Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification
Abstract
1. Introduction
2. Materials and LCA Methodology
2.1. Goal and Scope Definition
2.2. Life Cycle Inventory (LCI) Analysis
- Activity Level (Functional Unit, FU) = 1 kg H2;
- Input/Output per FU = amount of material or energy required or produced per FU (from the inventory data).
| Category | Component | Amount | Unit |
|---|---|---|---|
| Inputs from the Technosphere | AD plant operation | ||
| Manure | 25.51 | kg | |
| Waste maize silage | 8.5 | kg | |
| Fodder beet | 8.5 | kg | |
| Cheese whey | 8.5 | kg | |
| Electricity | 4054.96 | kJ | |
| Construction | |||
| Concrete | 500.47 | g | |
| Reinforced steel | 18.9 | g | |
| Chromium steel | 2.18 | g | |
| Copper | 0.2 | g | |
| Laminated timber | 9.22 | g | |
| High-density polyethylene | 0.08 | g | |
| High-impact polystyrene | 0.95 | g | |
| Polyvinyl chloride | 0.13 | g | |
| Synthetic rubber | 0.51 | g | |
| Outputs to the Technosphere | BG (Biogas) | 7.18 | Nm3 |
| Digestate | 42.82 | kg | |
| Avoided Products | Fertilizer, as N | 57.81 | g |
| Fertilizer, as P | 67.23 | g | |
| Fertilizer, as K | 179.84 | g | |
| Outputs to the Environment | CO2, biogenic | 90.42 | g |
| CH4, biogenic | 284.19 | g | |
| NH3 | 40.45 | g |
2.3. Life Cycle Impact Assessment (LCIA)
2.4. Interpretation
3. Results and Discussion
3.1. LCA of Hydrogen Production
3.1.1. Biogas Reforming
3.1.2. Agricultural Residue Gasification
3.2. Comparative LCA of Hydrogen Production: Biogas Reforming vs. Agricultural Residue Gasification
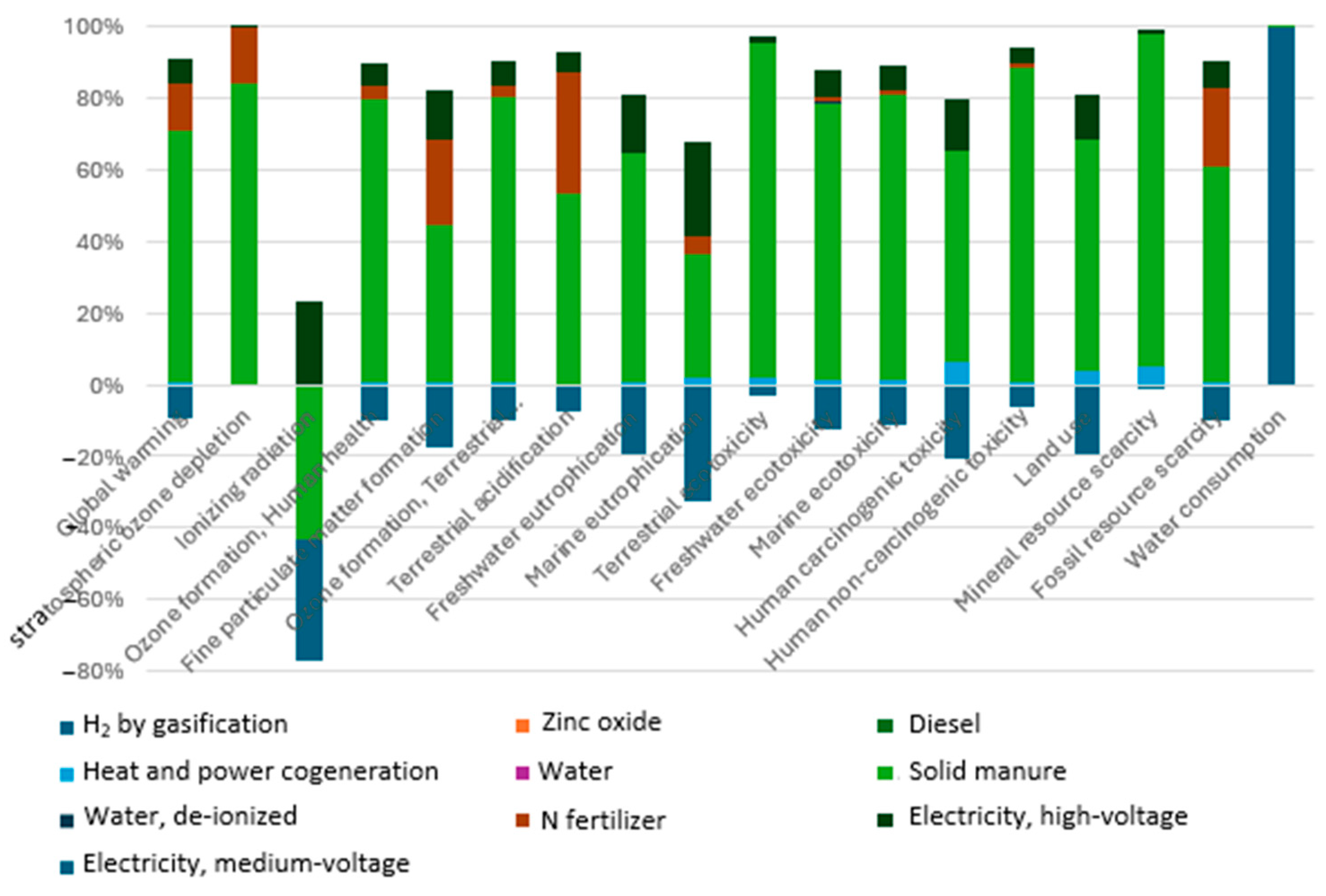
3.2.1. Global Warming Potential
3.2.2. Ozone Depletion and Ionizing Radiation Impact
3.2.3. Human and Ecosystem Toxicity
3.2.4. Particulate Matter and Acidification Effects
3.2.5. Fossil and Mineral Resource Scarcity
3.2.6. Water Consumption and Land Use
3.3. Socio-Economic Analysis of Hydrogen Production Pathways
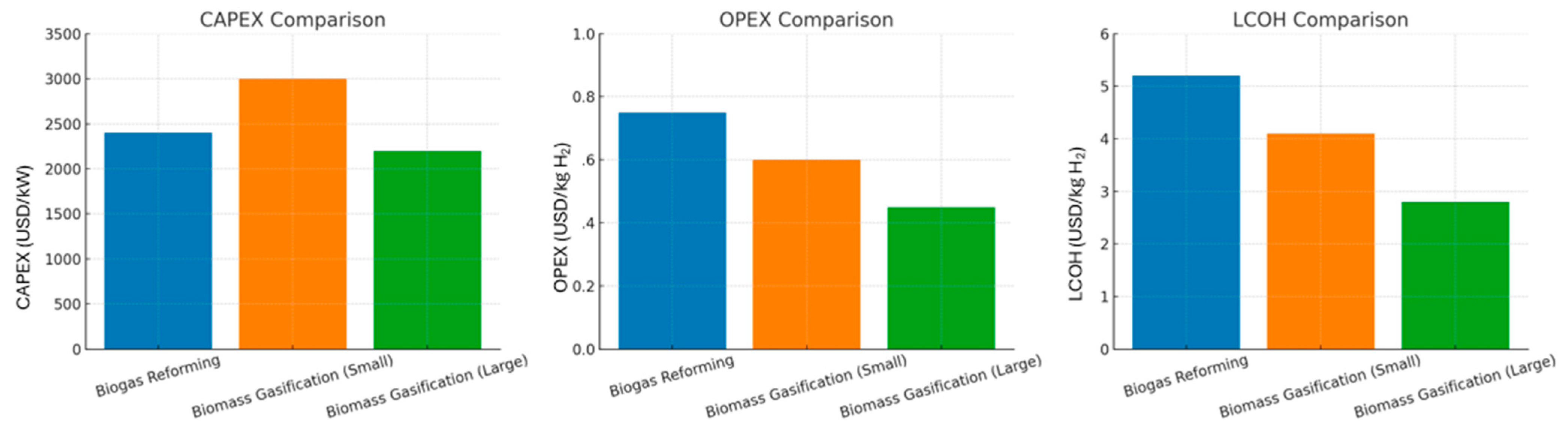
3.3.1. Capital Expenditure (CAPEX)
3.3.2. Operational Expenditure (OPEX)
- Fuel cost = biomass/biogas feedstock cost;
- Electricity = power consumption × unit price;
- Labor = operators and technicians;
- Maintenance = 2–5% of CAPEX annually (commonly used assumption);
- Miscellaneous = catalyst, consumables, and disposal fees.
3.3.3. Levelized Cost of Hydrogen (LCOH)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| ATR | autothermal reforming |
| BF | biogas reforming |
| CAPEX | capital expenditure |
| FPMF | fine particulate matter formation |
| FRS | fossil resource scarcity |
| FU | functional unit |
| FWE | freshwater eutrophication |
| FWET | freshwater ecotoxicity |
| GHG | greenhouse gas |
| GWP | global warming potential |
| HCT | human carcinogenic toxicity |
| HNCT | human non-carcinogenic toxicity |
| HTS | High-Temperature Shift |
| IR | ionizing radiation |
| LCA | life cycle assessment |
| LCI | life cycle inventory |
| LCIA | life cycle impact assessment |
| LCOH | levelized cost of hydrogen |
| LTS | Low-Temperature Shift |
| LU | land use |
| ME | marine eutrophication |
| MET | marine ecotoxicity |
| MRS | mineral resource scarcity |
| NG | natural gas |
| OFHH | ozone formation, human health |
| OFTES | ozone formation, terrestrial ecosystems |
| OPEX | operating expenditure |
| POX | Partial Oxidation |
| SG | synthesis gas |
| SMR | steam methane reforming |
| SOD | stratospheric ozone depletion |
| TA | terrestrial acidification |
| TET | terrestrial ecotoxicity |
| WGS | water–gas shift |
| WC | water consumption |
References
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Chipangamate, N.S.; Nwaila, G.T. Assessment of Challenges and Strategies for Driving Energy Transitions in Emerging Markets: A Socio-Technological Systems Perspective. Energy Geosci. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Awewomom, J.; Dzeble, F.; Takyi, Y.D.; Ashie, W.B.; Ettey, E.N.Y.O.; Afua, P.E.; Sackey, L.N.A.; Opoku, F.; Akoto, O. Addressing Global Environmental Pollution Using Environmental Control Techniques: A Focus on Environmental Policy and Preventive Environmental Management. Discov. Environ. 2024, 2, 8. [Google Scholar] [CrossRef]
- Angelico, R.; Giametta, F.; Bianchi, B.; Catalano, P. Green Hydrogen for Energy Transition: A Critical Perspective. Energies 2025, 18, 404. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an Alternative Fuel: A Comprehensive Review of Challenges and Opportunities in Production, Storage, and Transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Abdul Wahid, M.; Jamil, M.M.; Azli, A.A.M.; Misbah, M.F. A Review on Biomass-Based Hydrogen Production for Renewable Energy Supply. Int. J. Energy Res. 2015, 39, 1597–1615. [Google Scholar] [CrossRef]
- Methane Steam Reforming—An Overview ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/methane-steam-reforming (accessed on 11 March 2025).
- Timmerberg, S.; Kaltschmitt, M.; Finkbeiner, M. Hydrogen and Hydrogen-Derived Fuels through Methane Decomposition of Natural Gas—GHG Emissions and Costs. Energy Convers. Manag. X 2020, 7, 100043. [Google Scholar] [CrossRef]
- Akobi, C.O. Biohydrogen and Biomethane Production from Lignocellulosic Biomass. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2016. [Google Scholar]
- Hajjaji, N.; Martinez, S.; Trably, E.; Steyer, J.-P.; Helias, A. Life Cycle Assessment of Hydrogen Production from Biogas Reforming. Int. J. Hydrogen Energy 2016, 41, 6064–6075. [Google Scholar] [CrossRef]
- Zhou, Y. Life-Cycle Greenhouse Gas Emissions of Biomethane and Hydrogen Pathways in the European Union; The International Council of Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- Meena, P.K.; Kumar, D.; Singh, L.; Sharma, S.; Didwania, M. Evaluating the Sustainability of Biohydrogen, Biogas, and Biohythane Generation from Agricultural, Industrial, and Municipal Waste Sources. Biofuels Bioprod. Biorefining 2025, 19, 485–507. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Liao, X.; Dauriat, A.; Binotti, M.; Manzolini, G. Life Cycle Assessment and Economic Analysis of an Innovative Biogas Membrane Reformer for Hydrogen Production. Processes 2019, 7, 86. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Jameel, M.K.; Mustafa, M.A.; Ahmed, H.S.; Mohammed, A.J.; Ghazy, H.; Shakir, M.N.; Lawas, A.M.; Mohammed, S.K.; Idan, A.H.; Mahmoud, Z.H.; et al. Biogas: Production, Properties, Applications, Economic and Challenges: A Review. Results Chem. 2024, 7, 101549. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Iulianelli, A.; Manisco, M.; Bion, N.; Valant, A.L.; Epron, F.; Colpan, O.; Esposito, E.; Jansen, J.C.; Gensini, M.; Caravella, A. Sustainable H2 Generation via Steam Reforming of Biogas in Membrane Reactors: H2S Effects on Membrane Performance and Catalytic Activity. Int. J. Hydrogen Energy 2021, 46, 29183–29197. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Huang, Y.; Basile, A. Model Biogas Steam Reforming in a Thin Pd-Supported Membrane Reactor to Generate Clean Hydrogen for Fuel Cells. J. Power Sources 2015, 273, 25–32. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of Hydrogen Production from Biogas Reforming: Technological Advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Rubinsin, N.J.; Karim, N.A.; Timmiati, S.N.; Lim, K.L.; Isahak, W.N.R.W.; Pudukudy, M. An Overview of the Enhanced Biomass Gasification for Hydrogen Production. Int. J. Hydrogen Energy 2024, 49, 1139–1164. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Life Cycle Assessment of Hydrogen Production from Biomass Gasification Systems. Int. J. Hydrogen Energy 2012, 37, 14026–14039. [Google Scholar] [CrossRef]
- Muresan, M.; Cormos, C.C.; Agachi, P.S. Comparative Life Cycle Analysis for Gasification-Based Hydrogen Production Systems. J. Renew. Sustain. Energy 2014, 6, 013131. [Google Scholar] [CrossRef]
- Singh, A.; Shivapuji, A.M.; Dasappa, S. Hydrogen Production through Agro-Residue Gasification and Adsorptive Separation. Appl. Therm. Eng. 2023, 234, 121247. [Google Scholar] [CrossRef]
- Moreno, J.; Dufour, J. Life Cycle Assessment of Hydrogen Production from Biomass Gasification. Evaluation of Different Spanish Feedstocks. Int. J. Hydrogen Energy 2013, 38, 7616–7622. [Google Scholar] [CrossRef]
- Narnaware, S.L.; Panwar, N.L. Catalysts and Their Role in Biomass Gasification and Tar Abetment: A Review. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Kauffman, N.; Hayes, D.; Brown, R. A Life Cycle Assessment of Advanced Biofuel Production from a Hectare of Corn. Fuel 2011, 90, 3306–3314. [Google Scholar] [CrossRef]
- Christoforou, E.A.; Fokaides, P.A. Life Cycle Assessment (LCA) of Olive Husk Torrefaction. Renew. Energy 2016, 90, 257–266. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Hettiaratchi, P.; Siddiqui, O.; Razi, F.; Pokhrel, D.; Sadiq, R. Sustainability of Waste-to-Hydrogen Conversion Pathways: A Life Cycle Thinking-Based Assessment. Energy Convers. Manag. 2022, 270, 116218. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Huang, J.; Xu, X.; Tang, J.; Hou, P.; Han, W.; Li, H. Environmental Impact Assessment of a Combined Bioprocess for Hydrogen Production from Food Waste. Waste Manag. 2024, 173, 152–159. [Google Scholar] [CrossRef]
- Arfan, M.; Eriksson, O.; Wang, Z.; Soam, S. Life Cycle Assessment and Life Cycle Costing of Hydrogen Production from Biowaste and Biomass in Sweden. Energy Convers. Manag. 2023, 291, 117262. [Google Scholar] [CrossRef]
- Mio, A.; Barbera, E.; Massi Pavan, A.; Bertucco, A.; Fermeglia, M. Sustainability Analysis of Hydrogen Production Processes. Int. J. Hydrogen Energy 2024, 54, 540–553. [Google Scholar] [CrossRef]
- Kolahchian Tabrizi, M.; Famiglietti, J.; Bonalumi, D.; Campanari, S. The Carbon Footprint of Hydrogen Produced with State-of-the-Art Photovoltaic Electricity Using Life-Cycle Assessment Methodology. Energies 2023, 16, 5190. [Google Scholar] [CrossRef]
- Krishnan, S.; Corona, B.; Kramer, G.J.; Junginger, M.; Koning, V. Prospective LCA of Alkaline and PEM Electrolyser Systems. Int. J. Hydrogen Energy 2024, 55, 26–41. [Google Scholar] [CrossRef]
- Henriksen, M.S.; Matthews, H.S.; White, J.; Walsh, L.; Grol, E.; Jamieson, M.; Skone, T.J. Tradeoffs in Life Cycle Water Use and Greenhouse Gas Emissions of Hydrogen Production Pathways. Int. J. Hydrogen Energy 2023, 49, 1221–1234. [Google Scholar] [CrossRef]
- Salah, C.; Cobo, S.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Environmental Sustainability Assessment of Hydrogen from Waste Polymers. ACS Sustain. Chem. Eng. 2023, 11, 3238–3247. [Google Scholar] [CrossRef]
- Patel, G.H.; Havukainen, J.; Horttanainen, M.; Soukka, R.; Tuomaala, M. Climate Change Performance of Hydrogen Production Based on Life Cycle Assessment. Green Chem. 2024, 26, 992–1006. [Google Scholar] [CrossRef]
- Ji, M.; Shi, M.; Wang, J. Life Cycle Assessment of Nuclear Hydrogen Production Processes Based on High Temperature Gas-Cooled Reactor. Int. J. Hydrogen Energy 2023, 48, 22302–22318. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Environmental Impact Assessment of Hydrogen Production via Steam Methane Reforming Based on Emissions Data. Energy Rep. 2022, 8, 13585–13595. [Google Scholar] [CrossRef]
- Weidner, T.; Tulus, V.; Guillén-Gosálbez, G. Environmental Sustainability Assessment of Large-Scale Hydrogen Production Using Prospective Life Cycle Analysis. Int. J. Hydrogen Energy 2023, 48, 8310–8327. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Petrescu, L.; Galusnyak, S.C.; Cormos, C.-C. Environmental Evaluation of Hydrogen Production Employing Innovative Chemical Looping Technologies—A Romanian Case Study. Int. J. Hydrogen Energy 2023, 48, 12112–12128. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Mohamed, A.R.; Abdelhaleem, A.; Ayati, A.; Farghali, M.; Al-Muhtaseb, A.H.; Al-Fatesh, A.S.; Rooney, D.W. Life Cycle Assessment of Hydrogen Production, Storage, and Utilization toward Sustainability. Wiley Interdiscip. Rev. Energy Environ. 2024, 13, e526. [Google Scholar] [CrossRef]
- Abawalo, M.; Pikoń, K.; Landrat, M. Przegląd oceny cyklu życia produkcji biowodoru w procesie reformingu biogazu. Przem. Chem. 2024, 103, 1497–1501. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Villarini, M.; Colantoni, A.; Moretti, M.; Bocci, E. Life Cycle Performance of Hydrogen Production via Agro-Industrial Residue Gasification—A Small Scale Power Plant Study. Energies 2018, 11, 675. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Xue, L.; Zuo, J.; Chen, T.; Vuppaladadiyam, A.; Duan, H. Anaerobic Digestion-Based Waste-to-Energy Technologies Can Halve the Climate Impact of China’s Fast-Growing Food Waste by 2040. J. Clean. Prod. 2020, 277, 123490. [Google Scholar] [CrossRef]
- Bareiß, K.; De La Rua, C.; Möckl, M.; Hamacher, T. Life Cycle Assessment of Hydrogen from Proton Exchange Membrane Water Electrolysis in Future Energy Systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Life Cycle Assessment of Hydrogen Energy Systems: A Review of Methodological Choices. Int. J. Life Cycle Assess. 2017, 22, 346–363. [Google Scholar] [CrossRef]
- Spath, P.L.; Mann, M.K. Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming; National Renewable Energy Laboratory: Golden, CO, USA, 2000; p. NREL/TP-570-27637.
- Bhandari, R.; Trudewind, C.A.; Zap, P. Life Cycle Assessment of Hydrogen Production Methods—A Review; Institut für Energie-und Klimaforschung, Systemforschung und Technologische Entwicklung (IEF-STE): Jülich, Germany, 2012. [Google Scholar]
- Chang, A.C.C.; Chang, H.-F.; Lin, F.-J.; Lin, K.-H.; Chen, C.-H. Biomass Gasification for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
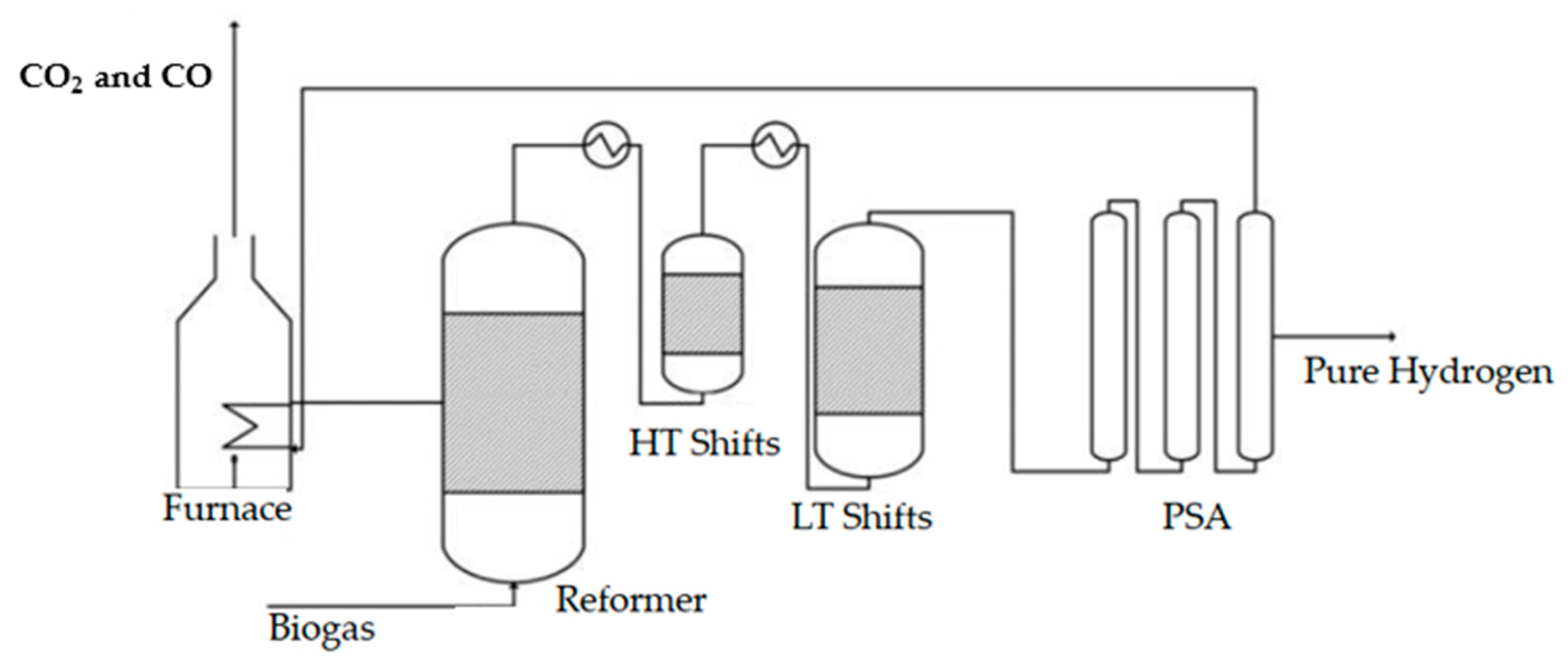
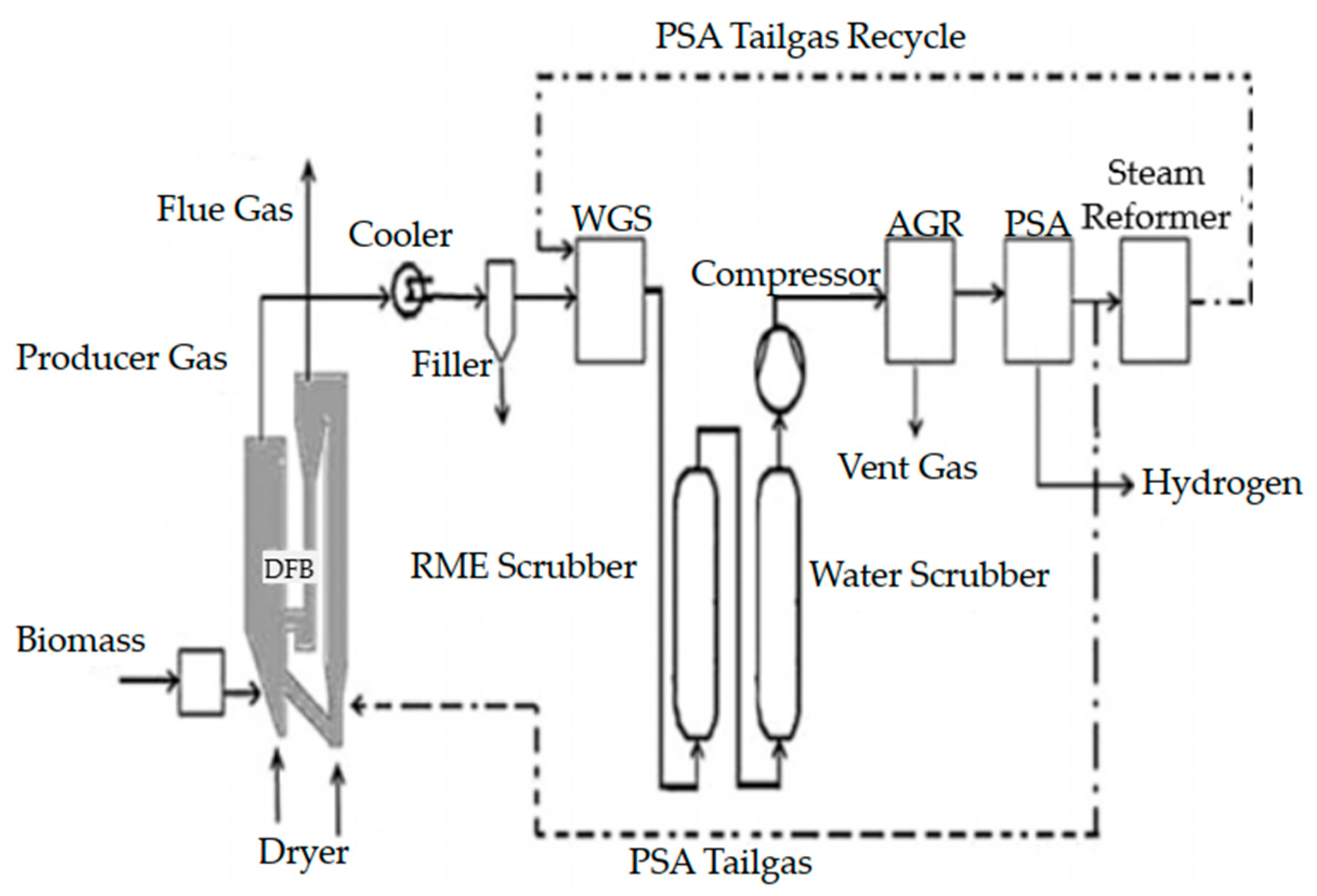
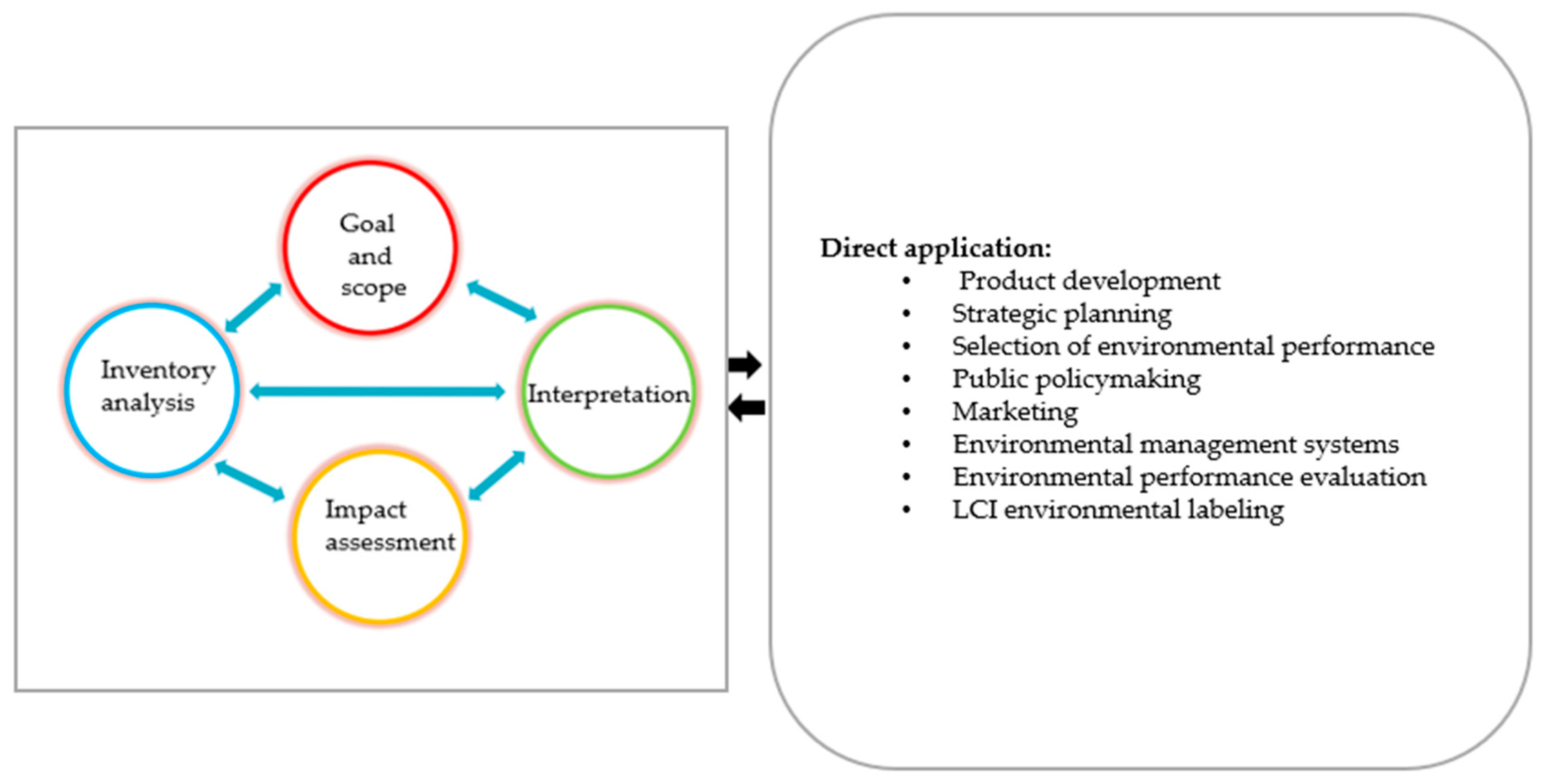
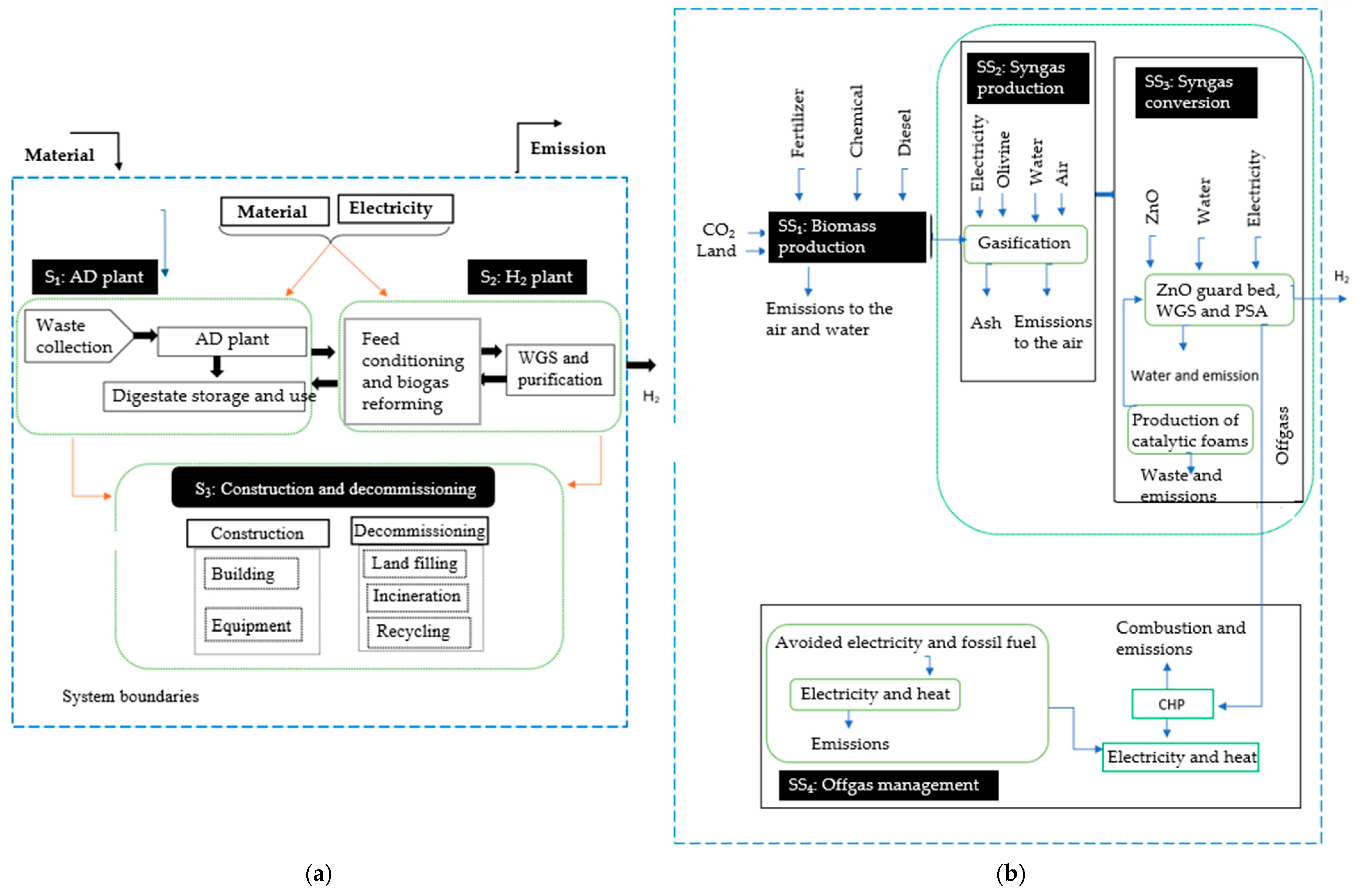

| Ref. | Software | Type of Data | Background Database | F.U. | System Boundaries | LCIA | Global Warming (kg CO2-eq)/FU |
|---|---|---|---|---|---|---|---|
| [30] | SimaPro 8.5.2 | Software sim. + secondary data | Background data source + ecoinvent + GREET 2021 | 1 kg H2 | cradle-to-gate | ReCiPe 2016 | 0.59 and 21.14 |
| [31] | SimaPro 9.0 | Software sim. + secondary data | Pilot scale + ecoinvent | 1 kg H2, 99.99% | cradle-to-gate | IPCC 2013 GWP 100a | 10.1 |
| [32] | SimaPro 9.13 | Primary (AD) + secondary data | ecoinvent 3.8 | 1 kg H2, 99.99% | cradle-to-gate | CML 2001 | 2.13 |
| [33] | undefined | Software sim. + secondary data | Background data source + ecoinvent | 1 kg H2 | cradle-to-gate | ReCiPe | 12.6 |
| [34] | SimaPro 9.4 | Secondary data | ecoinvent 3.5 | 1 kg H2, harmonized | cradle-to-gate | E. F. 3.0 | 3.78 and 4.28 |
| [35] | SimaPro | Secondary data | ecoinvent + literature + state of the art from producers | 1 kg H2 | cradle-to-gate | E. F. 3.0 | |
| [36] | OpenLCA v 1.11 | Secondary data | NETL Reports + literature | 1 kg H2, >99.99%; 925 psig | cradle-to-gate | IPCC 2013 GWP 100a; water balance | −15 to 31 |
| [37] | SimaPro 9.2 | Secondary data | ecoinvent 3.5 | 1 kg H2 | cradle-to-gate | IPCC 2013 GWP 100a | −101.12–12.66 |
| [38] | GaBi v 10.5.1.124 | Secondary data | GaBi database | 1 kg H2 | cradle-to-gate | undefined | 0.6–13 |
| [39] | GaBi | Primary (mining; fuel prep) + secondary data | Literature | 1 kg H2 | cradle-to-gate | CML 2001 | 296 |
| [40] | OpenLCA 1.10.2 | Primary (plant emission) + secondary data | Undefined | 1 kg H2 | gate-to-gate | ReCiPe | 11.2 |
| [41] | SimaPro 9.2 | Secondary data | ecoinvent 3.8 + literature | 1 kg H2, 25 bar | cradle-to-gate | E. F. 3.0 | 0.6–11 |
| [42] | GaBi 10 | Secondary data | Undefined | 1 kg H2 | cradle-to-gate | ReCiPe | 0.31–9.65 |
| Category | Component | Amount | Unit |
|---|---|---|---|
| Inputs from the Technosphere | Feed Conditioning and BG Reforming | ||
| BG | 7.18 | Nm3 | |
| Water | 7.5 | kg | |
| Air | 19.1 | kg | |
| Electricity | 1952.8 | kJ | |
| Construction | |||
| Concrete | 227.9 | g | |
| Steel | 72.81 | g | |
| Aluminum | 0.6 | g | |
| Iron | 0.89 | g | |
| Equipment | |||
| Steel | 0.72 | mg | |
| Steel high alloy | 2.78 | mg | |
| Alumina | 0.96 | mg | |
| Cast iron | 0.64 | mg | |
| Steel low alloy | 0.3 | mg | |
| WGS and Purification | |||
| Steel | 4 | mg | |
| Steel high alloy | 0.13 | mg | |
| Aluminum | 1.12 | mg | |
| Alumina | 2.13 | mg | |
| Iron | 1.65 | mg | |
| Cast iron | 0.3 | mg | |
| Nickel | 0.15 | mg | |
| Steel low alloy | 18.22 | mg | |
| Outputs to the Technosphere | H2 | 1.0 | kg |
| Outputs to the Environment | CO2, biogenic | 13.43 | kg |
| Category | Component | Amount | Unit |
|---|---|---|---|
| Inputs from the Technosphere | Rice husk | 1.54 | kg |
| Diesel | 2.6 × 10−5 | kg | |
| Water | 0.254 | kg | |
| De-ionized water | 0.209 | kg | |
| Zinc oxide | 1.61 × 10−4 | kg | |
| Cu foam | 2.97 × 10−7 | P | |
| Heat and power cogeneration unit | 4.21 × 10−8 | P | |
| Energy | Electricity | 0.19 | kWh |
| Input from Nature | Olivine | 2.7 × 10−5 | kg |
| Outputs to the Technosphere | Hydrogen | 1 | kg |
| Avoided Product | Electricity | 0.26 | kWh |
| Emissions to Air | Nitrogen oxide | 1 × 10−9 | kg |
| Methane | 1.7 × 10−8 | kg | |
| Carbon dioxide, biogenic | 0.16 | kg | |
| Water | 0.15 | kg | |
| Sulfur dioxide | 2 × 10−6 | kg | |
| Emissions to Water | Wastewater | 0.05 | kg |
| Solid Waste Flows | Ash | 1.6 × 10−3 | kg |
| Olivine | 2.7 × 10−4 | kg | |
| Insulation | 0.5 × 10−7 | kg | |
| Hydrogen sulfide | 5 × 10−5 | kg |
| Impact Category | H2 Production by Gasification (>99%) | H2 Production from Biogas Reforming (>99%) |
|---|---|---|
| GWP (kg CO2 eq) | 1.298778438 | 5.04713142 |
| SOD (kg CFC11 eq) | 2.14344 × 10−5 | 2.72936 × 10−5 |
| IR (kBq Co-60 eq) | −0.035526092 | 0.37295624 |
| OFHH (kg NOx eq) | 0.002332391 | 0.012577232 |
| FPMF (kg PM2.5 eq) | 0.001513217 | 0.02432786 |
| OFTES (kg NOx eq) | 0.002376209 | 0.012821774 |
| TA (kg SO2 eq) | 0.004978886 | 0.121065517 |
| FEW (kg P eq) | 0.000315479 | 0.00413299 |
| ME (kg N eq) | 6.98639 × 10−6 | 0.006735414 |
| TET (kg 1,4-DCB) | 4.148159856 | 33.44363458 |
| FWET (kg 1,4-DCB) | 0.03321721 | 0.413201296 |
| MET (kg 1,4-DCB) | 0.049880356 | 0.562196103 |
| HCT (kg 1,4-DCB) | 0.018535482 | 0.323963195 |
| HNCT (kg 1,4-DCB) | 1.508073488 | 23.28455044 |
| LU (m2 a crop eq) | 0.015720069 | 3.683536534 |
| MRS (kg Cu eq) | 0.0106641388 | 0.048885568 |
| FRS (kg oil eq) | 0.330117896 | 1.10519594 |
| WC (m3) | 5.373767301 | 0.040951105 |
| NRE (MJ eq) | 0.457 | 1.05 |
| CED (MJ eq) | 0.685 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abawalo, M.; Pikoń, K.; Landrat, M. Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification. Appl. Sci. 2025, 15, 5029. https://doi.org/10.3390/app15095029
Abawalo M, Pikoń K, Landrat M. Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification. Applied Sciences. 2025; 15(9):5029. https://doi.org/10.3390/app15095029
Chicago/Turabian StyleAbawalo, Mamo, Krzysztof Pikoń, and Marcin Landrat. 2025. "Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification" Applied Sciences 15, no. 9: 5029. https://doi.org/10.3390/app15095029
APA StyleAbawalo, M., Pikoń, K., & Landrat, M. (2025). Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification. Applied Sciences, 15(9), 5029. https://doi.org/10.3390/app15095029







