1. Introduction
Positron emission tomography (PET) has emerged as a powerful molecular imaging modality, offering unique functional insights that complement the anatomical information provided by computed tomography (CT) and magnetic resonance imaging (MRI) [
1,
2,
3,
4,
5]. This synergy enables clinicians and researchers to visualize molecular processes in both clinical practice and small animal studies, creating new opportunities to understand the mechanisms of the disease and the response to treatment.
Among the key performance characteristics of PET systems, spatial resolution is particularly critical for modern medical applications. The ability to resolve fine details in PET images directly affects diagnostic accuracy in numerous clinical scenarios, including early detection of lesions, precise definition of tumor boundaries, neurological investigations, cardiovascular studies, and evaluation of treatment efficacy and recurrence. Despite technological advancements, current commercial whole-body PET/CT and PET/MRI systems face inherent PET spatial resolution limitations [
6,
7,
8,
9,
10,
11]. These systems typically achieve spatial resolutions exceeding 4 mm at the center of the field of view (FOV), with the performance degrading radially due to variations in the depth of interaction of the annihilation photons in the system detectors (DOI) [
6].
This paper is organized as follows:
Section 2 discusses the key factors limiting PET spatial resolution, including detector geometry, scintillator design, and electronic signal processing.
Section 3 explores advanced detector designs achieving ultra-high resolution (<2 mm), focusing on pixelated and monolithic scintillator configurations as well as photon depth-of-interaction (DOI) techniques. In addition, this section also provides reviews of the selected ultra-high-resolution PET systems, including a comparison of commercial scanners with research prototypes described in the recent literature.
Section 4 provides in-depth details on a prototype 1 mm
3 resolution clinical PET system developed at Stanford University’s Molecular Imaging Instrumentation Laboratory (MIIL). This comprehensive review highlights key technological advances and provides practical information on advancing the capabilities of molecular imaging scanner designs.
This work constitutes a review of the literature focused on the design of ultra-high-resolution (<2 mm) clinical PET systems. A comprehensive search of peer-reviewed articles published between 2010 and 2024 was performed using databases such as PubMed, IEEE Xplore, Scopus, and Google Scholar. The search strategy used keywords including ‘high-resolution PET’, ‘depth of interaction PET’, ‘pixelated detectors’, ‘monolithic PET detectors’, and ‘ultra-high-resolution PET systems’. Articles were selected based on their relevance to technological innovations in the design and implementation of clinical PET detector systems. The primary objective of this review is to synthesize recent advancements in PET detector technology and spatial resolution enhancement strategies, providing a consolidated overview for researchers and developers in the field of molecular imaging.
2. Key Factors Contributing to Spatial Resolution in PET Imaging
Positron emission tomography (PET) is based on the detection of coincident 511-keV photon pairs resulting from positron–electron (
–
) annihilation [
12,
13]. A
, emitted by a radioactive tracer, travels a short distance in tissue before interacting with a nearby
, leading to an annihilation event. This process produces two 511-keV photons emitted in nearly opposite directions (≈180° apart). These photons are detected in temporal coincidence by detectors, and each event is assigned to the corresponding line of response (LOR). The accumulation of a large number of such events positioned on the system LORs enables tomographic reconstruction of the tracer distribution in three dimensions. The physical and technical characteristics involved in
emission, annihilation, and photon detection contribute to the overall spatial resolution of the reconstructed PET image.
The spatial resolution of the PET system is further determined by the design and performance of the PET detection system. A typical PET scanner consists of detector modules comprising of scintillation crystals optically coupled to photodetectors. Upon interaction with a 511-keV photon, the scintillator emits visible light, which is converted into electrical signals by the photodetector [
12,
14,
15]. Common scintillator materials include lutetium oxyorthosilicate (LSO), bismuth germanate (BGO), and gadolinium oxyorthosilicate (GSO), selected based on properties such as light yield, decay time, and stopping power [
16,
17]. The intrinsic resolution, energy and timing performance, and depth of interaction (DOI) handling of these detection elements significantly influence the PET system’s spatial resolution.
One metric to determine a PET scanner’s spatial resolution is its ability to distinguish two closely placed positron-emitting point sources. This resolution is influenced by several factors, both inherent and design-dependent. The inherent, unavoidable factors include the positron range [
18], determined by the characteristics of the radionuclide used and tissue traversed, and the non-collinearity of annihilation photons [
18], whose impact is influenced by the scanner’s geometry. Design-dependent factors, which can be mitigated through proper design and operation, include the detector dimensions and architecture, variation in photon penetration depth in the detector crystals (parallax error) [
19,
20], Compton scattering of photons between detector crystals, patient movement, the event position decoding method used, and the image reconstruction process, which can introduce further blurring due to factors such as noise level, sampling rate, and smoothing [
18,
21].
2.1. Annihilation Photon Accolinearity
Annihilation occurs when a positron (
) interacts with an electron (
), resulting in the emission of two photons, each with an energy of 511-keV, traveling in nearly opposite directions. However, owing to the residual initial inertia of the positron and electron at the moment of annihilation, their center of mass possesses kinetic energy. This potential motion causes a slight deviation from the ideal 180° emission angle of the two 511-keV photons [
21]. In a circular/cylindrical geometry PET scanner with radius
R, this angular deviation introduces Gaussian blurring, with an estimated full width at half maximum (FWHM) of approximately
[
18,
21,
22]; for a schematic illustration, see
Figure 1.
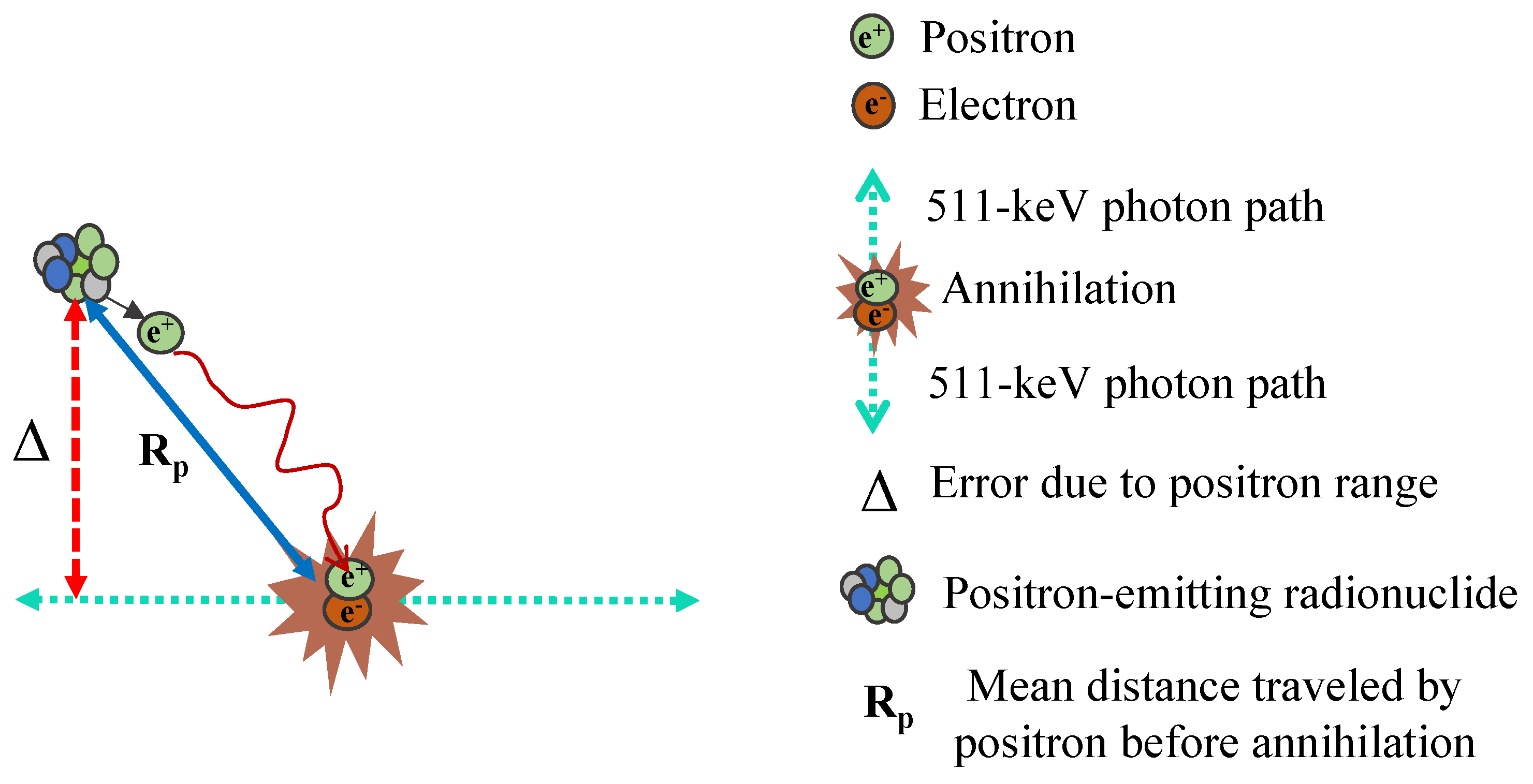
2.2. Positron Range
Within the human body, a positron (
) travels a certain distance before interacting with an electron (
) and undergoing annihilation. This results in a displacement between the annihilation site and the original location of the radionuclide, referred to as the positron range. This effect contributes to event localization errors in PET image reconstruction. The extent of blurring depends on the energy of the emitted positron and the properties of the tissue it traverses.
Figure 2 illustrates how the positron range phenomenon, denoted as
, while
Table 1 presents the positron ranges for various radionuclides in water-equivalent media.
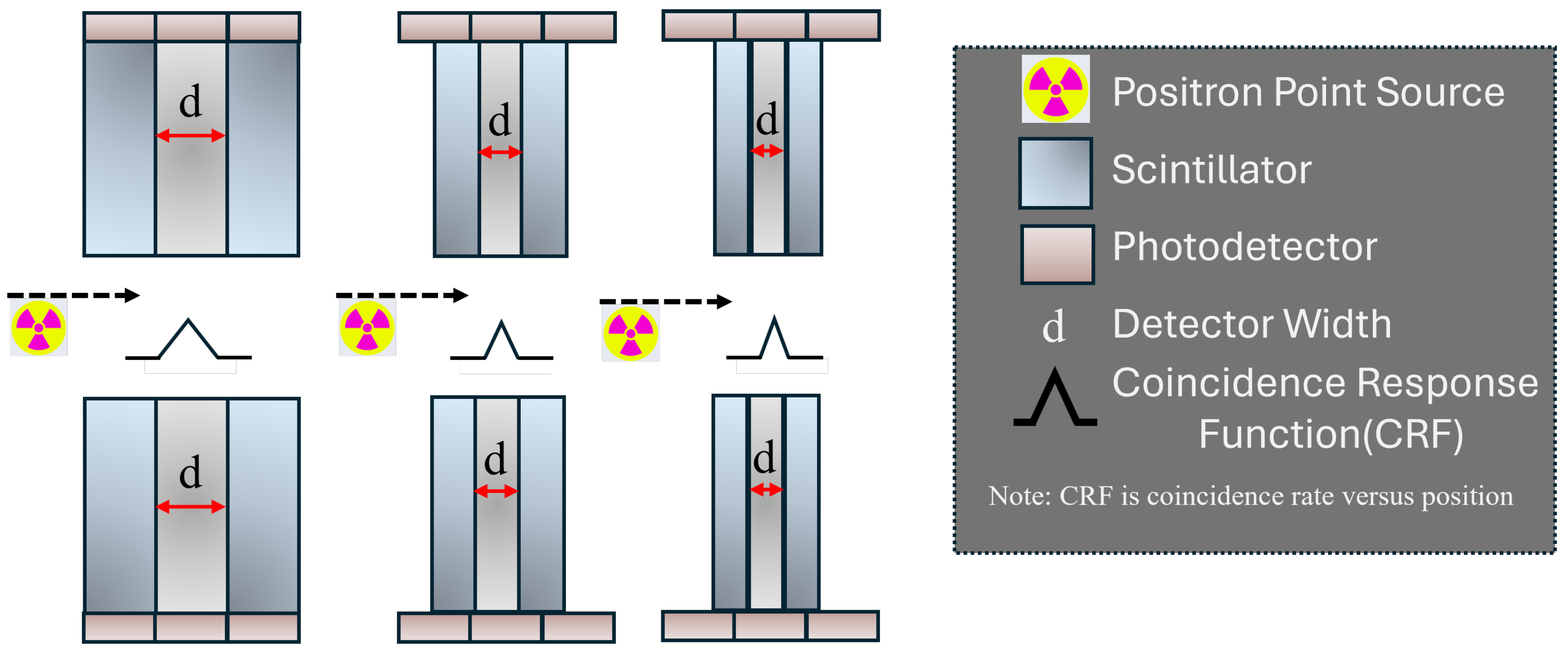
2.3. Detector Element Width
The detector element dimension also critically influences the PET scanner’s spatial resolution [
18,
27,
28]. Wider crystal elements within the detector arrays used to build the PET ring lead to increased uncertainty in locating the interaction points of 511-keV photons in the system, degrading the spatial resolution of the resultant images.
Figure 3 demonstrates the impact of scintillation crystal width on the system’s spatial resolution. The quantity
FWHM represents the theoretical limit of spatial resolution under the presumption that other influencing factors like the decoding process, sampling discrepancies, and detector penetration are minimal.
2.4. Design Strategies for Ultra-High Spatial Resolution PET Detectors
Although it is challenging to mitigate the spatial resolution degradations caused by inherent factors such as the positron range and non-collinearity, other factors can be refined, and an optimized solution can be achieved through advanced detector design and readout strategies. A common approach to improve the spatial resolution is by reducing the size of the scintillation crystals [
18,
29,
30]. Although employing narrower scintillation crystals improves spatial resolution, this approach introduces challenges such as more photodetector channels, complex readout configurations, and increased inter-crystal scatter. Optical and electronic multiplexing can simplify PET detector designs by reducing readout channels, lowering costs, and simplifying electronics. However, these methods complicate the data decoding process, potentially affecting the accuracy of energy, timing, and spatial information.
Additionally, scatter within the crystal and photon penetration in the detectors further contribute to spatial resolution uncertainties [
31]. The absence of depth-of-interaction (DOI) information for annihilation photons makes it difficult to precisely localize photon interactions within the crystals. This uncertainty can lead to image blurring, ultimately degrading the system’s spatial resolution.
Moreover, as the crystal element width decreases, the efficiency for collection of scintillating light photons reduces, and the variations in the light signals increase, further complicating the extraction of a sufficient number of photons for a high detection signal-to-noise ratio (SNR) [
30].
Thus, a fundamental design principle for high-resolution PET detectors is to employ a scintillation array with substantial light yield. Precise 3D localization of events within a scintillation crystal, especially for off-center interactions, can also augment spatial resolution. The one-to-one coupling approach, assigning a distinct photodetector to each crystal element in an array, can enhance the signal-to-noise ratio compared to multiplexing-based approaches, potentially boosting spatial resolution. However, this design necessitates an extensive array of photodetectors, significantly elevating costs and readout circuit complexity, introducing design challenges at the front-end and back-end readout electronics design. Therefore, a balanced degree of optical multiplexing in the scintillation arrays and electronic multiplexing in the readout circuit simplify high-resolution PET system design.
2.5. Advanced Photon Depth-of-Interaction (DOI) Capable PET Detector Design
Various detector designs have been proposed to achieve photon DOI measurement. One approach uses narrow scintillation crystal elements with a dual-end readout, where photodetectors are placed at both ends of the crystal to capture the DOI information [
32,
33,
34]. Alternatively, light-sharing techniques have been developed, allowing DOI positioning to be determined by analyzing light distribution across photodetectors [
31,
35,
36,
37]. This enables single-ended readout, where photodetectors are attached to only one end of the crystal element, a common setup in PET detector arrays employed in industry. Each method has its advantages and limitations. Dual-end readouts require twice as many photodetectors as single-ended designs, while single-ended readouts typically achieve DOI accuracy of around 2–3 mm.
Zhang et al. and Vandenbroucke et al. introduced an innovative side-readout approach using an
LYSO (lutetium-yttrium-oxyorthosilicate) scintillator array with each array coupled to a position-sensitive avalanche photodiode (PSAPD). Each crystal element in the array was measured as
mm
3. Instead of the usual configuration, they arranged the array “edge-on” with respect to incoming photons. In addition to clearly resolving the minuscule crystals in a flood histogram, this side-coupled array allows for a depth-of-interaction (DOI) resolution of ∼1 mm (the dimension of each crystal element) and an energy resolution of about 10% FWHM owing to reduced light collection efficiency variations. The PSAPD’s slim design also helps maximize the packing density of the crystal elements, making this novel configuration more efficient [
38,
39].
2.6. Image Reconstruction
Image reconstruction in PET plays a crucial role in determining the spatial resolution of the final reconstructed images [
40]. Reconstruction algorithms convert raw coincidence data into spatial maps of the radiotracer distribution within the body. The main purpose of the image reconstruction algorithm is to realize the desired reconstructed spatial resolution goal with a high reconstructed image SNR. Thus, for example, a higher-sensitivity system design can achieve a higher reconstruction resolution goal.
A common cause of resolution loss during reconstruction is the use of smoothing filters to reduce noise, which can blur edges and diminish fine details [
40]. Although smoothing improves image quality by suppressing statistical noise, it must be carefully balanced to preserve spatial resolution.
The sampling rate of the reconstructed image grid also impacts resolution. An insufficient sampling grid can lead to aliasing artifacts and detail loss, whereas a finer grid increases computational demands without necessarily improving resolution if it exceeds the scanner’s intrinsic capabilities [
41].
Advanced reconstruction algorithms like OSEM can incorporate models of the scanner’s point spread function (PSF) to compensate for resolution-degrading effects, provided there is adequate SNR. Including PSF modeling can enhance spatial resolution by iteratively deconvolving for detector blurring and system imperfections. However, excessive resolution recovery can amplify noise and introduce artifacts if not properly regularized.
3. Ultra-High Spatial Resolution (<2 mm) Clinical PET Systems
Translating a high-resolution detector design into a clinical system presents numerous hardware design challenges due to the increased crystal elements, phototectors, and number of electronic readout channels required. Typically, there is a trade-off between spatial resolution and scintillation light collection efficiency, which can affect achievable energy and temporality, in addition to spatial resolutions.Hence, detector designers often employ relatively short crystal elements to promote adequate light collection efficiency, which compromises 511-keV photon detection efficiency. In addition to describing such standard high-resolution system design approaches, we will discuss one design that mostly avoids this trade-off between system spatial resolution and sensitivity. Ultra-high spatial resolution systems often address these challenges by confining the FOV to specific organs (locoregional scanning), thereby reducing the number of detector elements and bringing the detectors in close proximity to the patient’s tissues.
One promising way to achieve high-resolution PET imaging is through advances in detector technology. However, direct-conversion detectors like cadmium zinc telluride (CZT) offer the potential for high spatial resolution by eliminating the need for manufacturing arrays of miniscule crystals and the associated scintillation light collection and photodetectors [
42,
43,
44,
45,
46], they face challenges such as charge carrier trapping, robust packaging, and lower detection efficiency for 511-keV photons. As a result, most clinical PET systems continue to rely on scintillator-based designs, where improvements in crystal segmentation, DOI correction, and optimized detector electronics have enabled spatial resolutions ≤ 2 mm while maintaining high sensitivity [
14,
47,
48,
49].
We specifically highlight several selected systems from two categories: brain-dedicated and breast-dedicated PET systems. Detailed specifications of a few selected systems are summarized in
Table 2.
3.1. Brain-Dedicated PET Systems
For the sake of comparison, we briefly describe the ECAT high-resolution research tomograph (HRRT), a well-known, commercially available brain-dedicated PET system from CTI PET Systems Inc. [
58] that for about two decades served as the highest resolution commercially available clinical PET system. This system incorporates a phoswich design, implemented with dual layers of 8 × 8 lutetium–oxy–orthosilicate (LSO) scintillation crystal arrays, each with an individual crystal size of 2.1 × 2.1 × 7.5 mm
3 [
51]. Spatial resolutions of less than 2.4 mm and 2.8 mm were reported at the center and 10 cm off-center, respectively. The transaxial plane’s spatial resolutions were relatively uniform compared to those along the axial direction due to the DOI information enabled by the phoswich design.
Over the years, several research groups have proposed unique designs for brain-dedicated PET systems to enhance PET image quality beyond the ECAT HRRT. One such system is a brain-dedicated scanner developed by Hamamatsu Photonics K.K., capable of producing images with a spatial resolution of less than 2.1 mm [
52]. This system employs a four-layer DOI detector module. Each layer is composed of five 32 × 32 LYSO scintillation crystal arrays with a 1.2 mm pitch, arranged together to form the detection area. Four different crystal layer thicknesses are used depending on the layer’s location relative to the front entrance face to uniformly distribute count rates across each layer. A 0.25 mm diameter
22Na point radiation source was used to evaluate reconstructed spatial resolutions according to NEMA NU 4-2008 standards for small animal PET. A spatial resolution of 1.8 mm to 2.1 mm, reconstructed with 2-D FBP, was reported throughout the entire FOV.
The MINDVIEW (Multimodal Imaging of Neurological Disorder) PET insert utilized monolithic scintillation crystals to improve spatial resolution [
53]. This MR-compatible PET system consists of 50 × 50 × 20 mm
3 monolithic crystal blocks. Black paint was applied to the lateral sides of the crystal arrays, and the front surface (50 × 50 mm
2) was treated with retroreflector (RR) layers. Initial results reported FWHM spatial resolutions ranging from 1.7 mm to 3.5 mm throughout the FOV using iterative algorithms with a 1 mm
22Na point source. Using the FBP algorithm, spatial resolutions were measured with a 0.25 mm diameter
22Na point source at various reconstruction voxel sizes (1 mm, 0.5 mm, and 0.25 mm), reporting resolutions of 1.5 mm to 4.9 mm at different positions in the FOV. Additionally, the system successfully resolved 2.5 mm rods in reconstructed images of a Derenzo phantom.
The NeuroEXPLORER (NX) PET/CT system [
57], developed collaboratively by Yale, UC Davis, and United Imaging Healthcare, features high sensitivity and high resolution with a LYSO-based U-shaped light-sharing detector design that provides DOI information. With a 49.5 cm axial field of view, its spatial resolution ranges from 1.63 mm to 3.05 mm, measured using a 0.25 mm
22Na point source and FBP reconstruction. Sensitivity was evaluated using a NEMA PET sensitivity phantom, yielding 46–47.6 cps/kBq. The system employs a 3D time-of-flight (TOF)-DOI-PSF OSEM algorithm for image reconstruction, offering enhanced imaging for both phantoms and human studies. The NX holds promise for advancing neuroimaging, particularly for detailed cortical and subcortical analyses.
3.2. Breast-Dedicated PET Systems
Breast-dedicated PET systems can achieve superior spatial resolutions by employing fine crystal elements and reducing the separation of opposing detectors compared to brain-dedicated PET systems; this smaller detector separation reduces the spatial resolution blurring from photon non-collinearity, but perhaps even more importantly, boosts geometric efficiency, enabling the realization of the desired improved resolution in terms of SNR in the reconstructed images.
Researchers at M.D. Anderson Cancer Center developed a positron emission mammography (PEM) camera composed of two planar detector modules [
54]. Each module contains 60 photomultiplier (PMT)-Quadrant-Sharing (PQS) LYSO blocks, incorporating two types of crystal sizes, 1.5 × 1.5 × 10 mm
3 and 1.5 × 1.9 × 10 mm
3, to minimize dead spaces. Due to the dual-panel geometry, the spatial resolutions depend on the distance between the two detector heads, with reported resolutions ranging from 1.19 mm to 4.82 mm along different axes at three different bank-to-bank distances (50, 70, and 90 mm).
Shimadzu Co. constructed a high-resolution, ring-shaped, breast-dedicated PET scanner using detectors with four-layer DOI capability [
55]. Each detector block consists of four layers of 32 × 32 LGSO (LU1.8Gd0.2SiO5:Ce) crystal arrays, with 1.4 × 1.4 × 4.5 mm
3 crystal elements, coupled to a position-sensitive PMT (PS-PMT). A unique reflector arrangement was applied to extract DOI information from the stacked crystal arrays [
59]. Reported spatial resolutions for FBP reconstruction were 1.6 to 2.7 mm radially, 1.7 to 2.2 mm tangentially, and 2.0 to 2.2 mm axially. With iterative algorithm reconstructions, spatial resolutions were reported to be 0.8 to 1.3 mm radially, 0.8 to 1.0 mm tangentially, and 0.8 to 1.0 mm axially.
The MAMMI breast PET, developed by Oncovision, Inc., also features a ring geometry and incorporates DOI-capable detector modules, each consisting of 40 × 40 × 10 mm
3 monolithic LYSO crystals coupled to PS-PMTs [
60]. Using 3D MLEM reconstruction, spatial resolutions of 2 mm, 1.9 mm, and 1.7 mm were reported in the radial, tangential, and axial directions, respectively.
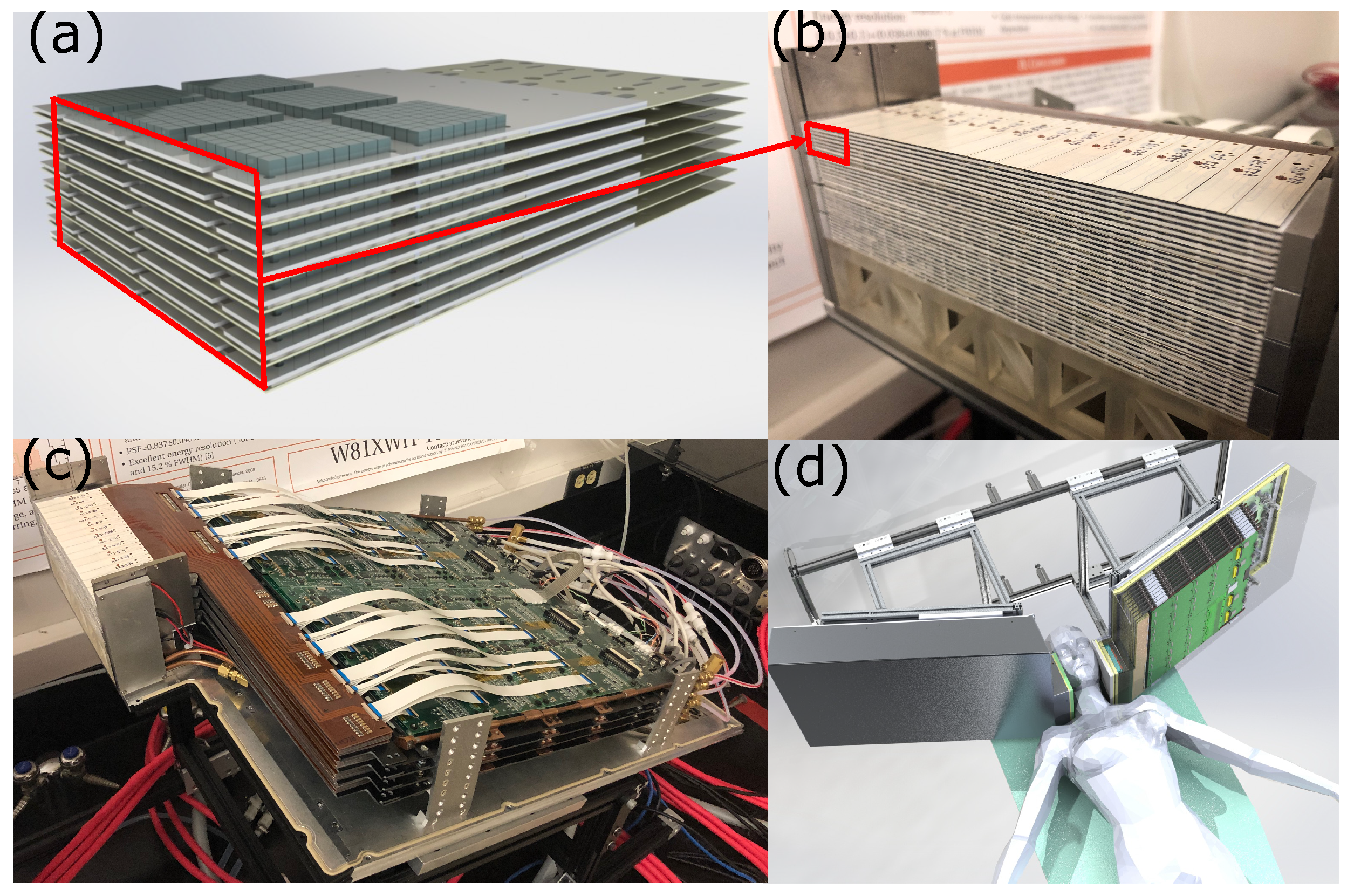
4. The 1 mm Resolution Clinical PET System
To further enhance reconstructed spatial resolution, it is essential to (1) reduce the crystal element width and (2) improve the DOI resolution while maintaining adequate crystal element length to promote high sensitivity. To address these challenges, we have been developing a prototype 1 mm resolution clinical PET system (see
Figure 4) [
38,
39,
50,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72], which aims to achieve sub-millimeter spatial resolutions with exceptionally high 511-keV photon sensitivity.
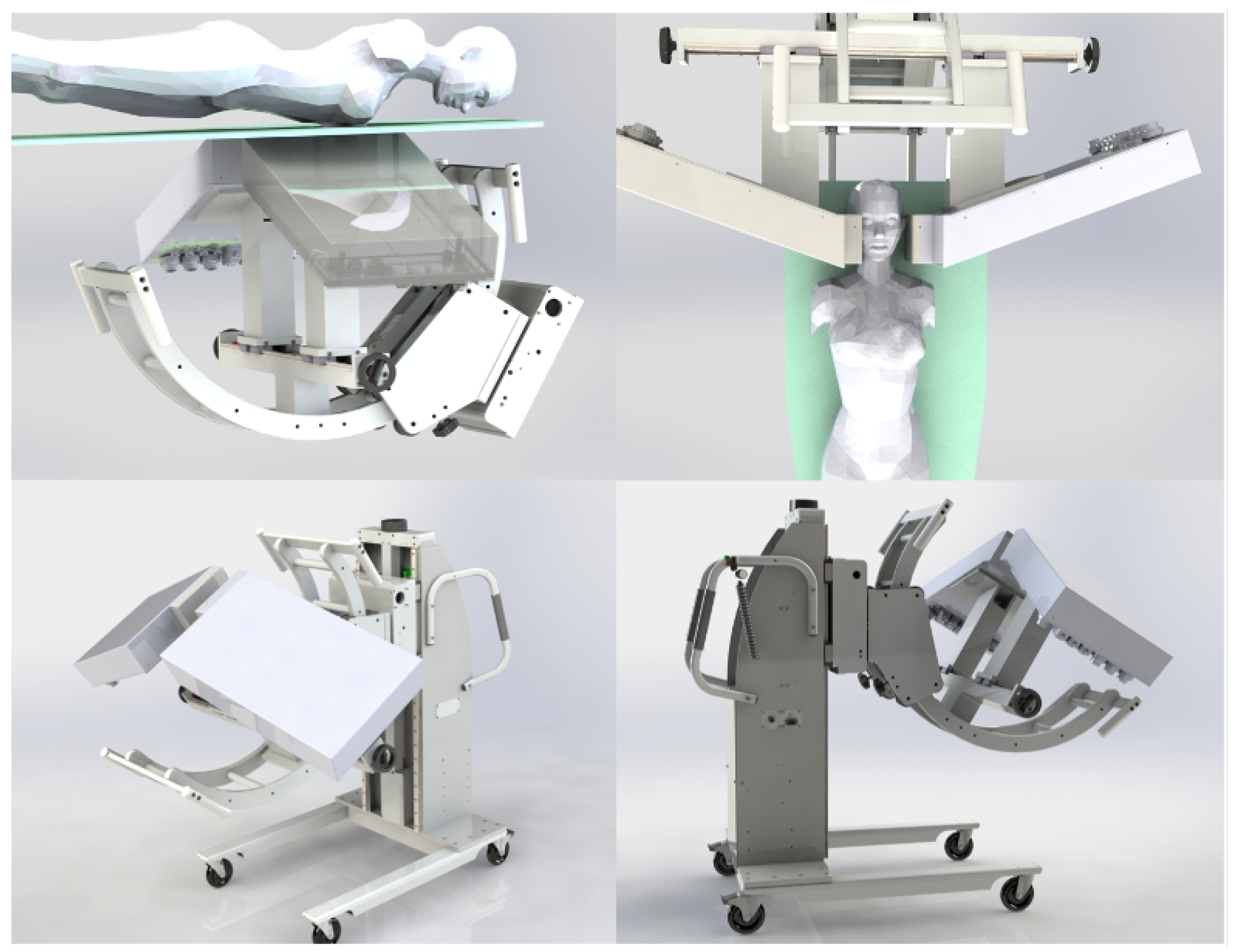
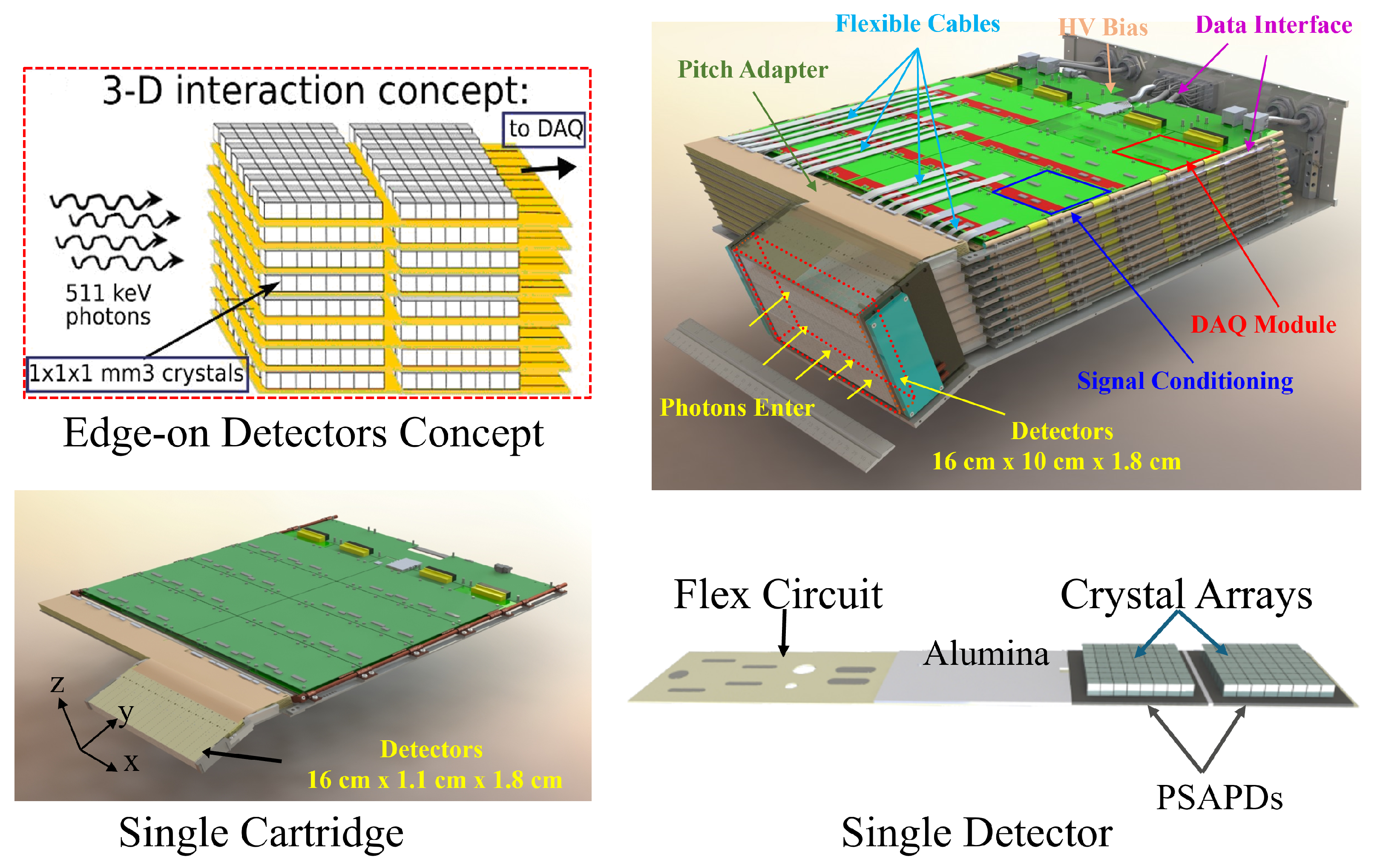
The system features two patient-adjustable panels to maximize sensitivity and enhance spatial resolution for visualization and quantification of smaller lesions. This dual-panel system is mounted on a custom-built mobile gantry capable of rotating the detector panels along three orthogonal axes to meet clinical requirements (
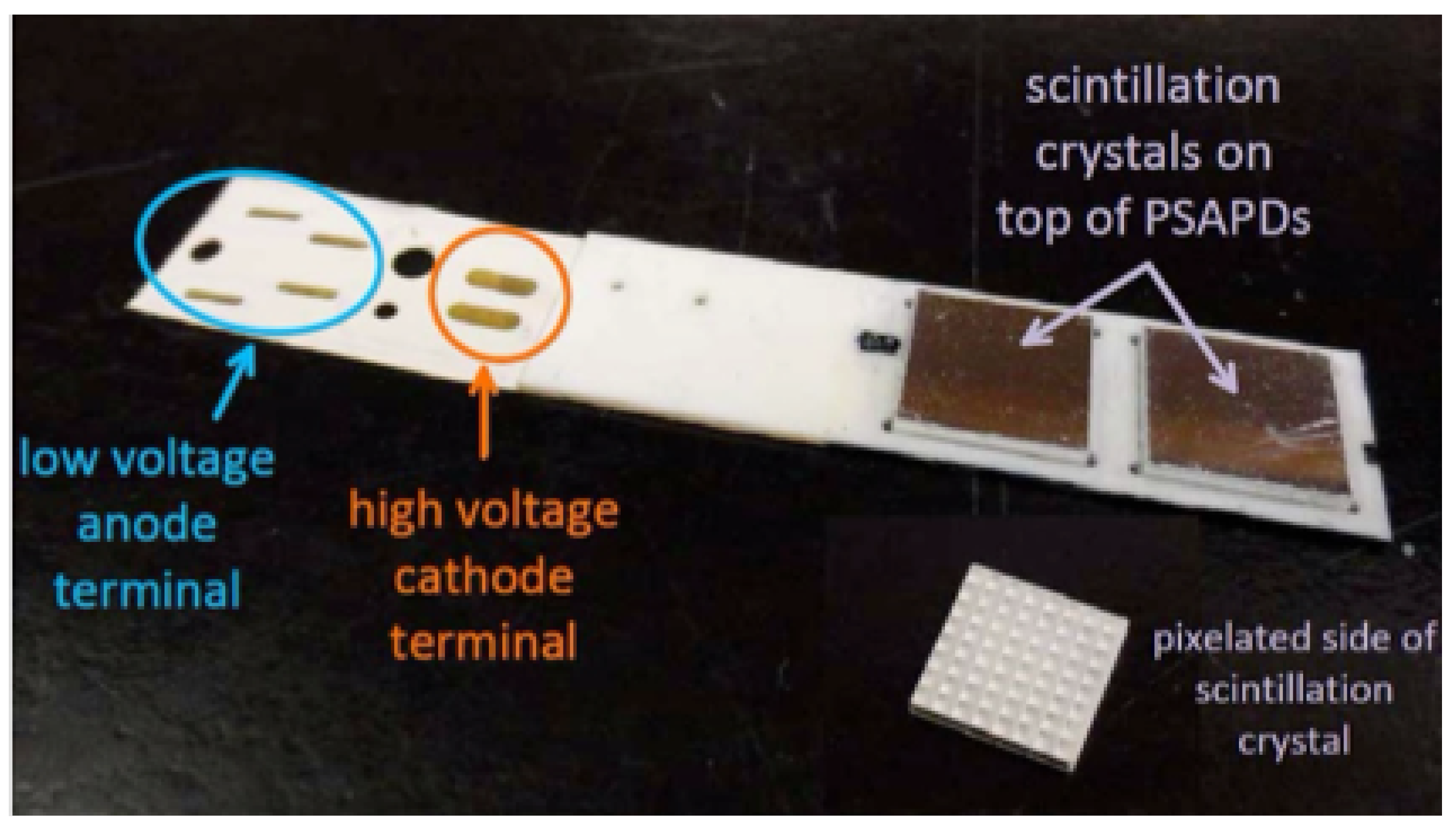
Figure 5). Each basic detector unit, termed a dual LYSO position-sensitive avalanche photodiode (dual-LYSO-PSAPD) detector (
Figure 6), embodies a 3D position-sensitive scintillation detector design concept that enables the measurement of photon energies, interaction locations, and arrival times in three dimensions (
Figure 7) [
65]. The dual-LYSO-PSAPD consists of two 8 × 8 arrays with individual crystal element dimensions of 0.9 mm × 0.9 mm × 1.0 mm, each coupled to a PSAPD, which is a high-gain, position-sensitive, large-area avalanche photodiode [
73]. Each detector layer is arranged in a novel “edge-on” orientation with respect to incoming photons.
Each panel houses multiple stackable detector layers, each comprising 16 dual-LYSO-PSAPD detectors (
Figure 7) [
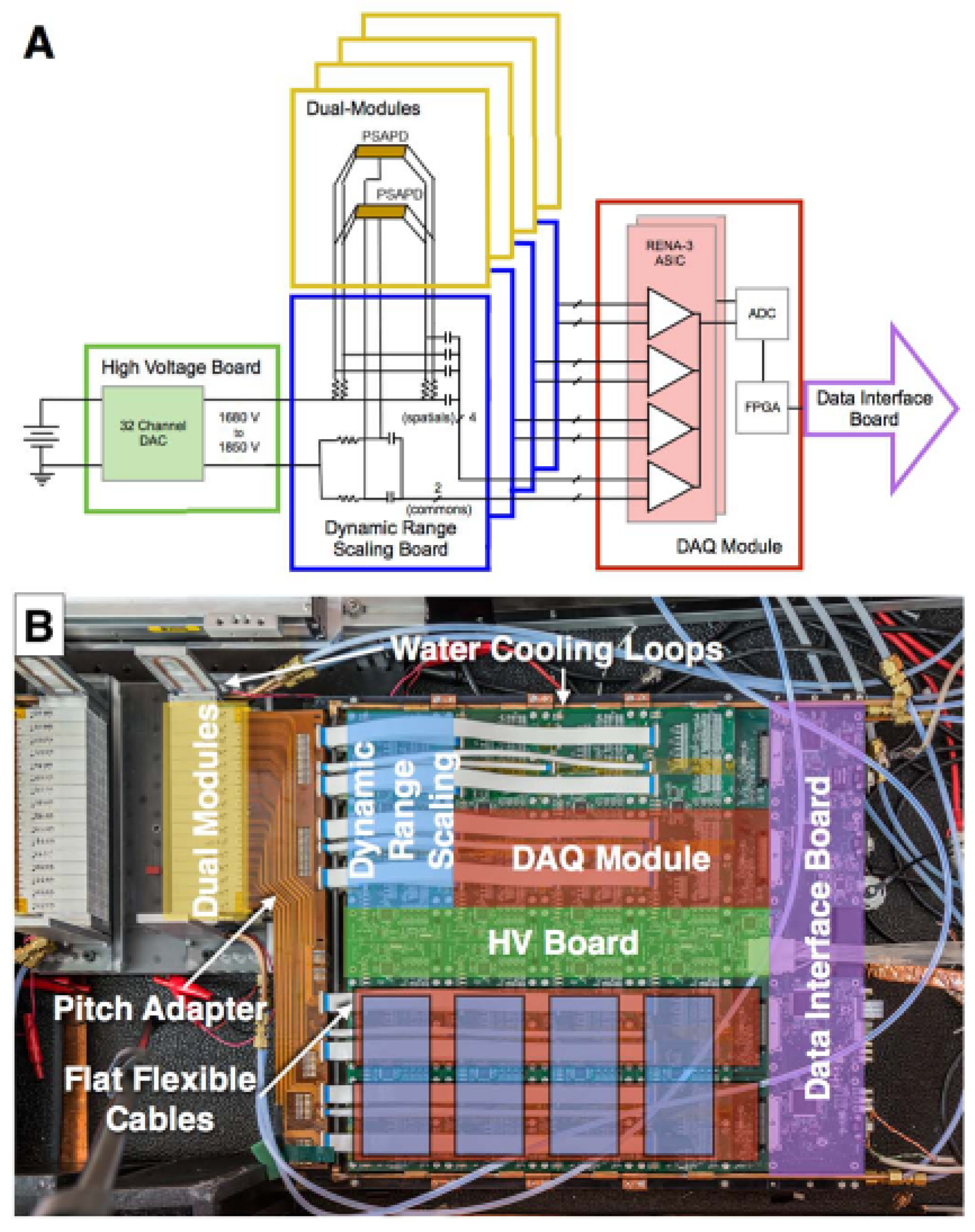
50]. The current prototype system has 32 detector layers, with the final system planned to have 48 layers per panel. The PSAPDs in the system are biased by a programmable high-voltage distribution circuit [
69], and the output signals from the dual-LYSO-PSAPD detectors are read out by RENA-3 ASIC chips [
74] after analog preprocessing [
66]. The RENA-3 outputs are then processed through FPGA-based backend boards, which send the event data to a master data-acquisition (DAQ) computer via Ethernet connections (
Figure 8) [
75]. The overall configuration of the system is summarized in
Table 3, and the system architecture is comprehensively discussed in Hsu et al. [
50,
71,
72].
The reconstructed spatial resolution, a primary focus of this section, is also detailed by Hsu et al. [
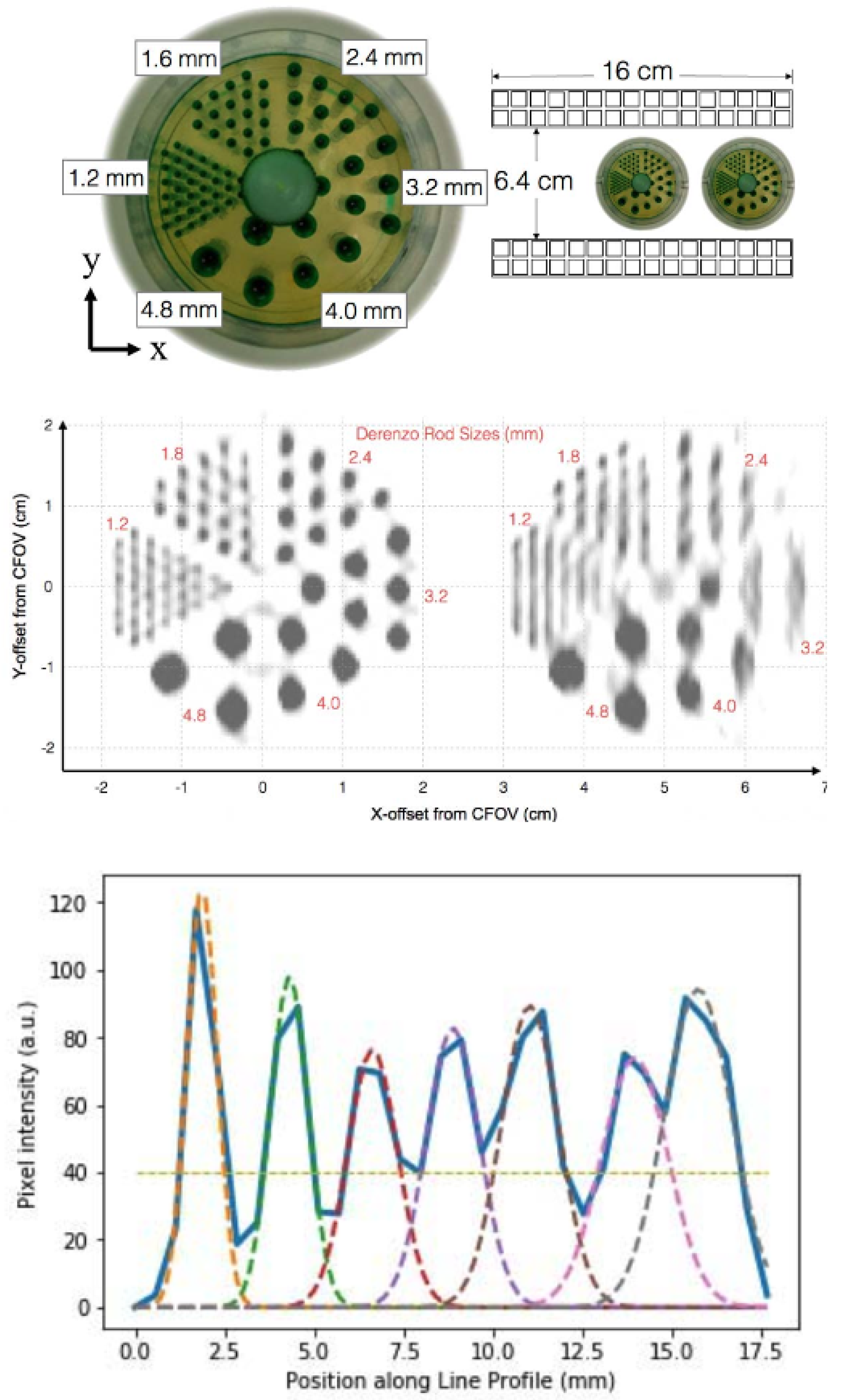
50]. A micro-Derenzo hot-rod phantom (Data Spectrum Corporation, Durham, NC) was scanned at the center of the FOV, and a separate scan was conducted with the same phantom placed at the edge of the FOV (
Figure 9). All rods in the phantom, including those with a 1.2 mm diameter, were clearly visible when positioned at the center. However, the spatial resolution along the axis orthogonal to the detector panels degraded when the phantom was placed at the FOV edge, due to the limited-angle tomographic sampling inherent to a dual-panel PET system design.
Limitations of 1 mm3 Spatial Resolution Clinical PET Systems
Achieving 1 mm
3 spatial resolution in clinical PET systems is a major step forward, but some important challenges remain, especially those related to the choice of radiotracer and how it is delivered to the body. One key factor is the positron range—the distance a positron travels in tissue before it interacts with a nearby electron and annihilates. In case of high-energy positron emitters such as
68Ga or
124I, this range can exceed 1 mm in soft tissue, causing a mismatch between where the radiotracer molecule is located and where the annihilation event occurs [
18,
23,
24,
25,
26,
76,
77]. This effect can lead to image blurring and minimize the benefit of high-resolution hardware.
Another challenge involves how the radiotracer is administered. Systemic delivery methods, such as intravenous injection, may lead to widespread tracer distribution or non-specific uptake, especially in areas with high blood flow or inflammation. This can make it harder to detect small lesions, even with advanced imaging systems.
5. Future Advancements, Clinical Impact, and Comparison of PET Systems with Standard Imaging Methods
Recent advancements in PET scanner design are driving a new era of molecular imaging with sub-2 mm, and even 1 mm3, isotropic spatial resolution. These improvements are motivated by the need for better early-stage disease detection, more accurate lesion localization, and personalized treatment planning. A key trend in this direction is the development of organ-targeted, or loco-regional, long axial FOV PET systems that optimize photon detection efficiency (PDE) while minimizing signal-to-noise ratio (SNR) degradation. Innovations include anatomically optimized geometries (e.g., for the head/neck and breast), high-performance scintillators, fast readout electronics, and image reconstruction algorithms that account for complex physical effects in the scanner.
These ultra-high-resolution PET systems complement traditional anatomical imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) [
78]. While CT and MRI detect changes in structure or density, PET provides molecular information by visualizing biological processes at the molecular level. This enables earlier disease detection, often before anatomical changes become visible. The latest PET technologies, including TOF and DOI configurations, further enhance contrast, reduce image noise, and improve the detection of small or low-contrast lesions.
For example, the 1 mm3 resolution PET scanner developed in our laboratory for head-and-neck or breast imaging demonstrates the clinical potential of these advances. With the ability to target specific molecular markers using radiotracers, PET offers unmatched sensitivity and versatility for applications in oncology, neurology, and cardiology. These advantages make next-generation PET systems a valuable addition to standard imaging, improving diagnostic precision and patient management across a range of clinical practices.
6. Conclusions
In this paper, the factors limiting the spatial resolution of PET systems, along with several selected PET detector designs and clinical locoregional PET systems, have been discussed with a focus on those achieving <2 mm resolution. Although not covered in detail in this paper, post-processing algorithms—such as inter-crystal scatter event positioning and image reconstruction strategies—can also play critical roles in improving spatial resolution. The overall goal of such ultra-high-resolution PET systems is to enable clinicians to better visualize and quantify disease earlier, make more accurate diagnoses, and more sensitively monitor the results of therapy.
Author Contributions
Conceptualization, M.C., M.N.U. and C.S.L.; Methodology, M.C., M.N.U. and C.S.L.; Data curation, M.C. and M.N.U.; Writing—original draft preparation, M.C. and M.N.U.; Writing—review and editing, M.C., M.N.U., D.I. and C.S.L.; 3D system design and rendering, D.I.; Supervision, C.S.L.; Project administration, C.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The reported Stanford research was supported in part by The Stanford-Coulter Translational Research Program, The Emerson Collective Cancer Research Fund, and National Institutes of Health, National Cancer Institute (NIH-NC) grant R01CA119056
Conflicts of Interest
None the funders of this research had a role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Muzic, R.F., Jr.; DiFilippo, F.P. Positron emission tomography-magnetic resonance imaging: Technical review. In Proceedings of the Seminars in Roentgenology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 49, pp. 242–254. [Google Scholar]
- Elangovan, A.; Jeyaseelan, T. Medical imaging modalities: A survey. In Proceedings of the 2016 International Conference on Emerging Trends in Engineering, Technology and Science (ICETETS), Pudukkottai, India, 24–26 February 2016; pp. 1–4. [Google Scholar]
- Torigian, D.A.; Zaidi, H.; Kwee, T.C.; Saboury, B.; Udupa, J.K.; Cho, Z.H.; Alavi, A. PET/MR imaging: Technical aspects and potential clinical applications. Radiology 2013, 267, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.E.; Lee, M.C.; Inoue, T.; Wong, W.H. Clinical PET and PET/CT: Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Dong, Q.; Ullah, M.N.; Innes, D.; Watkins, R.D.; Chang, C.M.; Zou, S.J.; Groll, A.; Sacco, I.; Chinn, G.; Levin, C.S. PETcoil: First results from a second-generation RF-penetrable TOF-PET brain insert for simultaneous PET/MRI. Phys. Med. Biol. 2024, 69, 185007. [Google Scholar] [CrossRef] [PubMed]
- Delso, G.; Fürst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance Measurements of the Siemens mMR Integrated Whole-Body PET/MR Scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.M.; Deller, T.W.; Khalighi, M.M.; Maramraju, S.H.; Delso, G.; Levin, C.S. NEMA NU 2-2012 performance studies for the SiPM-based ToF-PET component of the GE SIGNA PET/MR system. Med. Phys. 2016, 43, 2334–2343. [Google Scholar] [CrossRef]
- Reynés-Llompart, G.; Gámez-Cenzano, C.; Romero-Zayas, I.; Rodríguez-Bel, L.; Vercher-Conejero, J.L.; Martí-Climent, J.M. Performance Characteristics of the Whole-Body Discovery IQ PET/CT System. J. Nucl. Med. 2017, 58, 1155–1161. [Google Scholar] [CrossRef]
- Rausch, I.; Ruiz, A.; Valverde-Pascual, I.; Cal-González, J.; Beyer, T.; Carrio, I. Performance Evaluation of the Vereos PET/CT System According to the NEMA NU2-2012 Standard. J. Nucl. Med. 2019, 60, 561–567. [Google Scholar] [CrossRef]
- van Sluis, J.; de Jong, J.; Schaar, J.; Noordzij, W.; van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance Characteristics of the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef]
- Chicheportiche, A.; Marciano, R.; Orevi, M. Comparison of NEMA characterizations for Discovery MI and Discovery MI-DR TOF PET/CT systems at different sites and with other commercial PET/CT systems. EJNMMI Phys. 2020, 7, 4. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Townsend, D. Physical principles and technology of clinical PET imaging. Ann.-Acad. Med. Singap. 2004, 33, 133–145. [Google Scholar] [CrossRef]
- Gonzalez-Montoro, A.; Ullah, M.N.; Levin, C.S. Advances in Detector Instrumentation for PET. J. Nucl. Med. 2022, 63, 1138–1144. [Google Scholar] [CrossRef]
- El Ouaridi, A.; Ait Elcadi, Z.; Mkimel, M.; Bougteb, M.; El Baydaoui, R. The detection instrumentation and geometric design of clinical PET scanner: Towards better performance and broader clinical applications. Biomed. Phys. Eng. Express 2024, 10, 032002. [Google Scholar] [CrossRef] [PubMed]
- Melcher, C.L. Scintillation crystals for PET. J. Nucl. Med. 2000, 41, 1051–1055. [Google Scholar]
- Valais, I.; Michail, C.; David, S.; Nomicos, C.; Panayiotakis, G.; Kandarakis, I. A comparative study of the luminescence properties of LYSO: Ce, LSO: Ce, GSO: Ce and BGO single crystal scintillators for use in medical X-ray imaging. Phys. Medica 2008, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Hoffman, E.J. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys. Med. Biol. 1999, 44, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.N.; Park, J.H.; Pratiwi, E.; Kim, G.B.; Yeom, J.Y. Wavelength discrimination (WLD) detector optimization for time-of-flight positron emission tomography with depth of interaction information. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 982, 164498. [Google Scholar] [CrossRef]
- Rahmim, A.; Qi, J.; Sossi, V. Resolution modeling in PET imaging: Theory, practice, benefits, and pitfalls. Med. Phys. 2013, 40, 064301. [Google Scholar] [CrossRef]
- Moses, W.W. Fundamental limits of spatial resolution in PET. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 648, S236–S240. [Google Scholar] [CrossRef]
- Shibuya, K.; Yoshida, E.; Nishikido, F.; Suzuki, T.; Tsuda, T.; Inadama, N.; Yamaya, T.; Murayama, H. Annihilation photon acollinearity in PET: Volunteer and phantom FDG studies. Phys. Med. Biol. 2007, 52, 5249–5261. [Google Scholar] [CrossRef]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. National Institute of Standards and Technology. Available online: https://www.nist.gov (accessed on 4 April 2022).
- Laboratoire National Henri Becquerel. Laboratoire National Henri Becquerel. Available online: http://www.lnhb.fr/ (accessed on 4 April 2022).
- Brookhaven National Laboratory. Brookhaven National Laboratory. Available online: https://www.bnl.gov/ (accessed on 4 April 2022).
- Stickel, J.R.; Qi, J.; Cherry, S.R. Fabrication and Characterization of a 0.5-mm Lutetium Oxyorthosilicate Detector Array for High-Resolution PET Applications. J. Nucl. Med. 2007, 48, 115–121. [Google Scholar]
- Stickel, J.R.; Cherry, S.R. High-resolution PET detector design: Modelling components of intrinsic spatial resolution. Phys. Med. Biol. 2004, 50, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S. Design of a high resolution and high sensitivity scintillation crystal array with nearly perfect light collection. In Proceedings of the 2001 IEEE Nuclear Science Symposium Conference Record (Cat. No.01CH37310), San Diego, CA, USA, 4–10 November 2001; Volume 1, pp. 48–52. [Google Scholar] [CrossRef]
- Levin, C.S.; Foudray, A.M.K.; Olcott, P.D.; Habte, F. Investigation of position sensitive avalanche photodiodes for a new high resolution PET detector design. In Proceedings of the 2003 IEEE Nuclear Science Symposium. Conference Record (IEEE Cat. No.03CH37515), Portland, OR, USA, 19–25 October 2003; Volume 4, pp. 2262–2266. [Google Scholar] [CrossRef]
- Lewellen, T.K. Recent developments in PET detector technology. Phys. Med. Biol. 2008, 53, R287. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, X.; Lan, K.A.; Bircher, C.; Lou, K.; Deng, Z. Development of a prototype PET scanner with depth-of-interaction measurement using solid-state photomultiplier arrays and parallel readout electronics. Phys. Med. Biol. 2014, 59, 1223–1238. [Google Scholar] [CrossRef]
- Du, J.; Bai, X.; Cherry, S.R. Performance comparison of depth-encoding detectors based on dual-ended readout and different {SiPMs} for high-resolution {PET} applications. Phys. Med. Biol. 2019, 64, 15NT03. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, X.; Ren, N.; Wu, S.; Gao, J.; Zeng, T.; Gao, D.; Zhang, C.; Sang, Z.; Hu, Z.; et al. Design and performance of SIAT aPET: A uniform high-resolution small animal PET scanner using dual-ended readout detectors. Phys. Med. Biol. 2020, 65, 235013. [Google Scholar] [CrossRef] [PubMed]
- Lewellen, T.K.; Janes, M.; Miyaoka, R.S. DMice-a depth-of-interaction detector design for PET scanners. In Proceedings of the IEEE Symposium Conference Record Nuclear Science 2004, Rome, Italy, 16–22 October 2004; Volume 4, pp. 2388–2392. [Google Scholar] [CrossRef]
- Yoshida, E.; Obata, F.; Kamada, K.; Yamaya, T. A Crosshair Light Sharing PET Detector With DOI and TOF Capabilities Using Four-to-One Coupling and Single-Ended Readout. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 638–644. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Wang, L. Evaluation of high-resolution and depth-encoding PET detector modules based on single-ended readout with TOFPET2 ASIC. Radiat. Detect. Technol. Methods 2021, 5, 451–458. [Google Scholar] [CrossRef]
- Vandenbroucke, A.; Foudray, A.M.K.; Olcott, P.D.; Levin, C.S. Performance characterization of a new high resolution PET scintillation detector. Phys. Med. Biol. 2010, 55, 5895–5911. [Google Scholar] [CrossRef]
- Zhang, J.; Olcott, P.D.; Chinn, G.; Foudray, A.M.K.; Levin, C.S. Study of the performance of a novel resolution dual-panel PET camera design dedicated to breast cancer imaging using Monte Carlo simulation. Med. Phys. 2007, 34, 689–702. [Google Scholar] [CrossRef]
- Qi, J.; Leahy, R.M. Iterative reconstruction techniques in emission computed tomography. Phys. Med. Biol. 2006, 51, R541. [Google Scholar] [CrossRef]
- Vandenberghe, S.; Mikhaylova, E.; D’Hoe, E.; Mollet, P.; Karp, J.S. Recent developments in time-of-flight PET. EJNMMI Phys. 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. High SPATIAL Resolution Dedicated Head and Neck Positron Emission Tomography System Based on Cadmium Zinc Telluride Detectors; University of California: Santa Cruz, CA, USA, 2021. [Google Scholar]
- Jin, Y.; Streicher, M.; Yang, H.; Brown, S.; He, Z.; Meng, L.J. Experimental evaluation of a 3-D CZT imaging spectrometer for potential use in Compton-Enhanced PET imaging. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 7, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, F.T.; Dadgar, M.; Abbaszadeh, S. Assessment of CZT Dual Panel PET as total-body imaging in small animal with high spatial resolution and multi-sample capability. J. Nucl. Med. 2024, 65, 241928. [Google Scholar]
- Stanford-Hill, R.; Groll, A.; Levin, C.S. A simulation of a high-resolution cadmium zinc telluride positron emission tomography system. Med. Phys. 2024, 51, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Ueno, Y.; Takeuchi, W.; Kojima, S.; Matsuzaki, K.; Ishitsu, T.; Umegaki, K.; Kiyanagi, Y.; Kubo, N.; Katoh, C.; et al. Development of a 3D brain PET scanner using CdTe semiconductor detectors and its first clinical application. IEEE Trans. Nucl. Sci. 2011, 58, 2181–2189. [Google Scholar] [CrossRef]
- Cherlin, A.; Wirth, A.; Erlandsson, K.; Baistow, I.; Thielemans, K.; Hutton, B.F. A new concept for a low-dose stationary tomographic molecular breast imaging camera using 3D position sensitive CZT detectors. In Proceedings of the 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Virtual, 16–23 October 2021; pp. 1–3. [Google Scholar]
- Gu, Y.; Matteson, J.; Skelton, R.; Deal, A.; Stephan, E.; Duttweiler, F.; Gasaway, T.; Levin, C. Study of a high-resolution, 3D positioning cadmium zinc telluride detector for PET. Phys. Med. Biol. 2011, 56, 1563. [Google Scholar] [CrossRef]
- Jiang, W.; Chalich, Y.; Deen, M.J. Sensors for positron emission tomography applications. Sensors 2019, 19, 5019. [Google Scholar] [CrossRef]
- Hsu, D.F.C.; Freese, D.L.; Reynolds, P.D.; Innes, D.R.; Levin, C.S. Design and Performance of a 1 mm3 Resolution Clinical PET System Comprising 3-D Position Sensitive Scintillation Detectors. IEEE Trans. Med. Imaging 2018, 37, 1058–1066. [Google Scholar] [CrossRef]
- Wienhard, K.; Schmand, M.; Casey, M.E.; Baker, K.; Bao, J.; Eriksson, L.; Jones, W.F.; Knoess, C.; Lenox, M.; Lercher, M.; et al. The ECAT HRRT: Performance and first clinical application of the new high resolution research tomograph. IEEE Trans. Nucl. Sci. 2002, 49, 104–110. [Google Scholar] [CrossRef]
- Watanabe, M.; Saito, A.; Isobe, T.; Ote, K.; Yamada, R.; Moriya, T.; Omura, T. Performance evaluation of a high-resolution brain PET scanner using four-layer MPPC DOI detectors. Phys. Med. Biol. 2017, 62, 7148–7166. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Gonzalez-Montoro, A.; Vidal, L.F.; Barbera, J.; Aussenhofer, S.; Hernandez, L.; Moliner, L.; Sanchez, F.; Correcher, C.; Pincay, E.J.; et al. Initial Results of the MINDView PET Insert Inside the 3T mMR. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 343–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Ramirez, R.A.; Li, H.; Liu, S.; An, S.; Wang, C.; Baghaei, H.; Wong, W.H. The System Design, Engineering Architecture, and Preliminary Results of a Lower-Cost High-Sensitivity High-Resolution Positron Emission Mammography Camera. IEEE Trans. Nucl. Sci. 2010, 57, 104–110. [Google Scholar] [CrossRef]
- Miyake, K.K.; Matsumoto, K.; Inoue, M.; Nakamoto, Y.; Kanao, S.; Oishi, T.; Kawase, S.; Kitamura, K.; Yamakawa, Y.; Akazawa, A.; et al. Performance Evaluation of a New Dedicated Breast PET Scanner Using NEMA NU4-2008 Standards. J. Nucl. Med. 2014, 55, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Moliner, L.; González, A.J.; Soriano, A.; Sánchez, F.; Correcher, C.; Orero, A.; Carles, M.; Vidal, L.F.; Barberá, J.; Caballero, L.; et al. Design and evaluation of the MAMMI dedicated breast PET. Med. Phys. 2012, 39, 5393–5404. [Google Scholar] [CrossRef]
- Li, H.; Badawi, R.D.; Cherry, S.R.; Fontaine, K.; He, L.; Henry, S.; Hillmer, A.T.; Hu, L.; Khattar, N.; Leung, E.K.; et al. Performance Characteristics of the NeuroEXPLORER, a Next-Generation Human Brain PET/CT Imager. J. Nucl. Med. 2024, 65, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Wienhard, K.; Eriksson, M.; Casey, M.E.; Knoess, C.; Bruckbauer, T.; Hamill, J.; Schmand, M.; Gremillion, T.; Lenox, M.; et al. The ECAT HRRT: NEMA NEC evaluation of the HRRT system, the new high-resolution research tomograph. IEEE Trans. Nucl. Sci. 2002, 49, 2085–2088. [Google Scholar] [CrossRef]
- Tsuda, T.; Murayama, H.; Kitamura, K.; Yamaya, T.; Yoshida, E.; Omura, T.; Kawai, H.; Inadama, N.; Orita, N. A four-Layer depth of interaction detector block for small animal PET. IEEE Trans. Nucl. Sci. 2004, 51, 2537–2542. [Google Scholar] [CrossRef]
- García Hernández, T.; Vicedo González, A.; Ferrer Rebolleda, J.; Sánchez Jurado, R.; Roselló Ferrando, J.; Brualla González, L.; Granero Cabañero, D.; del Puig Cozar Santiago, M. Performance evaluation of a high resolution dedicated breast PET scanner. Med. Phys. 2016, 43, 2261–2272. [Google Scholar] [CrossRef]
- Chin, M.; Innes, D.; Levin, C.S. Results from a Further Scaled up Prototype of a 1-millimeter Resolution Clinical PET System. In Proceedings of the 2020 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Boston, MA, USA, 31 October–7 November 2020; pp. 1–2. [Google Scholar] [CrossRef]
- Zhang, J.; Olcott, P.D.; Levin, C.S. A New Positioning Algorithm for Position-Sensitive Avalanche Photodiodes. IEEE Trans. Nucl. Sci. 2007, 54, 433–437. [Google Scholar] [CrossRef]
- Lau, F.W.Y.; Vandenbroucke, A.; Reynolds, P.D.; Olcott, P.D.; Horowitz, M.A.; Levin, C.S. Analog signal multiplexing for PSAPD-based PET detectors: Simulation and experimental validation. Phys. Med. Biol. 2010, 55, 7149. [Google Scholar] [CrossRef]
- Reynolds, P.D.; Olcott, P.D.; Pratx, G.; Lau, F.W.Y.; Levin, C.S. Convex Optimization of Coincidence Time Resolution for a High-Resolution PET System. IEEE Trans. Med. Imaging 2011, 30, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S. Promising new photon detection concepts for high-resolution clinical and preclinical PET. J. Nucl. Med. 2012, 53, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.W.Y.; Vandenbroucke, A.; Reynolds, P.; Ho, H.; Innes, D.; Levin, C.S. Signal Conditioning Technique for Position Sensitive Photodetectors to Manipulate Pixelated Crystal Identification Capabilities. IEEE Trans. Nucl. Sci. 2012, 59, 1815–1822. [Google Scholar] [CrossRef]
- Vandenbroucke, A.; McLaughlin, T.J.; Levin, C.S. Influence of temperature and bias voltage on the performance of a high resolution {PET} detector built with position sensitive avalanche photodiodes. J. Instrum. 2012, 7, P08001. [Google Scholar] [CrossRef]
- Zhai, J.; Vandenbroucke, A.; Levin, C.S. Thermal regulation for APDs in a 1 mm3 resolution clinical PET camera: Design, simulation and experimental verification. Phys. Med. Biol. 2014, 59, 3951. [Google Scholar] [CrossRef]
- Lau, F.W.Y.; Vandenbroucke, A.; Yeom, J.; Reynolds, P.D.; Hsu, D.; Innes, D.; Levin, C.S. Programmable High Voltage Distribution for Photodetectors in a 1 mm Resolution Clinical PET System. IEEE Trans. Nucl. Sci. 2015, 62, 1989–1994. [Google Scholar] [CrossRef]
- Freese, D.L.; Vandenbroucke, A.; Innes, D.; Lau, F.W.Y.; Hsu, D.F.C.; Reynolds, P.D.; Levin, C.S. Thermal regulation of tightly packed solid-state photodetectors in a 1 mm3 resolution clinical PET system. Med. Phys. 2015, 42, 305–313. [Google Scholar] [CrossRef]
- Hsu, D.F.C.; Freese, D.L.; Innes, D.R.; Levin, C.S. Intercrystal scatter studies for a 1 mm3 resolution clinical PET system prototype. Phys. Med. Biol. 2019, 64, 95024. [Google Scholar] [CrossRef]
- Hsu, D.F.C.; Freese, D.L.; Innes, D.R.; Levin, C.S. Time Resolution Studies for a 1-mm Resolution Clinical PET System With a Charge Sharing Readout and Leading Edge Discrimination. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 285–291. [Google Scholar] [CrossRef]
- Shah, K.S.; Grazioso, R.; Farrell, R.; Glodo, J.; McClish, M.; Entine, G.; Dokhale, P.; Cherry, S.R. Position sensitive APDs for small Animal PET imaging. IEEE Trans. Nucl. Sci. 2004, 51, 91–95. [Google Scholar] [CrossRef]
- Tumer, T.O.; Cajipe, V.B.; Clajus, M.; Hayakawa, S.; Volkovskii, A. Multi-Channel Front-End Readout IC for Position Sensitive Solid-State Detectors. In Proceedings of the 2006 IEEE Nuclear Science Symposium Conference Record, San Diego, CA, USA, 29 October–4 November 2006; Volume 1, pp. 384–388, ISSN 1082-3654. [Google Scholar] [CrossRef]
- Reynolds, P.D.; Vandenbroucke, A.; Freese, D.; Hsu, D.; Innes, D.; Levin, C.S. Stackable electronics architecture for densely packed PET detectors. In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sauerzapf, S.; Thomas, L.; Behe, M.; Weber, W.; Zakhnini, A.; Pietrzyk, U.; Mix, M. Using Monte-Carlo simulations to implement corrections for I-124 as a non-pure positron emitter in small animal and human PET imaging. In Proceedings of the 2011 IEEE Nuclear Science Symposium Conference Record, Valencia, Spain, 23–29 October 2011; pp. 2688–2691. [Google Scholar]
- Alavi, A.; Werner, T.J.; Stępień, E.Ł.; Moskal, P. Unparalleled and revolutionary impact of PET imaging on research and day to day practice of medicine. Bio-Algorithms Med-Syst. 2022, 17, 203–212. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).