Analysis of a Mixture of Banana Peel and Rice Straw Extracts for Inhibiting Corrosion of Carbon Steel in Hydrochloric Acid Solution
Abstract
1. Introduction
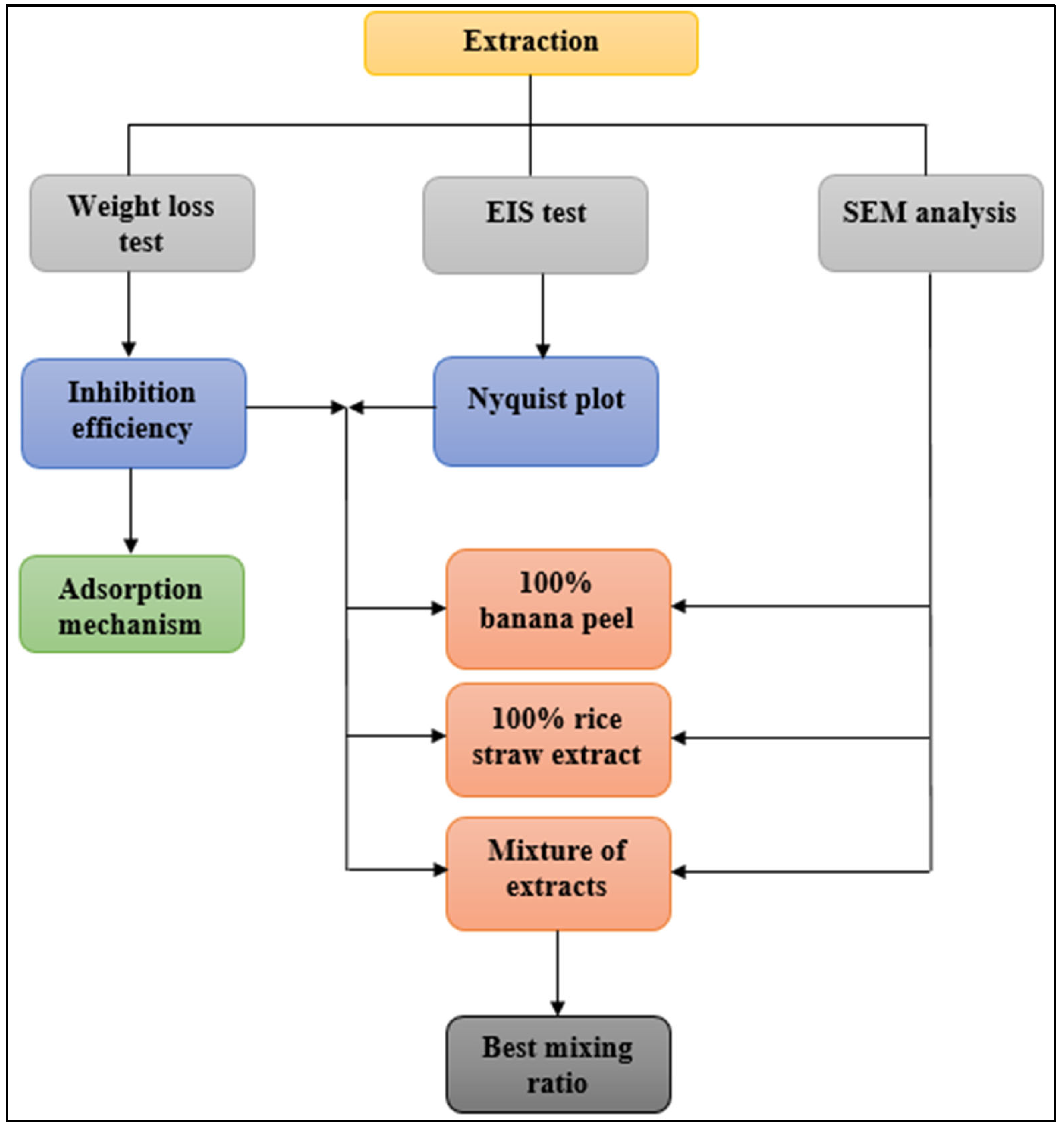
2. Material and Methods
2.1. Materials and Extract Preparation
2.2. Weight Loss Method
2.3. Electrochemical Tests
2.4. Scanning Electron Microscopy Analysis
2.5. Adsorption Analysis
3. Results and Discussion
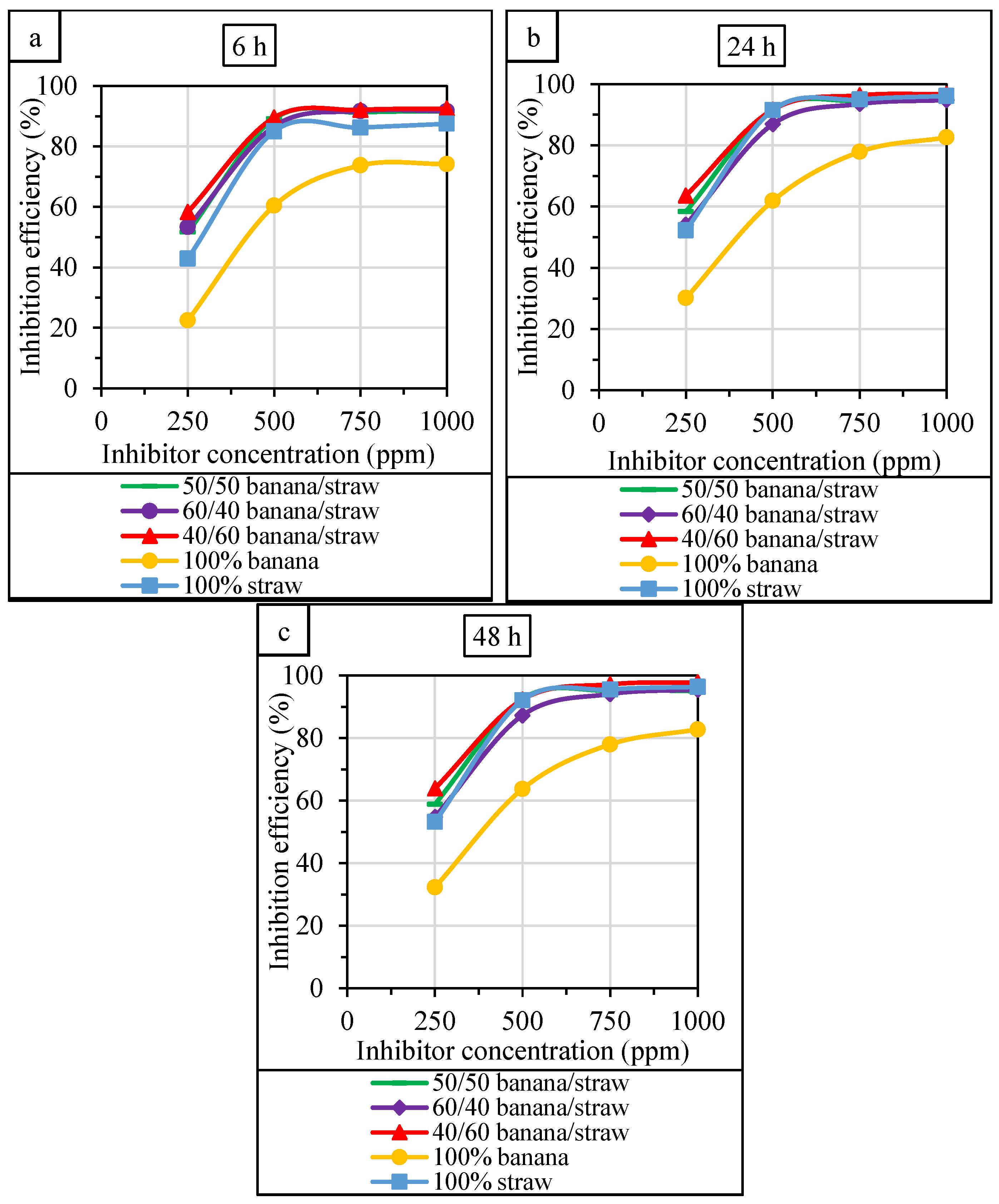
3.1. The Results of Weight Loss Tests
2H+ (aq) + 2e− ➝ H2 (g)
Fe (s) + 2HCl (aq) ➝ Fe2+ (aq) + H2 (g)+ 2Cl− (aq) ➝ FeCl2 (aq) + H2 (g)
2Fe (s) + O2 (g) + 2H2O (l) ➝ 2Fe2+ (aq) + 4OH− (aq) ➝ 2Fe(OH)2 (s)
2Fe(OH)2 (s)+ 0.5O2 (g) + H2O (l) ➝ 2Fe(OH)3 (s)
2Fe(OH)3 (s) ➝ Fe2O3 (s) + 3H2O (l)
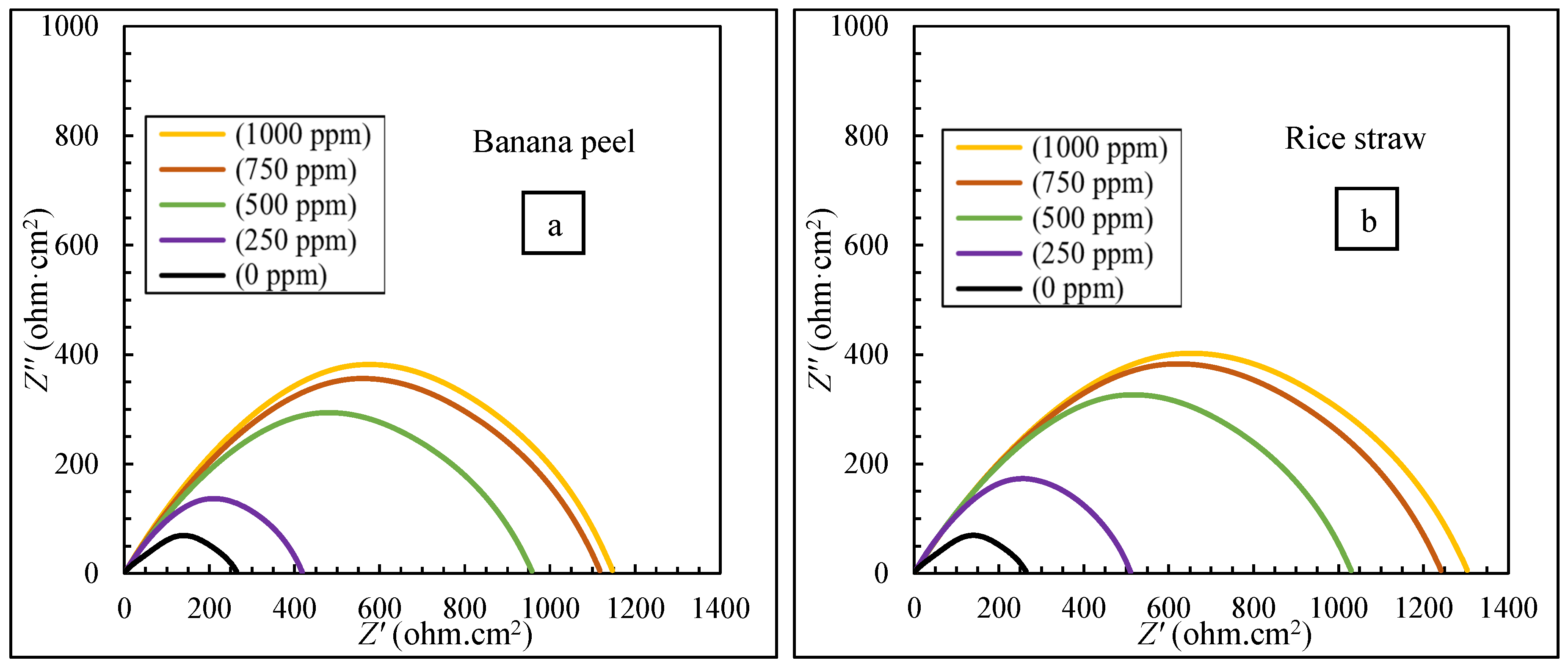
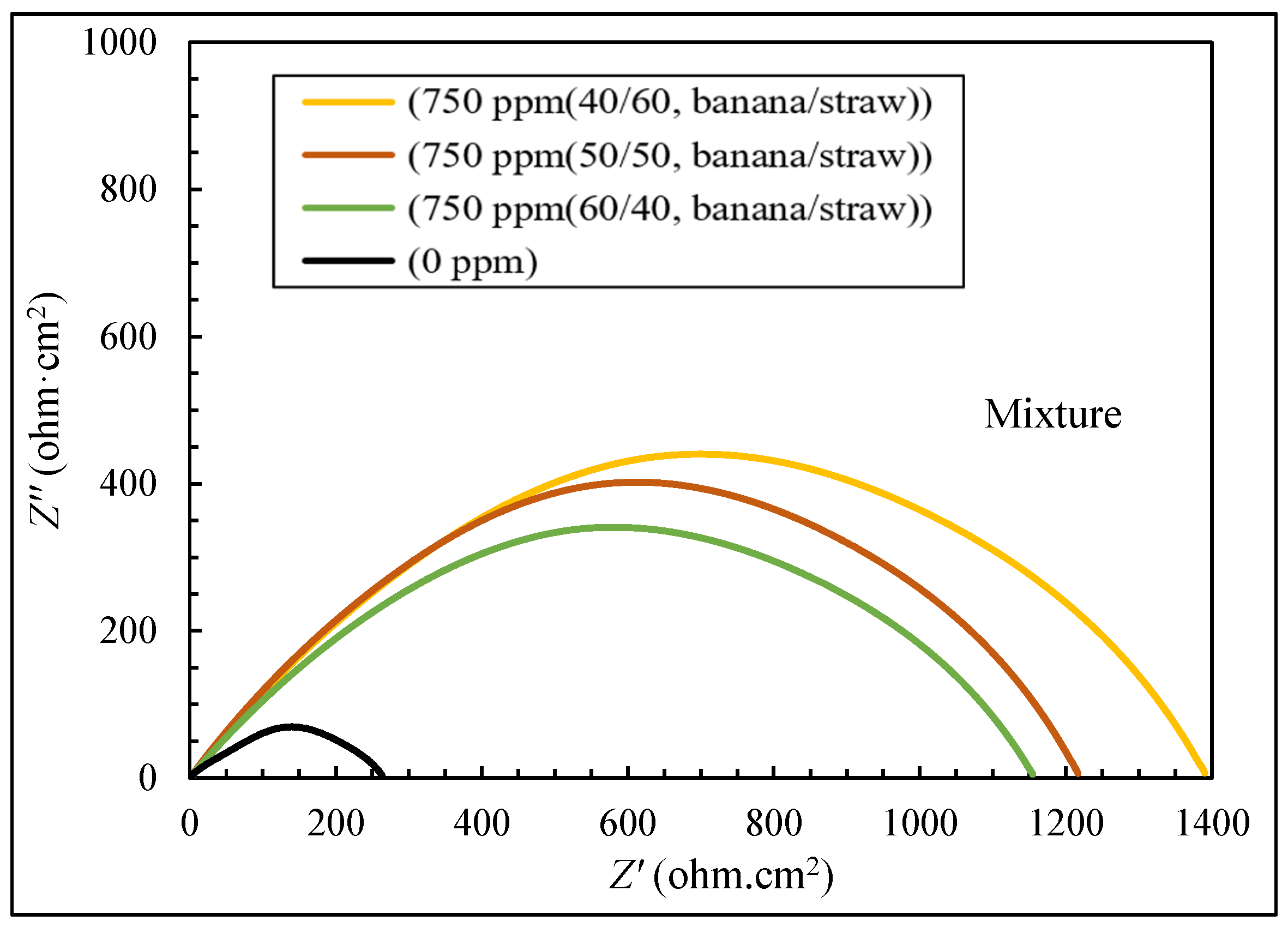
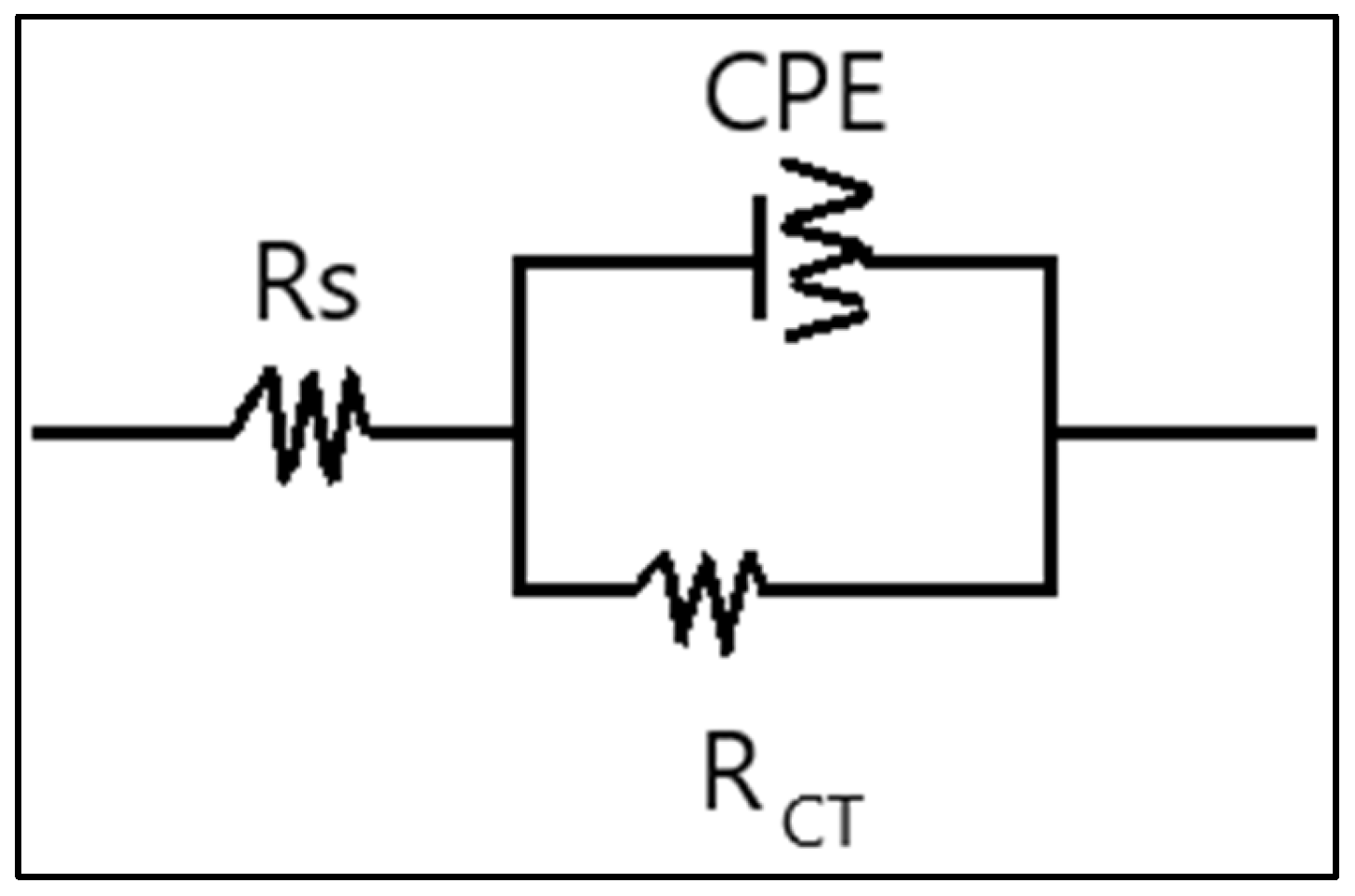
3.2. The Results of EIS Tests
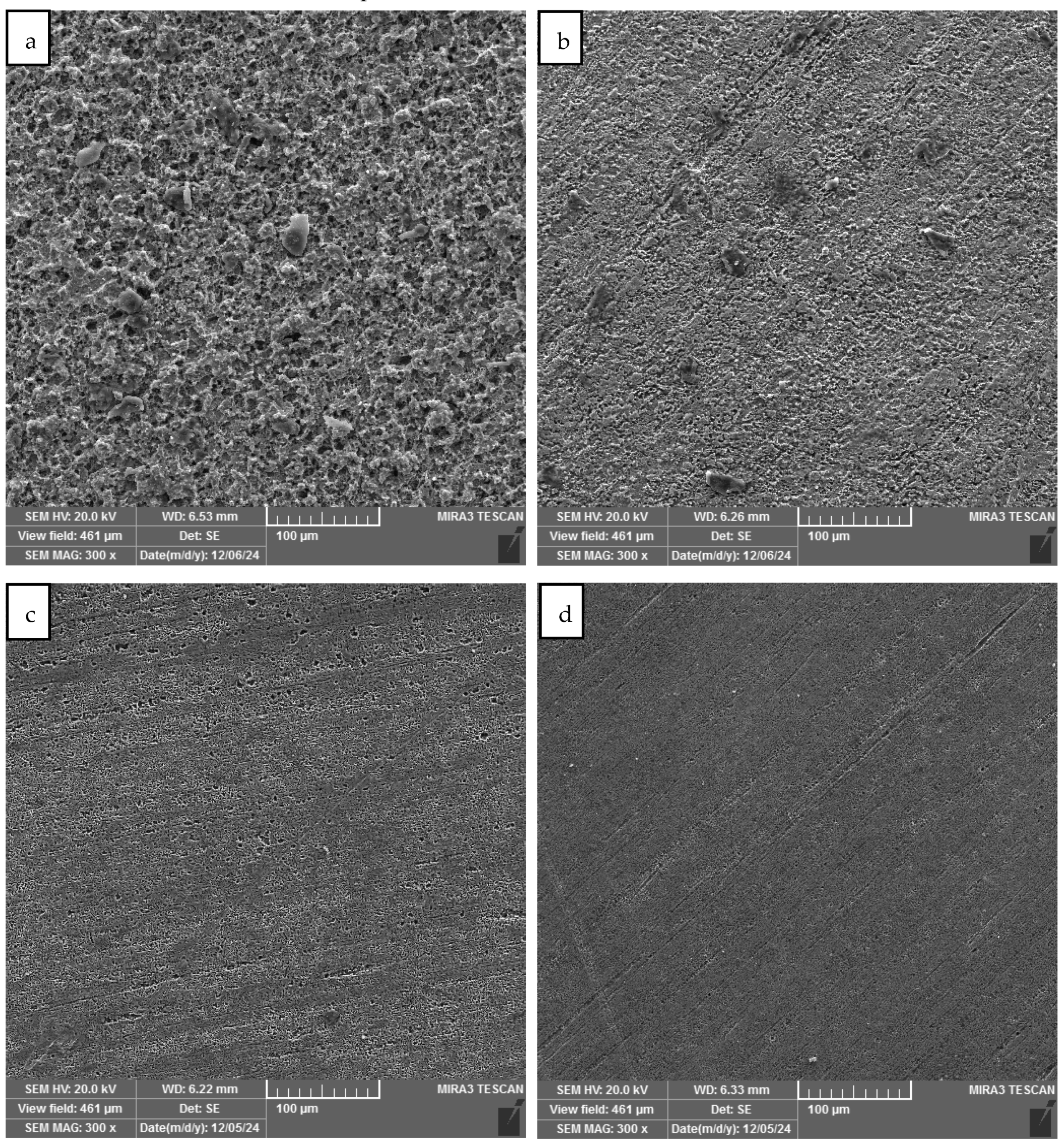
3.3. Results of SEM Analysis
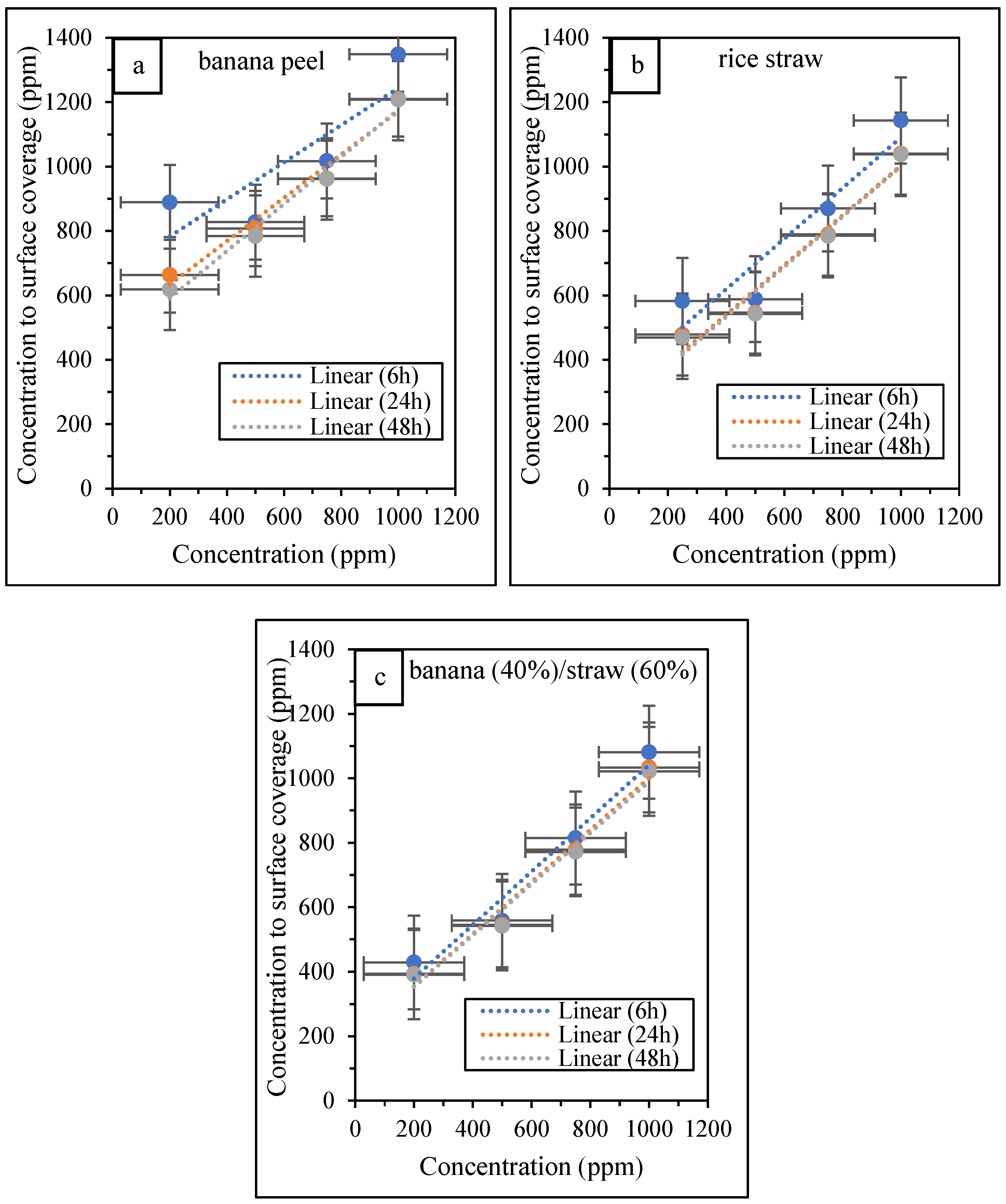
3.4. The Results of Analysis of Inhibitor Adsorption on Metal Surface
3.5. Inhibition Mechanism
4. Conclusions
- The inhibition efficiency of the mixture at a ratio of 40:60 (banana peel to rice straw) was higher than that of the individual extracts at all concentrations and immersion times based on the weight loss method.
- The inhibition efficiency was increased by increasing the concentration of the green corrosion inhibitors. Weight loss and electrochemical experiments showed that the optimum concentration of individual inhibitors and their mixture was 750 ppm, and the performance did not change significantly with further increase of the extract content in the HCl solution. Moreover, increasing the immersion time increased the corrosion inhibition efficiency, but this effect was weaker than the effect of inhibitor concentration.
- For all immersion times and inhibitor concentrations, a positive synergistic effect was observed at a mixing ratio of banana peel and rice straw of 40:60. The highest synergistic value was 1.65, indicating an average increase of 65% in inhibition efficiency for the mixture compared to the extract alone.
- The electrochemical tests confirmed the higher efficiency of the mixture (40:60 banana peel and rice straw) compared to 100% banana peel and 100% rice straw. When extracts were added to the corrosive environment, the resistance of the solution increased, and it became more difficult for ions to reach the metal surface, which led to an increase in the effectiveness of corrosion inhibition.
- Scanning electron microscope images clearly showed the formation of a stable film on the metal surface. The film formed in the presence of the banana peel and rice straw mixture was more stable and uniform than that in the presence of the individual extract.
- The Langmuir isotherm model most accurately describes the adsorption characteristics of the used extracts and their mixture. Both physical and chemical adsorption occurred for the banana peel and rice straw extracts, as well as for their mixture.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dehghani, A.; Berdimurodov, E.; Verma, C.; Verma, D.K.; Berdimuradov, K.; Quraishi, M.; Aliev, N. Constructing efficacy: A novel perspective on organic corrosion inhibitors and interfacial interactions. Chem. Pap. 2024, 78, 1367–1397. [Google Scholar] [CrossRef]
- Medupin, R.O.; Ukoba, K.O.; Yoro, K.O.; Jen, T.-C. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: A review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Khormali, A.; Ahmadi, S. Experimental and modeling analysis on the performance of 2-mercaptobenzimidazole corrosion inhibitor in hydrochloric acid solution during acidizing in the petroleum industry. J. Pet. Explor. Prod. Technol. 2023, 13, 2217–2235. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 2022, 364, 119992. [Google Scholar] [CrossRef]
- Groysman, A. Corrosion problems and solutions in oil, gas, refining and petrochemical industry. Koroze A Ochr. Mater. 2017, 61, 100. [Google Scholar] [CrossRef]
- Haldhar, R.; Kim, S.-C.; Berdimurodov, E.; Verma, D.K.; Hussain, C.M. Corrosion inhibitors: Industrial applications and commercialization. In Sustainable Corrosion Inhibitors II: Synthesis, Design, and Practical Applications; ACS Publications: Washington, DC, USA, 2021; pp. 219–235. [Google Scholar]
- Ahmadi, S.; Khormali, A.; Razmjooie, A. Experimental investigation on separation of water in crude oil emulsions using an oil-soluble demulsifier. Iran. J. Chem. Chem. Eng. 2023, 42, 2332–2343. [Google Scholar]
- Annon, I.A.; Abbas, A.S.; Al-Azzawi, W.K.; Hanoon, M.M.; Alamiery, A.; Isahak, W.N.R.; Kadhum, A.A.H. Corrosion inhibition of mild steel in hydrochloric acid environment using thiadiazole derivative: Weight loss, thermodynamics, adsorption and computational investigations. S. Afr. J. Chem. Eng. 2022, 41, 244–252. [Google Scholar] [CrossRef]
- Ma, I.W.; Ammar, S.; Kumar, S.S.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The effect of immersion corrosion time on electrochemical corrosion behavior and the corrosion mechanism of EH47 ship steel in seawater. Metals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A. Development of an RSM-based predictive model for evaluation of corrosion efficiency of ATMP in one molar HCl for carbon steel samples. Pet. Sci. Technol. 2024, 42, 4537–4555. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic compounds as corrosion inhibitors for carbon steel in HCl solution: A comprehensive review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and emerging trends of inorganic, organic and eco-friendly corrosion inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef]
- Shwetha, K.; Praveen, B.; Devendra, B.K. A review on corrosion inhibitors: Types, mechanisms, electrochemical analysis, corrosion rate and efficiency of corrosion inhibitors on mild steel in an acidic environment. Results Surf. Interfaces 2024, 16, 100258. [Google Scholar]
- Zomorodian, A.; Behnood, A. Review of corrosion inhibitors in reinforced concrete: Conventional and green materials. Buildings 2023, 13, 1170. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Vazquez-Delgado, R.; Salvador-Carlos, J.; Beltran-Partida, E.; Salinas-Martinez, R.; Cheng, N.; Curiel-Alvarez, M. Azadirachta indica leaf extract as green corrosion inhibitor for reinforced concrete structures: Corrosion effectiveness against commercial corrosion inhibitors and concrete integrity. Materials 2021, 14, 3326. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Maghraby, Y.R.; Ibrahim, A.H.; Shouier, K.R.; Alturki, A.M.; El-Shabasy, R.M. Role of green chemistry in sustainable corrosion inhibition: A review on recent developments. Mater. Today Sustain. 2022, 20, 100242. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Seman, A.A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Sobri, S.A.; Ali, A. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Miralrio, A.; Espinoza Vázquez, A. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: A review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Albo Hay Allah, M.A.; Balakit, A.A.; Salman, H.I.; Abdulridha, A.A.; Sert, Y. New heterocyclic compound as carbon steel corrosion inhibitor in 1 M H2SO4, high efficiency at low concentration: Experimental and theoretical studies. J. Adhes. Sci. Technol. 2023, 37, 525–547. [Google Scholar] [CrossRef]
- Răuță, D.-I.; Matei, E.; Avramescu, S.-M. Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technologies 2025, 13, 103. [Google Scholar] [CrossRef]
- Huang, M.; Wang, R.; Xiong, J.; Liu, C.; Wang, J.; Wang, Q. Inhibition effect and adsorption behavior of Michelia alba leaf extract as corrosion inhibitors for Cu in 0.5 M H2SO4. J. Dispers. Sci. Technol. 2025, 1–18. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Su, Y.; Wu, F.; Xiao, P.; Zhang, H.; Lv, G.; Zhu, M.; Liu, Q.; Zhao, C. Facile and green synthesis of Ag/Cu bimetallic nanoparticles using waste banana peel extract for effective corrosion inhibition of mixed sulfate-reducing bacteria. J. Environ. Manag. 2025, 373, 123713. [Google Scholar] [CrossRef]
- Manikandan, C.B.; Balamurugan, S.; Balamurugan, P.; Beneston, S.L. Corrosion inhibition of mild steel by using banana peel extract. Int. J. Innov. Technol. Explor. Eng 2019, 8, 1372–1375. [Google Scholar]
- Tambun, R.; Christamore, E.; Pakpahan, Y.F.; Haryanto, B. Banana peel utilization as the green corrosion inhibitor of Iron in NaCl medium. Mater. Sci. Eng. 2018, 420, 012059. [Google Scholar] [CrossRef]
- Fouda, A.; Gadow, H.; Abd Elal, E.; El-Tantawy, M. Corrosion inhibition of aluminium by rice straw extract in 2 M hydrochloric acid solution. J. Bio-Tribo-Corros. 2021, 7, 102. [Google Scholar] [CrossRef]
- Oyewole, O.; Abayomi, T.S.; Oreofe, T.A.; Oshin, T.A. Anti-corrosion using rice straw extract for mild steel in 1.5 M H2SO4 solution. Results Eng. 2022, 16, 100684. [Google Scholar] [CrossRef]
- Yahya, S.; Othman, N.; Ismail, M. Corrosion inhibition of steel in multiple flow loop under 3.5% NaCl in the presence of rice straw extracts, lignin and ethylene glycol. Eng. Fail. Anal. 2019, 100, 365–380. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Azzaoui, K.; Warad, I.; Hammouti, B.; Shityakov, S.; Sabbahi, R.; Saoiabi, S.; Youssoufi, M.; Akartasse, N.; Jodeh, S. Moroccan, Mauritania, and senegalese gum Arabic variants as green corrosion inhibitors for mild steel in HCl: Weight loss, electrochemical, AFM and XPS studies. J. Mol. Liq. 2022, 347, 118354. [Google Scholar] [CrossRef]
- Khormali, A.; Ahmadi, S.; Kazemzadeh, Y.; Karami, A. Evaluating the Efficacy of Binary Benzimidazole Derivatives as Corrosion Inhibitors for Carbon Steel Using Multi-Modal Analysis and Optimization Techniques. Results Eng. 2025, 26, 104671. [Google Scholar] [CrossRef]
- Khormali, A.; Ahmadi, S. Synergistic effect between oleic imidazoline and 2-mercaptobenzimidazole for increasing the corrosion inhibition performance in carbon steel samples. Iran. J. Chem. Chem. Eng. 2023, 42, 321–336. [Google Scholar]
- Vaszilcsin, C.G.; Putz, M.V.; Kellenberger, A.; Dan, M.L. On the evaluation of metal-corrosion inhibitor interactions by adsorption isotherms. J. Mol. Struct. 2023, 1286, 135643. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Zarrok, H.; Al-Deyab, S.; Messali, M. Temperature effect, activation energies and thermodynamic adsorption studies of L-cysteine methyl ester hydrochloride as copper corrosion inhibitor in nitric acid 2M. Int. J. Electrochem. Sci. 2011, 6, 6261–6274. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Wang, Y.; Tang, J.; Wang, Y.; Wang, H. Molecular dynamic simulation and experimental investigation on the synergistic mechanism and synergistic effect of oleic acid imidazoline and l-cysteine corrosion inhibitors. Corros. Sci. 2021, 185, 109414. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A review. J. Environ. Chem. Eng. 2017, 5, 246–273. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.; Taheri, P.; Mol, J.; Montemor, M. Comparison of the synergistic effects of inhibitor mixtures tailored for enhanced corrosion protection of bare and coated AA2024-T3. Surf. Coat. Technol. 2016, 303, 342–351. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kaya, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef]
- Zaidon, F.H.; Kassim, K.; Zaki, H.M.; Embong, Z.; Anouar, E.H.; Hashim, N.Z.N. Adsorption and corrosion inhibition accomplishment for thiosemicarbazone derivatives for mild steel in 1.0 M HCl medium: Electrochemical, XPS and DFT studies. J. Mol. Liq. 2021, 329, 115553. [Google Scholar] [CrossRef]
- Toghan, A.; Gadow, H.; Fawzy, A.; Alhussain, H.; Salah, H. Adsorption mechanism, kinetics, thermodynamics, and anticorrosion performance of a new thiophene derivative for C-steel in a 1.0 M HCl: Experimental and computational approaches. Metals 2023, 13, 1565. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Utami, S.P.; Fermi, M.I.; Aziz, Y.; Irianti, R.S. Corrosion control of carbon steel using inhibitor of banana peel extract in acid diluted solutions. Mater. Sci. Eng. 2018, 345, 012030. [Google Scholar]
- Verma, D.K.; Dewangan, Y.; Dewangan, A.K.; Asatkar, A. Heteroatom-based compounds as sustainable corrosion inhibitors: An overview. J. Bio-Tribo-Corros. 2021, 7, 15. [Google Scholar] [CrossRef]
- Mohd, N.K.; Kian, Y.S.; Ibrahim, N.A.; Nor, S.M.M.; Wan Yunus, W.M.Z.; Ghazali, M.J.; Huei, L.W. Corrosion inhibition, adsorption and thermodynamic properties of hydrophobic-tailed imines on carbon steel in hydrochloric acid solution: A comparative study. J. Adhes. Sci. Technol. 2021, 35, 2558–2579. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]

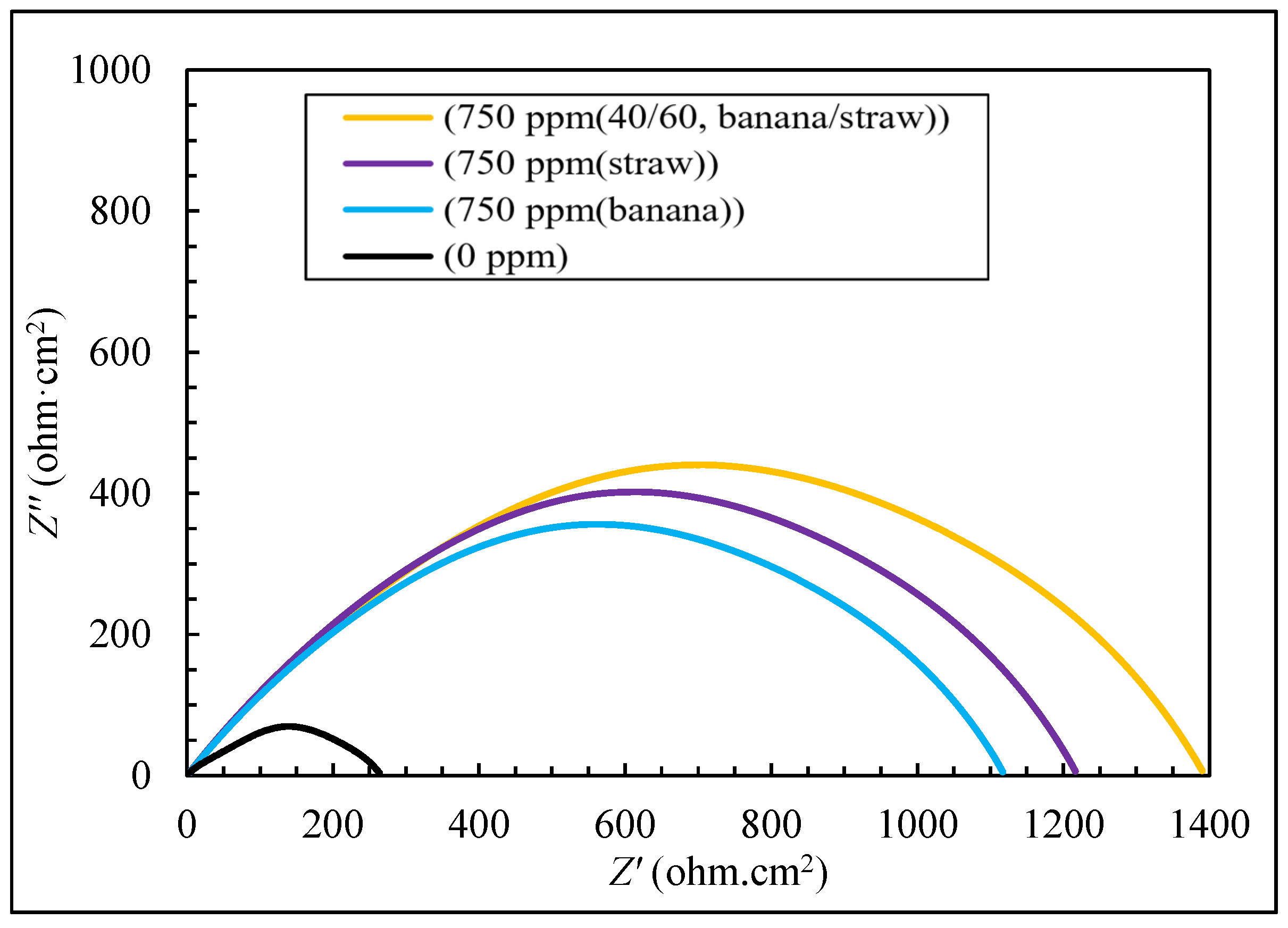

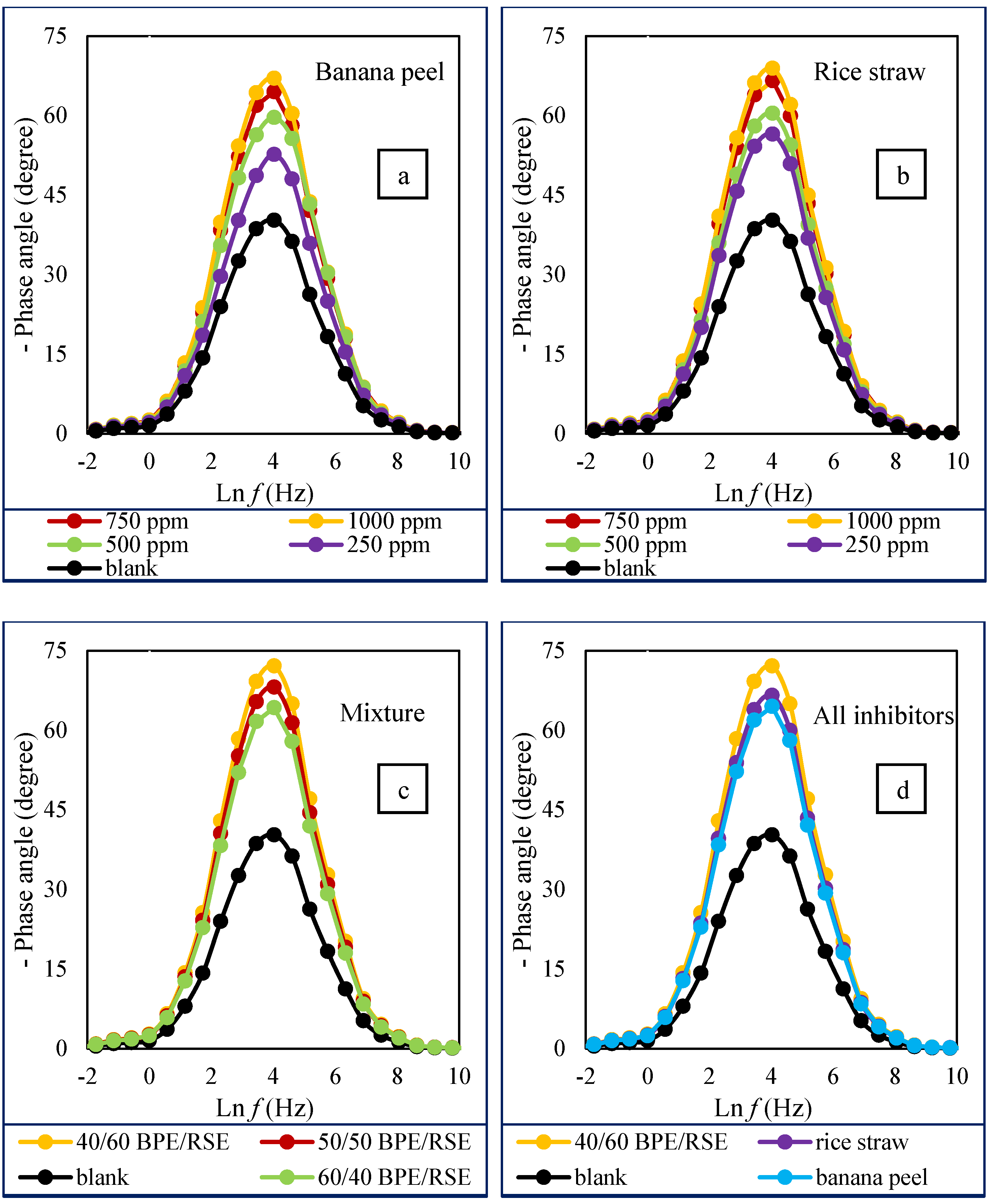

| Inhibition Efficiency (%) | RCT (ohm·cm2) | Concentration (ppm) | Inhibitor Type | |
|---|---|---|---|---|
| --- | 46.15 | 0 | blank | |
| 30.13 | 66.05 | 250 | banana peel | |
| 59.96 | 115.26 | 500 | ||
| 78.18 | 211.48 | 750 | ||
| 81.50 | 249.45 | 1000 | ||
| 50.34 | 92.94 | 250 | rice straw | |
| 90.56 | 488.62 | 500 | ||
| 95.19 | 958.57 | 750 | ||
| 95.52 | 1029.47 | 1000 | ||
| 96.34 | 1259.48 | 750 | 40/60 | banana peel/rice straw |
| 94.67 | 865.07 | 750 | 50/50 | |
| 93.41 | 700.18 | 750 | 60/40 | |
| Inhibition Efficiency (%) | Deviation (Comparison of Experimental and Theoretical Values) (%) | Causal Factor According to 3 | Deviation (Comparison of Experimental and Theoretical Values) (%) | Causal Factor According to 2 | icorr (μ·cm−2) | Concentration (ppm) | Inhibitor Type | |
|---|---|---|---|---|---|---|---|---|
| --- | 4.67 | 3.14 | 9.00 | 2.18 | 1065 | 0 | blank | |
| 30.70 | 1.67 | 3.05 | 2.50 | 1.95 | 738 | 250 | banana peel | |
| 61.03 | 4.00 | 2.88 | 1.00 | 2.02 | 415 | 500 | ||
| 78.40 | 1.00 | 2.97 | 4.00 | 2.08 | 230 | 750 | ||
| 80.19 | 3.33 | 3.10 | 2.00 | 2.04 | 211 | 1000 | ||
| 51.27 | 1.33 | 2.96 | 7.00 | 2.14 | 519 | 250 | rice straw | |
| 88.92 | 4.33 | 2.87 | 2.00 | 2.04 | 118 | 500 | ||
| 94.55 | 3.00 | 3.09 | 2.50 | 1.95 | 58 | 750 | ||
| 95.02 | 7.00 | 3.21 | 5.50 | 1.89 | 53 | 1000 | ||
| 95.59 | 5.33 | 3.16 | 8.50 | 2.17 | 47 | 750 | 40/60 | banana peel/rice straw |
| 95.87 | 1.67 | 3.05 | 6.50 | 2.13 | 44 | 750 | 50/50 | |
| 92.86 | 3.67 | 3.11 | 8.50 | 2.17 | 76 | 750 | 60/40 | |
| ΔGads (kJ·mol−1) | Kads (M−1) | Kads (ppm−1) | Kads−1 (ppm) | Time (h) | Inhibitor Type |
|---|---|---|---|---|---|
| −38.7156 | 4.89298 × 10−9 | 0.001497 | 667.89 | 6 | banana peel |
| −37.9684 | 6.55088 × 10−9 | 0.002005 | 498.86 | 24 | |
| −37.6778 | 7.33799 × 10−9 | 0.002245 | 445.35 | 48 | |
| −38.3650 | 5.61107 × 10−9 | 0.003277 | 305.17 | 6 | rice straw |
| −37.6571 | 7.39763 × 10−9 | 0.00432 | 231.47 | 24 | |
| −37.5484 | 7.71841 × 10−9 | 0.004508 | 221.85 | 48 | |
| −37.1160 | 9.1383 × 10−9 | 0.004321 | 231.45 | 6 | banana peel (40%): rice straw (60%) |
| −36.6501 | 1.09617 × 10−8 | 0.005183 | 192.95 | 24 | |
| −36.6689 | 1.08816 × 10−8 | 0.005145 | 194.37 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dazdari, M.; Khormali, A.; Taleghani, A. Analysis of a Mixture of Banana Peel and Rice Straw Extracts for Inhibiting Corrosion of Carbon Steel in Hydrochloric Acid Solution. Appl. Sci. 2025, 15, 5026. https://doi.org/10.3390/app15095026
Dazdari M, Khormali A, Taleghani A. Analysis of a Mixture of Banana Peel and Rice Straw Extracts for Inhibiting Corrosion of Carbon Steel in Hydrochloric Acid Solution. Applied Sciences. 2025; 15(9):5026. https://doi.org/10.3390/app15095026
Chicago/Turabian StyleDazdari, Maral, Azizollah Khormali, and Akram Taleghani. 2025. "Analysis of a Mixture of Banana Peel and Rice Straw Extracts for Inhibiting Corrosion of Carbon Steel in Hydrochloric Acid Solution" Applied Sciences 15, no. 9: 5026. https://doi.org/10.3390/app15095026
APA StyleDazdari, M., Khormali, A., & Taleghani, A. (2025). Analysis of a Mixture of Banana Peel and Rice Straw Extracts for Inhibiting Corrosion of Carbon Steel in Hydrochloric Acid Solution. Applied Sciences, 15(9), 5026. https://doi.org/10.3390/app15095026






