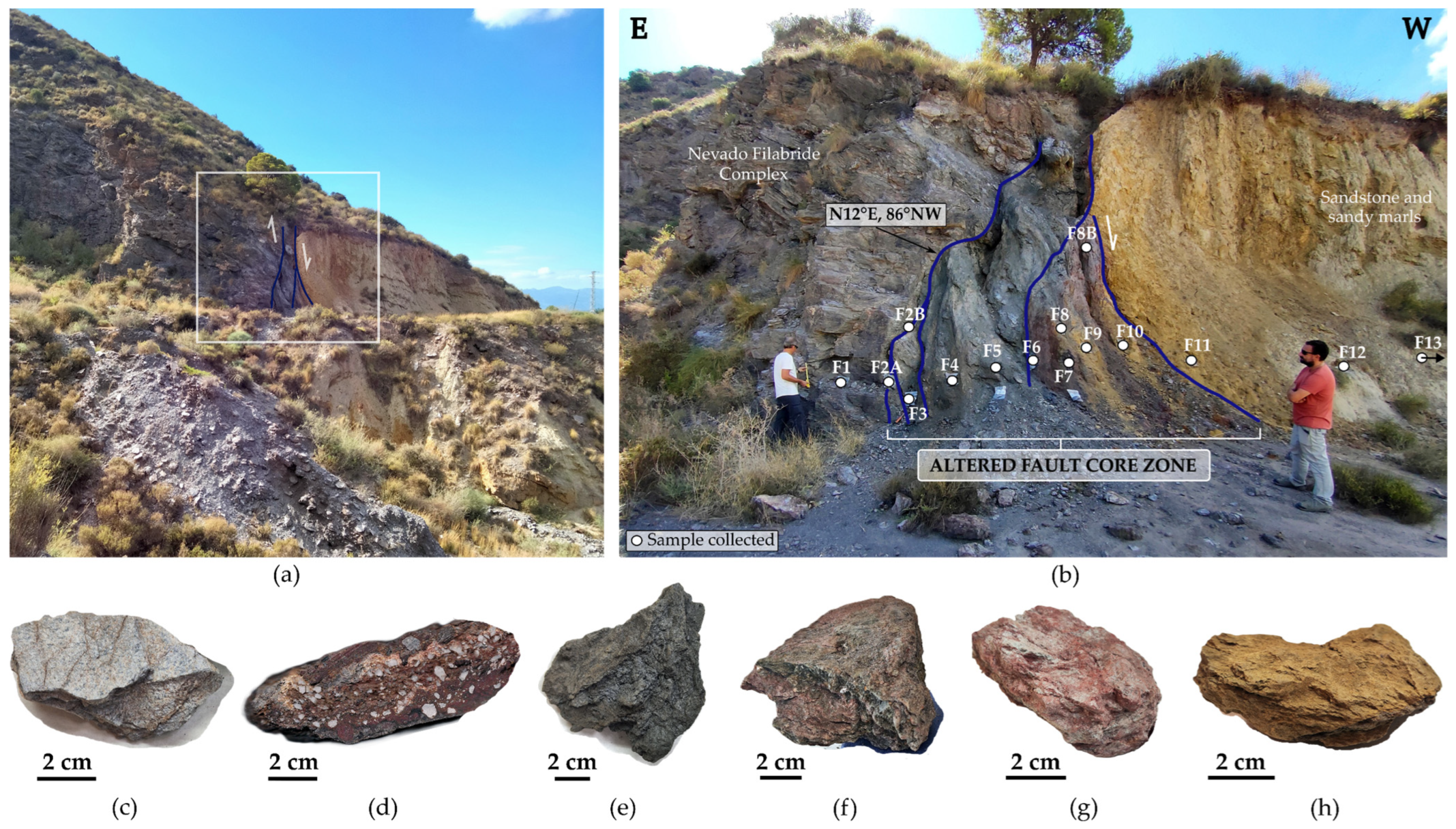
2.1. The PFZ and El Arteal Fault
2.2. Historical Mining
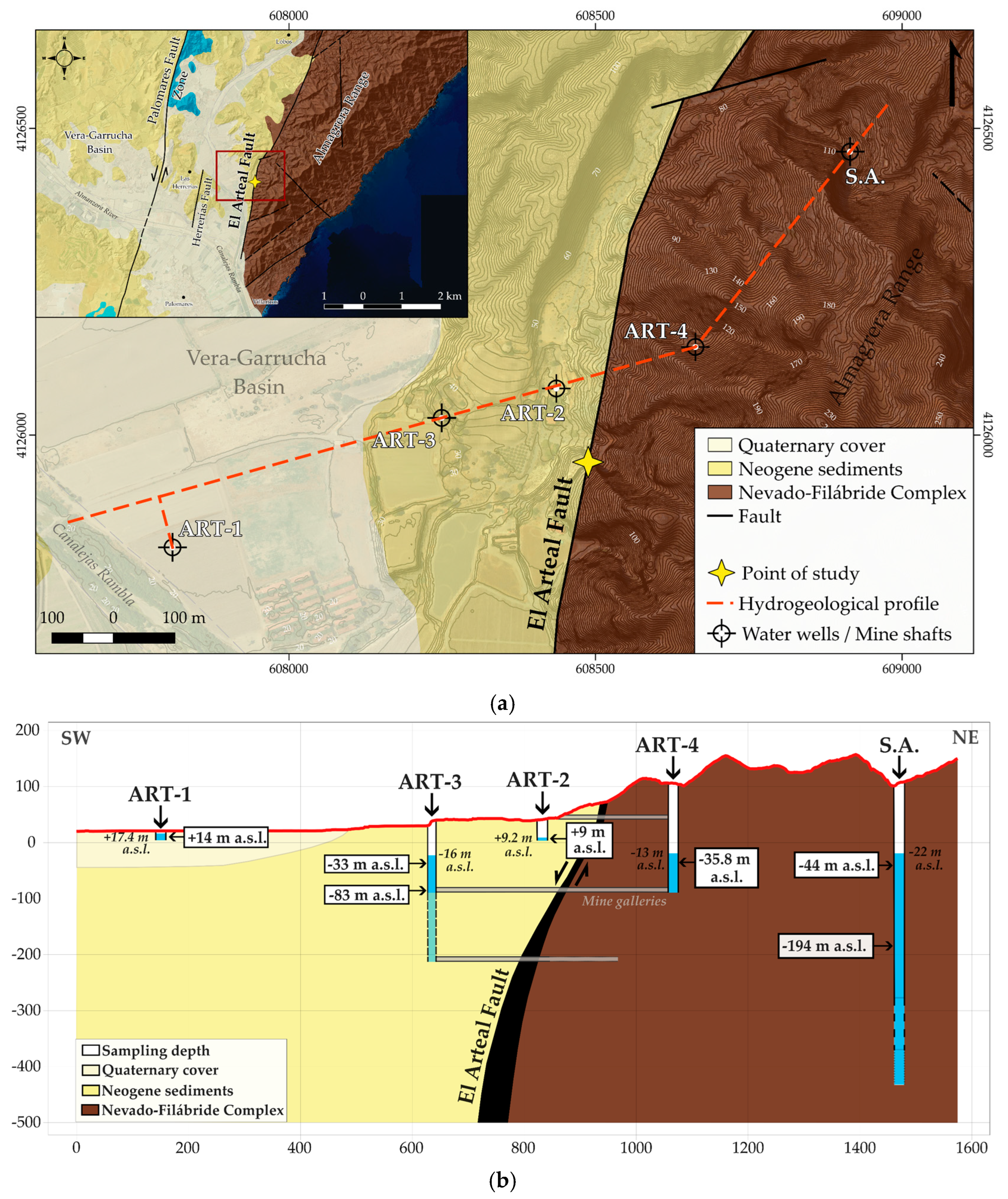
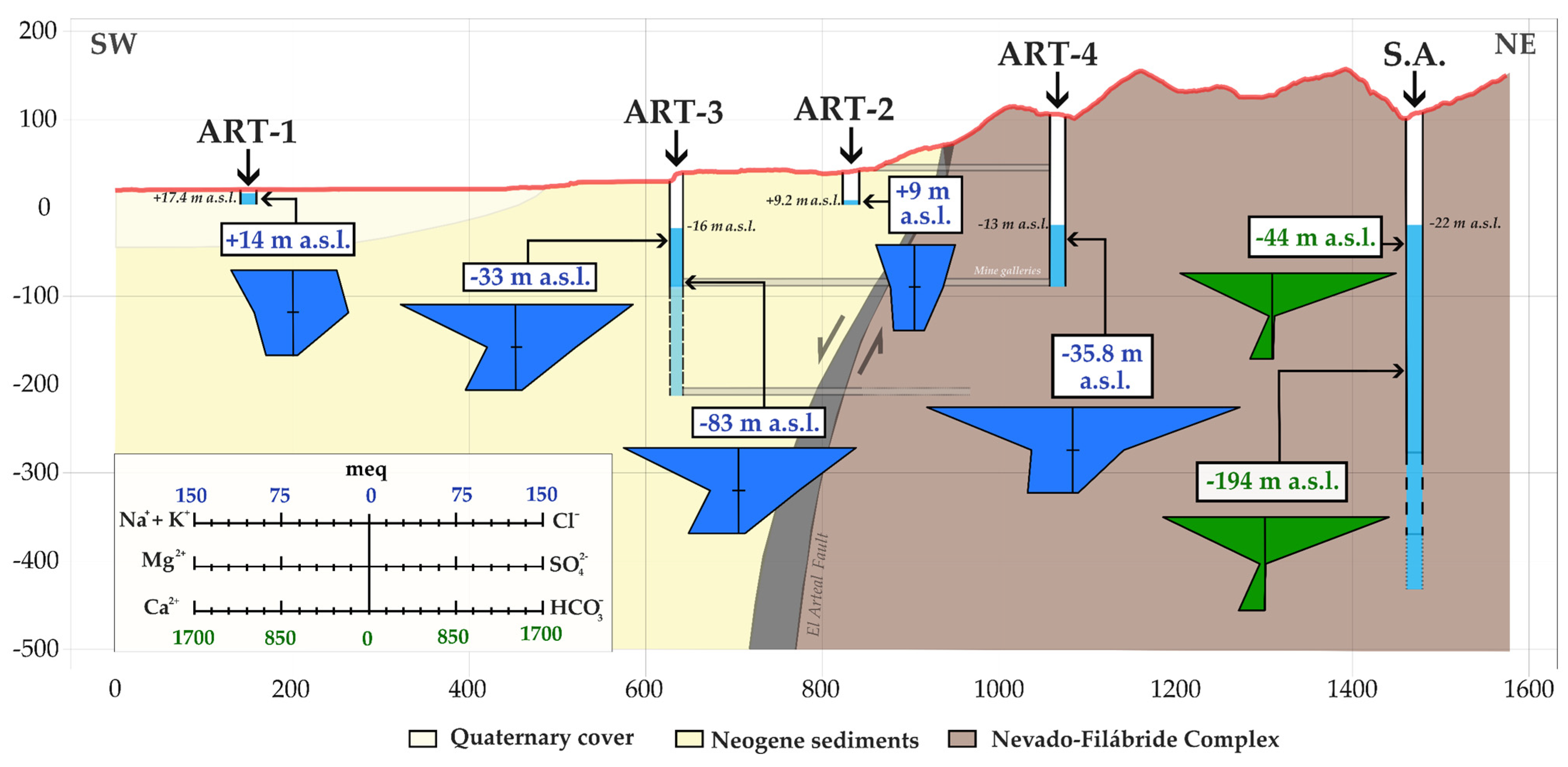
3. Hydrogeological Context
4. Materials and Methods
5. Results
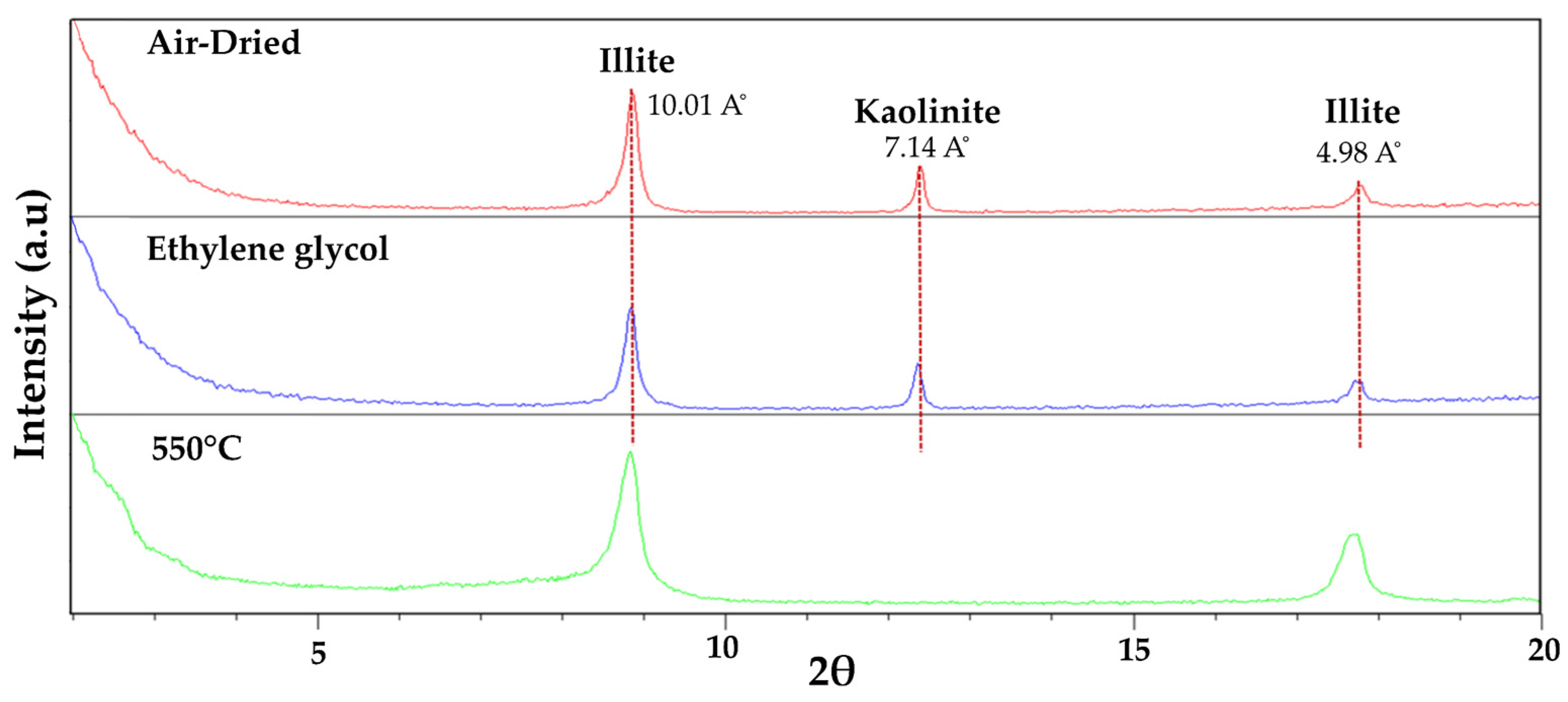
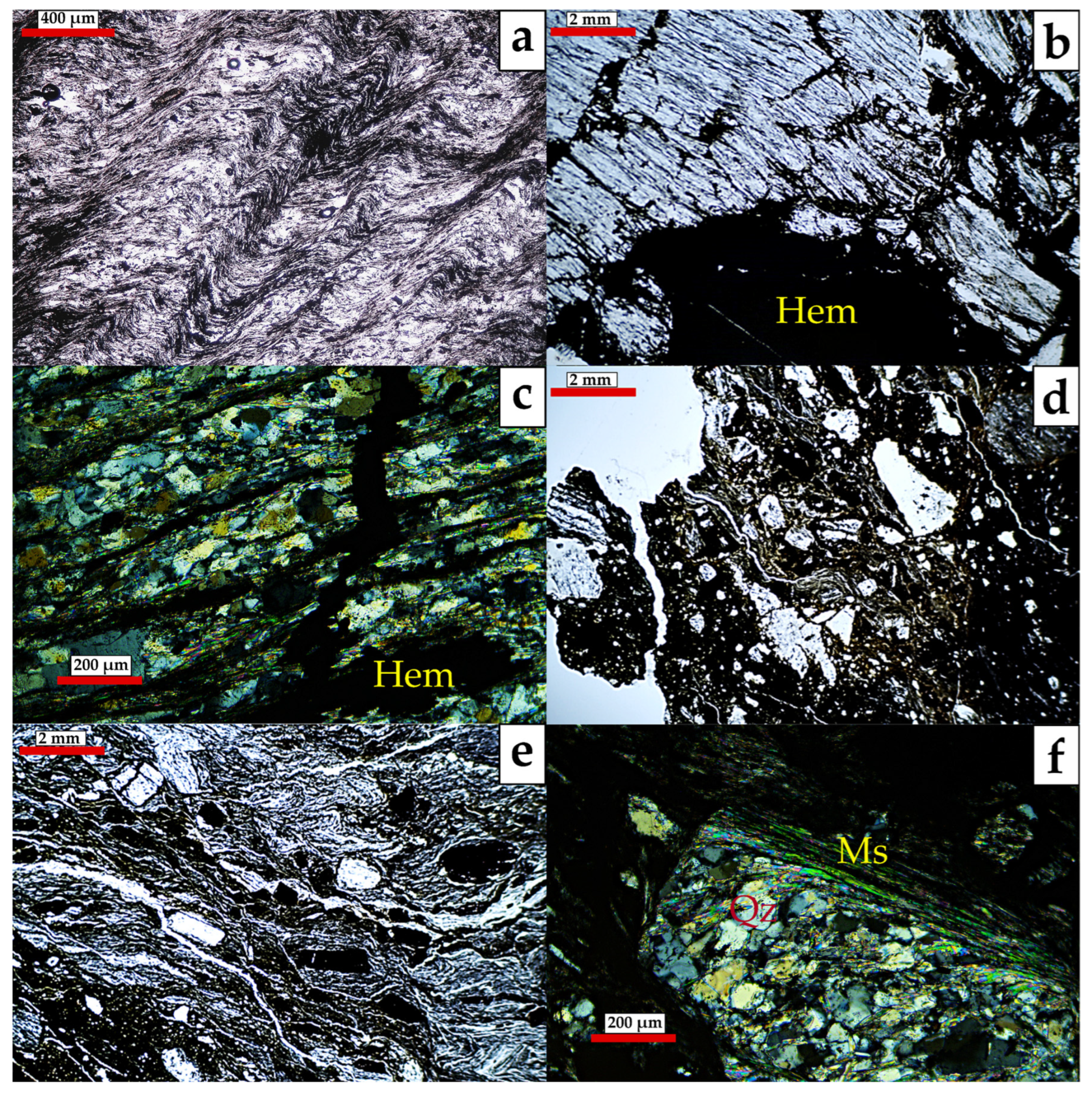
5.1. Mineralogy
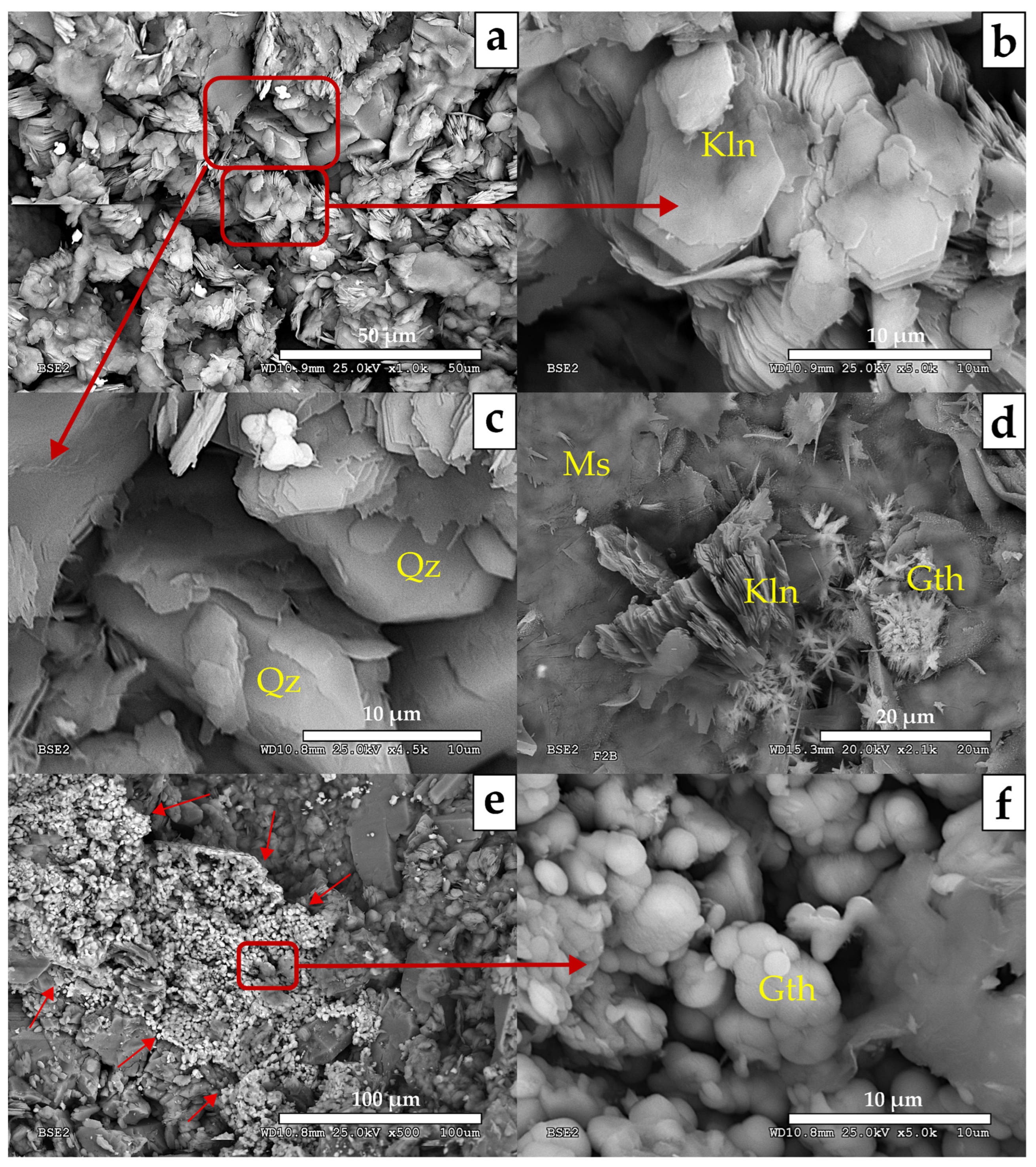
- MA-1 (Nevado-Filábride Complex reference rock (uplifted block): F1 sample). The assemblage is dominated by quartz (71%) and phyllosilicates (25%) with contents below 5% of potassium feldspar and goethite. Phyllosilicates are dominated by illite-muscovite, with traces of kaolinite. The presence of halite was not detected.
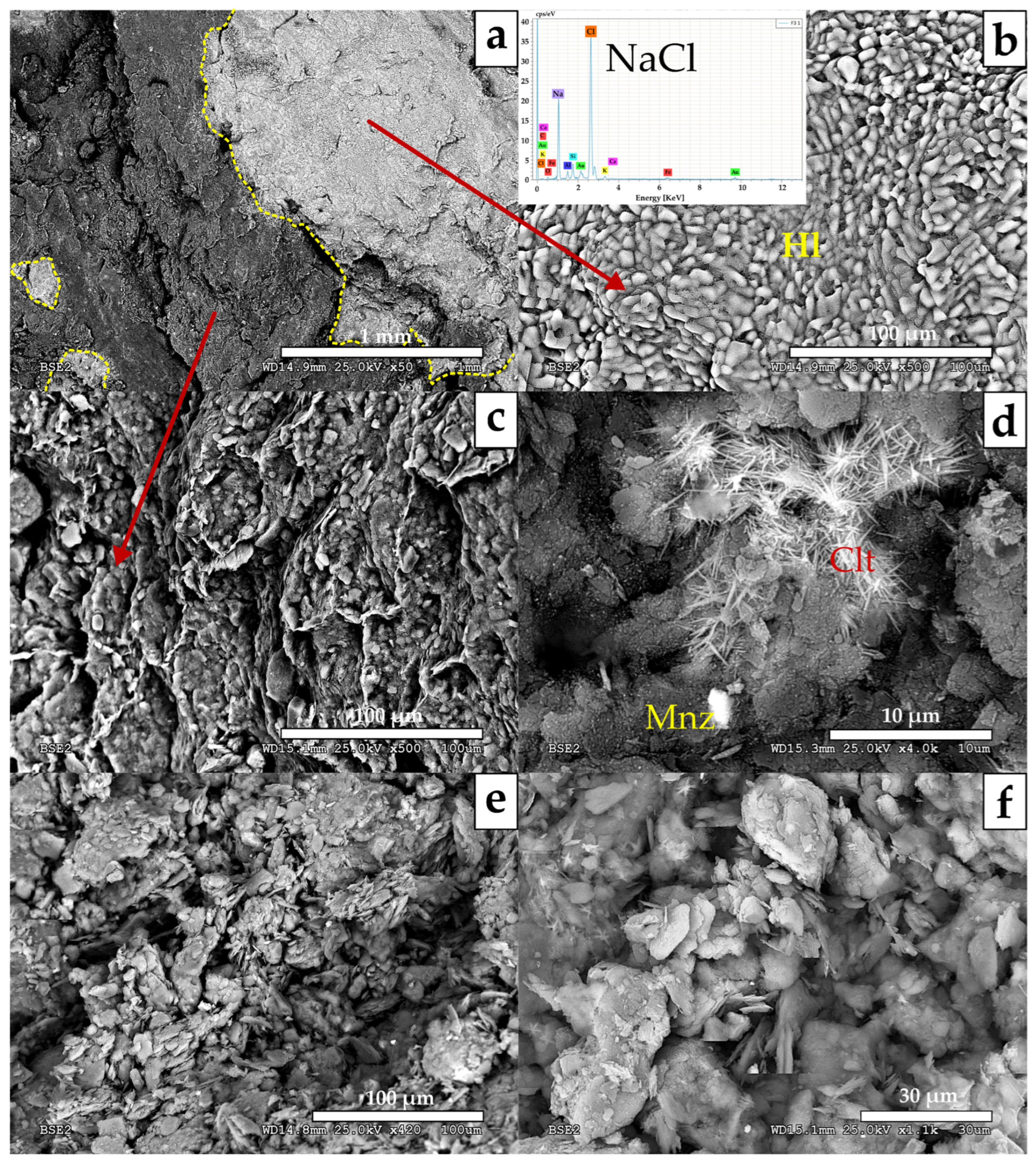
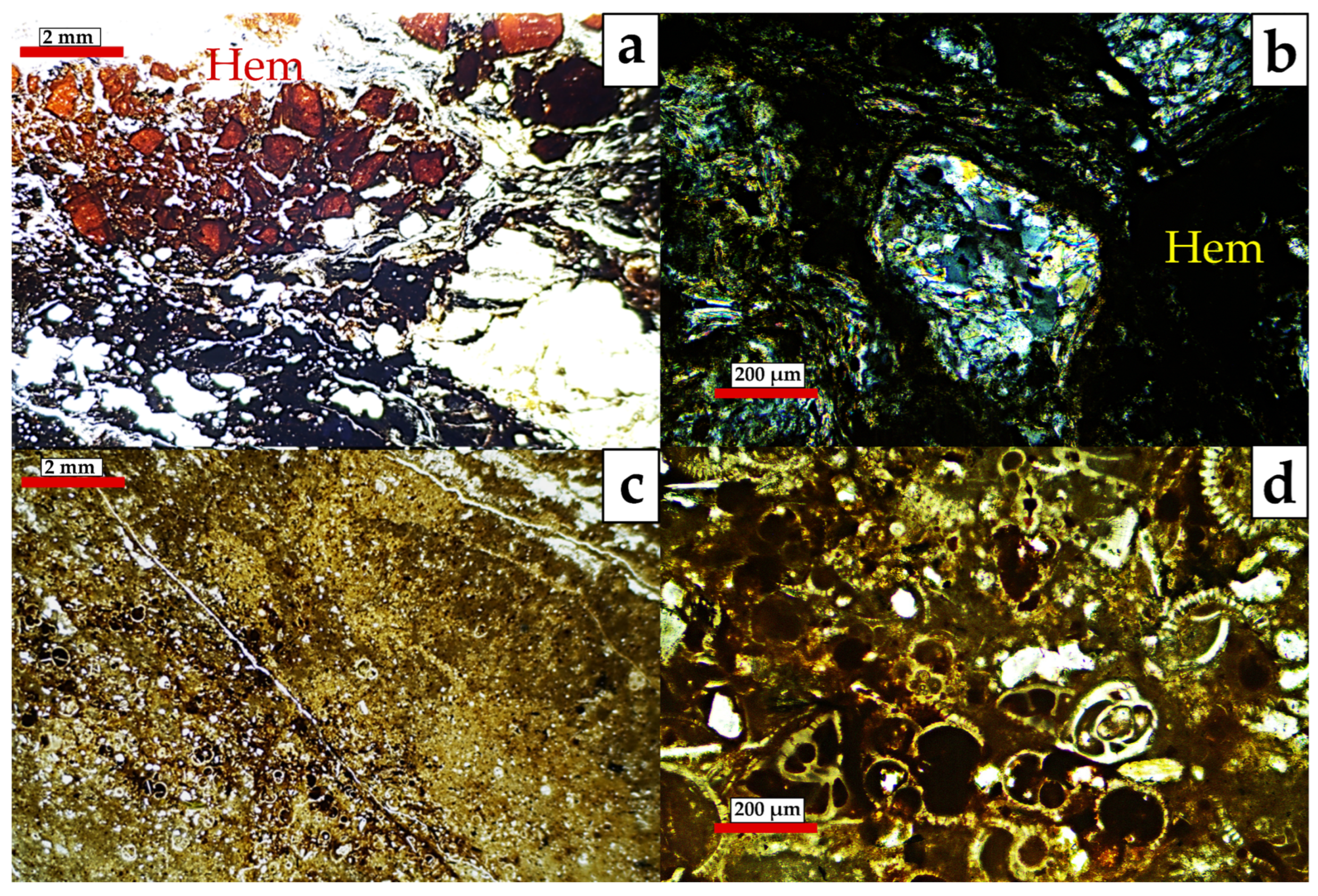
- MA-2 (Fault core: F2A and F2B samples; Figure 5). It is dominated by quartz (55–68%) and phyllosilicates (23–30%) with a relevant content of hematite (1–14%) and goethite (1–6%) together with traces of halite and rutile. Petrographic analysis and SEM observations also identified tourmaline, zircon, plagioclase, and gypsum. Among the phyllosilicates, illite-muscovite and kaolinite were both detected. The study of the clay fraction (<2 µm) revealed contents of 90% illite and 10% kaolinite.
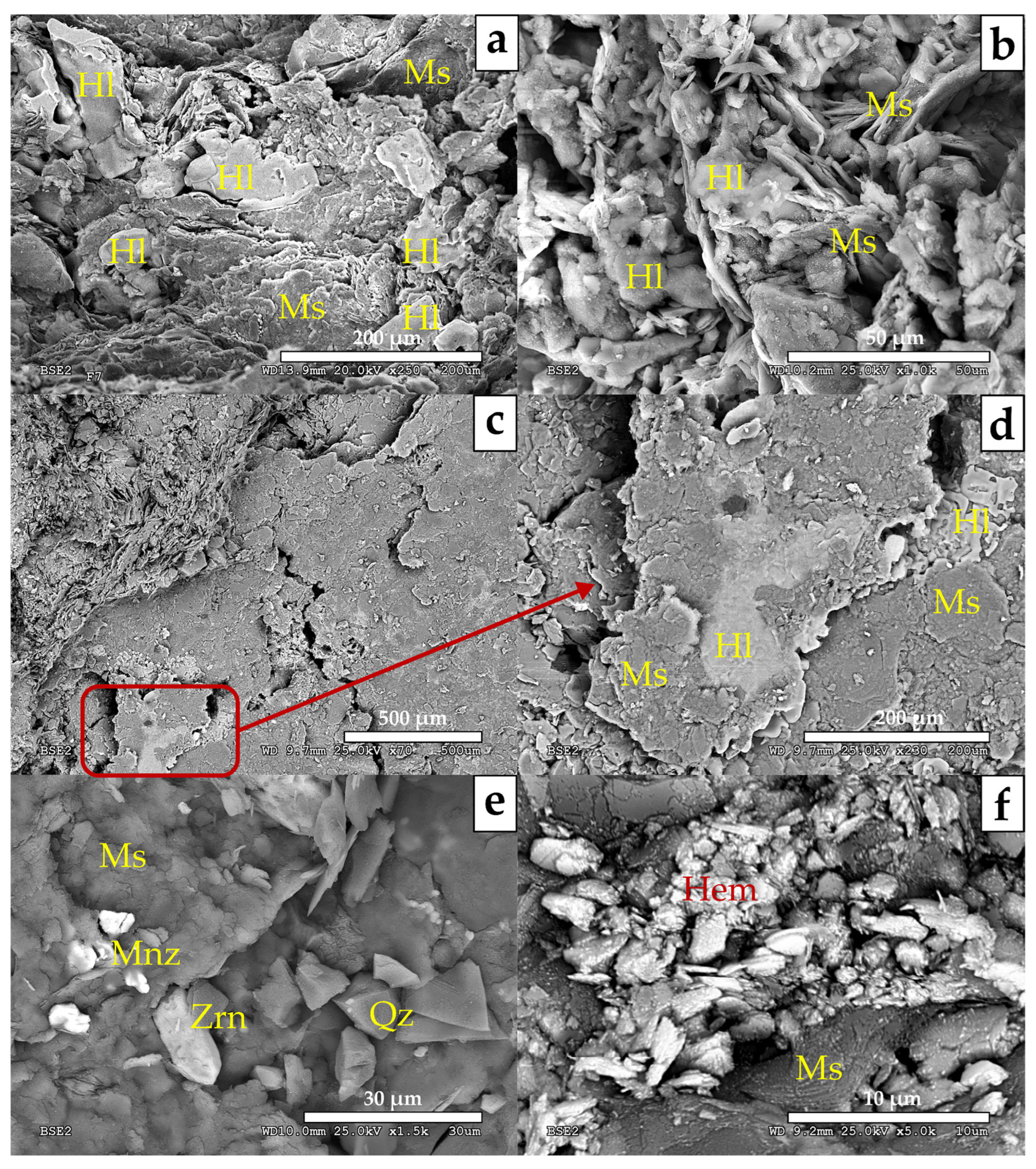
- MA-3 (Fault core: F3, F4 and F5 samples; Figure 6). It is characterized by a decrease in quartz (39–52%) and an increase in phyllosilicates (42–43%), with variable amounts of goethite (3–8%), plagioclase (0–9%), halite (1–2%), and traces of potassium feldspar. Petrographic analysis and SEM observations also identified the presence of rutile, celestine, monazite, gypsum, and barite. Phyllosilicates are dominated by illite-muscovite and kaolinite, with minor indications of paragonite. The clay fraction (<2 µm) consisted of approximately 96% illite and 4% kaolinite.
- MA-4 (Fault core: F6, F7, F8 and F8B samples; Figure 7). It is primarily composed of phyllosilicates (49–64%) with a minor amount of quartz (23–41%) with variable contents of goethite (0–6%), hematites (0–9%), halite (traces < 1–9%), dolomite (0–6%), some feldspars (0–5%) and with traces of gypsum. Petrographic analysis and SEM observations additionally have detected rutile, monazite, tourmaline, trydimite, and zircon. Phyllosilicates are dominated by illite-muscovite and kaolinite, with minor indications of paragonite. The clay fraction (<2 µm) consisted of approximately 93% illite and 7% kaolinite.
- MA-5 (Calcareous fault core: F9, F10, and F11 samples). The assemblage is dominated by phyllosilicates (33–46%), calcite (21–36%), quartz (10–23%), and halite (2–11%). Dolomite, hematite, goethite, and gypsum are present with contents below 5%. SEM observations additionally have detected chromite and zircon. Phyllosilicates identified were illite-muscovite, paragonite, and kaolinite with traces of chlorite. The study of the clay fraction (<2 µm) revealed contents of 91% illite and 9% kaolinite.
- MA-6 (Neogene sediments (downthrown block): F12 and F13 samples). It is primarily composed of phyllosilicates (40–57%) and quartz (28–45%) and, in amounts equal to or less than 5%, of gypsum, calcite, and dolomite. Phyllosilicates identified were illite-muscovite, paragonite, chlorite, and kaolinite. The presence of halite was not detected.
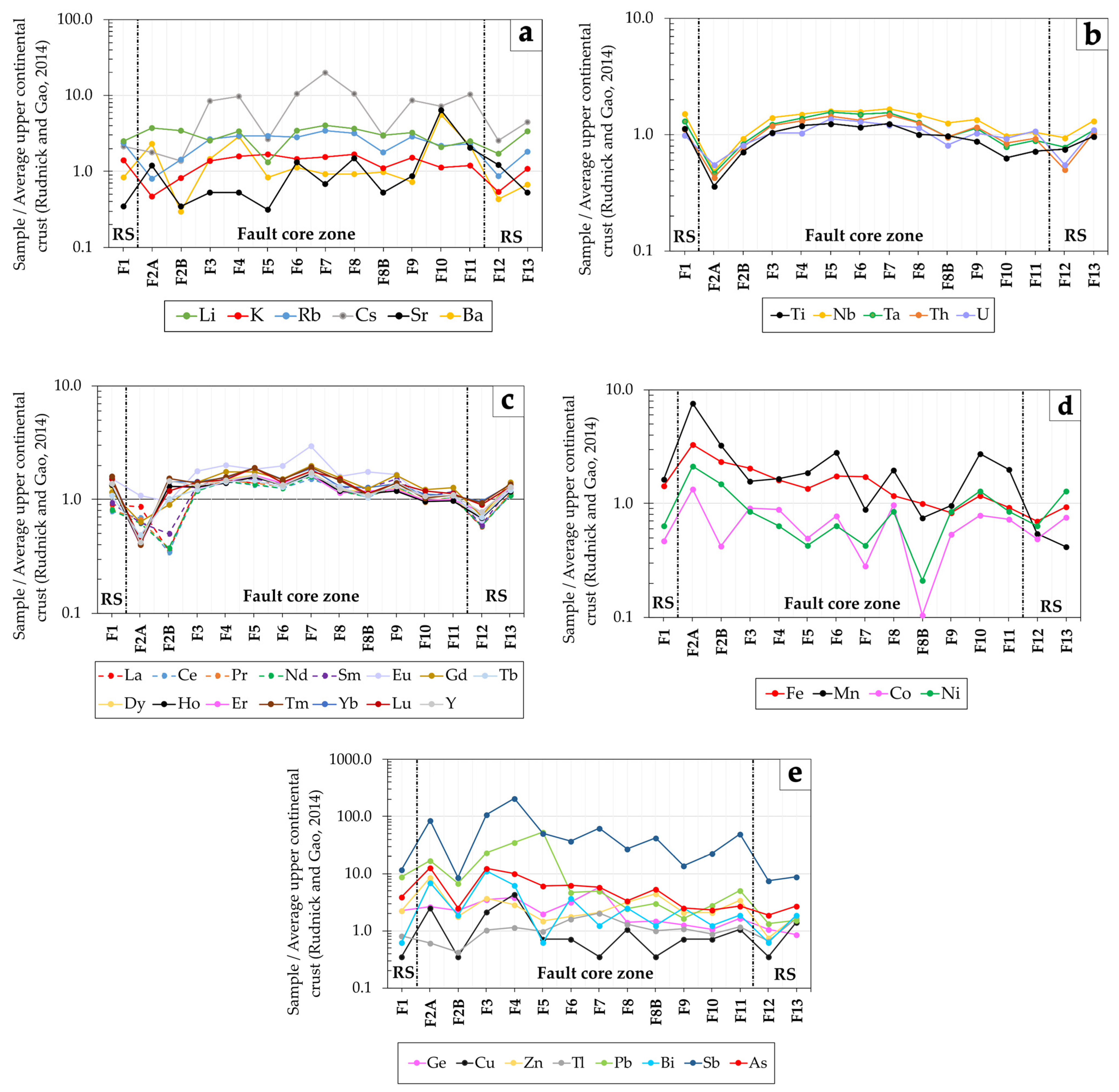
5.2. Geochemistry
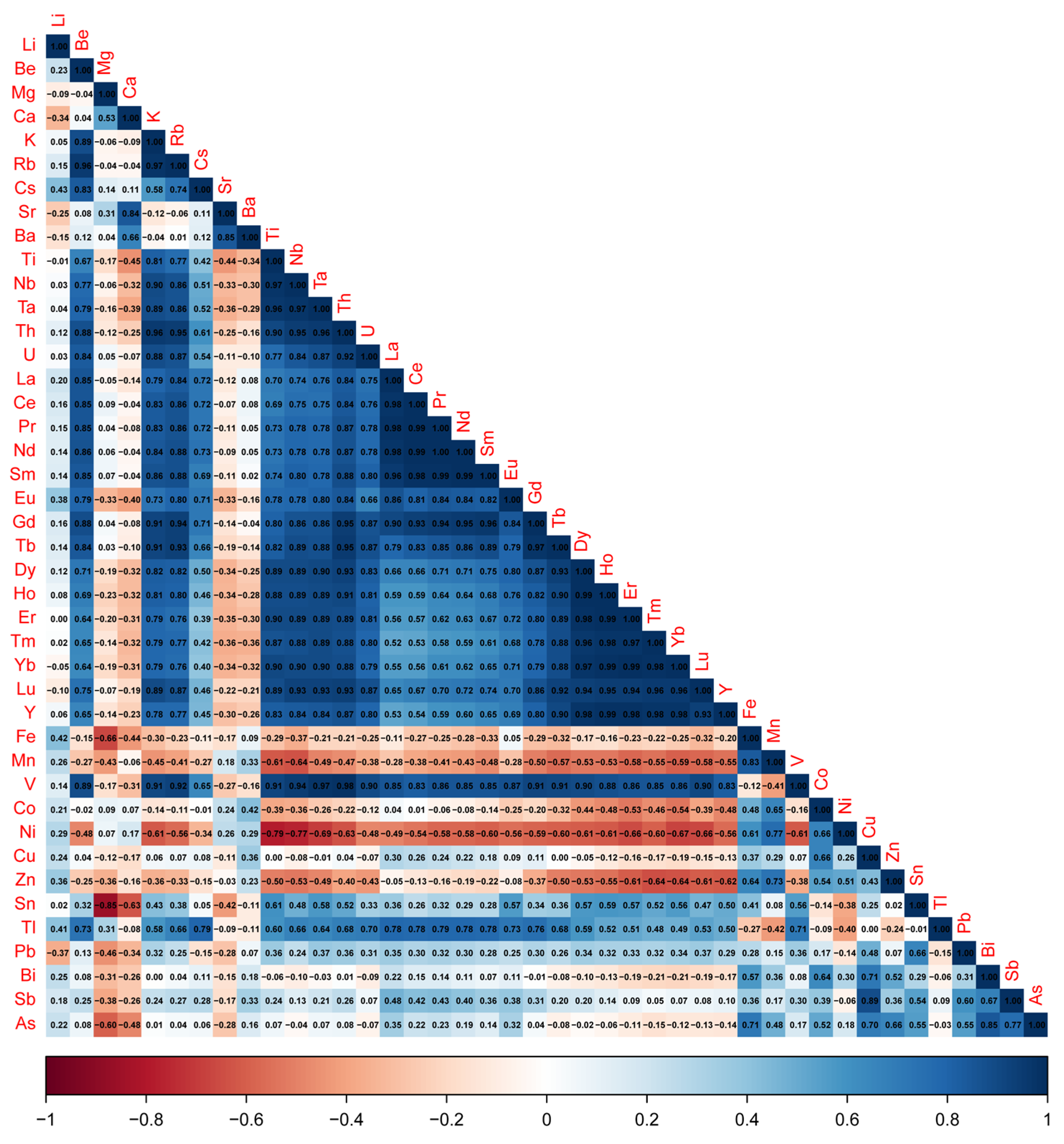
5.3. Element Associations
5.4. Hydrochemical Data
6. Discussion
6.1. Fault Rock Mineralogy: Implications for Hydrothermal (Re)Activation
6.2. Geochemical Signature in the Fault Segment
6.3. The Hydrogeological Key
6.4. Comparative Analysis of Hydrochemistry and Geochemistry in El Arteal Fault Segment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFZ | Palomares Fault Zone |
| TASZ | Trans-Alboran Shear Zone |
| EBSZ | Eastern Betic Shear Zone |
| HP–LT | High-Pressure/Low-Temperature |
| NVP | Neogene Volcanic Province |
| LILE | Large Ion Lithophile Elements |
| HFSE | High Field Strength Elements |
| LREE | Light Rare-Earth Elements |
| HREE | Heavy Rare-Earth Elements |
| TTE | Transition Trace Elements |
| SEM | Scanning Electron Microscopy |
| BSE | Backscattered Electrons |
| SE | Secondary Electrons |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| ICP-AES | Inductively Coupled Plasma–Atomic Emission Spectroscopy |
| XRD | X-Ray Diffraction |
| D.L. | Detection Limits |
| MA | Mineral Assemblages |
| TDS | Total Dissolved Solids |
| RS | Reference Samples |
References
- Liu, M.; Kong, Y.; Guo, Q. Sources and Enrichment Mechanisms of Lithium, Rubidium, and Cesium in Waters of Magmatic-Hydrothermal Systems. Earth-Sci. Rev. 2025, 270, 105241. [Google Scholar] [CrossRef]
- Sanjuan, B.; Gourcerol, B.; Millot, R.; Rettenmaier, D.; Jeandel, E.; Rombaut, A. Lithium-Rich Geothermal Brines in Europe: An up-Date about Geochemical Characteristics and Implications for Potential Li Resources. Geothermics 2022, 101, 102385. [Google Scholar] [CrossRef]
- Szanyi, J.; Rybach, L.; Abdulhaq, H.A. Geothermal Energy and Its Potential for Critical Metal Extraction—A Review. Energies 2023, 16, 7168. [Google Scholar] [CrossRef]
- Shu, Q.; Deng, J. The Composition of Magmatic-Hydrothermal Fluids and Their Related Metal Mineralization. Sci. China Earth Sci. 2025, 68, 208–225. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.; Zhu, Y.S.; Ge, W.C.; Ji, Z.; Jing, Y.; Zhang, Z.C. How Do Fluids Control Beryllium Mineralization in a Magmatic-Hydrothermal System: Evidence from Mica Geochemistry and Quartz-Beryl O Isotopes. Am. Mineral. 2025. [Google Scholar] [CrossRef]
- Addleman, R.S. Recovery of Rare Earths, Precious Metals and Other Critical Materials from Geothermal Waters with Advanced Sorbent Structures—CRADA 355 (Abstract); Pacific Northwest National Laboratory: Richland, WA, USA, 2025; p. 5. [Google Scholar]
- Pawar, R.; Santara, S.; Sircar, A.; Kumari, R.; Bist, N.; Yadav, K. Extraction of Salt and Base Metals from Geothermal Water: Kinetic Modeling and Mechanism. MRS Energy Sustain. 2023, 10, 219–237. [Google Scholar] [CrossRef]
- Audétat, A. The Metal Content of Magmatic-Hydrothermal Fluids and Its Relationship to Mineralization Potential. Econ. Geol. 2019, 114, 1033–1056. [Google Scholar] [CrossRef]
- Faulds, J.; Coolbaugh, M.; Bouchot, V.; Moeck, I.; Oğuz, K. Characterizing Structural Controls of Geothermal Reservoirs in the Great Basin, USA, and Western Turkey: Developing Successful Exploration Strategies in Extended Terranes; World Geothermal Congress: Bali, Indonesia, 2010; p. 11. [Google Scholar]
- Faulkner, D.R.; Mitchell, T.M.; Rutter, E.H.; Cembrano, J. On the Structure and Mechanical Properties of Large Strike-Slip Faults. In The Internal Structure of Fault Zones: Implications for Mechanical and Fluid-Flow Properties; Wibberley, C.A.J., Kurz, W., Imber, J., Holdsworth, R.E., Collettini, C., Eds.; Geological Society of London: London, UK, 2008; Volume 299, pp. 139–150. [Google Scholar]
- Faulkner, D.R.; Jackson, C.A.L.; Lunn, R.J.; Schlische, R.W.; Shipton, Z.K.; Wibberley, C.A.J.; Withjack, M.O. A Review of Recent Developments Concerning the Structure, Mechanics and Fluid Flow Properties of Fault Zones. J. Struct. Geol. 2010, 32, 1557–1575. [Google Scholar] [CrossRef]
- Schleicher, A.M.; Van Der Pluijm, B.A.; Warr, L.N. Nanocoatings of Clay and Creep of the San Andreas Fault at Parkfield, California. Geology 2010, 38, 667–670. [Google Scholar] [CrossRef]
- Sibson, R.H. Structural Permeability of Fluid-Driven Fault-Fracture Meshes. J. Struct. Geol. 1996, 18, 1031–1042. [Google Scholar] [CrossRef]
- Audétat, A.; Edmonds, M. Magmatic-Hydrothermal Fluids. Elements 2020, 16, 401–406. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Isotopic Shifts in Waters from Geothermal and Volcanic Systems along Convergent Plate Boundaries and Their Origin. Earth Planet. Sci. Lett. 1992, 113, 495–510. [Google Scholar] [CrossRef]
- Abad, I.; Jiménez-Millán, J.; Sánchez-Roa, C.; Nieto, F.; Velilla, N. Neocrystallization of Clay Minerals in the Alhama de Murcia Fault (Southeast Spain): Implications for Fault Mechanics. Clay Miner. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Abad, I.; Nieto, F.; Reolid, M.; Jiménez-Millán, J. Evidence of Phyllosilicate Alteration Processes and Clay Mineral Neoformation Promoted by Hydrothermal Fluids in the Padul Fault Area (Betic Cordillera, SE Spain). Appl. Clay Sci. 2022, 230, 106669. [Google Scholar] [CrossRef]
- Dyja, V.; Hibsch, C.; Tarantola, A.; Cathelineau, M.; Boiron, M.-C.; Marignac, C.; Bartier, D.; Carrillo-Rosúa, J.; Ruano, S.M.; Boulvais, P. From Deep to Shallow Fluid Reservoirs: Evolution of Fluid Sources during Exhumation of the Sierra Almagrera, Betic Cordillera, Spain. Geofluids 2016, 16, 103–128. [Google Scholar] [CrossRef]
- Haines, S.; Lynch, E.; Mulch, A.; Valley, J.W.; van der Pluijm, B. Meteoric Fluid Infiltration in Crustal-Scale Normal Fault Systems as Indicated by δ18O and δ2H Geochemistry and 40Ar/39Ar Dating of Neoformed Clays in Brittle Fault Rocks. Lithosphere 2016, 8, 587–600. [Google Scholar] [CrossRef]
- Hanson, R.B. Hydrodynamics of Magmatic and Meteoric Fluids in the Vicinity of Granitic Intrusions. Earth Environ. Sci. Trans. R. Soc. Edinb. 1996, 87, 251–259. [Google Scholar] [CrossRef]
- Weis, P. The Dynamic Interplay between Saline Fluid Flow and Rock Permeability in Magmatic-Hydrothermal Systems. Geofluids 2015, 15, 350–371. [Google Scholar] [CrossRef]
- Coppier, G.; Griveaud, P.; De Larouziere, F.-D.; Montenat, C.; Ott d’Estevou, P. Example of Neogene Tectonic Indentation in the Eastern Betic Cordilleras: The Arc of Aguilas (Southeastern Spain). Geodin. Acta 1989, 3, 37–51. [Google Scholar] [CrossRef]
- Sanz De Galdeano, C. Geologic Evolution of the Betic Cordilleras in the Western Mediterranean, Miocene to the Present. Tectonophysics 1990, 172, 107–119. [Google Scholar] [CrossRef]
- Sanz de Galdeano, C.; Azañón, J.M.; Cabral, J.; Ruano, P.; Alfaro, P.; Canora, C.; Ferrater, M.; García-Tortosa, F.J.; García-Myordomo, J.; Grácia, E.; et al. Active Faults in the Betic Cordillera. In The Geology of Iberia: A Geodynamic Approach: Volume 5: Active Processes: Seismicity, Active Faulting and Relief; Quesada, C., Oliveira, J.T., Eds.; Regional Geology Reviews; Springer Nature: Cham, Switzerland, 2020; Volume 5, pp. 56–75. [Google Scholar]
- Tendero-Salmerón, V.; Ercilla, G.; González-Castillo, L.; Madarieta-Txurruka, A.; Martínez-Moreno, F.J.; Estrada, F.; Galindo-Zaldivar, J. Gravimetric Survey and Modelling of a Tectonic Indenter Boundary: The Palomares Fault Zone (Betic Cordillera, Iberia). Tectonophysics 2024, 872, 230147. [Google Scholar] [CrossRef]
- Weijermars, R. The Palomares Brittle—Ductile Shear Zone of Southern Spain. J. Struct. Geol. 1987, 9, 139–157. [Google Scholar] [CrossRef]
- Instituto Geológico y Minero de España (IGME); Empresa Nacional Adaro de Investigaciones Mineras (ENADIMSA). Investigación de las Posibilidades de Existencia de Energía Geotérmica en la Comarca de Mazarrón–Águilas (Murcia). Tomo I.; IGME: Madrid, Spain, 1985; pp. 1–29. [Google Scholar]
- De Larouzière, F.D.; Bolze, J.; Bordet, P.; Hernandez, J.; Montenat, C.; Ott d’Estevou, P. The Betic Segment of the Lithospheric Trans-Alboran Shear Zone during the Late Miocene. Tectonophysics 1988, 152, 41–52. [Google Scholar] [CrossRef]
- Somoza, L.; Medialdea, T.; Terrinha, P.; Ramos, A.; Vázquez, J.-T. Submarine Active Faults and Morpho-Tectonics Around the Iberian Margins: Seismic and Tsunamis Hazards. Front. Earth Sci. 2021, 9, 653639. [Google Scholar] [CrossRef]
- López Ruiz, J.; Rodríguez Badiola, E. La Region Volcánica Neogena del Sureste de España. Estud. Geológicos 1980, 36, 5–63. [Google Scholar]
- Casalini, M.; Avanzinelli, R.; Tommasini, S.; Natali, C.; Bianchini, G.; Prelević, D.; Mattei, M.; Conticelli, S. Petrogenesis of Mediterranean Lamproites and Associated Rocks: The Role of Overprinted Metasomatic Events in the Post-Collisional Lithospheric Upper Mantle. In Lamprophyres, Lamproites and Related Rocks: Tracers to Supercontinent Cycles and Metallogenesis; Krmíček, L., Chalapathi Rao, N.V., Eds.; Geological Society of London: London, UK, 2022; ISBN 978-1-78620-543-8. [Google Scholar]
- Torne, M.; Jiménez-Munt, I.; Negredo, A.M.; Fullea, J.; Vergés, J.; Marzán, I.; Alcalde, J.; Gómez-Rivas, E.; de la Noceda, C.G. Advances in the Modeling of the Iberian Thermal Lithosphere and Perspectives on Deep Geothermal Studies. Geotherm Energy 2023, 11, 3. [Google Scholar] [CrossRef]
- Alonso-Chaves, F.M.; Andreo, B.; Arias, C.; Azañón, J.M.; Balanyá, J.C.; Barón, A.; Booth-Rea, G.; Castro, J.M.; Chacón, B.; Company, M.; et al. Cordillera Bética y Baleares. In Geología de España; Vera, J.A., Ed.; IGME: Madrid, Spain, 2004; pp. 346–464. [Google Scholar]
- Colmenar-Santos, A.; Folch-Calvo, M.; Rosales-Asensio, E.; Borge-Diez, D. The Geothermal Potential in Spain. Renew. Sustain. Energy Rev. 2016, 56, 865–886. [Google Scholar] [CrossRef]
- Instituto Geológico y Minero de España (IGME). La Energía Geotérmica; IGME: Madrid, Spain, 1985. [Google Scholar]
- Navarro, A.; Carulla, N. Evaluation of Geothermal Potential in the Vicinity of the Flooded Sierra Almagrera Mines (Almeria, SE Spain). Mine Water Env. 2018, 37, 137–150. [Google Scholar] [CrossRef]
- Booth-Rea, G.; Azañón, J.M.; Garcı́a-Dueñas, V.; Augier, R. Uppermost Tortonian to Quaternary Depocentre Migration Related with Segmentation of the Strike-Slip Palomares Fault Zone, Vera Basin (SE Spain). C. R. Geosci. 2003, 335, 751–761. [Google Scholar] [CrossRef]
- Booth-Rea, G.; Azañón, J.-M.; Azor, A.; Garcı́a-Dueñas, V. Influence of Strike-Slip Fault Segmentation on Drainage Evolution and Topography. A Case Study: The Palomares Fault Zone (Southeastern Betics, Spain). J. Struct. Geol. 2004, 26, 1615–1632. [Google Scholar] [CrossRef]
- Arribas Rosado, A.; Arribas Moreno, A. Caracteres metalogénicos y geoquímica isotópica del azufre y el plomo de los yacimientos de minerales metálicos del sureste de España. Boletín Geológico Y Min. 1995, 106, 23–62. [Google Scholar]
- Martinez-Frías, J. Sulphide and sulphosalt mineralogy and paragenesis from the Sierra Almagrera veins, Betic Cordillera (SE Spain). Estud. Geológicos 1991, 47, 271–279. [Google Scholar] [CrossRef]
- López-Gutiérrez, J.; Martínez-Frías, J.; Lunar, R.; López-García, J.A. El Rombohorst mineralizado de Las Herrerias: Un caso de «doming» e hidrotermalismo submarino mioceno en el SE ibérico. Estud. Geológicos 1993, 49, 13–19. [Google Scholar] [CrossRef]
- Mata Perelló, J.M. Recorrido geológico y minero por las comarcas de Sorbas, Vera y Cuevas de Almanzora: Desde la Venta del Pobre a Cuevas de Almanzora y a la Sierra de Almagrera. Algeps Rev. Geol. 2011, XXXVIII, 1–8. [Google Scholar] [CrossRef]
- Navarro, A. Posible aprovechamiento geotérmico del drenaje minero en Sierra Almagrera (Almería). Re Met. 2018, 30, 89–102. [Google Scholar]
- Navarro, A. Las Aguas Termales de Sierra Almagrera: De Un Problema Histórico En El Desarrollo de La Actividad Minera a Un Recurso Por Explotar. In Geología y Minería en Los Siglos XIX y XX: Paisajes, Historia y Patrimonio. Actas Del XIX Congreso Internacional Sobre Patrimonio Geológico y Minero; Ayarzagüen Sanz, M., Fernández Bolea, E., López Cidad, J.F., Sebastián Pérez, M.Á., Eds.; Sociedad Española para la Defensa del Patrimonio Geológico y Minero (SEDPGYM): Almería, Spain, 2023; pp. 60–80. [Google Scholar]
- Sánchez Martos, F.; Alonso Blanco, J.M.; Calaforra Chordi, J.M. Encuadre geológico de la provincia de Almería. In Problemática de la Gestión del Agua en Regiones Semiáridas; Instituto de Estudios Almerienses: Almería, Spain, 2001; pp. 15–28. ISBN 84-8108-240-6. [Google Scholar]
- Artero García, J.M. Síntesis geológico-minera de la provincia de Almería. Boletín Inst. Estud. Almerienses. Cienc. 1986, 57–79. [Google Scholar]
- Booth-Rea, G.; Azañón, J.M.; Goffé, B.; Vidal, O.; Martı́nez-Martı́nez, J.M. High-Pressure, Low-Temperature Metamorphism in Alpujarride Units of Southeastern Betics (Spain). C. R. Géosci. 2002, 334, 857–865. [Google Scholar] [CrossRef]
- Stokes, M. Plio-Pleistocene Drainage Development in an Inverted Sedimentary Basin: Vera Basin, Betic Cordillera, SE Spain. Geomorphology 2008, 100, 193–211. [Google Scholar] [CrossRef]
- Augier, R.; Jolivet, L.; Robin, C. Late Orogenic Doming in the Eastern Betic Cordilleras: Final Exhumation of the Nevado-Filabride Complex and Its Relation to Basin Genesis. Tectonics 2005, 24, TC4003. [Google Scholar] [CrossRef]
- Booth-Rea, G.; Azañón, J.M.; Martínez-Martínez, J.M.; Vidal, O.; García-Dueñas, V. Contrasting Structural and P-T Evolution of Tectonic Units in the Southeastern Betics: Key for Understanding the Exhumation of the Alboran Domain HP/LT Crustal Rocks (Western Mediterranean). Tectonics 2005, 24, TC2009. [Google Scholar] [CrossRef]
- Montenat, C.; Bizon, G.; Bizon, J.-J.; Carbonnel, G.; Muller, C.; Reneville, P.D. Continuité ou discontinuité de sémentation marine mio-pliocène en Méditerranée occidentale. L’example du bassin de vera (Espagne méridionale). Rev. Inst. Fr. Pét. 1976, 31, 613–664. [Google Scholar] [CrossRef]
- Ott d’Estevou, P.; Montenat, C.; Alvado, J.C. Le Bassin de Vera-Garrucha. In Les Bassins Néogeènes du Domaine Beétique Orientale (Espagne); Documents et Travaux du Institut Géologique Albert-de-Lapparent 12–13; Institut Géologique Albert-de-Lapparent: Paris, France, 1990; pp. 165–187. [Google Scholar]
- Sánchez-Bellón, A.; Mosser, C.; Roquin, C.; Pardo-Sebastián, E. Geochemical Characterization of Sedimentary Basins by Statistical Analysis: The Mio-Pliocene Sequences of the Vera Basin, SE Spain. Chem. Geol. 1994, 116, 229–243. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.M.; Azañón, J.M. Mode of Extensional Tectonics in the Southeastern Betics (SE Spain): Implications for the Tectonic Evolution of the Peri-Alborán Orogenic System. Tectonics 1997, 16, 205–225. [Google Scholar] [CrossRef]
- Platt, J.P.; Vissers, R.L.M. Extensional Collapse of Thickened Continental Lithosphere: A Working Hypothesis for the Alboran Sea and Gibraltar Arc. Geology 1989, 17, 540–543. [Google Scholar] [CrossRef]
- Weijermars, R.; Roep, T.B.; Eeckhout, B.V.d.; Postma, G.; Kleverlaan, K. Uplift History of a Betic Fold Nappe Inferred from Neogene-Quaternary Sedimentation and Tectonics (in the Sierra Alhamilla and Almeria, Sorbas and Tabernas Basins of the Betic Cordilleras, SE Spain). Neth. J. Geosci. 1985, 56, 397–411. [Google Scholar]
- Weijermars, R. Geology and Tectonics of the Betic Zone, SE Spain. Earth-Sci. Rev. 1991, 31, 153–236. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.M.; Soto, J.I.; Balanyá, J.C. Orthogonal Folding of Extensional Detachments: Structure and Origin of the Sierra Nevada Elongated Dome (Betics, SE Spain). Tectonics 2002, 21, 3-1-3-20. [Google Scholar] [CrossRef]
- Tendero-Salmerón, V.; Ercilla, G.; González-Castillo, L.; Madarieta-Txurruka, A.; Martínez-Moreno, F.J.; Estrada, F.; Galindo-Zaldívar, J. Influencia de la zona de falla de Palomares en las cuencas adyacentes mediante prospección gravimétrica. Geotemas 2024, 600. [Google Scholar]
- Booth Rea, G.; Azañón, J.M.; Martínez-Martínez, J.M.; Vidal, O.; García-Dueñas, V. Análisis estructural y evolución tectonometamórfica del basamento de las cuencas neógenas de Vera y Huércal-Overa, Béticas orientales. Rev. Soc. Geológica España 2003, 16, 193–211. [Google Scholar]
- Álvarez, F.; Aldaya, F. Las unidades de la Zona Bética en la región de Aguilas-Mazarrón (prov. de Murcia). Estud. Geológicos 1985, 41, 139–148. [Google Scholar] [CrossRef]
- Martinez-Frías, J.; García-Guinea, J.; López-Ruiz, J.; López-García, J.Á.; Benito-García, R. Las mineralizaciones epitermales de Sierra Almagrera y de la cuenca de Herrerías, Cordilleras Béticas. Boletín Soc. Española Mineral. 1989, 12, 261–271. [Google Scholar]
- Martínez-Frías, J.; García-Guinea, J. Yacimientos de plata y chimeneas submarinas asociadas del área de Cuevas de Almanzora. In Recursos Naturales y Medio Ambiente de Cuevas del Almanzora; García Rossell, L., Martínez-Frías, J., Eds.; Instituto de Estudios Almerienses: Almería, Spain, 1993; pp. 237–262. [Google Scholar]
- Morales Ruano, S. Mineralogía, Geoquímica y Metalogenia de Los Yacimientos Hidrotermales del Sureste de España (Águilas-Sierra Almagrera). Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1994. [Google Scholar]
- Font, X.; Rodríguez, P.; Navarro Flores, A.; Viladevall Solé, M. Las mineralizaciones auríferas de Sierra Almagrera (Almería): Estudio geoquímico y modelos de yacimientos. Boletín Geológico Min. 1994, 105, 85–101. [Google Scholar]
- Trio Maseda, M.; Guillermo Ortuño, M. BARIO06: Panorama Nacional de Producción Minera de Barita; Instituto Geológico y Minero de España (IGME): Madrid, Spain, 2006; pp. 1–6. [Google Scholar]
- Carulla, N. Contribución al Conocimiento de la Dinámica Hidrogeólogica en Clima Semiárido Depresion de Vera (Almería). Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 1978. [Google Scholar]
- Gómez Iribarne, B. Estadística Minera de España; Consejo de Minería, Ministerio de Fomento, Dirección General de Agricultura, Industria y Comercio: Madrid, Spain, 1908; pp. 142–149. [Google Scholar]
- Carulla, N. Las salmueras mineras, termales y litiníferas de Sierra Almagrera (N.E. Almería). Geotemas 2012, 930–933. [Google Scholar]
- Ovejero, G. Aguas Mineralizadas de Almagrera—Sondeo de Investigación. Informe Anual; Peñarroya—MASA: Almería, Spain, 1982; pp. 1–4. [Google Scholar]
- Alcalá, F.J.; Custodio, E. Using the Cl/Br Ratio as a Tracer to Identify the Origin of Salinity in Aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Santamaría-López, Á.; Abad, I.; Nieto, F.; Galdeano, C.S.d.; Santamaría-López, Á.; Abad, I.; Nieto, F.; Galdeano, C.S. de Early Mylonitization in the Nevado-Filábride Complex (Betic Cordillera) during the High-Pressure Episode: Petrological, Geochemical and Thermobarometric Data. Minerals 2022, 13, 24. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: New York, NY, USA, 1989; ISBN 10: 0195087135. [Google Scholar]
- Schultz, L.G. Quantitative Interpretation of Mineralogical Composition from X-Ray and Chemical Data for the Pierre Shale; U.S. Geological Survey Professional Paper 391; United States Government Printing Office: Washington, DC, USA, 1964; pp. C1–C31. [CrossRef]
- Van der Marei, H.W. Quantitative Analysis of Clay Minerals and Their Admixtures. Contr. Mineral. Petrol. 1966, 12, 96–138. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for Names of Rock-Forming Minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Priem, H.N.A.; Boelrijk, N.A.J.M.; Hebeda, E.H.; Verschure, R.M. Isotopic Age Determinations on Tourmaline Granite-Gneiss and a Metagranite in the Eastern Betic Cordilleras, SE Spain. Geol. Mijnb. 1966, 45, 184–187. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–51. [Google Scholar]
- Putnis, A. Mineral Replacement Reactions: From Macroscopic Observations to Microscopic Mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Wintsch, R.P.; Christoffersen, R.; Kronenberg, A.K. Fluid-Rock Reaction Weakening of Fault Zones. J. Geophys. Res. Solid Earth 1995, 100, 13021–13032. [Google Scholar] [CrossRef]
- Inoue, A.; Kitagawa, R. Morphological Characteristics of Illitic Clay Minerals from a Hydrothermal System. Am. Mineral. 1994, 79, 700–711. [Google Scholar]
- Fulignati, P. Clay Minerals in Hydrothermal Systems. Minerals 2020, 10, 919. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 1980; Volume 5, ISBN 978-0-903056-37-3. [Google Scholar]
- Ece, Ö.I.; Ercan, H.Ü. Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits. Minerals 2024, 14, 353. [Google Scholar] [CrossRef]
- Abad, I.; Jiménez-Millán, J.; Schleicher, A.M.; van der Pluijm, B.A. Mineral Characterization, Clay Quantification and Ar–Ar Dating of Faulted Schists in the Carboneras and Palomares Faults (Betic Cordillera, SE Spain). Eur. J. Mineral. 2017, 29, 17–34. [Google Scholar] [CrossRef]
- Jiménez-Millán, J.; Abad, I.; Hernández-Puentes, P.; Jiménez-Espinosa, R. Influence of Phyllosilicates and Fluid–Rock Interaction on the Deformation Style and Mechanical Behaviour of Quartz-Rich Rocks in the Carboneras and Palomares Fault Areas (SE Spain). Clay Miner. 2015, 50, 619–638. [Google Scholar] [CrossRef]
- Gruner, J.W. Conditions for the Formation of Paragonite. Am. Mineral. 1942, 27, 131–134. [Google Scholar]
- Tekin, E.; Varol, B.; Ayan, Z.; Satir, M. Epigenetic Origin of Celestite Deposits in the Tertiary Sivas Basin: New Mineralogical and Geochemical Evidence. Neues Jahrb. Für Mineral.-Monatshefte 2002, 2002, 289–318. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Formation. In The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 345–364. ISBN 978-3-527-60209-4. [Google Scholar]
- Schwertmann, U.; Friedl, J.; Stanjek, H. From Fe(III) Ions to Ferrihydrite and Then to Hematite. J. Colloid Interface Sci. 1999, 209, 215–223. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Crystal Morphology and Size. In The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 59–94. ISBN 978-3-527-60209-4. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Bruhn, R.L.; Parry, W.T.; Yonkee, W.A.; Thompson, T. Fracturing and Hydrothermal Alteration in Normal Fault Zones. Pure Appl. Geophys. 1994, 142, 609–644. [Google Scholar] [CrossRef]
- Zheng, Y.-F. Subduction Zone Geochemistry. Geosci. Front. 2019, 10, 1223–1254. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, M.; Yu, C.; Cui, H. The Fluid Mobilities of K and Zr in Subduction Zones: Thermodynamic Constraints. Minerals 2021, 11, 394. [Google Scholar] [CrossRef]
- Środoń, J.; Jewuła, K. Controls over Cesium and Rubidium Contents of Sedimentary Rocks. Chem. Geol. 2025, 683, 122745. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The Role of Magmas in the Formation of Hydrothermal Ore Deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Heinrich, C.A. The Physical and Chemical Evolution of Low-Salinity Magmatic Fluids at the Porphyry to Epithermal Transition: A Thermodynamic Study. Min. Depos. 2005, 39, 864–889. [Google Scholar] [CrossRef]
- Cherniak, D.J. Diffusion in Accessory Minerals: Zircon, Titanite, Apatite, Monazite and Xenotime. Rev. Mineral. Geochem. 2010, 72, 827–869. [Google Scholar] [CrossRef]
- Poitrasson, F.; Chenery, S.; Bland, D.J. Contrasted Monazite Hydrothermal Alteration Mechanisms and Their Geochemical Implications. Earth Planet. Sci. Lett. 1996, 145, 79–96. [Google Scholar] [CrossRef]
- Abdel-Halim, A.H. Hydrothermal Alteration of Ni-Rich Sulfides in Peridotites of Abu Dahr, Eastern Desert, Egypt: Relationships among Minerals in the Fe-Ni-Co-O-S System, fO2 and fS2. Am. Mineral. 2023, 108, 614–633. [Google Scholar] [CrossRef]
- Berger, B.R. Hydrothermal Alteration. In Geochemistry; Springer: Dordrecht, The Netherlands, 1998; pp. 331–333. ISBN 978-1-4020-4496-0. [Google Scholar]
- Sanjuan, B.; Millot, R.; Innocent, C.; Dezayes, C.; Scheiber, J.; Brach, M. Major Geochemical Characteristics of Geothermal Brines from the Upper Rhine Graben Granitic Basement with Constraints on Temperature and Circulation. Chem. Geol. 2016, 428, 27–47. [Google Scholar] [CrossRef]
- Arnórsson, S.; Andrésdóttir, A. Processes Controlling the Distribution of Boron and Chlorine in Natural Waters in Iceland. Geochim. Et Cosmochim. Acta 1995, 59, 4125–4146. [Google Scholar] [CrossRef]
- Burazer, N.; Šajnović, A.; Spahić, D.; Tančić, P.; Grba, N.; Jovančićević, B. Unveiling the Paleosalinity Constraints on Southern Peri-Pannonian Lower Miocene Lacustrine Systems in Serbia and Bosnia and Herzegovina: Lopare (Dinaride Lake System) versus Toplica Basin (Serbian Lake System). Chem. Geol. 2025, 671, 122475. [Google Scholar] [CrossRef]
- Trompetter, W.J.; Reyes, A.G.; Vickridge, I.C.; Markwitz, A. Lithium and Boron Distributions in Geological Samples. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 158, 568–574. [Google Scholar] [CrossRef]
- Ellis, A.J.; Mahon, W.A.J. Chemistry and Geothermal Systems; Denton, J., Ed.; Energy Science and Engineering: Resources, Techonology, Management; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Cinti, D.; Tassi, F.; Procesi, M.; Brusca, L.; Cabassi, J.; Capecchiacci, F.; Delgado Huertas, A.; Galli, G.; Grassa, F.; Vaselli, O.; et al. Geochemistry of Hydrothermal Fluids from the Eastern Sector of the Sabatini Volcanic District (Central Italy). Appl. Geochem. 2017, 84, 187–201. [Google Scholar] [CrossRef]
- Elders, W.A.; Cohen, L.H. Salton Sea Geothermal Field, Imperial Valley, California as a Site for Continental Scientific Drilling. [Abstract Only]. Geol. Soc. Am. Abstr. Programs 1983, 15. [Google Scholar]
- McKibben, M.A.; Williams, A.E.; Elders, W.A.; Eldridge, C.S. Saline Brines and Metallogenesis in a Modern Sediment-Filled Rift: The Salton Sea Geothermal System, California, U.S.A. Appl. Geochem. 1987, 2, 563–578. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Johir, M.A.H.; Fane, A.G.; Kandasamy, J.; Vigneswaran, S. Rubidium Extraction from Seawater Brine by an Integrated Membrane Distillation-Selective Sorption System. Water Res. 2017, 123, 321–331. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Chen, Y.; Ma, B. Rubidium Extraction from Mineral and Brine Resources: A Review. Hydrometallurgy 2021, 203, 105644. [Google Scholar] [CrossRef]
- del Villar, A.; Melgarejo, J.; García-López, M.; Fernández-Aracil, P.; Montano, B. The Economic Value of the Extracted Elements from Brine Concentrates of Spanish Desalination Plants. Desalination 2023, 560, 116678. [Google Scholar] [CrossRef]
| Parameters | El Arteal Mine | Ramo De Flores | Guzmana | Exp. Deep Well | ||
|---|---|---|---|---|---|---|
| Data year | 1971 | 1973 | 1971 | 1971 | 1983 | 1983 |
| Piezometric head (m) | −167 | −171.3 | −137 | −98 | −176.8 | −195 |
| pH | 6.9 | 3.6 | - | 2.9 | - | - |
| T(°C) | 33 | 34 | 35 | 28 | 51 | 51 |
| Salinity (g/L) 110° | 10,210 | - | 40,460 | 25,130 | - | - |
| Ca (mg/L) | 648 | 830 | 1400 | 240 | 7200 | 6750 |
| K (mg/L) | 134 | 160 | 670 | 197 | 1700 | 1750 |
| Mg (mg/L) | 282 | 1180 | 1035 | 1244 | 600 | 600 |
| Na (mg/L) | 2450 | 1700 | 9150 | 4450 | 28,200 | 27,400 |
| Cl (mg/L) | 3410 | 3800 | 1711 | 8252 | 58,900 | 57,500 |
| SO4 (mg/L) | 3275 | 2500 | 4300 | 7575 | 820 | 1010 |
| HCO3 (mg/L) | 283 | - | 0 | 0 | 260 | 260 |
| Br (mg/L) | 3.9 | - | 3.05 | 1.8 | - | - |
| F (mg/L) | - | 2.1 | - | - | - | - |
| Fe (mg/L) | 1.9 | 17.2 | 206 | 1400 | 48 | 33 |
| Li (mg/L) | 6 | 6.4 | 27 | 13.2 | 65 | 66 |
| Rb (mg/L) | - | - | - | - | 17 | 18 |
| Zn (mg/L) | - | 2.3 | - | - | 2 | 1 |
| Si (mg/L) | 4 | 1.7 | 2 | 2 | 6.5 | 7.1 |
| Sr (mg/L) | - | - | - | - | 275 | 260 |
| Cu (mg/L) | - | 1.7 | - | - | - | - |
| As (μg/L) | - | 55 | - | - | - | - |
| Ni (μg/L) | - | 60 | - | - | - | - |
| Bulk Sample | Clay Fraction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Type of Rock | Qz | PSIc | Mc | Pl | Gth | Hem | Gp | Hl | Dol | Cal | Others | Ilt | Kln |
| F1 | Phyllite (RS) | 71 | 25 | 1 | - | 3 | - | - | - | - | - | - | 96 | 4 |
| F2A | Fault breccia | 55 | 30 | - | - | 1 | 14 | - | - | - | - | - | - | - |
| F2B | Phyllite altered | 68 | 23 | - | 2 | 6 | - | - | 1 | - | - | Rt, Tur, Zrn | 90 | 10 |
| F3 | Fault gouge | 39 | 42 | 1 | 9 | 8 | - | <1 | 1 | - | - | Rt, Clt, Mnz | - | - |
| F4 | Fault gouge | 52 | 43 | - | - | 3 | - | <1 | 2 | - | - | Brt, Rt | - | - |
| F5 * | Fault gouge | 52 | 42 | - | - | 5 | - | - | 1 | - | - | - | 96 | 4 |
| F6 * | Fault gouge | 28 | 64 | - | - | 3 | - | <1 | 5 | - | - | - | - | - |
| F7 * | Fault gouge | 26 | 49 | - | 5 | 1 | 9 | 1 | 9 | - | - | Rt, Trd, Mnz, Zrn | 93 | 7 |
| F8 * | Fault gouge | 23 | 53 | 3 | - | 6 | - | <1 | 9 | 6 | - | - | - | - |
| F8B | Mylonitic fault rock | 41 | 51 | - | - | - | 8 | - | <1 | - | - | Mnz, Zrn, Tur | - | - |
| F9 * | Calcareous Fault gouge | 23 | 43 | - | - | <1 | 3 | - | 8 | 2 | 21 | Chr, Zrn | 91 | 9 |
| F10 * | Calcareous fault gouge | 10 | 46 | - | - | - | - | 3 | 2 | 3 | 36 | Ank | - | - |
| F11 * | Calcareous fault gouge | 20 | 33 | - | - | - | - | 1 | 11 | 2 | 33 | - | - | - |
| F12 * | Calcareous sands (RS) | 48 | 40 | 2 | - | - | - | 5 | - | 3 | 2 | - | - | - |
| F13 * | Calcareous sandstone and sandy marl (RS) | 25 | 57 | - | - | - | - | <1 | - | 5 | 5 | - | - | - |
| F1 | F2A | F2B | F3 | F4 | F5 | F6 | F7 | F8 | F8B | F9 | F10 | F11 | F12 | F13 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | ppm | 53 | 79 | 73 | 54 | 71 | 28 | 73 | 84 | 77 | 63 | 68 | 44 | 53 | 36 | 71 |
| Be | ppm | 2.8 | 1.4 | 1.6 | 3.4 | 3.6 | 3.4 | 4.4 | 4.8 | 4 | 2.2 | 3.6 | 3.2 | 3.2 | 1.1 | 2.2 |
| Mg | % | 0.15 | 0.08 | 0.13 | 0.27 | 0.32 | 0.25 | 0.29 | 0.45 | 1.1 | 0.17 | 0.99 | 0.96 | 1.12 | 1.18 | 1.76 |
| Ca | % | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 1 | 0.2 | 7.1 | 14.6 | 10 | 3.2 | 2.3 |
| K | % | 3.23 | 1.09 | 1.91 | 3.21 | 3.67 | 3.88 | 3.41 | 3.62 | 3.91 | 2.58 | 3.5 | 2.62 | 2.8 | 1.26 | 2.49 |
| Rb | ppm | 198.5 | 67.3 | 120 | 223 | 249 | 245 | 235 | 291 | 265 | 149.5 | 242 | 181.5 | 193.5 | 72.3 | 153.5 |
| Cs | ppm | 10.4 | 8.7 | 6.8 | 41.6 | 48 | 13 | 51.8 | 98.8 | 52 | 14.5 | 42.5 | 35 | 50.2 | 12.6 | 21.9 |
| Sr | ppm | 110 | 380 | 110 | 170 | 170 | 100 | 420 | 220 | 480 | 170 | 280 | 2030 | 660 | 390 | 170 |
| Ba | ppm | 517 | 1450 | 185 | 918 | 1790 | 518 | 703 | 578 | 575 | 610 | 455 | 3460 | 1350 | 271 | 419 |
| Ti | % | 0.538 | 0.172 | 0.336 | 0.499 | 0.571 | 0.593 | 0.555 | 0.59 | 0.476 | 0.462 | 0.416 | 0.301 | 0.343 | 0.358 | 0.458 |
| Nb | ppm | 18.3 | 5.8 | 11.2 | 16.8 | 18 | 19.3 | 19.1 | 20 | 17.8 | 15.2 | 16.2 | 11.8 | 12.6 | 11.3 | 15.8 |
| Ta | ppm | 1.18 | 0.42 | 0.75 | 1.11 | 1.24 | 1.4 | 1.36 | 1.38 | 1.16 | 0.86 | 1.02 | 0.71 | 0.8 | 0.7 | 0.98 |
| Th | ppm | 11.8 | 4.5 | 8.2 | 12.6 | 13.9 | 15.2 | 14 | 15.6 | 13.2 | 10 | 12.3 | 8.9 | 9.8 | 5.3 | 10.8 |
| U | ppm | 2.7 | 1.5 | 2.2 | 2.8 | 2.8 | 3.7 | 3.5 | 3.3 | 3.1 | 2.2 | 2.8 | 2.5 | 2.9 | 1.5 | 3 |
| La | ppm | 27.7 | 26.6 | 11 | 40 | 46.3 | 43.5 | 43.3 | 51.6 | 37.3 | 32.8 | 39.8 | 29.9 | 32.2 | 17.9 | 34.5 |
| Ce | ppm | 49.4 | 43.5 | 21.8 | 74.5 | 90.4 | 84.1 | 79.1 | 95.3 | 79.5 | 65.5 | 82.8 | 60.4 | 65.5 | 37.4 | 68.5 |
| Pr | ppm | 5.84 | 4.67 | 2.65 | 8.79 | 10.45 | 9.89 | 9.23 | 11.4 | 8.53 | 7.8 | 9.2 | 6.77 | 7.5 | 4.16 | 7.97 |
| Nd | ppm | 21.9 | 16.75 | 10.05 | 31.9 | 38.7 | 36.9 | 33.9 | 42.8 | 32 | 28.3 | 35.9 | 25.9 | 28 | 15.85 | 29.4 |
| Sm | ppm | 4.41 | 2.95 | 2.35 | 6.14 | 7.32 | 7.07 | 6.52 | 7.9 | 6.04 | 5.89 | 7.07 | 4.98 | 5.52 | 2.78 | 5.99 |
| Eu | ppm | 1.53 | 1.08 | 0.96 | 1.77 | 2 | 1.85 | 1.98 | 2.95 | 1.6 | 1.76 | 1.67 | 1.03 | 1.1 | 0.65 | 1.31 |
| Gd | ppm | 4.61 | 2.53 | 3.59 | 5.69 | 7.02 | 6.99 | 6.08 | 7.9 | 6.17 | 4.97 | 6.55 | 4.9 | 5.07 | 2.88 | 5.63 |
| Tb | ppm | 0.76 | 0.34 | 0.71 | 0.86 | 1 | 1.03 | 0.9 | 1.14 | 0.91 | 0.73 | 1 | 0.73 | 0.76 | 0.49 | 0.84 |
| Dy | ppm | 5.05 | 1.83 | 5.01 | 5.09 | 5.91 | 6.37 | 5.47 | 6.86 | 4.79 | 4.57 | 4.89 | 3.85 | 3.96 | 2.76 | 4.96 |
| Ho | ppm | 1.12 | 0.34 | 1.08 | 1.07 | 1.16 | 1.31 | 1.11 | 1.4 | 0.99 | 0.93 | 0.99 | 0.8 | 0.81 | 0.57 | 0.97 |
| Er | ppm | 3.17 | 0.91 | 2.99 | 2.92 | 3.21 | 3.79 | 3.21 | 3.78 | 2.64 | 2.73 | 2.79 | 2.26 | 2.31 | 1.78 | 2.87 |
| Tm | ppm | 0.48 | 0.12 | 0.46 | 0.42 | 0.47 | 0.57 | 0.45 | 0.58 | 0.44 | 0.33 | 0.42 | 0.31 | 0.33 | 0.28 | 0.41 |
| Yb | ppm | 3.16 | 0.87 | 2.86 | 2.85 | 3.09 | 3.75 | 3.01 | 3.76 | 2.62 | 2.53 | 2.75 | 2.21 | 2.18 | 1.92 | 2.68 |
| Lu | ppm | 0.47 | 0.14 | 0.37 | 0.44 | 0.46 | 0.59 | 0.44 | 0.55 | 0.47 | 0.35 | 0.43 | 0.37 | 0.35 | 0.28 | 0.4 |
| Y | ppm | 28.9 | 8.8 | 30.4 | 27.8 | 30.8 | 34.1 | 28.3 | 36.5 | 25.5 | 23.1 | 27.3 | 22.5 | 23.1 | 16.2 | 27.1 |
| Fe | % | 5.57 | 12.9 | 9.15 | 8.05 | 6.28 | 5.32 | 6.83 | 6.75 | 4.61 | 3.93 | 3.26 | 4.61 | 3.6 | 2.73 | 3.67 |
| Mn | ppm | 1260 | 5850 | 2530 | 1220 | 1280 | 1440 | 2190 | 690 | 1520 | 580 | 740 | 2130 | 1550 | 420 | 320 |
| V | ppm | 131 | 51 | 79 | 144 | 152 | 163 | 166 | 182 | 138 | 96 | 123 | 90 | 100 | 61 | 113 |
| Co | ppm | 8.1 | 23.2 | 7.3 | 15.8 | 15.4 | 8.6 | 13.4 | 4.9 | 16.8 | 1.8 | 9.3 | 13.6 | 12.6 | 8.4 | 13 |
| Ni | ppm | 30 | 100 | 70 | 40 | 30 | 20 | 30 | 20 | 40 | 10 | 40 | 60 | 40 | 30 | 60 |
| Mo | ppm | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | <2 | <2 | <2 | <2 | <2 | <2 |
| W | ppm | 8.4 | 5.6 | 10.4 | 9.6 | 11 | 6.7 | 12.2 | 32.3 | 6.3 | 13.1 | 4.3 | 3 | 2.5 | 1.6 | 2.5 |
| Re | ppm | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.01 | 0.03 | <0.01 | 0.03 | 0.02 | 0.02 | 0.01 | <0.01 |
| Ga | ppm | 24.3 | 9 | 14.2 | 24.4 | 26.9 | 29.2 | 29.3 | 31.3 | 26.2 | 18.9 | 24.2 | 16.8 | 19.3 | 10.9 | 20.5 |
| Ge | ppm | 3.2 | 3.7 | 3.2 | 4.9 | 5.3 | 2.8 | 4.5 | 6.4 | 2 | 2.1 | 1.8 | 1.5 | 2.3 | 1.5 | 1.2 |
| Cu | ppm | <20 | 70 | <20 | 60 | 120 | 20 | 20 | <20 | 30 | <20 | 20 | 20 | 30 | <20 | 40 |
| Zn | ppm | 150 | 570 | 120 | 250 | 190 | 100 | 120 | 140 | 210 | 300 | 140 | 140 | 230 | 50 | 40 |
| Ag | ppm | <5 | <5 | <5 | 6 | 7 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 120 |
| Cd | ppm | <0.8 | 1 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 | 1.4 | <0.8 | <0.8 | <0.8 | <0.8 | <0.8 |
| In | ppm | <0.3 | 0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 |
| Sn | ppm | 15 | 9 | 13 | 15 | 19 | 18 | 16 | 13 | 7 | 13 | 5 | 5 | 4 | 4 | <3 |
| Tl | ppm | 0.82 | 0.61 | 0.42 | 1.02 | 1.16 | 0.99 | 1.6 | 2.01 | 1.32 | 1 | 1.09 | 0.88 | 1.17 | 0.67 | 1.66 |
| Pb | ppm | 149.5 | 287 | 114 | 392 | 594 | 900 | 80.1 | 83.2 | 41.4 | 50.6 | 28.3 | 46.9 | 86.3 | 22.5 | 26.2 |
| Bi | ppm | 0.1 | 1.1 | 0.3 | 1.8 | 1 | 0.1 | 0.6 | 0.2 | 0.4 | 0.2 | 0.4 | 0.2 | 0.3 | 0.1 | 0.3 |
| Sb | ppm | 4.7 | 34.3 | 3.4 | 43.3 | 82.5 | 20 | 14.7 | 24.8 | 10.9 | 16.7 | 5.5 | 8.9 | 19.8 | 3 | 3.5 |
| As | ppm | 19 | 61 | 12 | 60 | 48 | 29 | 30 | 28 | 16 | 26 | 12 | 11 | 13 | 9 | 13 |
| Te | ppm | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Se | ppm | <3 | <3 | <3 | 3 | <3 | <3 | 4 | <3 | <3 | <3 | <3 | <3 | <3 | 3 | <3 |
| Parameters | ART-1 | ART-2 | ART-3 | ART-3-120 | ART-4 | S.A.-150 | S.A.-300 |
|---|---|---|---|---|---|---|---|
| Well depth (m) | 12.4 | 33.1 | 122.5 1 | 122.5 1 | 192.5 | 550 | 550 |
| Well depth (m a.s.l.) | 8 | 8 | −86 | −86 | −86 | −86 | −444 |
| Piezometric head (m a.s.l.) | 17.4 | 9.2 | −16.7 | −16 | −13 | −22 | −22 |
| Sampling depth (m a.s.l.) | 14 | 9 | −33 | −83 | −35.8 | −44 | −194 |
| T (°C) | 22.3 | 22.9 | 32 | ND | 34.5 | 34 | 42.7 |
| pH | 6.02 | 7.16 | 7.24 | 7.13 | 6.73 | 7.26 | 5.64 |
| Conductivity (µS/cm) | 9018 | 6261 | 13,400 | 9980 | 16,600 | 121,498 | 138,126 |
| TDS (mg/L) | 5970 | 4550 | 9780 | 9870 | 12,800 | 96,900 | 99,100 |
| Na (mg/L) | 1230 | 750 | 2230 | 2170 | 2830 | 20,600 | 20,600 |
| Ca (mg/L) | 479 | 362 | 871 | 895 | 779 | 5160 | 5140 |
| K (mg/L) | 48.5 | 38.6 | 147 | 151.5 | 193 | 1380 | 1415 |
| Mg (mg/L) | 416 | 253 | 296 | 298 | 431 | 542 | 543 |
| Cl (mg/L) | 1360 | 1290 | 3700 | 3340 | 5280 | 43,600 | 40,600 |
| SO4 (mg/L) | 2340 | 1230 | 2590 | 2000 | 2220 | 600 | 573 |
| HCO3 (mg/L) | 237 | 550 | 398 | 448 | 359 | 33 | 1.22 |
| NO3 (mg/L) | 46.7 | 0.341 | <0.5 | <0.5 | <0.5 | <5 | <5 |
| B (mg/L) | 2.84 | 1.33 | 5.12 | 5.3 | 5.69 | 24 | 24 |
| Ba (mg/L) | 0.019 | 0.015 | 0.030 | 0.033 | 0.028 | 0.34 | 0.4 |
| Br (mg/L) | 3.9 | 2.6 | <5 | <5 | 5.8 | 40 | 36.9 |
| Cs (mg/L) | 0.001 | 0.003 | 0.221 | 0.246 | 0.529 | - | - |
| F (mg/L) | <1 | <1 | <2 | <2 | <2 | <10 | <10 |
| Fe (mg/L) | 0.072 | 0.027 | 0.047 | 0.145 | 0.004 | <0.5 | 4.3 |
| Li (mg/L) | 0.66 | 0.76 | 5.35 | 5.53 | 7.39 | 59.5 | 59.4 |
| Mn (mg/L) | 0.04 | 0.02 | 0.098 | 1.06 | 22 | 3.9 | 4.7 |
| Rb (mg/L) | 0.064 | 0.024 | 0.765 | 1.035 | 1.345 | - | - |
| Si (mg/L) | 11.4 | 11.3 | 15.5 | 13.6 | 10.6 | 0.1 | 0.1 |
| Sr (mg/L) | 13.4 | 7.2 | 21.4 | 21.9 | 18.9 | 185 | 186 |
| Ag (µg/L) | 0.831 | 0.219 | <0.005 | 0.014 | 0.079 | <10 | <10 |
| Al (µg/L) | 12 | 7 | 4 | 19 | <3 | 700 | 700 |
| As (µg/L) | 1.13 | 0.38 | 0.71 | 1.02 | 2.21 | <100 | <100 |
| Be (µg/L) | 0.006 | 0.032 | <0.005 | 0.087 | <0.005 | <0.5 | <0.5 |
| Bi (µg/L) | 0.02 | 0.05 | <0.01 | 0.03 | <0.01 | - | - |
| Cd (µg/L) | 0.055 | 0.059 | 0.009 | 0.034 | 8.15 | <5 | <5 |
| Ce (µg/L) | <0.005 | <0.005 | 0.062 | 0.015 | 0.013 | - | - |
| Co (µg/L) | 0.943 | 0.153 | 0.325 | 1.055 | 89.7 | 70 | 70 |
| Cr (µg/L) | 2 | 0.5 | <0.5 | 1 | <0.5 | <0.5 | <0.5 |
| Cu (µg/L) | 7.5 | 1.5 | 0.7 | 1.2 | 1.5 | 10 | <10 |
| Dy (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Er (µg/L) | <0.005 | 0.007 | <0.005 | 0.005 | <0.005 | - | - |
| Eu (µg/L) | <0.005 | <0.005 | 0.005 | 0.008 | 0.005 | - | - |
| Ga (µg/L) | <0.05 | <0.05 | 0.08 | <0.05 | 0.06 | - | - |
| Gd (µg/L) | 0.01 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Hf (µg/L) | <0.005 | <0.005 | 0.008 | 0.015 | 0.008 | - | - |
| Hg (µg/L) | <0.05 | <0.05 | 0.13 | <0.05 | 0.08 | - | - |
| Ho (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| La (µg/L) | 0.007 | 0.018 | 0.005 | 0.016 | <0.005 | - | - |
| Lu (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Mo (µg/L) | 1.11 | 0.95 | 1.99 | 2.22 | 0.59 | - | - |
| Nb (µg/L) | 0.007 | 0.007 | <0.005 | 0.017 | <0.005 | - | - |
| Nd (µg/L) | 0.012 | 0.007 | <0.005 | 0.009 | <0.005 | - | - |
| Ni (µg/L) | 13.6 | 3.2 | 7.9 | 4.6 | 260 | 100 | 100 |
| P (µg/L) | 96 | 107 | 757 | 762 | <5 | <500 | <500 |
| Pb (µg/L) | 1.34 | 0.76 | 0.27 | 1.02 | <0.05 | <50 | <50 |
| Pd (µg/L) | 0.103 | 0.015 | 0.103 | 0.033 | 0.043 | - | - |
| Pt (µg/L) | 0.006 | 0.005 | 0.006 | <0.005 | 0.006 | - | - |
| Pr (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Sb (µg/L) | 4.4 | 0.32 | 0.55 | 0.05 | 0.66 | <100 | <100 |
| Sc (µg/L) | 1.89 | 1.38 | 0.43 | 0.77 | <0.01 | <50 | <50 |
| Se (µg/L) | 12.4 | 7.9 | 0.14 | <0.05 | 0.49 | - | - |
| Sm (µg/L) | 0.009 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Sn (µg/L) | 0.19 | <0.05 | <0.05 | 0.11 | <0.05 | - | - |
| Ta (µg/L) | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | - | - |
| Tb (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| Te (µg/L) | 0.02 | 0.02 | 0.02 | 0.07 | <0.01 | - | - |
| Th (µg/L) | 0.011 | <0.005 | 0.014 | 0.101 | 0.005 | - | - |
| Ti (µg/L) | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <5 | <5 |
| Tl (µg/L) | 0.091 | 3.2 | 0.13 | 0.047 | 48.4 | - | - |
| Tm (µg/L) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | - | - |
| U (µg/L) | 20.7 | 8.9 | 2.31 | 2.48 | 3.44 | - | - |
| V (µg/L) | 0.45 | 0.71 | 0.43 | 0.7 | 0.18 | <10 | <10 |
| W (µg/L) | 0.01 | 0.01 | 0.33 | 0.21 | 0.01 | - | - |
| Yb (µg/L) | 0.005 | 0.007 | <0.005 | <0.005 | <0.005 | - | - |
| Y (µg/L) | 0.098 | 0.072 | 0.011 | 0.074 | 0.116 | - | - |
| Zn (µg/L) | 34.2 | 22.4 | 26.7 | 1.6 | 3660 | <20 | 50 |
| Zr (µg/L) | 0.11 | 0.02 | 0.07 | 0.05 | <0.02 | - | - |
| Cl/Br | 349 | 500 | 1480 2 | 1336 2 | 910 | 1090 | 1100 |
| Rb/Cs | 65.6 | 7.1 | 3.5 | 4.2 | 2.5 | - | - |
| Ca/Cl | 0.35 | 0.28 | 0.24 | 0.27 | 0.15 | 0.12 | 0.13 |
| Mg/Cl | 0.31 | 0.20 | 0.08 | 0.09 | 0.08 | 0.01 | 0.1 |
| Na/Cl | 0.90 | 0.58 | 0.60 | 0.65 | 0.54 | 0.47 | 0.51 |
| 1 During mining operations, ART-3 was re-deepened to 250–257 m, currently collapsed at 122.5 m. 2 Br below detection limit; half the D.L value assigned. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Real-Fernández, E.; Pozo, M.; De Ignacio, C.; Sánchez-Malo, Á.; Sanz-Rubio, E.; Villa, L. Fluid-Rock Interaction Signature in Palomares Fault Zone—New Mineralogical and Geochemical Insights into the Tectono-Magmatic Águilas Arc Geothermal System (SE Spain). Appl. Sci. 2026, 16, 1420. https://doi.org/10.3390/app16031420
Real-Fernández E, Pozo M, De Ignacio C, Sánchez-Malo Á, Sanz-Rubio E, Villa L. Fluid-Rock Interaction Signature in Palomares Fault Zone—New Mineralogical and Geochemical Insights into the Tectono-Magmatic Águilas Arc Geothermal System (SE Spain). Applied Sciences. 2026; 16(3):1420. https://doi.org/10.3390/app16031420
Chicago/Turabian StyleReal-Fernández, Elena, Manuel Pozo, Cristina De Ignacio, Ángel Sánchez-Malo, Enrique Sanz-Rubio, and Luis Villa. 2026. "Fluid-Rock Interaction Signature in Palomares Fault Zone—New Mineralogical and Geochemical Insights into the Tectono-Magmatic Águilas Arc Geothermal System (SE Spain)" Applied Sciences 16, no. 3: 1420. https://doi.org/10.3390/app16031420
APA StyleReal-Fernández, E., Pozo, M., De Ignacio, C., Sánchez-Malo, Á., Sanz-Rubio, E., & Villa, L. (2026). Fluid-Rock Interaction Signature in Palomares Fault Zone—New Mineralogical and Geochemical Insights into the Tectono-Magmatic Águilas Arc Geothermal System (SE Spain). Applied Sciences, 16(3), 1420. https://doi.org/10.3390/app16031420







