The Microstructures of Tetradesmus obliquus Biofilms Formed Under Different Nutrient Supplies
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Biofilm Culture
2.2. Characterization of Biofilm Microstructures
2.3. Measurements of the Microalgae Cell Properties
3. Results
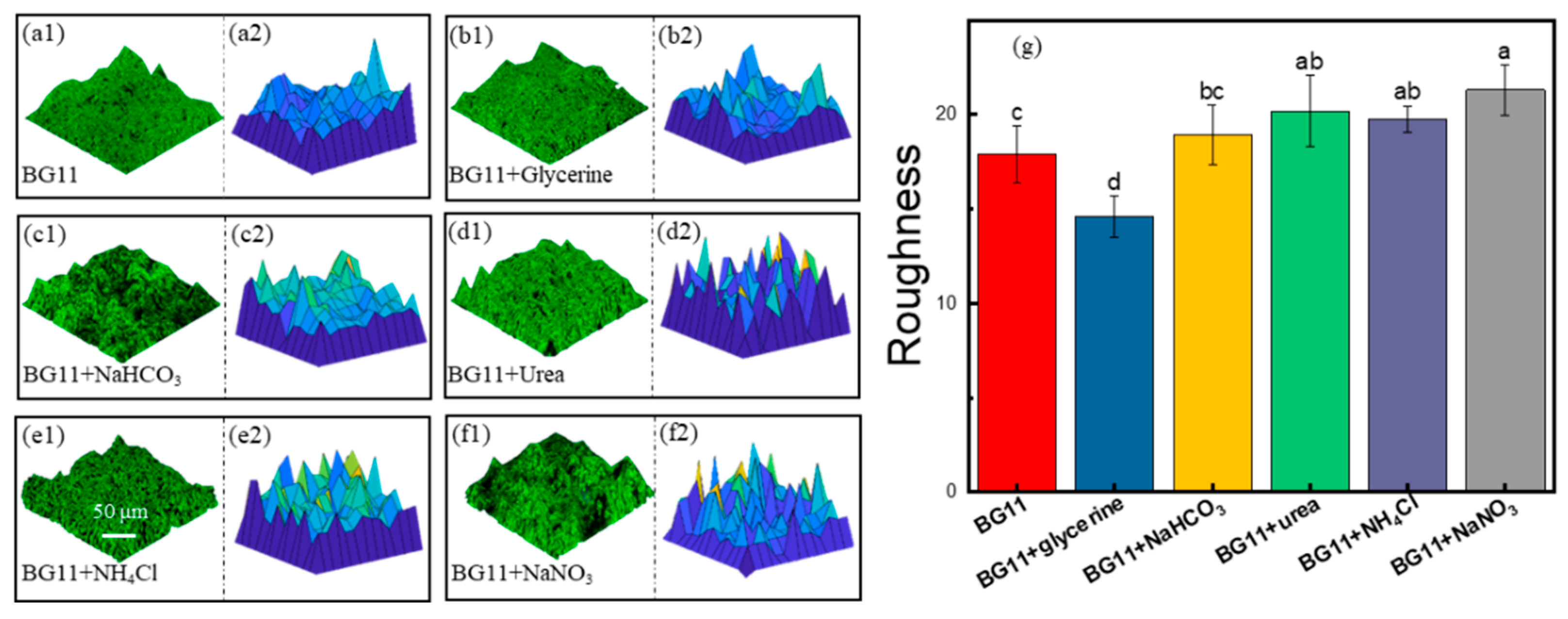
3.1. The Surface Microstructure of Microalgae Biofilms
3.2. The Pore Structure Characteristics of Microalgae Biofilms
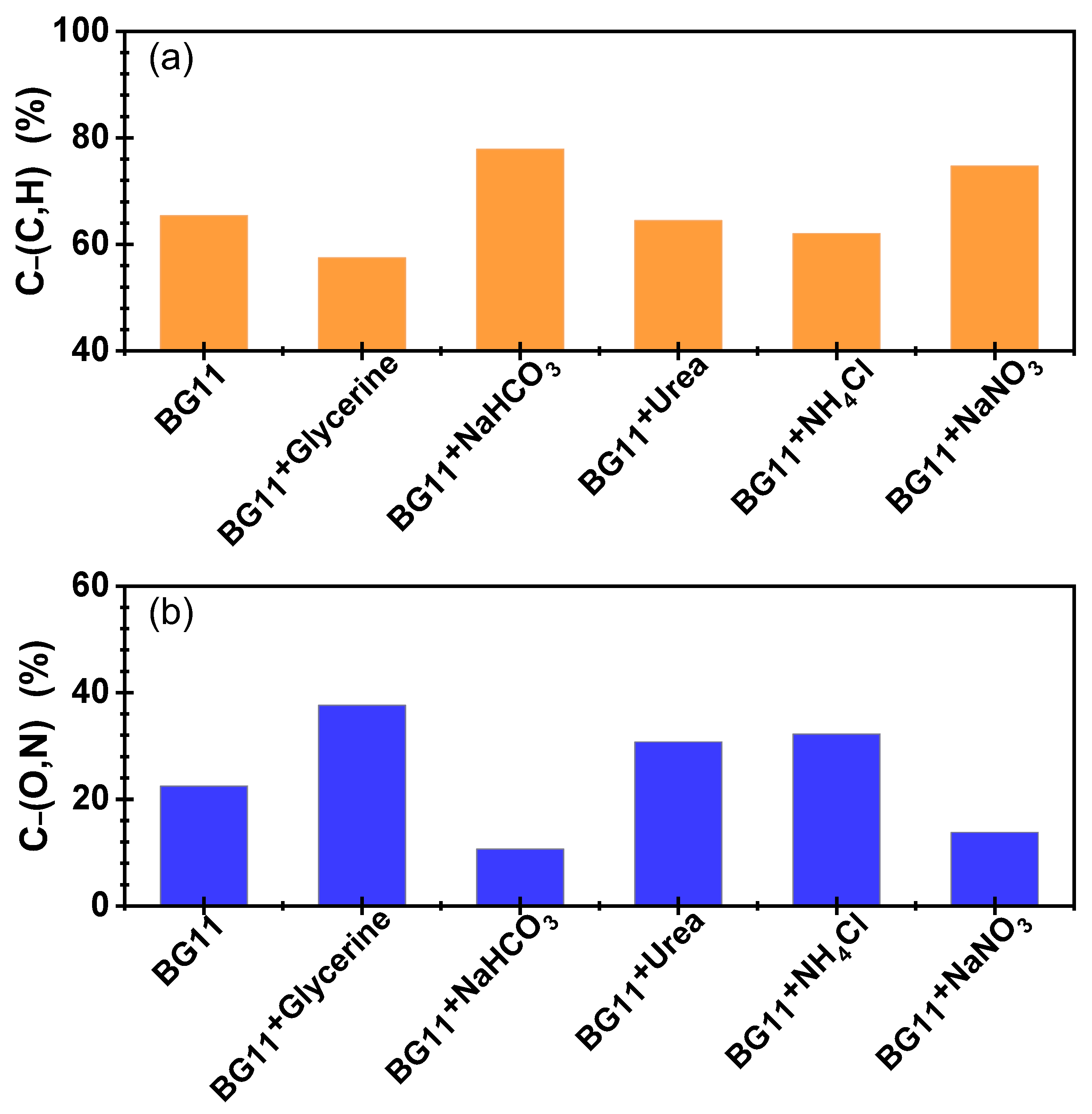
3.3. Surface Properties of Microalgae Cells Cultured with Different Nutrients
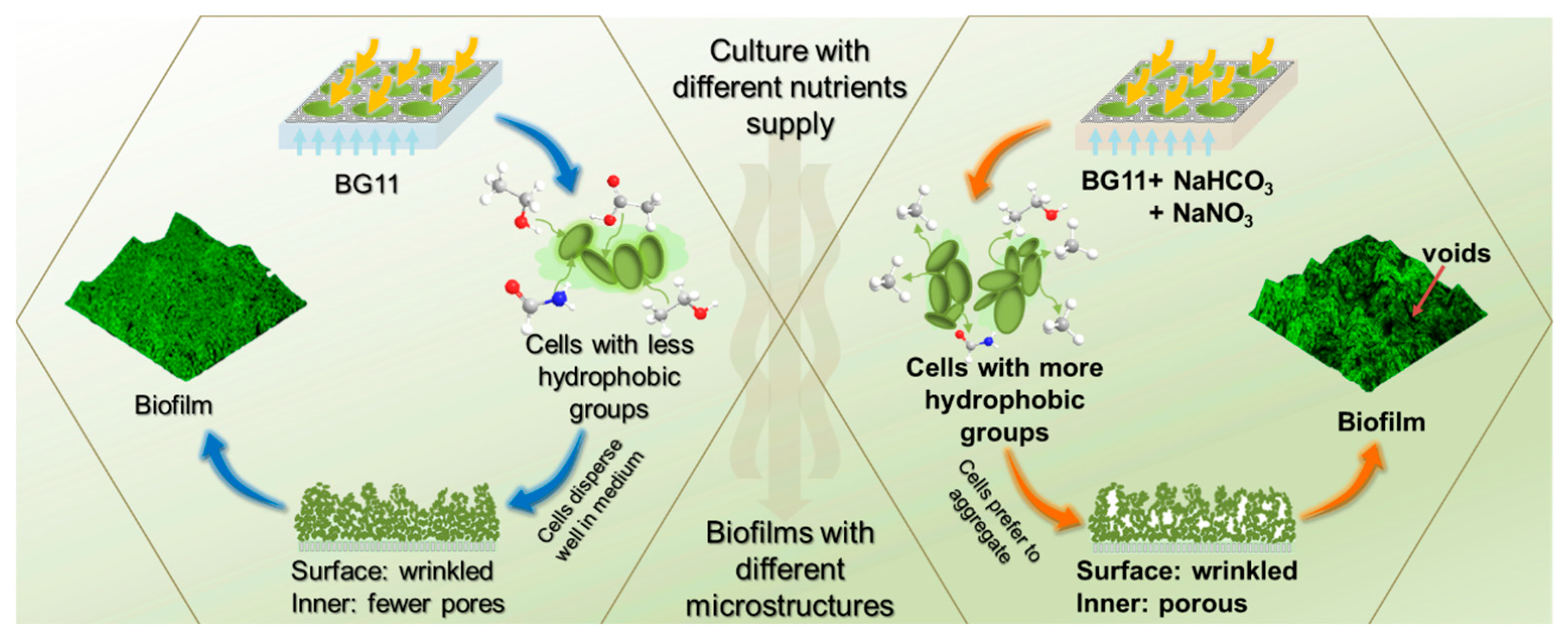
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current Bottlenecks and Challenges of the Microalgal Biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef]
- Lam, G.P.; Vermue, M.H.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Multi−Product Microalgae Biorefineries: From Concept Towards Reality. Trends Biotechnol. 2018, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Koruyucu, A.; Peest, T.; Korzin, E.; Gröninger, L.; Patricia; Brück, T.; Weuster−Botz, D. Cell Disruption and Hydrolysis of Microchloropsis salina Biomass as a Feedstock for Fermentation. Appl. Sci. 2024, 14, 9667. [Google Scholar] [CrossRef]
- Sharma, R.; Mishra, A.; Pant, D.; Malaviya, P. Recent advances in microalgae−based remediation of industrial and non−industrial wastewaters with simultaneous recovery of value−added products. Bioresour. Technol. 2022, 344, 126129. [Google Scholar] [CrossRef]
- Mofijur, M.; Rahman, S.M.A.; Nguyen, L.N.; Mahlia, T.M.I.; Nghiem, L.D. Selection of microalgae strains for sustainable production of aviation biofuel. Bioresour. Technol. 2022, 345, 126408. [Google Scholar] [CrossRef] [PubMed]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic digestion of microalgal biomass for bioenergy production, removal of nutrients and microcystin: Current status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef]
- Katsimichas, A.; Katsouli, M.; Spantidos, N.; Giannakourou, M.C.; Taoukis, P. Effect of High−Pressure Homogenization on the Functional and Emulsifying Properties of Proteins Recovered from Auxenochlorella pyrenoidosa. Appl. Sci. 2024, 15, 131. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, X.R.; Jiang, Z.Y.; Chen, X.H.; He, H.Z.; Zhang, X.X. Light/dark cycle of microalgae cells in raceway ponds: Effects of paddlewheel rotational speeds and baffles installation. Bioresour. Technol. 2016, 219, 387–391. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhuang, L.L.; Xu, X.Q.; Deantes−Espinosa, V.M.; Wang, X.X.; Hu, H.Y. Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew. Sustain. Energy Rev. 2018, 92, 331–342. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, W.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X.; Sun, Y. Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour. Technol. 2016, 222, 367–373. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Liu, T. The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae. Biotechnol. Biofuels 2015, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Jarboe, D.; Wen, Z.Y. biofilm-based algal cultivation systems. Appl. Microbiol. Biotechnol. 2015, 99, 5781–5789. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Microalgae−bacteria biofilms: A sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2018, 102, 1131–1144. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, Y.; Xia, A.; Fu, Q.; Liao, Q.; Zeng, W.; Zheng, Y.; Zhu, X. Optimizing culture conditions for heterotrophic−assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity. Bioresour. Technol. 2018, 270, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.; Derek, C.J.C. A Methodological Review on the Characterization of Microalgal Biofilm and Its Extracellular Polymeric Substances. J. Appl. Microbiol. 2022, 132, 3490–3514. [Google Scholar] [CrossRef]
- Karimi, Z.; Laughinghouse, H.D.; Davis, V.A.; Blersch, D.M. Substrate properties as controlling parameters in attached algal cultivation. Appl. Microbiol. Biotechnol. 2021, 105, 1823–1835. [Google Scholar] [CrossRef]
- Polizzi, B.; Bernard, O.; Ribot, M. A time−space model for the growth of microalgae biofilms for biofuel production. J. Theor. Biol. 2017, 432, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, Y.; Lai, Z.; Zhang, X.; Jiang, Z.; Zhang, X. Analyzing microalgal biofilm structures formed under different light conditions by evaluating cell–cell interactions. J. Colloid Interface Sci. 2021, 583, 563–570. [Google Scholar] [CrossRef]
- Fanesi, A.; Paule, A.; Bernard, O.; Briandet, R.; Lopes, F. The architecture of monospecific microalgae biofilms. Microorganisms 2019, 7, 352. [Google Scholar] [CrossRef]
- Wilking, J.N.; Zaburdaev, V.; De Volder, M.; Losick, R.; Brenner, M.P.; Weitz, D.A. Liquid transport facilitated by channels in Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2013, 110, 848–852. [Google Scholar] [CrossRef]
- Fanesi, A.; Lavayssiere, M.; Breton, C.; Bernard, O.; Briandet, R.; Lopes, F. Shear stress affects the architecture and cohesion of Chlorella vulgaris biofilms. Sci. Rep. 2021, 11, 4002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Z.; Lai, Z.; Yuan, H.; Zhang, X.; Jia, Y.; Zhang, X. The self−adaption capability of microalgal biofilm under different light intensities: Photosynthetic parameters and biofilm microstructures. Algal Res. 2021, 58, 102383. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Wang, Y.; Guan, L.; Zeng, Z.; Jiang, Z.; Zhang, X. Cell Surface Energy Affects the Structure of Microalgal Biofilm. Langmuir 2020, 36, 3057–3063. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Liu, T. Effects of nitrogen source and nitrogen supply model on the growth and hydrocarbon accumulation of immobilized biofilm cultivation of B. braunii. Bioresour. Technol. 2014, 166, 527–533. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Osei−Wusu, D.; Wang, Y.; Liu, T. Development of nitrogen supply strategy for Scenedesmus rubescens attached cultivation toward growth and lipid accumulation. Bioprocess Biosyst. Eng. 2018, 41, 435–442. [Google Scholar] [CrossRef]
- Wu, X.; Cen, Q.; Addy, M.; Zheng, H.; Luo, S.; Liu, Y.; Cheng, Y.; Zhou, W.; Chen, P.; Ruan, R. A novel algal biofilm photobioreactor for efficient hog manure wastewater utilization and treatment. Bioresour. Technol. 2019, 292, 121925. [Google Scholar] [CrossRef] [PubMed]
- Blanken, W.; Schaap, S.; Theobald, S.; Rinzema, A.; Wijffels, R.H.; Janssen, M. Optimizing carbon dioxide utilization for microalgae biofilm cultivation. Biotechnol. Bioeng. 2017, 114, 769–776. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X. Impact of the accumulation and adhesion of released oxygen during Scenedesmus obliquus photosynthesis on biofilm formation and growth. Bioresour. Technol. 2017, 244 Pt. 1, 198–205. [Google Scholar] [CrossRef]
- Asgharnejad, H.; Sarrafzadeh, M.-H.; Abhar−Shegofteh, O.; Khorshidi Nazloo, E.; Oh, H.-M. Biomass quantification and 3−D topography reconstruction of microalgal biofilms using digital image processing. Algal Res. 2021, 55, 102243. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Ozkan, A.; Berberoglu, H. Physico−chemical surface properties of microalgae. Colloids Surf. B Biointerfaces 2013, 112, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, F.; Latrache, H.; Zahir, H.; Elghmari, A.; Timinouni, M.; Ellouali, M. The relation between Escherichia coli surface functional groups’ composition and their physicochemical properties. Braz. J. Microbiol. 2008, 39, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Van der Mei, H.; Busscher, H. The use of X−ray photoelectron spectroscopy for the study of oral streptococcal cell surfaces. Adv. Dent. Res. 1997, 11, 388–394. [Google Scholar] [CrossRef]
- Boonaert, C.J.; Rouxhet, P.G. Surface of lactic acid bacteria: Relationships between chemical composition and physicochemical properties. Appl. Environ. Microbiol. 2000, 66, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Podola, B.; Melkonian, M. Investigating dynamic processes in a porous substrate biofilm photobioreactor—A modeling approach. Algal Res. 2016, 13, 30–40. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, G.; Sun, Z.; Zhang, X.; Jiang, Z.; Zhang, X. The Microstructures of Tetradesmus obliquus Biofilms Formed Under Different Nutrient Supplies. Appl. Sci. 2025, 15, 5104. https://doi.org/10.3390/app15095104
Zhang H, Chen G, Sun Z, Zhang X, Jiang Z, Zhang X. The Microstructures of Tetradesmus obliquus Biofilms Formed Under Different Nutrient Supplies. Applied Sciences. 2025; 15(9):5104. https://doi.org/10.3390/app15095104
Chicago/Turabian StyleZhang, Hu, Guan Chen, Zhiying Sun, Xinru Zhang, Zeyi Jiang, and Xinxin Zhang. 2025. "The Microstructures of Tetradesmus obliquus Biofilms Formed Under Different Nutrient Supplies" Applied Sciences 15, no. 9: 5104. https://doi.org/10.3390/app15095104
APA StyleZhang, H., Chen, G., Sun, Z., Zhang, X., Jiang, Z., & Zhang, X. (2025). The Microstructures of Tetradesmus obliquus Biofilms Formed Under Different Nutrient Supplies. Applied Sciences, 15(9), 5104. https://doi.org/10.3390/app15095104





