Slow Pyrolysis as a Method of Treating Household Biowaste for Biochar Production
Abstract
1. Introduction
- To characterize the biowaste materials used in the investigation;
- To evaluate the influence of the slow pyrolysis temperature on the yield and physicochemical properties of the resulting biochar;
- To analyze the properties of the produced biochar.
2. Materials and Methods
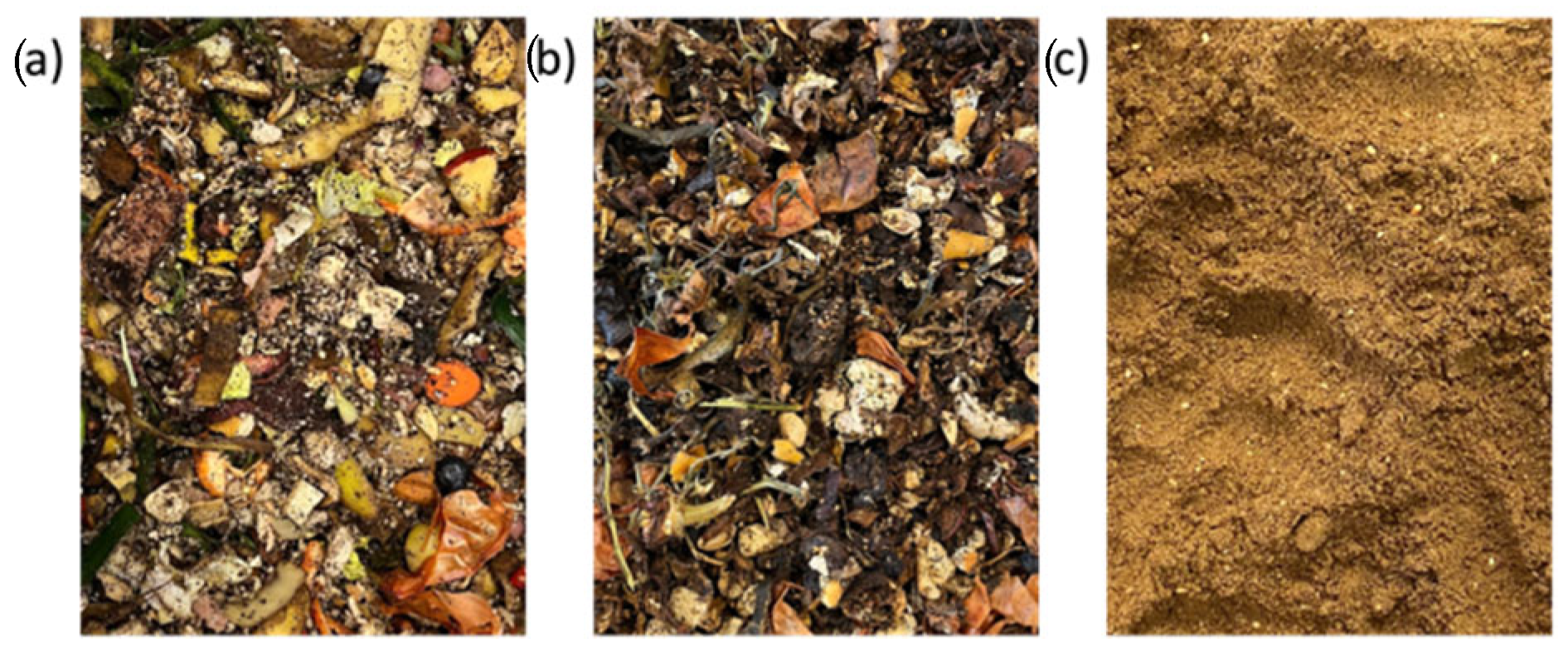
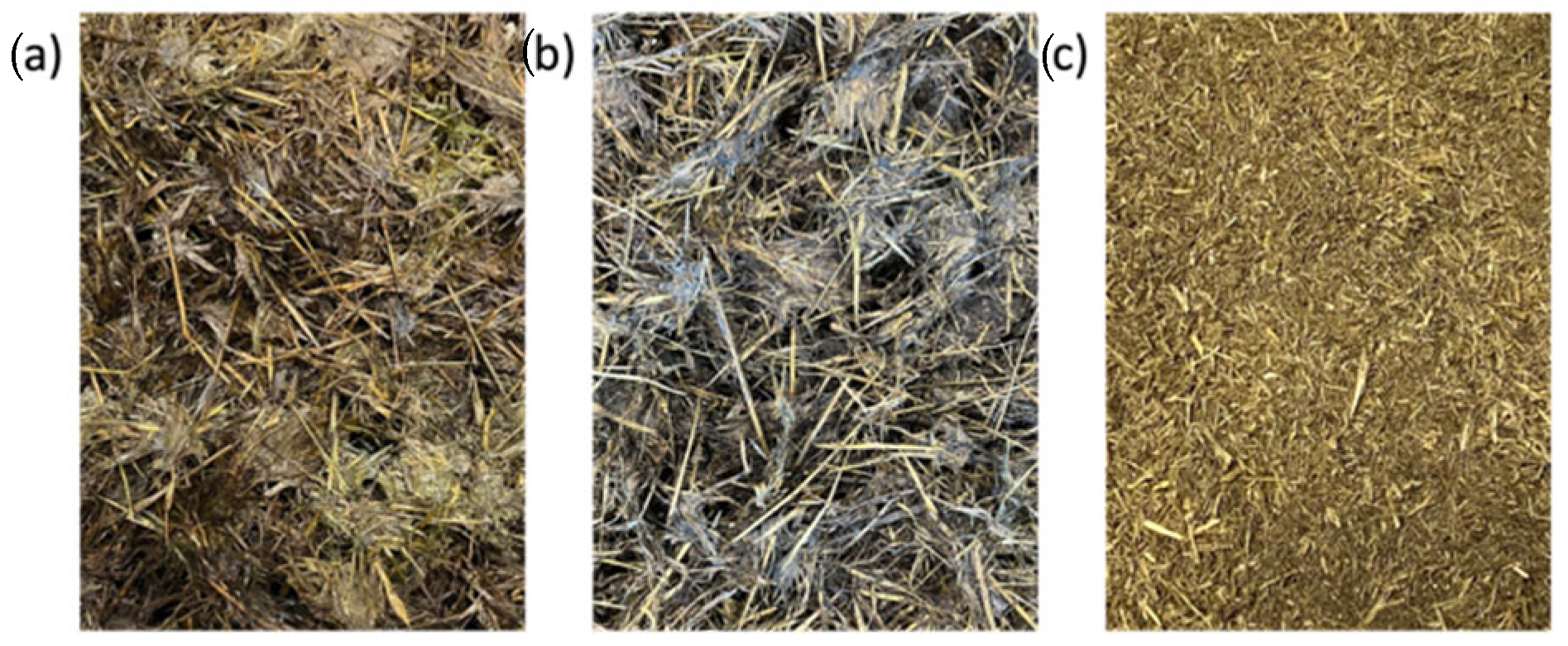
2.1. Biowaste Samples
2.2. Characterization of the Biowaste Samples
2.3. Slow Pyrolysis of Biowaste
2.4. Characterization of the Biochar
3. Results and Discussion
3.1. Properties of the Biowaste
3.1.1. Proximate Analysis Results of Tested Biowaste
3.1.2. Ultimate Analysis Results of Tested Biowaste
3.1.3. Results of Other Biowaste Measurements
3.1.4. Summary of Biowaste Properties
3.2. Biochar Yield
3.3. Properties of Biochar
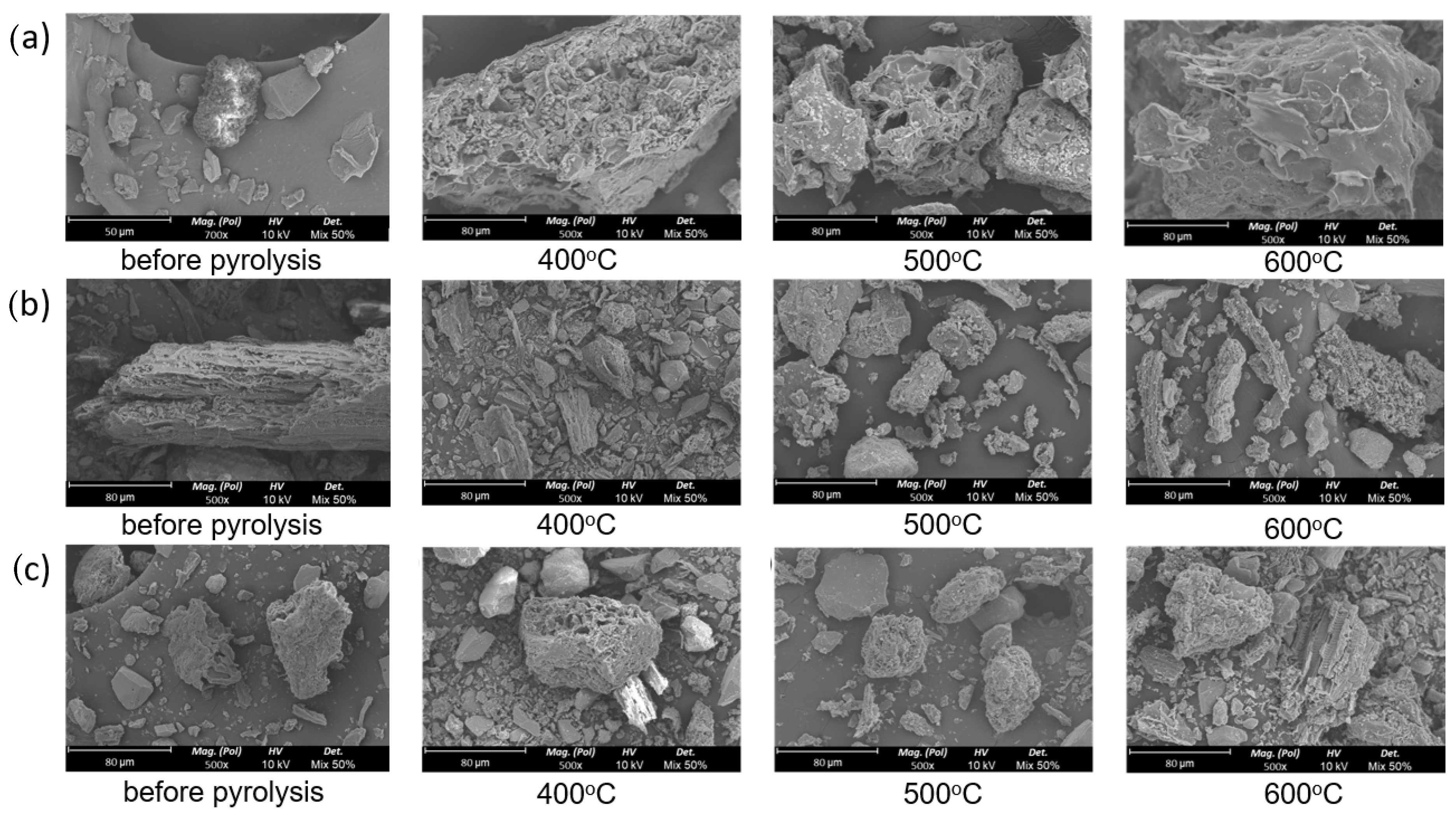
3.4. Scanning Electron Microscope (SEM) Analysis
4. Conclusions
- All biowaste samples had a high moisture content, ranging from 63.51% to 81.53%, indicating the need for drying prior to undergoing slow pyrolysis;
- Property analysis and SEM imaging revealed that the biowaste types are heterogeneous and differ significantly from each other. Therefore, those biowastes should be collected and processed separately;
- The kitchen biowaste properties were found to be similar to those of food waste studied in other regions of the world and are comparable to those of the typical biomasses used for biochar production through slow pyrolysis;
- Both garden biowaste types may have been contaminated with materials such as soil or rocks during collection. This likely contributed to the high ash content observed (17.75% in spring garden biowaste and 43.83% in autumn garden biowaste), leading to significant deviations from both the literature values for similar wastes and from standard biomass feedstocks used in pyrolysis;
- For all three types of biowaste, an increase in the pyrolysis temperature resulted in a decrease in the biochar yield. The highest mass yields recorded were 36.64% for kitchen biowaste, 66.53% for spring garden biowaste, and 66.99% for autumn garden biowaste;
- Kitchen biochar, in comparison to its raw biowaste, showed an increase in carbon content, fixed carbon, and a higher HHV. The SEM images also indicated that higher temperatures led to larger, more porous biochar particles. These properties make kitchen biochar suitable for applications such as carbon sequestration or enhancing soil by improving water and nutrient retention;
- In contrast, both garden-derived biochars had a lower carbon content and HHV than the original garden biowastes. Furthermore, their ash content was over three times higher than that of the kitchen biochar.
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission, Directorate General for Environment; Dubois, M.; Sims, E.; Moerman, T.; Watson, D.; Bauer, B.; Bel, J.B.; Mehlhart, G. Guidance for Separate Collection of Municipal Waste. Publications Office, 2020. Available online: https://data.europa.eu/doi/10.2779/691513 (accessed on 20 November 2022).
- European Parliament and Council. Directive 2008/98/EC on Waste and Repealing Certain Directives. Official Journal of the European Union L312. 19 November 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02008L0098-20180705&from=EN (accessed on 20 November 2022).
- European Union; European Regional Development Fund; Policy Learning Platform on Environment and Resource Efficiency. The Biowaste Management Challenge: A Policy Brief; Interreg Europe: Lille, France, 2021. [Google Scholar]
- Pavlas, M.; Dvořáček, J.; Pitschke, T.; Peche, R. Biowaste Treatment and Waste to Energy Environmental Benefits. Energies 2020, 13, 1994. [Google Scholar] [CrossRef]
- European Commission. Green Paper on the Management of Bio Waste in the European Union, COM(2008) 811 Final; European Commission: Brussels, Belgium, 2008.
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental impacts of food waste in Europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef]
- Bhakta Sharma, H.; Panigrahi, S.; Dubey, B.K. Food waste hydrothermal carbonization: Study on the effects of reaction severities, pelletization and framework development using approaches of the circular economy. Bioresour. Technol. 2021, 333, 125187. [Google Scholar] [CrossRef]
- European Environment Agency. Bio Waste in Europe Turning Challenges into Opportunities, No. 04/2020; European Environment Agency: Copenhagen, Denmark, 2020; ISBN 978-92-9480-223-1. [Google Scholar] [CrossRef]
- Amer, M.; Elwardany, A. Biomass Carbonization; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Rex, P.; Meenakshisundaram, N.; Barmavatu, P. Sustainable valorisation of kitchen waste through greenhouse solar drying and microwave pyrolysis—Technology readiness level for the production of biochar. J. Environ. Health Sci. Eng. 2024, 22, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Awasthi, S.K.; Liu, T.; Duan, Y.; Jain, A.; Sindhu, R.; Binod, P.; et al. Techno-economics and life-cycle assessment of biological and thermochemical treatment of bio-waste. Renew. Sustain. Energy Rev. 2021, 144, 110837. [Google Scholar] [CrossRef]
- Chandraratne, M.R.; Daful, A.G. Recent Advances in Thermochemical Conversion of Biomass. In Recent Perspectives in Pyrolysis Research; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2015, 57, 1126–1140. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Suero, S.R.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications—A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 2nd ed.; Academic Press: Oxford, UK, 2013; pp. 1–530. [Google Scholar]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Biotechnol. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Hong, Z.; Zhong, F.; Niu, W.; Zhang, K.; Su, J.; Liu, J.; Li, L.; Wu, F. Effects of temperature and particle size on the compositions, energy conversions and structural characteristics of pyrolysis products from different crop residues. Energy 2020, 190, 116413. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Pütün, E.; Pütün, A.E. Slow pyrolysis of pistachio shell. Fuel 2007, 86, 1892–1899. [Google Scholar] [CrossRef]
- Somerville, M.; Deev, A. The effect of heating rate, particle size and gas flow on the yield of charcoal during the pyrolysis of radiata pine wood. Renew. Energy 2020, 151, 419–425. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Slow pyrolysis of chemically treated walnut shell for valuable products: Effect of process parameters and in-depth product analysis. Energy 2019, 181, 665–676. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cai, L.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhang, J.; Zhu, Z. Process simulation of preparing biochar by biomass pyrolysis via Aspen Plus and its economic evaluation. Waste Biomass Valor. 2022, 13, 2609–2622. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Chong, K.; Bridgwater, A.V. A techno-economic analysis of energy recovery from organic fraction of municipal solid waste (MSW) by an integrated intermediate pyrolysis and combined heat and power (CHP) plant. Energy Convers. Manag. 2018, 174, 406–416. [Google Scholar] [CrossRef]
- Chhabra, V.; Bhattacharya, S.; Shastri, Y. Pyrolysis of mixed municipal solid waste: Characterisation, interaction effect and kinetic modelling using the thermogravimetric approach. Waste Manag. 2019, 90, 152–167. [Google Scholar] [CrossRef]
- Tokmurzin, D.; Kuspangaliyeva, B.; Aimbetov, B.; Abylkhani, B.; Inglezakis, V.; Anthony, E.J.; Sarbassov, Y. Characterization of solid char produced from pyrolysis of the organic fraction of municipal solid waste, high volatile coal and their blends. Energy 2020, 191, 116562. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Nannou, T.; Anguilano, L.; Krzyzyńska, R.; Ghazal, H.; Spencer, N.; Jouhara, H. Potentials of pyrolysis processes in the waste management sector. Energy Procedia 2017, 123, 387–394. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of banana peels wastes as potential slow pyrolysis feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Heuer, S.; Ammendola, P. Slow pyrolysis of walnut shells in nitrogen and carbon dioxide. Fuel 2018, 225, 419–425. [Google Scholar] [CrossRef]
- PN-EN ISO 18134-1:2023-02; Biopaliwa Stałe: Oznaczanie Zawartości Wilgoci: Część 1: Metoda Referencyjna. The Polish Committee for Standardization: Warszawa, Poland, 2023.
- PN-EN ISO 18123:2016-01; Solid Biofuels: Determination of the Content of Volatile Matter. The Polish Committee for Standardization: Warszawa, Poland, 2018. (In Polish)
- PN-Z-15008-03:1993; Odpady Komunalne Stałe: Badania Właściwości Paliwowych: Oznaczanie Zawartości Części Palnych i Niepalnych. The Polish Committee for Standardization: Warszawa, Poland, 1993.
- Speight, J.G. Handbook of Coal Analysis: Chemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- ISO 609:1996; Solid Mineral Fuels—Determination of Carbon and Hydrogen—High Temperature Combustion Method. ISO: Geneva, Switzerland, 1996.
- PN-G-04523:1992; Paliwa Stałe: Oznaczanie Zawartości Azotu Metodą Kjeldahla. The Polish Committee for Standardization: Warszawa, Poland, 1992.
- PN-ISO 351:1999; Paliwa stałe—Oznaczanie Zawartości Siarki—Metodą Spalania w Wysokiej Temperaturze. The Polish Committee for Standardization: Warszawa, Poland, 1999.
- PN-ISO 587:2000; Paliwa Stałe: Oznaczanie Zawartości Chloru z Zastosowaniem Mieszaniny Eschki. The Polish Committee for Standardization: Warszawa, Poland, 2000.
- Kotlicki, T. Oznaczanie Ciepła Spalania Węgla za Pomocą Kalorymetru—Instrukcja do Ćwiczenia Laboratoryjnego; The Polish Committee for Standardization: Warszawa, Poland, 2007. Available online: http://www.i15.p.lodz.pl/strony/elektrownie/spalanie_wegla.pdf (accessed on 12 December 2022).
- PN-ISO 1928:2020-05; Paliwa Stałe: Oznaczanie Ciepła Spalania Metodą Spalania w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej. The Polish Committee for Standardization: Warszawa, Poland, 2020.
- Biegańska, J. (Ed.) Metody Analizy w Gospodarce Odpadami: Zbiór Instrukcji do Ćwiczeń Laboratoryjnych; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2008. (In Polish) [Google Scholar]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2006; pp. 1–17. [Google Scholar] [CrossRef]
- Rago, Y.P.; Surroop, D.; Mohee, R. Assessing the potential of biofuel (biochar) production from food wastes through thermal treatment. Bioresour. Technol. 2018, 248, 258–264. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Simulation and Performance Analysis of Integrated Gasification-Syngas Fermentation Plant for Lignocellulosic Ethanol Production. Fermentation 2020, 6, 68. [Google Scholar] [CrossRef]
- Ward, J.; Rasul, M.G.; Bhuiya, M.M.K. Energy Recovery from Biomass by Fast Pyrolysis. Procedia Eng. 2014, 90, 669–674. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Liu, K.; Yu, H.; Zhu, C. Chemical, Energetic, and Structural Characteristics of Hydrothermal Carbonization Solid Products for Lawn Grass. BioResources 2015, 10, 4613–4625. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical Properties of Solid Biofuels—Significance and Impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Oštarić, F.; Kalit, S.; Curik, I.; Mikulec, N. Influence of Sodium and Potassium Chloride on Rennet Coagulation and Curd Firmness in Bovine Milk. Foods 2023, 12, 2293. [Google Scholar] [CrossRef]
- Ilakovac, B.; Voca, N.; Pezo, L.; Cerjak, M. Quantification and Determination of Household Food Waste and Its Relation to Sociodemographic Characteristics in Croatia. Waste Manag. 2020, 102, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of Binders on the Properties of Bio-Char Pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; Alaoui, F.E.M.; Elkhouakhi, M.; Sahibeddine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (Co)-Combustion Kinetics of Biomass, Coal and Municipal Solid Wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef]
- Seah, C.C.; Tan, C.H.; Arifin, N.A.; Hafriz, R.S.R.M.; Salmiaton, A.; Nomanbhay, S.; Shamsuddin, A.H. Co-Pyrolysis of Biomass and Plastic: Circularity of Wastes and Comprehensive Review of Synergistic Mechanism. Results Eng. 2023, 17, 100989. [Google Scholar] [CrossRef]
- Nyambura, M.; Li, C.; Xu, J.; Wang, J.; Li, H.; Zhang, Z.; Bertrand, G.; Ndumia, J.N.; Li, X.; Atsbha, M.W.; et al. Techno-Economic Assessment of Co-Pyrolyzed Kitchen Waste and Rice Straw for Co-Generation of Heat and Power. Sustain. Prod. Consum. 2023, 42, 434–445. [Google Scholar] [CrossRef]
- Cusenza, M.A.; Longo, S.; Cellura, M.; Guarino, F.; Messineo, A.; Mistretta, M.; Volpe, M. Environmental Assessment of a Waste-to-Energy Practice: The Pyrolysis of Agro-Industrial Biomass Residues. Sustain. Prod. Consum. 2021, 28, 866–876. [Google Scholar] [CrossRef]
- Petrova, T.; Naydenova, I.; Ribau, J.; Ferreira, A. Biochar from Agro-Forest Residue: Application Perspective Based on Decision Support Analysis. Appl. Sci. 2023, 13, 3240. [Google Scholar] [CrossRef]
- Saksiwi, N.; Rijo, B.; Zăvoianu, R.; Pavel, O.D.; Ferreira, O.; Galhano dos Santos, R.; Soares Dias, A.P.; Pereira, M. Biofuels from Pyrolysis of Third-Generation Biomass from Household and Garden Waste Composting Bin: Kinetics Analysis. Reactions 2023, 4, 18. [Google Scholar] [CrossRef]
- Khodaei, H.; Álvarez-Bermúdez, C.; Chapela, S.; Olson, C.; MacKenzie, M.D.; Gómez, M.A.; Porteiro, J. Eulerian CFD Simulation of Biomass Thermal Conversion in an Indirect Slow Pyrolysis Rotary Kiln Unit to Produce Biochar from Recycled Waste Wood. Energy 2024, 288, 129895. [Google Scholar] [CrossRef]
- Khodaei, H.; Gonzalez, L.; Chapela, S.; Porteiro, J.; Nikrityuk, P.; Olson, C. CFD-Based Coupled Multiphase Modeling of Biochar Production Using a Large-Scale Pyrolysis Plant. Energy 2021, 217, 119325. [Google Scholar] [CrossRef]
- Dahawi, Y.; Abdulrazik, A.; Abu Seman, M.N.; Abd Aziz, M.A.; Mohd Yunus, M.Y. Aspen Plus Simulation of Bio-Char Production from a Biomass-Based Slow Pyrolysis Process. Key Eng. Mater. 2019, 797, 336–341. [Google Scholar] [CrossRef]
- Safarian, S.; Rydén, M.; Janssen, M. Development and Comparison of Thermodynamic Equilibrium and Kinetic Approaches for Biomass Pyrolysis Modeling. Energies 2022, 15, 3999. [Google Scholar] [CrossRef]
- Kumar, M.; Srivastava, N.; Upadhyay, S.; Mishra, P. Thermal Degradation of Dry Kitchen Waste: Kinetics and Pyrolysis Products. Biomass Convers. Biorefin. 2021, 13, 2779–2796. [Google Scholar] [CrossRef]
- Gupta, A.; Mahajani, S. Kinetic Studies in Pyrolysis of Garden Waste in the Context of Downdraft Gasification: Experiments and Modeling. Energy 2020, 208, 118427. [Google Scholar] [CrossRef]



| Kitchen Biowaste | Spring Garden Biowaste | Autumn Garden Biowaste | |
|---|---|---|---|
| Proximate analysis (% wt.) | |||
| MC (total moisture content) (wet basis) | 68.10 | 81.53 | 63.51 |
| VM (volatile matter content) (dry basis) | 76.55 | 42.43 | 43.75 |
| A (ash content) (dry basis) | 6.81 | 17.75 | 43.83 |
| FC (fixed carbon) * (dry basis) | 16.64 | 39.82 | 12.43 |
| Ultimate analysis (% wt.) (dry basis) | |||
| C (carbon content) | 43.36 | 31.94 | 29.99 |
| H (hydrogen content) | 7.03 | 4.53 | 4.02 |
| N (nitrogen content) | 3.12 | 2.84 | 1.19 |
| S (sulfur content) | 0.10 | 0.07 | 0.03 |
| O (oxygen content) * | 38.87 | 42.82 | 20.92 |
| Cl (chlorine content) | 0.71 | 0.05 | 0.01 |
| Other measurements (dry basis) | |||
| HHV (higher heating value), MJ/kg | 17.24 | 10.60 | 11.16 |
| LHV (lower heating value), MJ/kg | 15.69 | 9.60 | 10.27 |
| CCs (combustible compounds) (%wt.) | 93.19 | 82.25 | 56.17 |
| OS (total organic matter content) (%wt.) | 90.06 | 81.77 | 55.32 |
| MS (total mineral substances content) (%wt.) | 9.94 | 18.23 | 44.68 |
| Corg (total organic carbon content) (%wt.) | 37.47 | 35.29 | 23.18 |
| pH (wet basis) | 5.18 | 9.20 | 8.62 |
| Kitchen Biochar | Spring Garden Biochar | Autumn Garden Biochar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | 400 °C | 500 °C | 600 °C | 400 °C | 500 °C | 600 °C | |
| Ym (mass yield of biochar) (% wt.) (dry basis) | 36.64 | 32.02 | 28.71 | 66.53 | 58.13 | 60.07 | 66.99 | 63.15 | 60.68 |
| Proximate analysis (% wt.) (dry basis) | |||||||||
| VM (volatile matter content) | 20.49 | 14.88 | 11.14 | 12.59 | 11.65 | 5.89 | 15.42 | 12.10 | 5.76 |
| A (ash content) | 18.57 | 21.81 | 21.09 | 64.69 | 65.38 | 72.57 | 61.71 | 64.38 | 71.32 |
| FC (fixed carbon) * | 60.94 | 63.31 | 67.77 | 22.73 | 22.96 | 21.54 | 22.87 | 23.52 | 22.93 |
| Ultimate analysis (% wt.) (dry basis) | |||||||||
| C (carbon content) | 58.02 | 54.81 | 55.55 | 25.34 | 26.60 | 21.00 | 27.84 | 27.45 | 25.62 |
| H (hydrogen content) | 4.00 | 3.02 | 2.67 | 2.41 | 1.71 | 1.41 | 2.80 | 2.54 | 1.47 |
| N (nitrogen content) | 3.64 | 3.46 | 3.01 | 1.44 | 1.52 | 1.05 | 1.15 | 1.00 | 1.02 |
| O (oxygen content) ** | 15.77 | 16.90 | 17.68 | 6.12 | 4.79 | 3.97 | 6.50 | 4.63 | 0.57 |
| Other measurements (dry basis) | |||||||||
| HHV (higher heating value), MJ/kg | 22.68 | 20.84 | 21.90 | 9.04 | 8.95 | 7.51 | 9.96 | 9.02 | 8.40 |
| LHV (lower heating value), MJ/kg | 21.80 | 20.17 | 21.31 | 8.51 | 8.58 | 7.20 | 9.35 | 8.46 | 8.07 |
| CCs (combustible compounds), (% wt.) | 81.43 | 78.19 | 78.91 | 35.31 | 34.62 | 27.43 | 38.29 | 35.62 | 28.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezuszko, A.; Landrat, M.; Pikoń, K.; Ferreira, A.F.; Rodrigues, A.; Olejarz, G.; Lewandowski, M. Slow Pyrolysis as a Method of Treating Household Biowaste for Biochar Production. Appl. Sci. 2025, 15, 7858. https://doi.org/10.3390/app15147858
Bezuszko A, Landrat M, Pikoń K, Ferreira AF, Rodrigues A, Olejarz G, Lewandowski M. Slow Pyrolysis as a Method of Treating Household Biowaste for Biochar Production. Applied Sciences. 2025; 15(14):7858. https://doi.org/10.3390/app15147858
Chicago/Turabian StyleBezuszko, Agnieszka, Marcin Landrat, Krzysztof Pikoń, Ana F. Ferreira, Abel Rodrigues, Gabor Olejarz, and Max Lewandowski. 2025. "Slow Pyrolysis as a Method of Treating Household Biowaste for Biochar Production" Applied Sciences 15, no. 14: 7858. https://doi.org/10.3390/app15147858
APA StyleBezuszko, A., Landrat, M., Pikoń, K., Ferreira, A. F., Rodrigues, A., Olejarz, G., & Lewandowski, M. (2025). Slow Pyrolysis as a Method of Treating Household Biowaste for Biochar Production. Applied Sciences, 15(14), 7858. https://doi.org/10.3390/app15147858







