Dynamic Simulation and Characteristic Analysis on Freezing Process in Ballast Tanks of Polar LNG Carriers
Abstract
1. Introduction
2. Materials and Methods
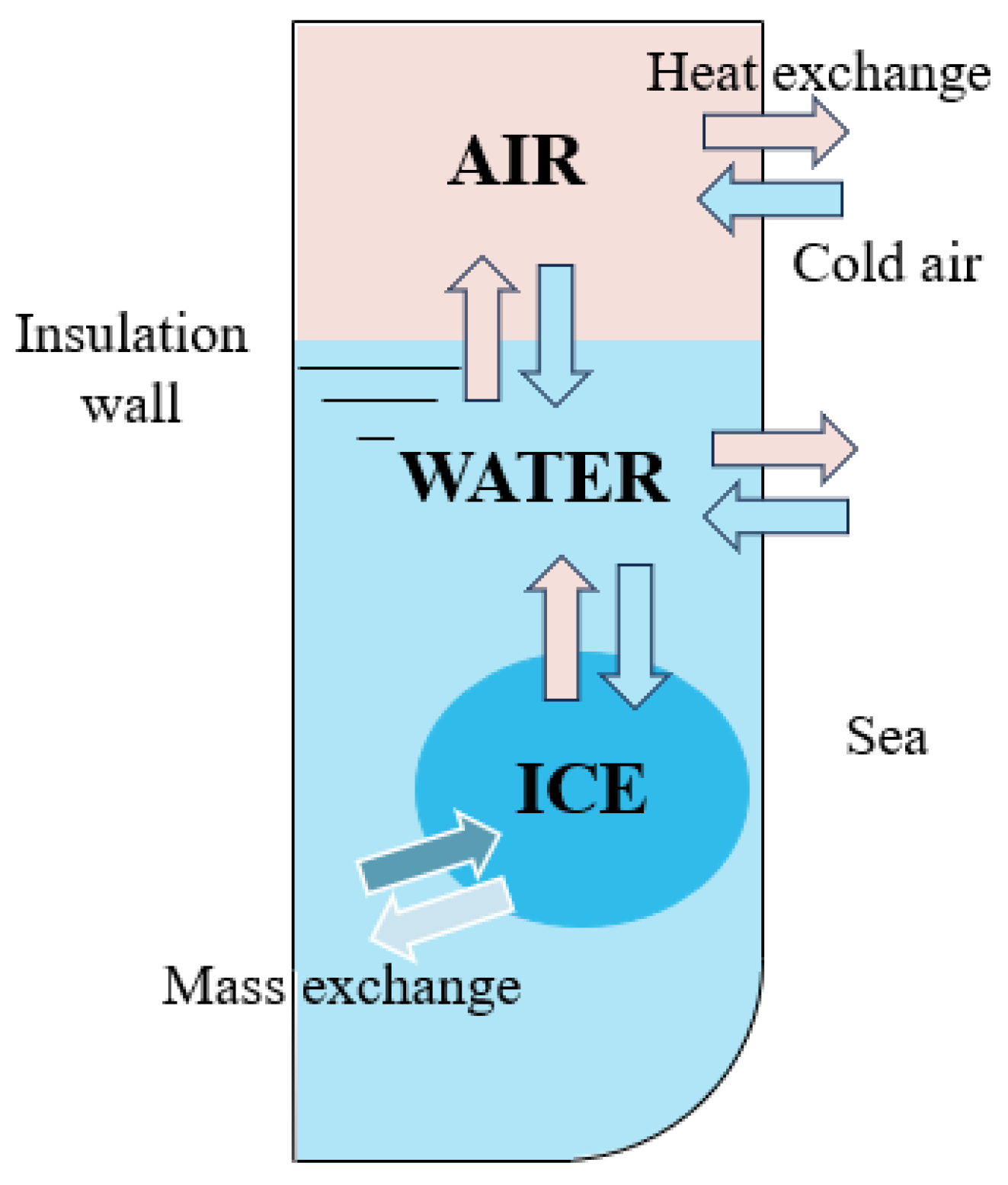
2.1. Presentation of Heat Transfer Model in Ballast Tank
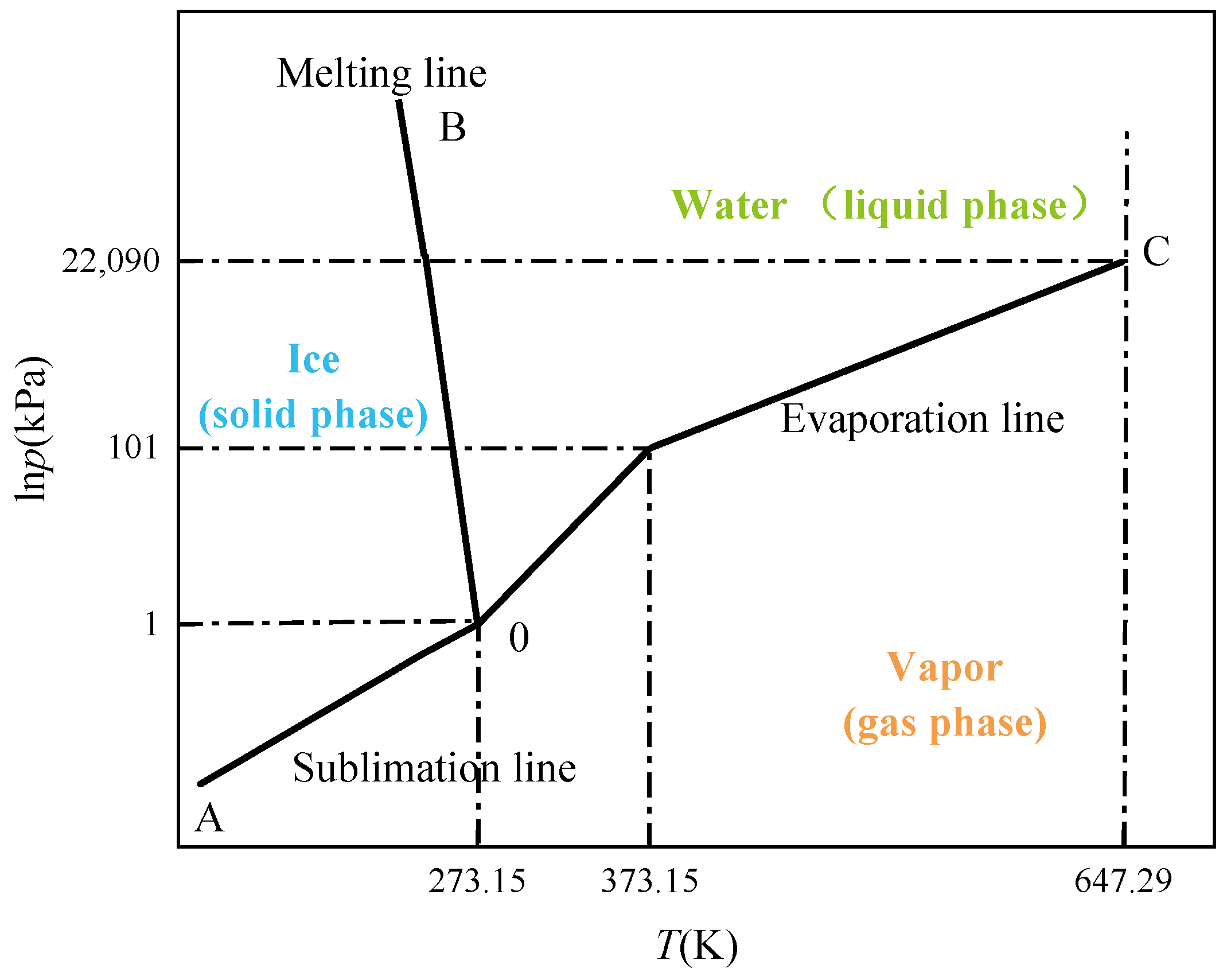
2.2. Freezing Theories
2.2.1. Two-Phase Flow Control Equation
2.2.2. Phase Transformation Model
2.2.3. Composition Equation
2.3. Physical Model and Boundary Conditions of Ballast Tank Freezing
2.3.1. Physical Model of Ballast Tank Freezing
2.3.2. Boundary Condition
2.3.3. Material Physical Parameters
2.4. Numerical Calculation Method and Verification
2.4.1. Numerical Analysis Process
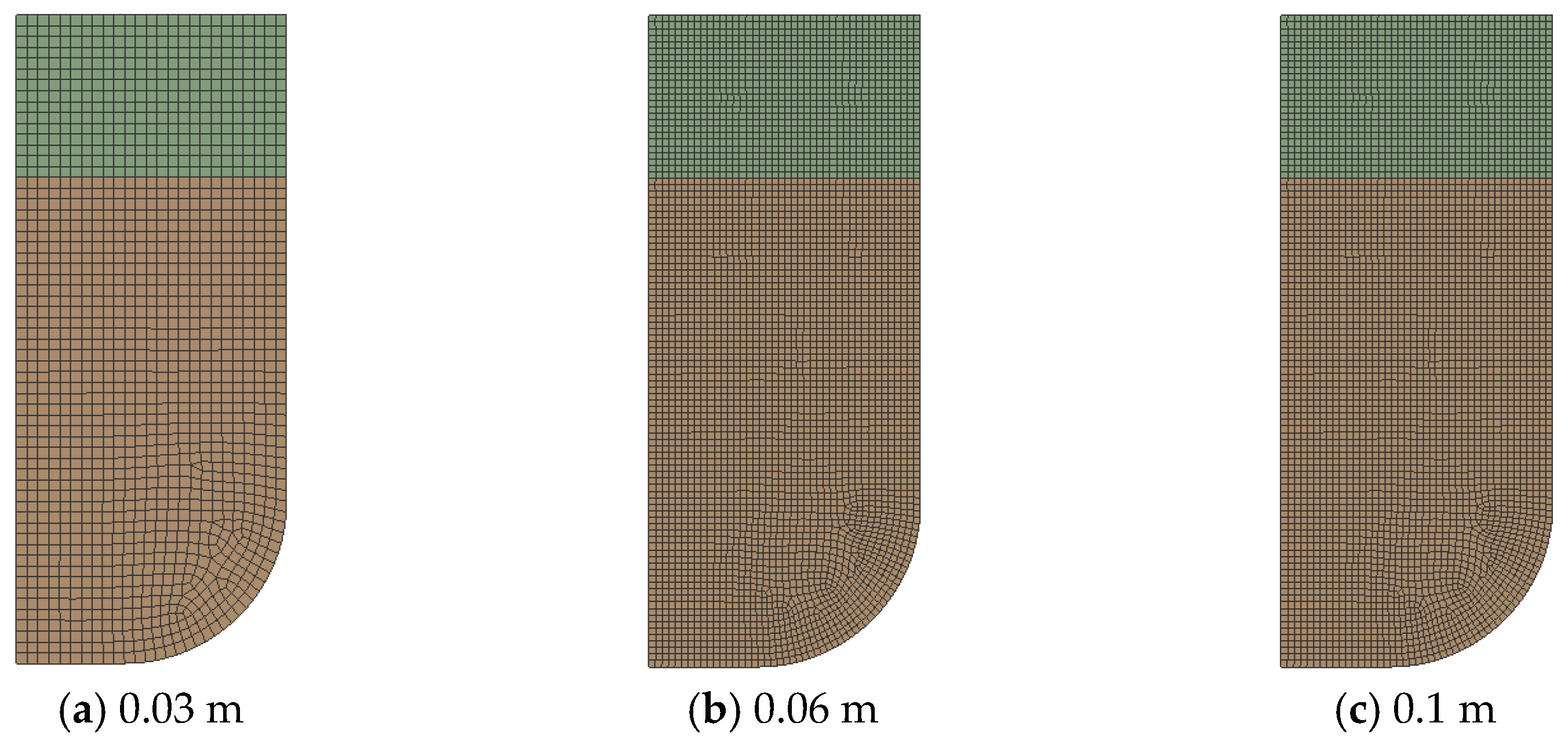
2.4.2. Grid Independence Analysis
2.4.3. Numerical Model Validation
3. Discussion and Analysis
3.1. Influence of Environmental Parameters on the Temperature Field Evolution
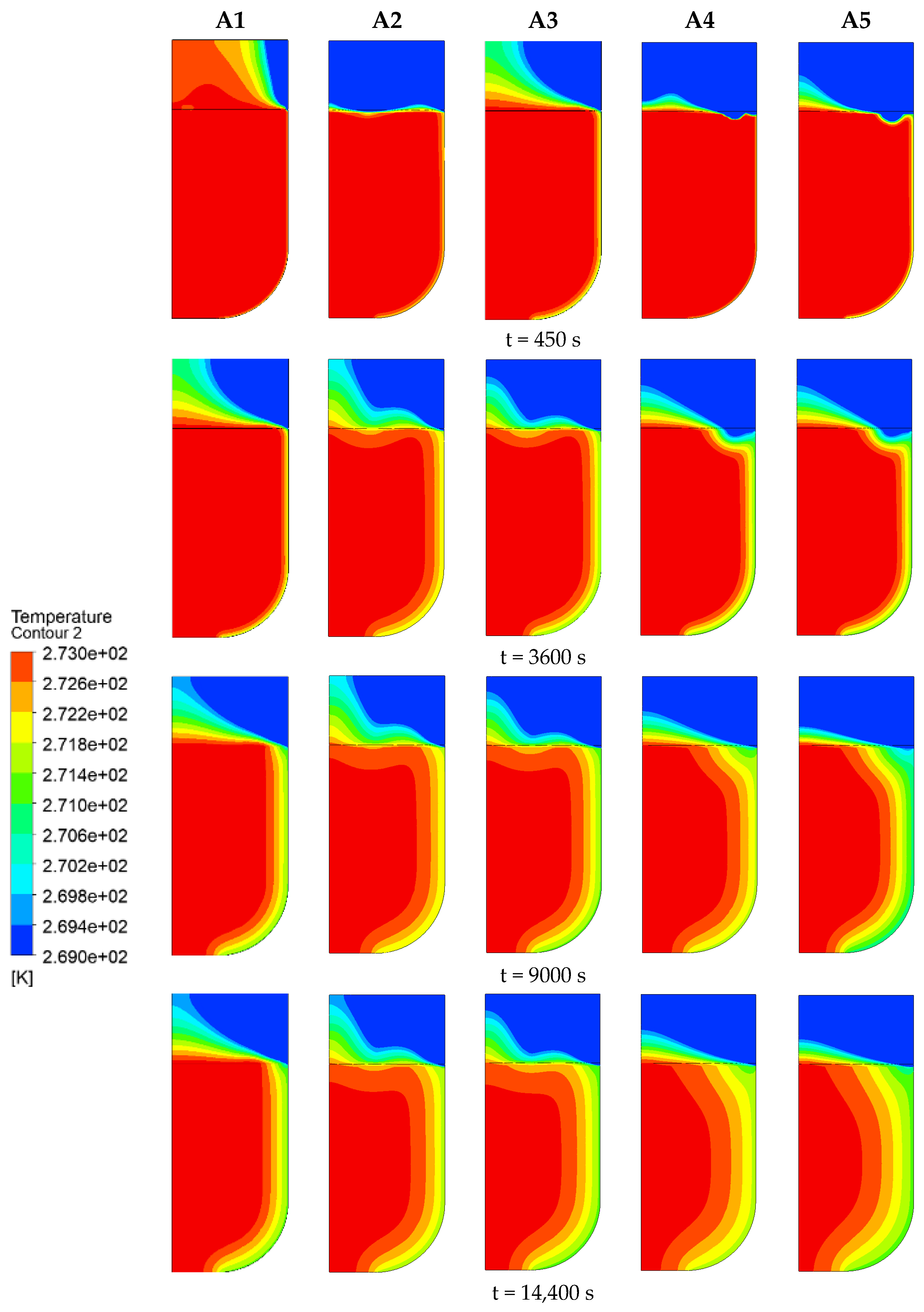
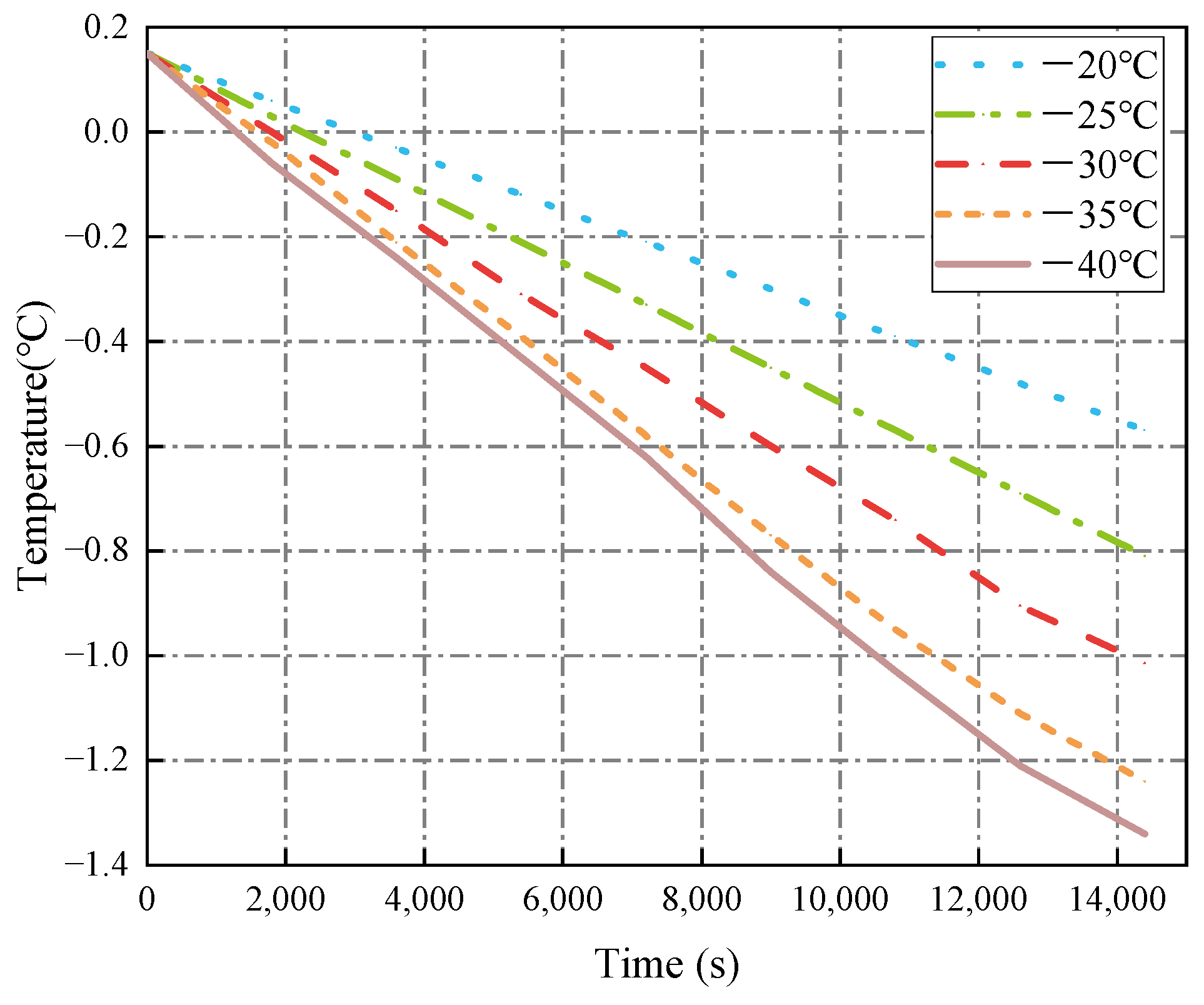
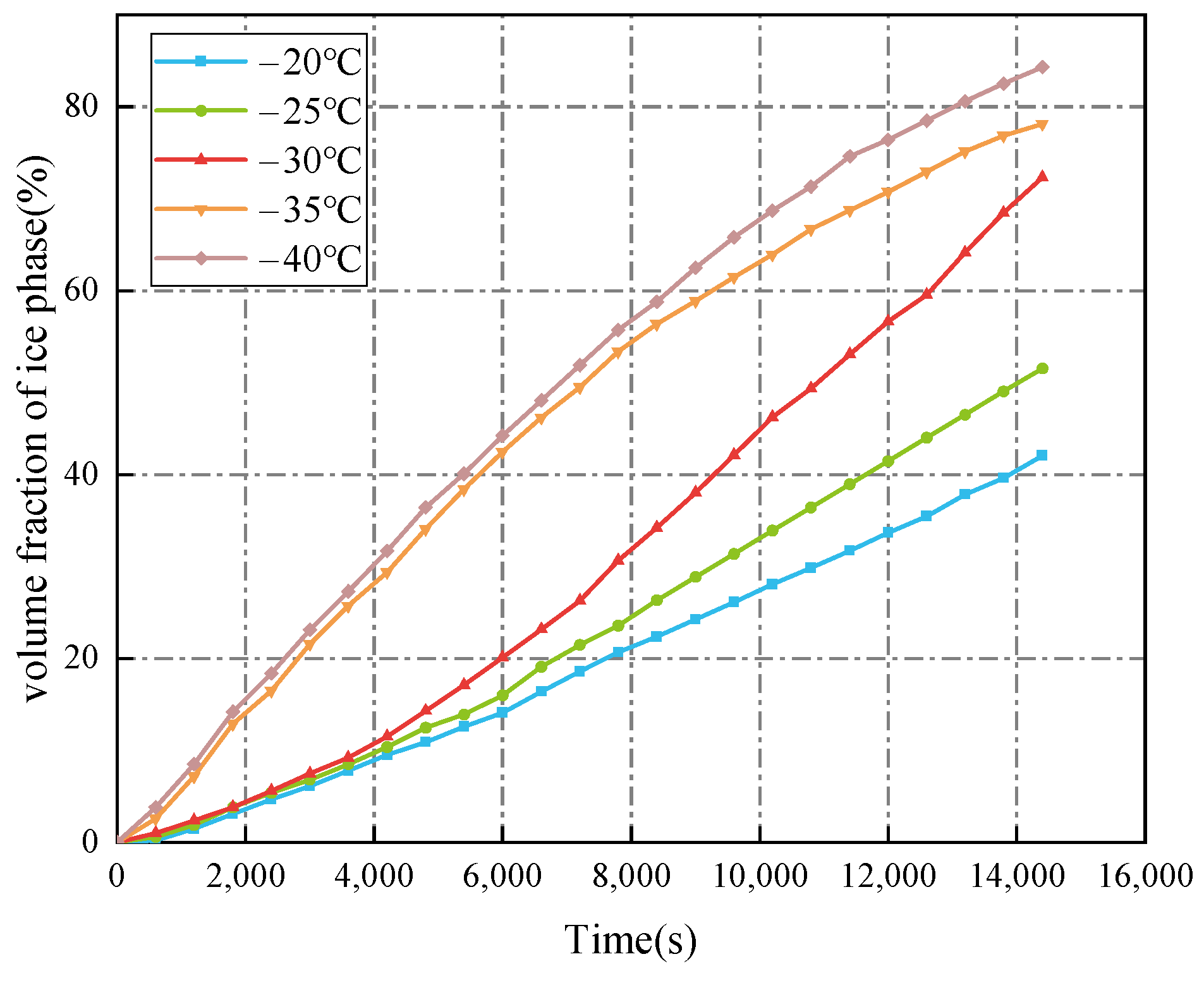
3.1.1. Cold Air Temperature
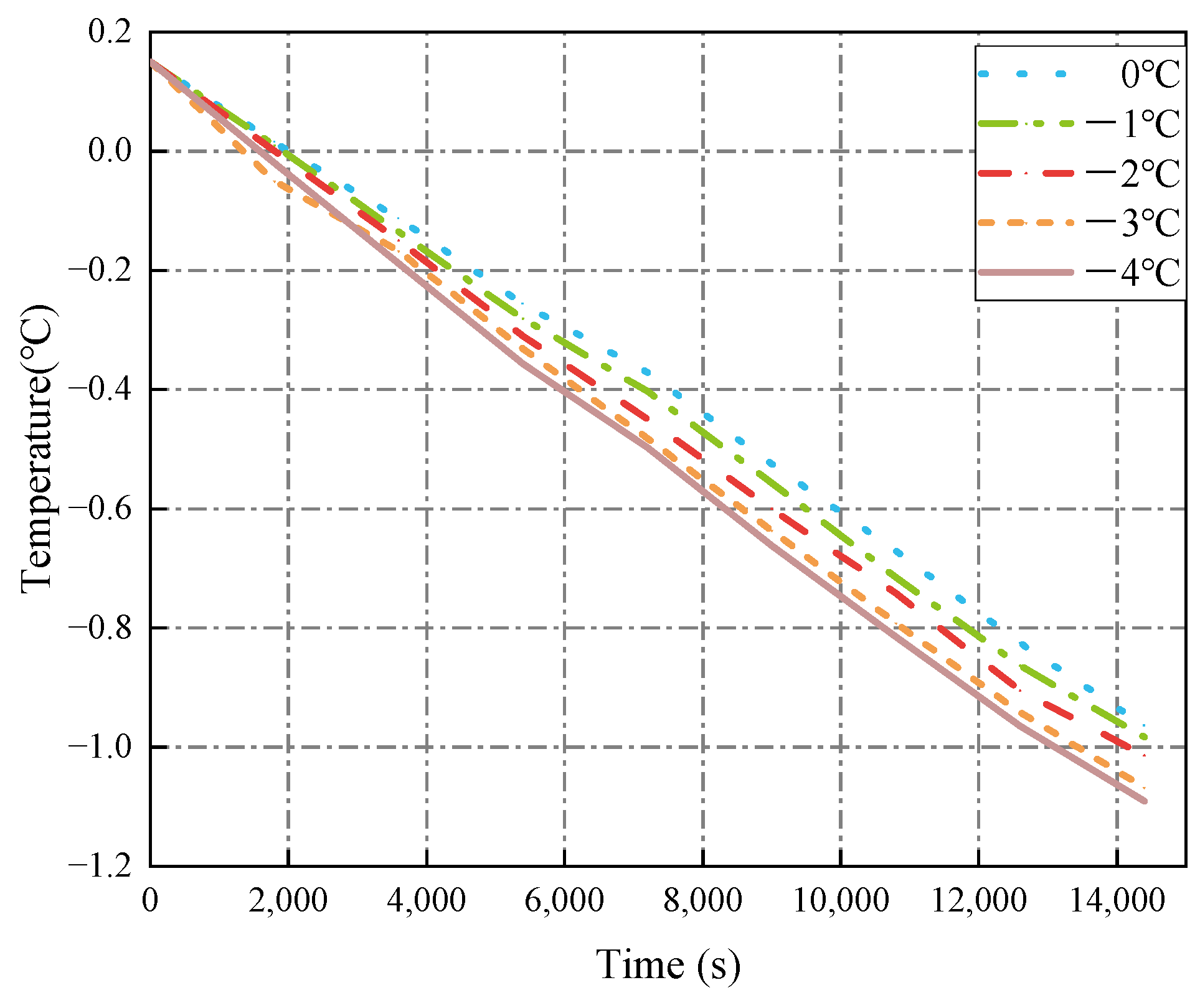
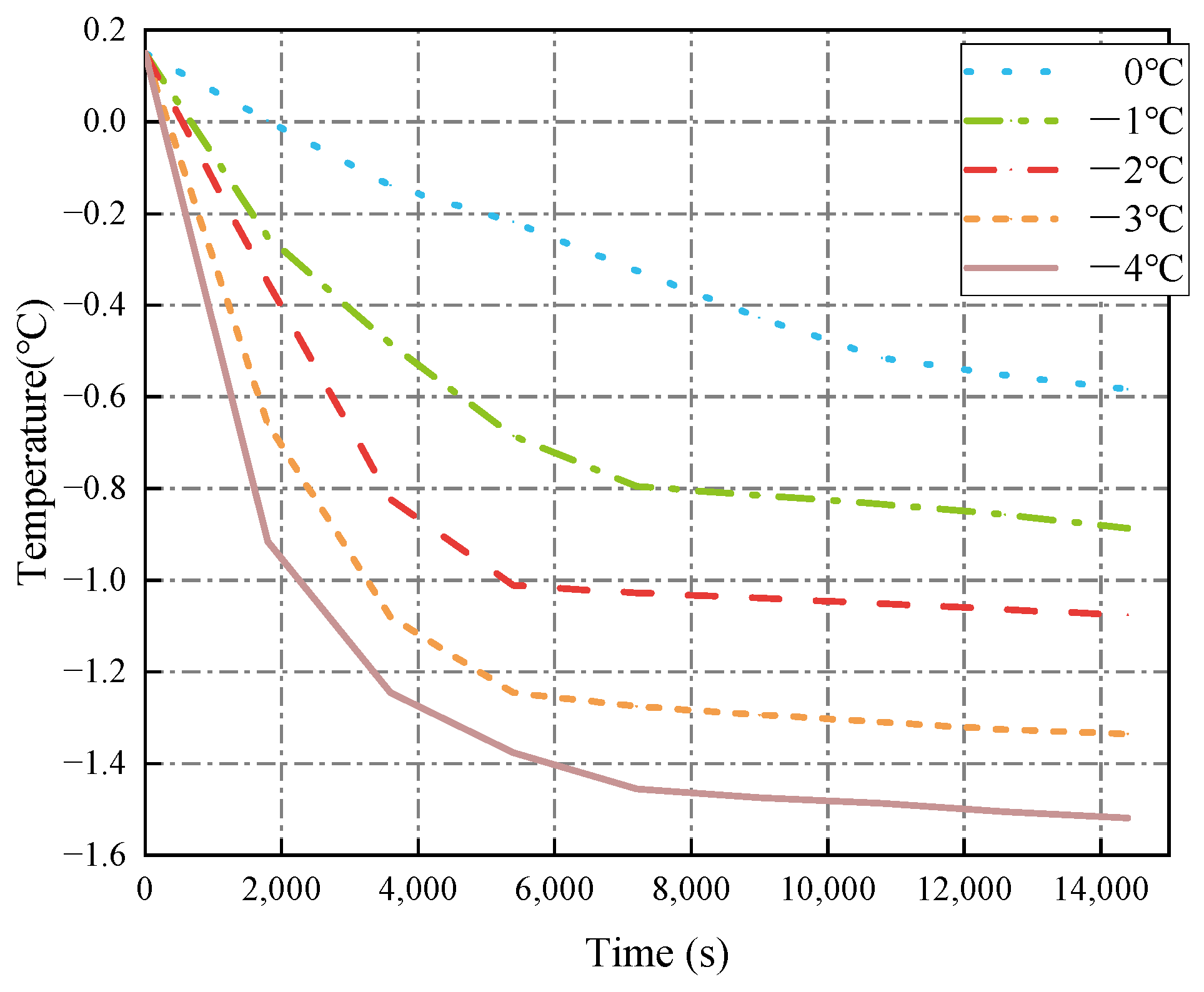
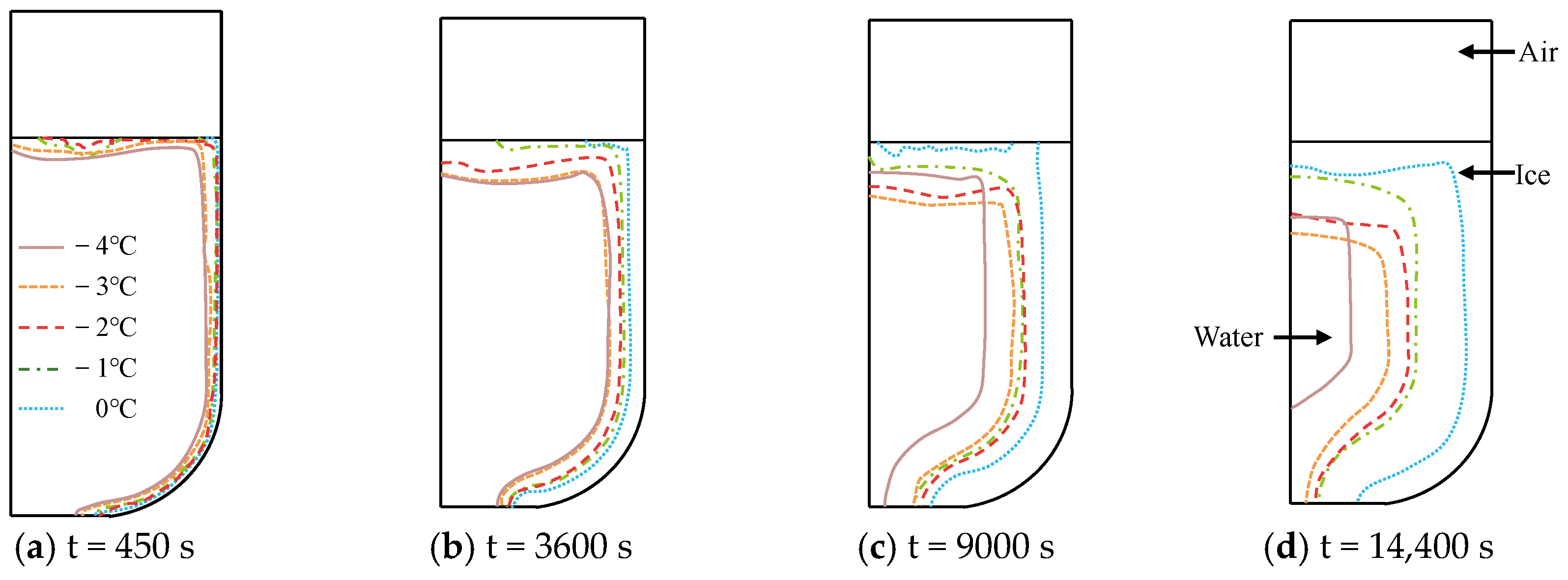
3.1.2. Seawater Temperature
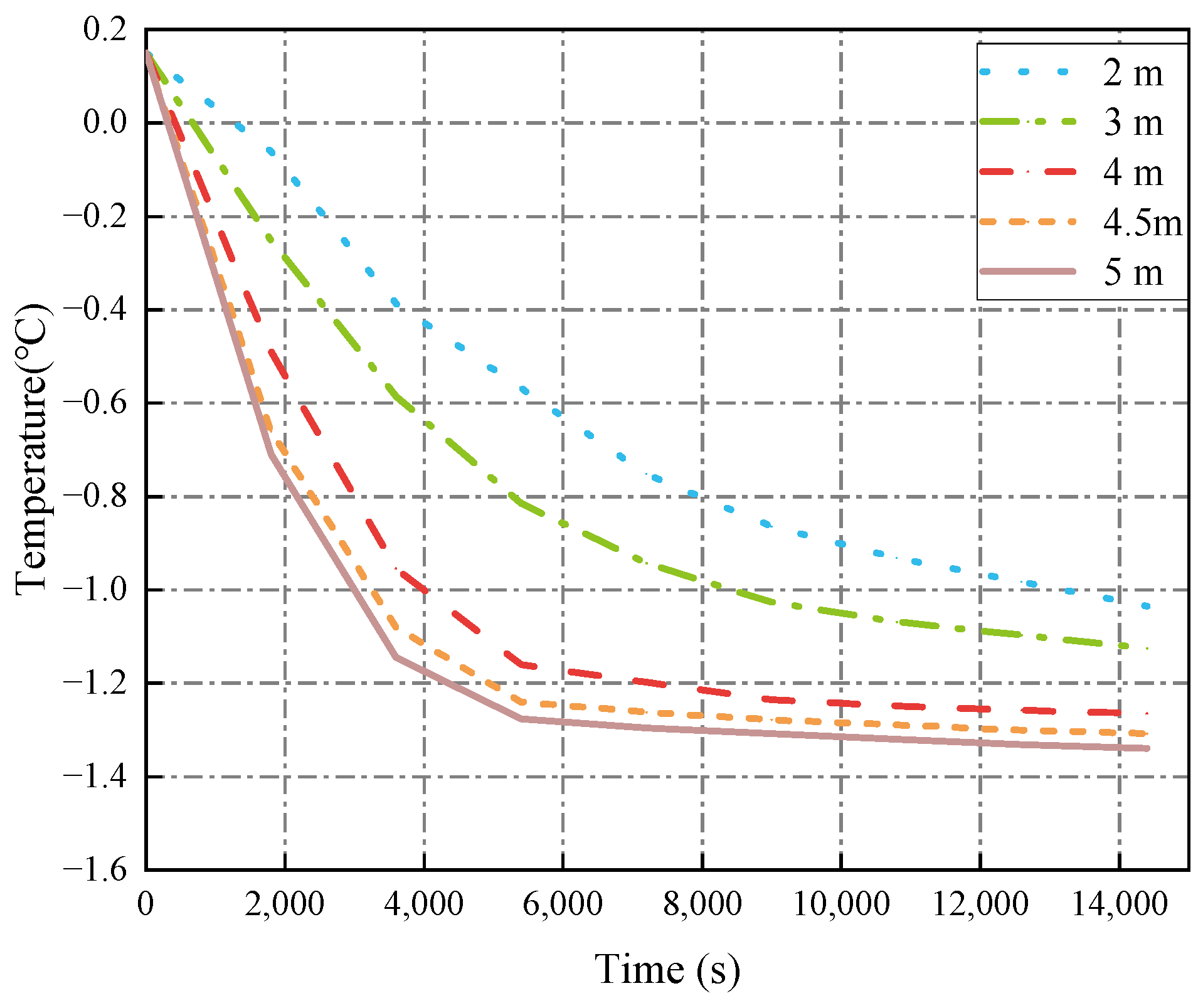
3.1.3. Ballast Water Height
3.2. Influence of Environmental Parameters on the Ice Shape Evolution
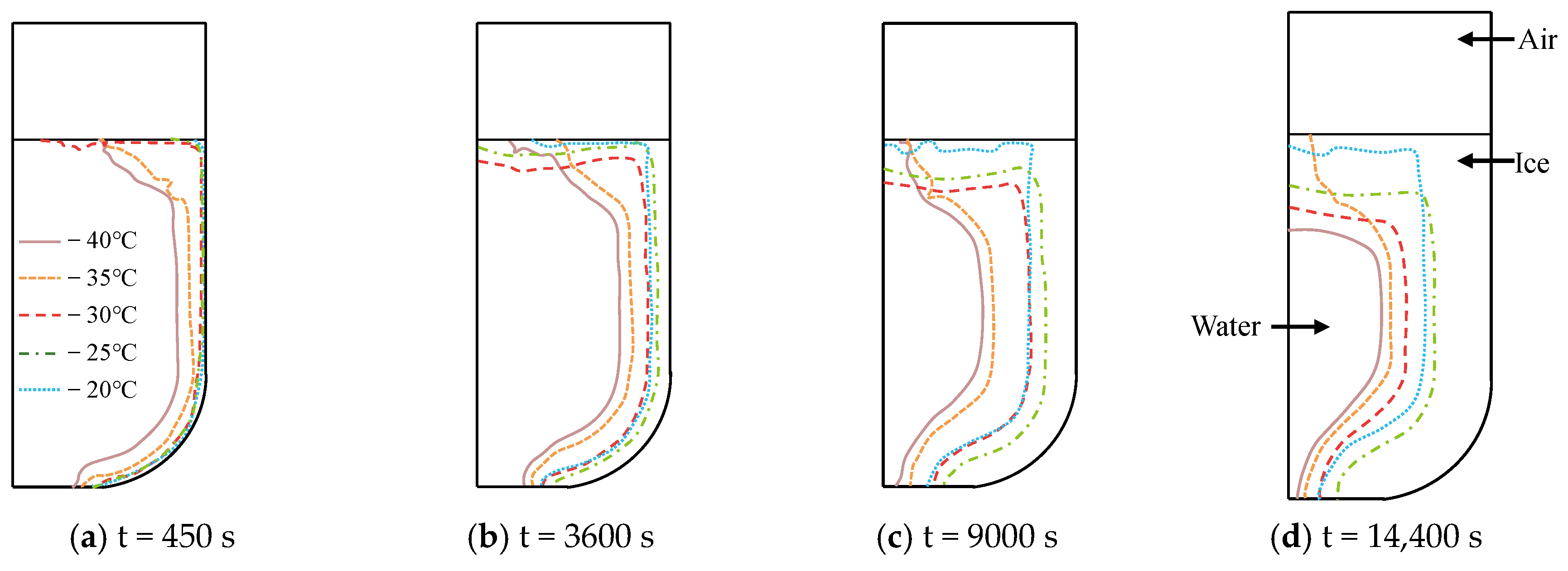
3.2.1. Cold Air Temperature
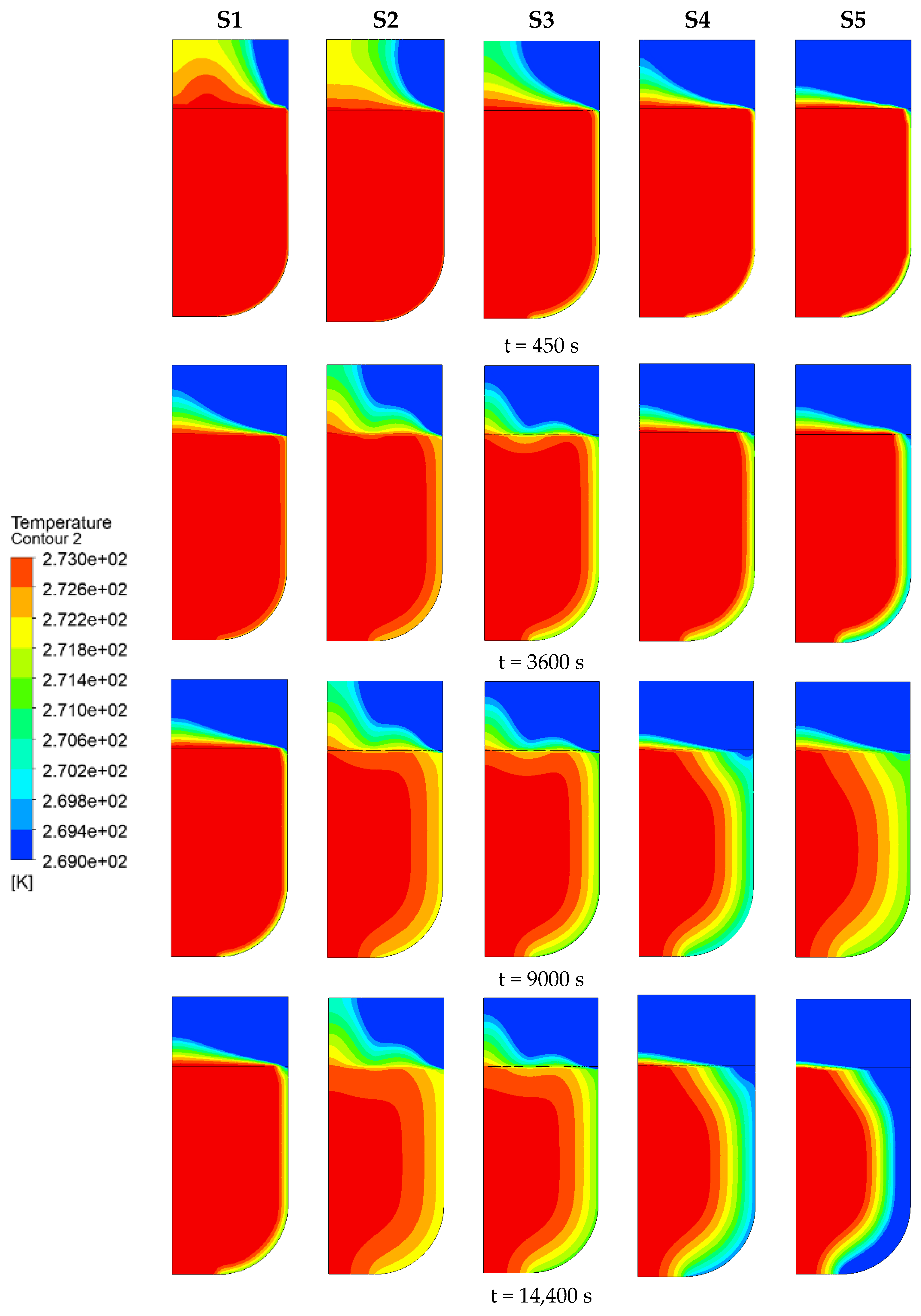
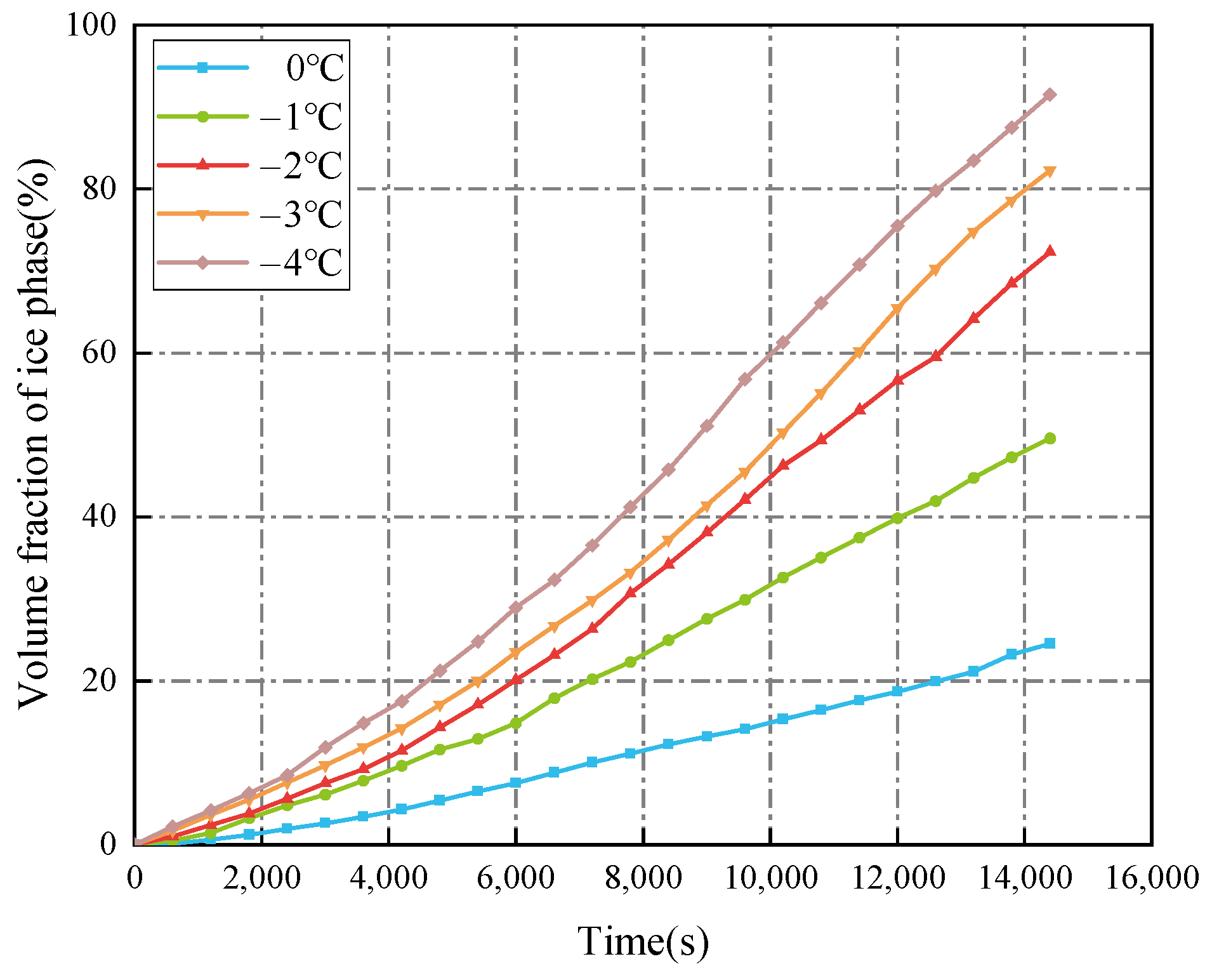
3.2.2. Seawater Temperature
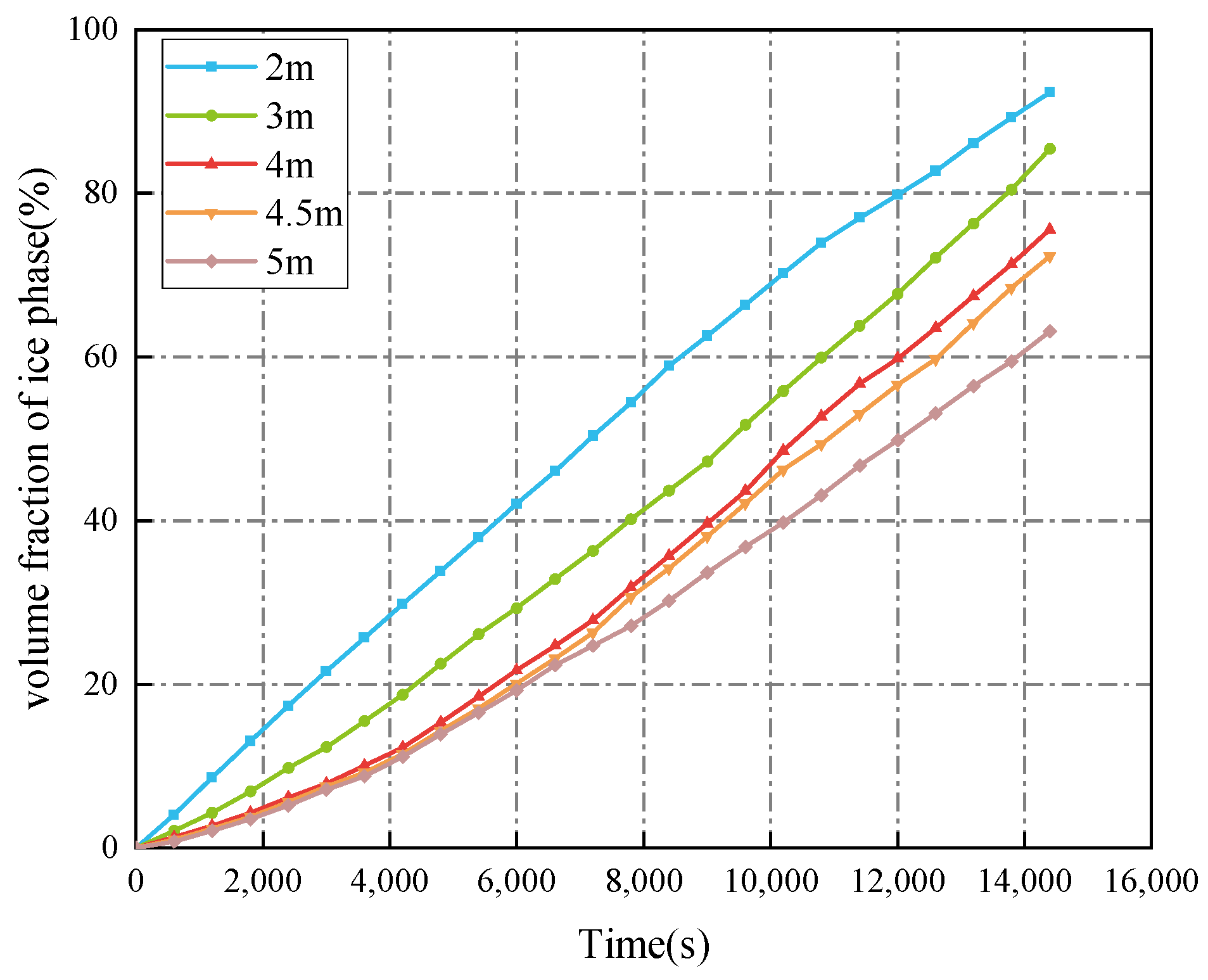
3.2.3. Ballast Water Height
3.3. The Limitations of the Numerical Model
4. Conclusions
- (1)
- The decrease in cold air temperature significantly impacts the temperature of ballast water. As the cold air temperature drops, the rate of temperature reduction at the ballast water level accelerates markedly. When the cold air temperature reaches −40 °C, the temperature of the ballast water reaches its lowest point at −1.34 °C. Additionally, changes in the seawater temperature greatly affect the temperature near the bulkhead. As the seawater temperature decreases, the rate of temperature reduction in the ballast water at the right bulkhead also accelerates significantly. When the seawater temperature is −4 °C, the ballast water temperature at the right bulkhead reaches its lowest point at −1.518 °C. The change in the height of the ballast water level has a substantial influence on the heat transfer rate. As the height of the liquid level decreases, the time required for heat transfer increases, causing the heat transfer rate to slow down. When the height of the liquid level is 5 m, the heat transfer rate is at its fastest, and the temperature of the ballast water at that level is the lowest, measuring −1.14 °C.
- (2)
- The decrease in the cold air temperature significantly impacts the evolution of ice formation at the ballast water level. As the cold air temperature drops, the thickness of the ice layer formed by freezing at the surface of the ballast water increases. When the ambient temperature reaches −40 °C, the volume fraction of the ice phase reaches its maximum, approximately 84.33%. Additionally, changes in the seawater temperature primarily influence the evolution of ice formation at the right bulkhead. As the seawater temperature decreases, the thickness of the ice layer formed by freezing at the right bulkhead also increases significantly. When the seawater temperature is −4 °C, the freezing degree of the ballast tank is at its highest, with the volume fraction of the ice phase in the ballast tank reaching approximately 91.53%. The change in the height of the ballast water level primarily affects the volume of ballast water. As the liquid level decreases, the volume of ballast water diminishes, resulting in a shorter time required for the ballast water to freeze and an increase in the degree of freezing. When the liquid level height is 2 m, the volume fraction of the ice phase is approximately 92.35%.
- (3)
- The variation in the temperature field has the most significant impact on the evolution of ice shape. As the rate of change in the ballast water temperature increases, the rate of ice shape evolution also shows an upward trend. Notably, in areas where the temperature field experiences substantial fluctuations, a faster freezing rate in ballast water leads to a thicker ice layer and a greater degree of freezing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.S.; An, I.S. Emergence of a climate oscillation in the Arctic ocean due to global warming. Nat. Clim. Change 2024, 14, 1267–1274. [Google Scholar] [CrossRef]
- Nieuwejaar, P.; Mazauric, V.; Betzler, C.; Carapuco, M.; Cattrijsse, A.; Coren, F.; Danobeitia, J.; Day, C.; Fitzgerald, A.; Florescu, S.; et al. Next generation European research vessels: Current status and foreseeable evolution. In Position Paper 25 of the European Marine Board; European Marine Board: Ostend, Belgium, 2019. [Google Scholar]
- Cai, W.Q.; Lin, J.D. Navigation practice and Prospect of normalized navigation in the Northeast Arctic channel. China Water Transp. 2016, 37, 21–24. [Google Scholar]
- Bagaev, D.V.; Syraleva, M.N.; Kudinovich, I.V. Comparative analysis of energy efficiency of ballast tank anti-freezing systems. J. Phys. Conf. Ser. 2020, 2, 022023. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, K.P.; Ahn, S.W. Numerical investigation on freezing in ballast tank of ship navigating in ice-bound sea. J. Korean Soc. Power Syst. Eng. 2013, 17, 95–102. [Google Scholar] [CrossRef]
- Wan, Z.; Yuan, Y.C.; Tang, W.Y. Experimental investigation on ice resistance of an arctic LNG carrier under multiple ice breaking conditions. Ocean. Eng. 2023, 267, 113264. [Google Scholar] [CrossRef]
- Zhu, X.S.; Han, X.L.; Xie, B.; Wang, J.R.; Yu, X.C. Application of simplified thermal transfer analysis in FLNG tank optimization. In Proceedings of the 28th International Ocean and Polar Engineering Conference; ISOPE International Ocean and Polar Engineering Conference, ISOPE-I-18-178, Sapporo, Japan, 10–15 June 2018. [Google Scholar]
- Ding, S.F.; Zhou, L.; Gu, Y.J.; Zhou, Y.J. Research on temperature field of the support structure for the independent LNG tank. Hydro Sci. Mar. Eng. 2020, 2, 33–39. [Google Scholar] [CrossRef]
- Li, R.; Shan, X.D.; Zhang, J.Y. Thermal Design of LNG Cabin on Sea Transport. In Proceedings of the 2015 International Conference on Industrial Technology and Management Science, Tianjin, China, 27–28 March 2015; pp. 1543–1546. [Google Scholar]
- Wang, C.; Qin, H.D.; Shen, J.; Hao, X.Z. The Temperature Distribution Analysis of the Large LNG-FSRU. In Proceedings of the ASME 2016 35th International Conference on Ocean, Offshore and Arctic Engineering, Busan, Republic of Korea, 19–24 June 2016; p. 49989. [Google Scholar]
- Lu, J.S.; Xu, S.; Deng, J.J.; Wu, W.F.; Wu, H.X.; Yang, Z.B. Numerical prediction of temperature field for cargo containment system (CCS) of LNG carriers during pre-cooling operations. J. Nat. Gas Sci. Eng. 2016, 29, 382–391. [Google Scholar] [CrossRef]
- Wu, S.X.; Ju, Y.L.; Lin, J.C.; Fu, Y.Z. Numerical simulation and experiment verification of the static boil-off rate and temperature field for a new independent type B liquefied natural gas ship mock up tank. Appl. Therm. Eng. 2020, 173, 115265. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.X.; Wang, J.L. CFD simulation of airflow organization in the ship accommodation. Appl. Mech. Mater. 2015, 711, 91–95. [Google Scholar] [CrossRef]
- Rosenau, S.G.; Reimer, N.K.; Notz, D.; Von, R.U.F. A growth model for aqueous model ice. In Proceedings of the International Conference on Port and Ocean Engineering under Arctic Conditions, POAC, Glasgow, UK, 12–14 June 2023. [Google Scholar]
- Fatahillah, A.; Setiawan, T.B.; Sholihin, A. Numerical analysis of ice freezing processes of block ice production in a brine tank factory using the finite volume method. J. Phys. Conf. Ser. 2021, 1832, 012023. [Google Scholar] [CrossRef]
- Ding, S.F.; Cai, J.Y.; Zhou, L.; Zhou, Y.J. Numerical simulation analysis of icing process in ice model tank. Chin. J. Ship Res. 2021, 16, 137–142. [Google Scholar]
- Huo, P.Z.; Lu, P.; Xie, F.; Wang, Q.K.; Li, Z.J.; Arvola, L. Numerical simulation of the complete process of lake ice formation and melting in Lake Ulansu. Adv. Water Sci. 2024, 35, 145–155. [Google Scholar]
- Zhang, Y.W.; Guo, W.F.; Li, Y.; Chi, H.D.; Zhao, P.Y.; Tagawa, K. An experimental study of icing distribution on a symmetrical airfoil for wind turbine blade in the offshore environmental condition. Ocean. Eng. 2023, 273, 113960. [Google Scholar] [CrossRef]
- Chuang, Z.J.; Li, C.Z.; Liu, S.W.; Li, X.; Li, Z.Y.; Zhou, L. Numerical analysis of blade icing influence on the dynamic response of an integrated offshore wind turbine. Ocean. Eng. 2022, 257, 111593. [Google Scholar] [CrossRef]
- Gao, L.Y.; Liu, Y.; Zhou, W.W.; Hui, H. An experimental study on the aerodynamic performance degradation of a wind turbine blade model induced by ice accretion process. Renew. Energy 2019, 133, 663–675. [Google Scholar] [CrossRef]
- Xu, L.; Chen, S.H.; Jiang, H.B.; Zhang, L.L. The phase field simulation of the influence of heat exchanger performance on ice crystals in sea water piping system of polar ship. China Shiprep. 2017, 30, 45–48. [Google Scholar]
- Mintu, S.; Molyneux, D. Ice accretion for ships and offshore structures. Part 1—State of the art review. Ocean. Eng. 2022, 258, 111501. [Google Scholar] [CrossRef]
- Bai, X.; Shen, J.; Xue, Y.Z.; Shu, Y.C. Numerical Forecasting of Icing on Structural Components of Offshore Platforms in Polar Region. China Ocean. Eng. 2021, 35, 588–597. [Google Scholar] [CrossRef]
- Liu, J.N.; Xia, H.B.; Zhao, H.J.; Wei, X.Q.; Wu, H. Temperature Field Analysis of 175000m3 Membrane FSRU. Ship Ocean. Eng. 2021, 50, 13–16. [Google Scholar]
- Liu, J.F.; Wang, J.Y.; Liu, T. Temperature Field Distribution of LNG Carrier for Type B Independent Tank. Ship Eng. 2017, 39, 33–38. [Google Scholar]
- Zhang, Y.K. Simulation Study on Heat and Mass Transfer Characteristic of Droplet Vacuum Freezing Process. Ph.D. Thesis, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- Hirt, C.W.; Nichol, B.D. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Wang, Y.K. Numerical Research on the Freezing Process of Supercooled Saline Water Droplets Impacting on the Cold Wall. Ph.D. Thesis, Harbin Engineering University, Harbin, China, 2022. [Google Scholar]
- Clyne, T.W.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Zhao, P.B.; Dai, R.K.; Li, W.; Wang, Q.W.; Zeng, M. Modeling the mushy zone during the melting process under Neumann boundary condition using the improved enthalpy-porosity method. Numer. Heat Transf. Part A Appl. 2020, 78, 423–442. [Google Scholar] [CrossRef]
- Chen, M. Characteristics of the LNG carrier with high ice class. J. Ship Des. 2020, 160, 7–12. [Google Scholar]
- Xie, Q.; Chen, H.L.; Zhang, J.F. Research progress of anti-icing and de-icing technology for polar navigation ships and offshore platforms. Chin. J. Ship Res. 2017, 12, 45–53. [Google Scholar]
- Luo, G.L. Temperature Field Analysis and Application of Independent Tank LNG Carrier. Mar. Technol. 2021, 49, 17–22. [Google Scholar]
- Horjen, I. Offshore drilling rig ice accretion modeling including a surficial brine film. Cold Reg. Sci. Technol. 2015, 119, 84–110. [Google Scholar] [CrossRef]
- Hu, Y.F.; Ding, S.F.; Liu, Z.B.; Zhou, L.; Wu, G.; Cao, J. Temperature Field Analysis and Freezing Process Simulation of Polar Ship Tanks. Ship Boat 2023, 34, 110–118. [Google Scholar]
- Zhu, J.H.; Dai, Z.; Wang, Y.H.; Wang, M.; Wang, Z.Y. Rapid freezing process of static salt-containing droplets under salt exclusion. Int. J. Heat Mass Transf. 2024, 220, 124927. [Google Scholar] [CrossRef]
- Barma, M.C.; Peng, Z.B.; Moghtaderi, B.; Doroodchi, E. Freeze desalination of drops of saline solutions. Desalination 2021, 514, 115265. [Google Scholar] [CrossRef]
- Qu, K.Y.; Jiang, Y. Study on the factors affecting freezing of the water flowing inside a circular tube. Acta Energiae Solaris Sin. 2001, 7, 250–255. [Google Scholar]







| Parameter | Value |
|---|---|
| Density/(kg/m3) | 1000 |
| Thermal conductivity/(W/m·K) | 0.6 |
| Specific heat capacity/(J/kg·K) | 4182 |
| Solidus temperature/°C | 0 |
| Grid Size/m | Temperature/°C | Volume of Ice Phase/% | |
|---|---|---|---|
| a | b | ||
| 0.1 | −1.009 | −1.036 | 68.470 |
| 0.06 | −1.014 | −1.042 | 69.049 |
| 0.03 | −1.015 | −1.043 | 69.336 |
| Case | Cold Air Temperature/°C | Seawater Temperature/°C | Ballast Water Height/m |
|---|---|---|---|
| A1 | −20 | −2 | 4.5 |
| A2 | −25 | −2 | 4.5 |
| A3 | −30 | −2 | 4.5 |
| A4 | −35 | −2 | 4.5 |
| A5 | −40 | −2 | 4.5 |
| Case | Cold Air Temperature/°C | Seawater Temperature/°C | Ballast Water Height/m |
|---|---|---|---|
| S1 | −30 | 0 | 4.5 |
| S2 | −30 | −1 | 4.5 |
| S3 | −30 | −2 | 4.5 |
| S4 | −30 | −3 | 4.5 |
| S5 | −30 | −4 | 4.5 |
| Case | Cold Air Temperature/°C | Seawater Temperature/°C | Ballast Water Height/m |
|---|---|---|---|
| B1 | −30 | −2 | 5 |
| B2 | −30 | −2 | 4.5 |
| B3 | −30 | −2 | 4 |
| B4 | −30 | −2 | 3 |
| B5 | −30 | −2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Xu, C.; Wu, D. Dynamic Simulation and Characteristic Analysis on Freezing Process in Ballast Tanks of Polar LNG Carriers. Appl. Sci. 2025, 15, 5192. https://doi.org/10.3390/app15095192
Bai X, Xu C, Wu D. Dynamic Simulation and Characteristic Analysis on Freezing Process in Ballast Tanks of Polar LNG Carriers. Applied Sciences. 2025; 15(9):5192. https://doi.org/10.3390/app15095192
Chicago/Turabian StyleBai, Xu, Cao Xu, and Daolei Wu. 2025. "Dynamic Simulation and Characteristic Analysis on Freezing Process in Ballast Tanks of Polar LNG Carriers" Applied Sciences 15, no. 9: 5192. https://doi.org/10.3390/app15095192
APA StyleBai, X., Xu, C., & Wu, D. (2025). Dynamic Simulation and Characteristic Analysis on Freezing Process in Ballast Tanks of Polar LNG Carriers. Applied Sciences, 15(9), 5192. https://doi.org/10.3390/app15095192






