The Use of Copper Slag in the Thermolysis Process for Solar Hydrogen Production—A Novel Alternative for the Circular Economy
Abstract
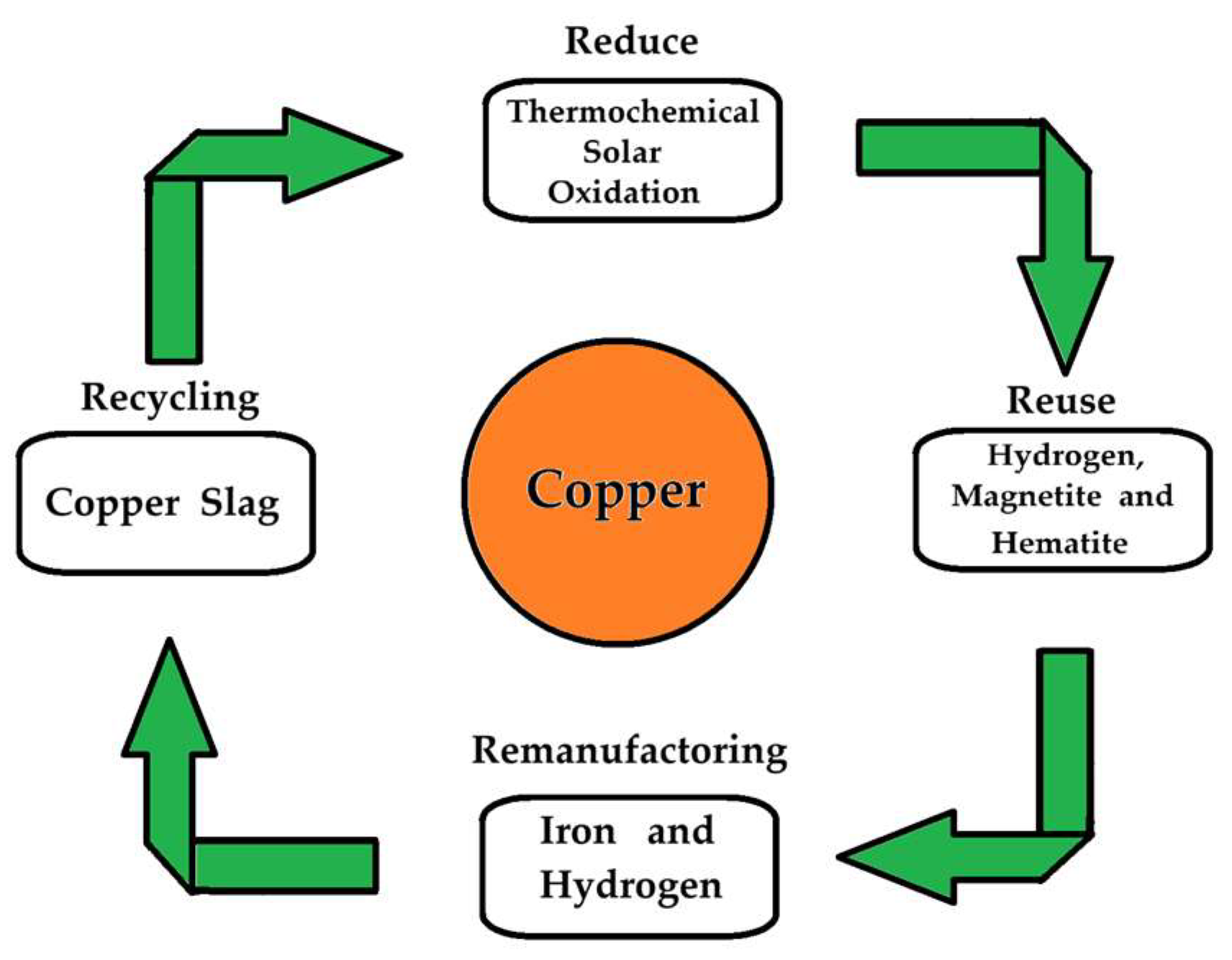
1. Introduction
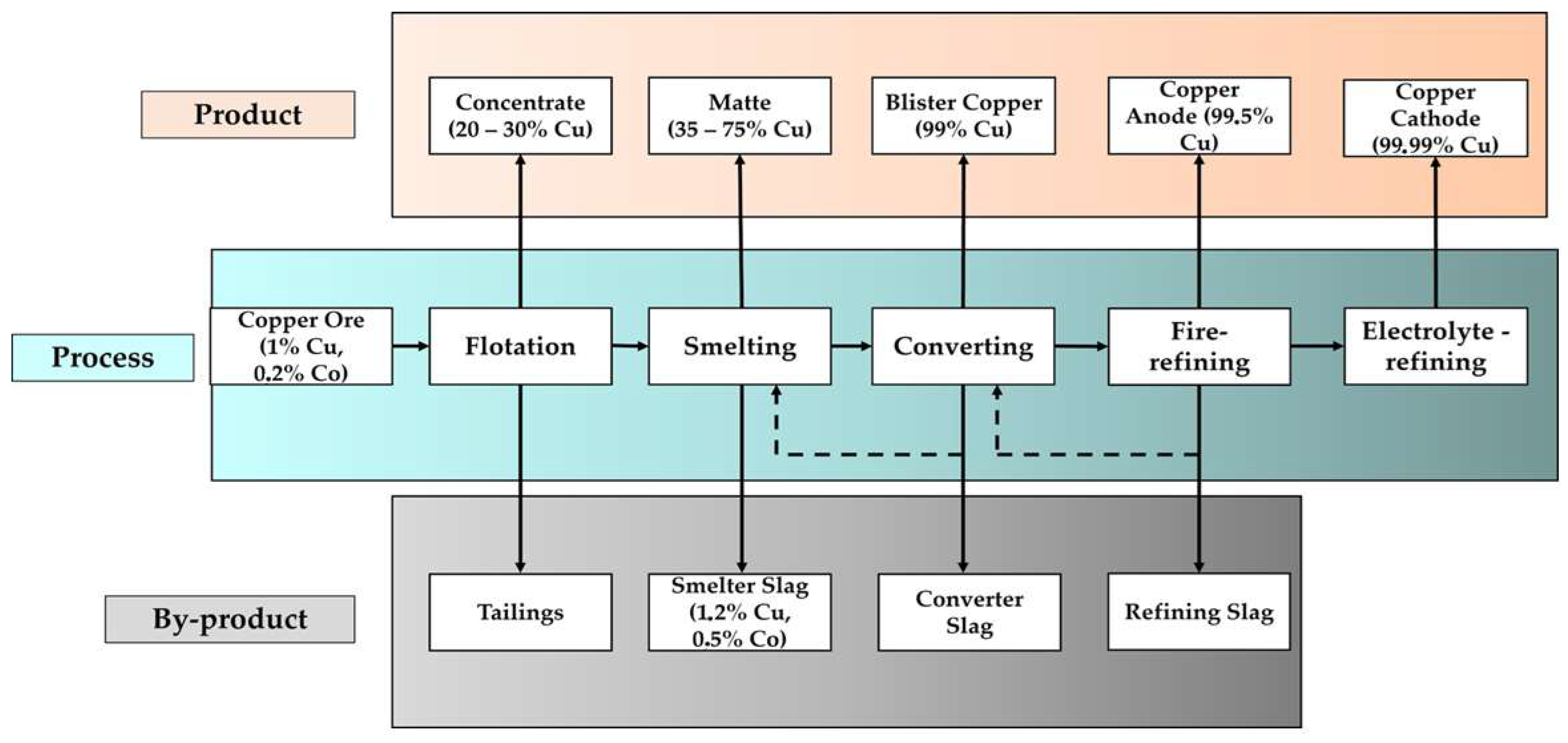
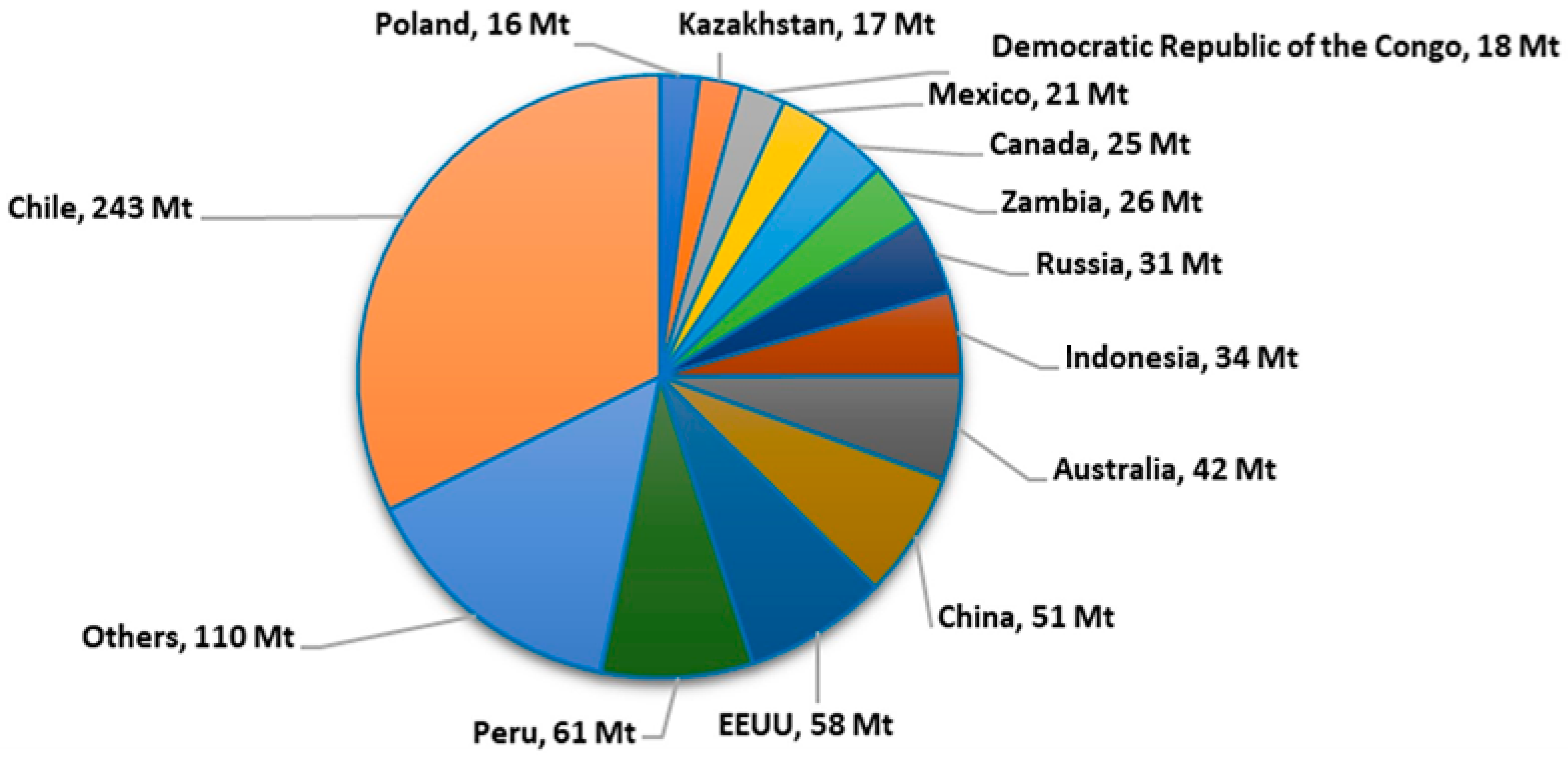
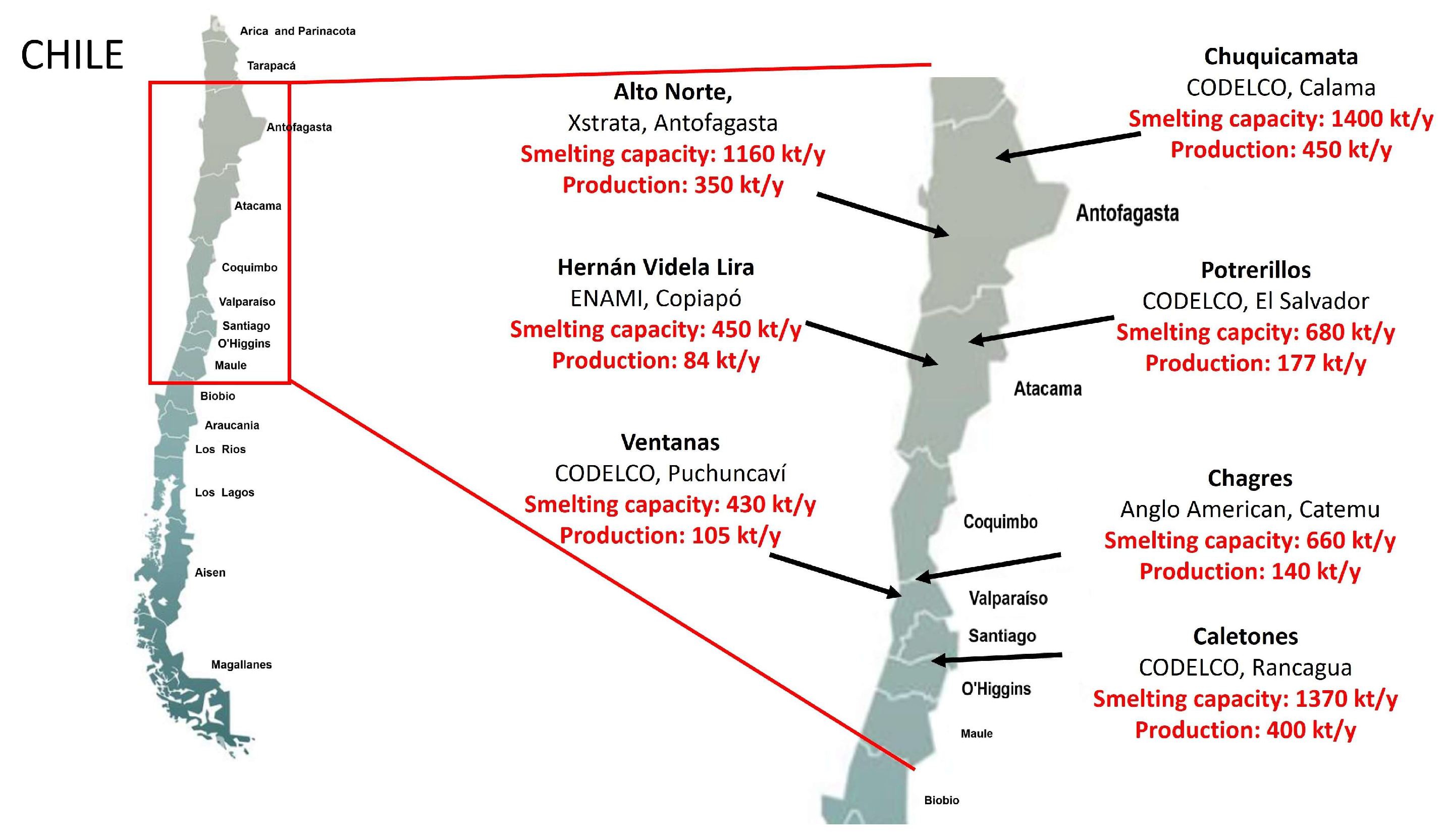
2. Production of Copper Slag
Copper Slag
3. Discussion
| Country | Company | Cu | Fe | S | K | P | Fe2O3 | FeO | SiO2 | CaO | Al2O3 | MgO | P2O5 | SO3 | CuO | K2O | Na2O | MnO | TiO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chile | O’Higgins. El Teniente.Converter, Rancagua | 4.56 | 40.30 | 1.53 | 0.80 | - | - | - | 20.30 | 0.33 | 2.86 | 0.75 | - | - | - | - | 1.20 | - | - | [21] |
| Chile | O’Higgins.El Teniente. Reververo furnace, Rancagua | 0.83 | 33.30 | 0.50 | 1.10 | - | - | - | 37.90 | 7.70 | 7.50 | 1.40 | - | - | - | - | 2.10 | - | - | [21] |
| Chile | Atacama, Century scoriales XIX Playa Negra, Copiapo | - | - | - | - | - | 14.23 | 18.99 | 35.00 | 17.05 | 6.69 | 1.41 | 0.49 | 0.40 | 0.77 | 1.21 | 0.81 | 0.17 | 0.27 | [19] |
| Chile | Atacama, Century scoriales XIX Canto del Agua, Copiapo | - | - | - | - | - | 12.87 | 29.60 | 39.39 | 4.32 | 4.62 | 1.30 | 0.75 | 1.14 | 1.40 | 0.32 | 0.33 | 0.05 | 0.14 | [19] |
| Chile | Atacama, Century scoriales XIX Nantoco, Copiapo | - | - | - | - | - | 13.58 | 22.71 | 38.58 | 7.66 | 6.57 | 1.13 | 0.70 | 0.53 | 1.83 | 1.01 | 0.58 | 0.30 | 0.13 | [19] |
| China | Tongling Non-ferrous Metals Group Holding Co., Ltd., Tongling, Anhui | 0.80 | 39.10 | 0.45 | - | 0.10 | - | 42.85 | 31.11 | 2.44 | 2.29 | 1.79 | - | - | - | - | - | - | - | [56] |
| Spain | Cabezo Juré archeological settlement, Alosno, Provincia de Huelva. | 20.1 | - | - | - | - | 50.60 | - | 13.81 | 0.20 | 1.79 | 0.20 | 0.058 | - | - | 0.01 | 0.26 | - | 0.307 | [57] |
| Mexico | Company and location unknown | - | - | - | - | - | 45.46 | - | 33.12 | 11.68 | 4.67 | 0.42 | 0.10 | - | - | 0.77 | 2.64 | 0.39 | 0.22 | [58] |
| Mexico | Smelter of the Mining-Metallurgical Complex of La Caridad, Sonora | 1.05 | 44.57 | - | - | - | - | - | 25.12 | - | - | - | 9.42 | - | - | - | - | - | - | [59] |
| Mexico | Industrias Magnelec SA de CV, Saltillo, Coah | - | - | - | - | - | 0.13 | - | 0.2 | 1.00 | 0.15 | 98.5 | - | - | - | - | - | - | - | [60] |
| Italy | Campigilia Marittima Century scoriales XI-XIII. Capattoli | - | - | - | - | - | - | 42.53 | 20.06 | 8.26 | 2.93 | 0.72 | - | - | - | 0.74 | 0.02 | - | - | [61] |
| Italy | Campigilia Marittima Century scoriales XI-XIII. Capattoli | - | - | - | - | - | - | 52.17 | 30.60 | 15.62 | 5.06 | 0.90 | - | - | - | 1.20 | 0.07 | - | - | [61] |
| Portugal | District Sao Domingos Mining Centre, Century XX, Moitinha, Achada do Gamo | - | - | - | - | - | 52.86 | - | 37.70 | 1.14 | 3.93 | 0.40 | 0.20 | - | - | 1.04 | 0.25 | 0.07 | 0.688 | [62,63] |
| Namibia | Dundee Precious Metals Ltd., Tsumeb smelting complex, Windhoek | - | - | 6.51 | - | - | 32.47 | 9.49 | 14.68 | 4.50 | 4.70 | 1.43 | 0.59 | - | - | 0.30 | 0.26 | 0.08 | 0.25 | [64,65] |
| India | Sterlite Industries India Limited, Tuticorin, Tamil Nadu | - | - | - | - | - | 61.24 | - | 19.72 | 5.65 | 3.03 | 0.90 | 0.63 | 3.31 | 1.49 | 1.23 | - | 0.08 | 0.44 | [10] |
| Australia | Tropical coastal zone of north Queensland. | 0.52 | - | - | - | - | - | 32.88 | 34.80 | 15.53 | 5.41 | 0.91 | 0.27 | - | - | 0.60 | 0.26 | 0.39 | - | [3] |
| Canada | INCO’s flash smelting furnace, Sudbury, Ontario | 0.58 | 38.6 | 0.93 | - | - | 45.40 | 52.20 | 17.40 | 1.11 | 2.51 | 1.65 | - | - | - | - | - | 0.03 | 0.15 | [56,66] |
- Costs of collecting and transporting copper slag from smelters to H2 production plants.
- Pretreatment costs (impurity removal, structural modification).
- Capital expenditures (CAPEXs) and operating costs (OPEXs) for high temperature thermolysis reactors and solar concentrators.
- Costs of purifying water for the process and hydrogen for industrial applications.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Hu, C.; Gao, W.; Lu, M. Recovery of Iron from Copper Slag Using Coal-Based Direct Reduction: Reduction Characteristics and Kinetics. Minerals 2020, 10, 973. [Google Scholar] [CrossRef]
- Wang, J.-P.; Erdenebold, U. A Study on Reduction of Copper Smelting Slag by Carbon for Recycling into Metal Values and Cement Raw Material. Sustainability 2020, 12, 1421. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K. Premchand Characteristics and Utilisation of Copper Slag—A Review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- dos Anjos, M.A.G.; Sales, A.T.C.; Andrade, N. Blasted Copper Slag as Fine Aggregate in Portland Cement Concrete. J. Env. Manag. 2017, 196, 607–613. [Google Scholar] [CrossRef]
- Murari, K.; Siddique, R.; Jain, K.K. Use of Waste Copper Slag, a Sustainable Material. J. Mater. Cycles Waste Manag. 2015, 17, 13–26. [Google Scholar] [CrossRef]
- González, C.; Parra, R.; Klenovcanova, A.; Imris, I.; Sánchez, M. Reduction of Chilean Copper Slags: A Case of Waste Management Project. Scand. J. Metall. 2005, 34, 143–149. [Google Scholar] [CrossRef]
- Nazer, A.; Pavez, O.; Rojas, F.; Aguilar, C. Una Revisión de Los Usos de Las Escorias de Cobre; Iberomet XI. X Conamet/sam: Viña del Mar, Chile, 2010. [Google Scholar]
- Huanosta-Gutiérrez, T.; Dantas, R.F.; Ramírez-Zamora, R.M.; Esplugas, S. Evaluation of Copper Slag to Catalyze Advanced Oxidation Processes for the Removal of Phenol in Water. J. Hazard. Mater. 2012, 213–214, 325–330. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An Overview of Recovery of Metals from Slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Wang, X.; Geysen, D.; Padilla Tinoco, S.V.; D’Hoker, N.; Van Gerven, T.; Blanpain, B. Characterisation of Copper Slag in View of Metal Recovery. Miner. Process. Extr. Metall. 2015, 124, 83–87. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Z.; Zhu, D.; Pan, J.; Wang, Y.; Zhan, R. Efficient Utilization of Copper Slag in an Innovative Sintering Process for Fe–Ni–Cu Alloy Preparation and Valuable Elements Recovery. J. Mater. Res. Technol. 2022, 18, 3115–3129. [Google Scholar] [CrossRef]
- Montoya-Bautista, C.V.; Acevedo-Peña, P.; Zanella, R.; Ramírez-Zamora, R.-M. Characterization and Evaluation of Copper Slag as a Bifunctional Photocatalyst for Alcohols Degradation and Hydrogen Production. Top. Catal. 2021, 64, 131–141. [Google Scholar] [CrossRef]
- Leiva-Guajardo, S.I.; Toro, N.; Fuentealba, E.; Morel, M.J.; Soliz, Á.; Portillo, C.; Galleguillos Madrid, F.M. Contribution of Copper Slag to Water Treatment and Hydrogen Production by Photocatalytic Mechanisms in Aqueous Solutions: A Mini Review. Materials 2024, 17, 5434. [Google Scholar] [CrossRef]
- Segovia, A.V.; Cardemil, J.M.; Sancy, M.; Escobar, R. Analysis of Copper Slag from a Chilean Foundry for Application as Filler Material in Thermal Energy Storage Systems. J. Energy Storage 2025, 111, 115281. [Google Scholar] [CrossRef]
- David-Hernández, M.A.; Calderon-Vásquez, I.; Battisti, F.G.; Cardemil, J.M.; Cazorla-Marín, A. Design and Assessment of a Concentrating Solar Thermal System for Industrial Process Heat with a Copper Slag Packed-Bed Thermal Energy Storage. Appl. Energy 2024, 376, 124280. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, Í.; González-Gasca, C.; Gómez-Rodríguez, C.; Verdeja, L. Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP). Metals 2021, 11, 1032. [Google Scholar] [CrossRef]
- Bellan, S.; Kodama, T.; Seok Cho, H.; Kim, J.S. Hydrogen Production by Solar Fluidized Bed Reactor Using Ceria: Euler-Lagrange Modelling of Gas-Solid Flow to Optimize the Internally Circulating Fluidized Bed. J. Therm. Sci. Technol. 2022, 17, 22–76. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Cho, H.S.; Bellan, S.; Matsubara, K.; Inoue, K. Particle Fluidized Bed Receiver/Reactor with a Beam-down Solar Concentrating Optics: Performance Test of Two-Step Water Splitting with Ceria Particles Using 30-KWth Sun-Simulator. In Proceedings of the AIP Conference Proceedings; AIP Publishing, M.N.U., Ed.; American Institute of Physics Inc.: College Park, MD, USA, 2018; Volume 2033. [Google Scholar]
- Nazer, A.P.; Fuentes, S.; Pavez, O.; Varela, O.; Lanas, O. Baldosas de Escorias de Cobre. Innovación En Producción Limpia. Iberoam. J. Proj. Manag. 2012, 3, 12. [Google Scholar]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The Potential for Copper Slag Waste as a Resource for a Circular Economy: A Review–Part I. Min. Eng. 2022, 180, 107474. [Google Scholar]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Gálvez, E.; Robles, P.; Wilson, R.; Navarra, A. Environmental, Economic and Technological Factors Affecting Chilean Copper Smelters—A Critical Review. J. Mater. Res. Technol. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Alexander, C.; Johto, H.; Lindgren, M.; Pesonen, L.; Roine, A. Comparison of Environmental Performance of Modern Copper Smelting Technologies. Clean. Environ. Syst. 2021, 3, 100052. [Google Scholar] [CrossRef]
- Nazer, A.; Payá, J.; Borrachero, M.V.; Monzó, J. Caracterización de Escorias de Cobre de Fundiciones Chilenas Del Siglo XIX. Rev. De. Metal. 2016, 52, e083. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The Potential for Copper Slag Waste as a Resource for a Circular Economy: A Review—Part II. Min. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Lyu, X.; Gao, W.; Su, H.; Li, C. Extraction and Separation of Copper and Iron from Copper Smelting Slag: A Review. J. Clean. Prod. 2022, 133095. [Google Scholar] [CrossRef]
- Zhai, Q.; Liu, R.; Wang, C.; Wen, X.; Li, X.; Sun, W. A Novel Scheme for the Utilization of Cu Slag Flotation Tailings in Preparing Internal Electrolysis Materials to Degrade Printing and Dyeing Wastewater. J. Hazard. Mater. 2022, 424, 127537. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Innovative Methodology for Comprehensive and Harmless Utilization of Waste Copper Slag via Selective Reduction-Magnetic Separation Process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Kim, B.S.; Jo, S.K.; Shin, D.; Lee, J.C.; Jeong, S.B. A Physico-Chemical Separation Process for Upgrading Iron from Waste Copper Slag. Int. J. Min. Process 2013, 124, 124–127. [Google Scholar] [CrossRef]
- Jin, Q.; Chen, L. A Review of the Influence of Copper Slag on the Properties of Cement-Based Materials. Materials 2022, 15, 8594. [Google Scholar] [CrossRef]
- Rohini, I.; Padmapriya, R. Properties of Bacterial Copper Slag Concrete. Buildings 2023, 13, 290. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, W.; Ma, G. Optimum Content of Copper Slag as a Fine Aggregate in High Strength Concrete. Mater. Des. 2010, 31, 2878–2883. [Google Scholar] [CrossRef]
- Lavanya, C.; Rao, A.S.; Kumar, N.D. Study on Coefficient of Permeability of Copper Slag When Admixed with Lime and Cement. IOSR J. Mech. Civ. Eng. 2013, 7, 19–25. [Google Scholar]
- Salleh, S.; Shaaban, M.G.; Mahmud, H.B.; Kang, J.; Looi, K.T. Production of Bricks from Shipyard Repair and Maintenance Hazardous Waste. Int. J. Environ. Sci. Dev. 2014, 5, 52. [Google Scholar] [CrossRef]
- Arivalagan, S. Experimental Study on the Flexural Behavior of Reinforced Concrete Beams as Replacement of Copper Slag as Fine Aggregate. J. Civ. Eng. Urban. 2013, 3, 176–182. [Google Scholar]
- Ambily, P.S.; Umarani, C.; Ravisankar, K.; Prem, P.R.; Bharatkumar, B.H.; Iyer, N.R. Studies on Ultra High Performance Concrete Incorporating Copper Slag as Fine Aggregate. Constr. Build. Mater. 2015, 77, 233–240. [Google Scholar] [CrossRef]
- Dzinomwa, G.; Mapani, B.; Nghipulile, T.; Maweja, K.; Kurasha, J.T.; Amwaama, M.; Chigayo, K. Mineralogical Characterization of Historic Copper Slag to Guide the Recovery of Valuable Metals: A Namibian Case Study. Materials 2023, 16, 6126. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, W.; Ma, G. Mechanical Properties of Copper Slag Reinforced Concrete under Dynamic Compression. Constr. Build. Mater. 2010, 24, 910–917. [Google Scholar] [CrossRef]
- Brindha, D.; Sureshkumar, P. Buckling Strength of RCC Columns Incorporating Copper Slag as Partial Replacement of Cement. In Proceedings of the National Conference on Emerging Trends in Civil Engineering, Tamil Nadu, India, 23 February 2010. [Google Scholar]
- Zhang, Q.; Zhang, B.; Wang, D. Environmental Benefit Assessment of Blended Cement with Modified Granulated Copper Slag. Materials 2022, 15, 5359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, Y.; Wang, D.; Qi, C.; Zeng, X. Influence of CaO on Physical and Environmental Properties of Granulated Copper Slag: Melting Behavior, Grindability and Leaching Behavior. Int. J. Env. Res. Public Health 2022, 19, 13543. [Google Scholar] [CrossRef]
- Pérez, K.; Villegas, Á.; Saldaña, M.; Jeldres, R.I.; González, J.; Toro, N. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part II. Sep. Sci. Technol. 2021, 56, 389–394. [Google Scholar] [CrossRef]
- Anudeep, R.; Ramesh, K.V.; SowjanyaVani, V. Study on Mechanical Properties of Concrete with Various Slags as Replacement to Fine Aggregate. Concr. Aggreg. Replace. 2015, 86–90. [Google Scholar]
- Shi, Y.; Wei, Y.; Zhou, S.; Li, B.; Yang, Y.; Wang, H. Effect of B2O3 Content on the Viscosity of Copper Slag. J. Alloys Compd. 2020, 822, 153478. [Google Scholar] [CrossRef]
- Lewowicki, S.; Rajczyk, J. The Usefulness of Copper Metallurgical Slag as a Micro-Aggregate Additive in Mortars and Concrete Mixtures. In Proceedings of the 13 International Conference on Solid Waste Technology and Management, Philadelphia, PA, USA, 16 November 1997; Volume 2. [Google Scholar]
- Alanoca, S. El Mercado Mundial de Fundiciones de Cobre. Situación, Cambios y Desafíos Para Chile. Available online: https://d1wqtxts1xzle7.cloudfront.net/111040568/El_mercado_mundial_de_fundiciones_de_Cobre._Situacion_cambios_y_desafios_para_Chile._SA_46_pag._09.2022_-libre.pdf (accessed on 11 January 2025).
- Chun, T.; Ning, C.; Long, H.; Li, J.; Yang, J. Mineralogical Characterization of Copper Slag from Tongling Nonferrous Metals Group China. JOM 2016, 68, 2332–2340. [Google Scholar] [CrossRef]
- Ma, Q.; Du, H.; Zhou, X.; He, K.; Lin, Z.; Yan, F.; Huang, L.; Guo, R. Performance of Copper Slag Contained Mortars after Exposure to Elevated Temperatures. Constr. Build. Mater. 2018, 172, 378–386. [Google Scholar] [CrossRef]
- Fuentes, M.; Pulido, D.; Fuentealba, E.; Soliz, A.; Toro, N.; Sagade, A.; Galleguillos Madrid, F.M. Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review. Appl. Sci. 2024, 14, 7056. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Li, X.; Yan, B.; Chen, D.; Guo, H. Hydrogen Generation Characteristics of Steel Slag-Steam High Temperature Reaction in Terms of Particle Size. Int. J. Hydrogen Energy 2020, 45, 17140–17152. [Google Scholar] [CrossRef]
- Li, P.; Guo, H.; Gao, J.; Min, J.; Yan, B.; Chen, D.; Seetharaman, S. Novel Concept of Steam Modification towards Energy and Iron Recovery from Steel Slag: Oxidation Mechanism and Process Evaluation. J. Clean. Prod. 2020, 254, 119952. [Google Scholar] [CrossRef]
- Perez, N. Electrochemistry and Corrosion Science; Perez, N., Ed.; Kluwer Academic Publishers: New York, NY, USA, 2004; ISBN 1-4020-7744-0. [Google Scholar]
- Etievant, C. Solar Energy Materials Solar High-Temperature Direct Water Splitting a Review of Experiments in France. Sol. Energy Mater. 1991, 24, 413–440. [Google Scholar] [CrossRef]
- Meredig, B.; Wolverton, C. First-Principles Thermodynamic Framework for the Evaluation of Thermochemical H2O- Or CO2- Splitting Materials. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 80, 245119. [Google Scholar] [CrossRef]
- Johansen, E.-B.; Rosenqvist, T.; Torgersen, P.T. On the Thermodynamics of Continuous Copper Smelting. JOM 1970, 22, 39–47. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive Review on Metallurgical Recycling and Cleaning of Copper Slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Saez, R.; Nocete, F.; Nieto, J.M.; Capitan, M.A.; Rovira, S. The Extractive Metallurgy of Copper from Cabezo Jure, Huelva, Spain: Chemical and Mineralogical Study of Slags Dated to the Third Millenium b.c. Can. Mineral. 2003, 41, 627–638. [Google Scholar] [CrossRef]
- Salmerón, C.; Fernando, O.; García, C.; De Lourdes, M.; Mejía, G.; Ariadna, T. Estudio Preliminar de Escoria de Cobre Como Precursor Potencial Para La Síntesis de Geopolímeros 2015. Available online: https://zaloamati.azc.uam.mx/bitstreams/6a85c67a-8689-42ea-9f2e-24cb164521fa/download (accessed on 10 January 2025).
- Pérez Segura, E.; Coronado López, H.; Robles Vega, A.; Brown Bojórquez, F. Mineralogía y Posibilidades de Recuperación de Cobre de La Escoria de La Planta Fundidora Del Complejo Minero-Metalúrgico de La Caridad, Sonora. RIIIT. Rev. Int. Investig. E Innovación Tecnológica 2016, 4, 1–20. [Google Scholar]
- Gómez-Rodríguez, C.; Antonio-Zárate, Y.; Revuelta-Acosta, J.; Verdeja, L.F.; Fernández-González, D.; López-Perales, J.F.; Rodríguez-Castellanos, E.A.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A. Research and Development of Novel Refractory of MgO Doped with ZrO2 Nanoparticles for Copper Slag Resistance. Materials 2021, 14, 2277. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M.; Viti, C. The Copper Slags of the Capattoli Valley, Campiglia Marittima, Italy. Eur. J. Mineral. 2001, 13, 949–960. [Google Scholar] [CrossRef]
- Sánchez, M.; Sudbury, M. Physicochemical Characterization of Copper Slag and Alternatives of Friendly Environmental Management. J. Min. Metall. Sect. B Metall. 2013, 49, 161–168. [Google Scholar] [CrossRef]
- Mateus, A.; Pinto, A.; Alves, L.C.; Matos, J.X.; Figueiras, J.; Neng, N.R. Roman and Modern Slag at S. Domingos Mine (IPB, Portugal): Compositional Features and Implications for Their Long-Term Stability and Potential Reuse. Int. J. Env. Waste Manag. 2011, 8, 133. [Google Scholar] [CrossRef]
- Gyurov, S.; Rabadjieva, D.; Kovacheva, D.; Kostova, Y. Kinetics of Copper Slag Oxidation under Nonisothermal Conditions. J. Therm. Anal. Calorim. 2014, 116, 945–953. [Google Scholar] [CrossRef]
- Lohmeier, S.; Lottermoser, B.G.; Schirmer, T.; Gallhofer, D. Copper Slag as a Potential Source of Critical Elements—A Case Study from Tsumeb, Namibia. J. S. Afr. Inst. Min. Met. 2021, 121, 129–142. [Google Scholar] [CrossRef]
- Gbor, P.K.; Mokri, V.; Jia, C.Q. Characterization of Smelter Slags. J. Environ. Sci. Health Part. A 2000, 35, 147–167. [Google Scholar] [CrossRef]
- Barin, I.; Platzhi, G. Thermochemical Data of Pure Substances; Wiley: Wienheim, Germany, 1989; Volume 304, ISBN 3527287450. [Google Scholar]
- Opila, E.J.; Jacobson, N.S.; Myers, D.L.; Copland, E.H. Predicting Oxide Stability in High-Temperature Water Vapor. JOM 2006, 58, 22–28. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Xing, X. Recovery of Iron from Nickel Slag in Water Vapor at High Temperature. JOM 2023, 75, 331–339. [Google Scholar] [CrossRef]
- Chen, J.H.; Mi, W.J.; Chen, H.Y.; Li, B.; Chou, K.C.; Hou, X.M. Iron Oxide Recovery from Fayalite in Water Vapor at High Temperature. J. Min. Metall. Sect. B Metall. 2018, 54, 1–8. [Google Scholar] [CrossRef]
- Nakamura, T. Hydrogen Production from Water Utilizing Solar Heat at High Temperatures. Sol. Energy 1977, 19, 467–475. [Google Scholar] [CrossRef]
- Steinfeld, A.; Sanders, S.; Palumbo, R. Design Aspects of Solar Thermochemical Engineering-a Case Study: Two-Step Water-Splitting Cycle Using the Fe3O4/FeO Redox System. Solar Energy 1999, 65, 43–53. [Google Scholar] [CrossRef]
- Kodama, T. High-Temperature Solar Chemistry for Converting Solar Heat to Chemical Fuels. Prog. Energy Combust. Sci. 2003, 29, 567–597. [Google Scholar]
- Kodama, T.; Gokon, N. Thermochemical Cycles for High-Temperature Solar Hydrogen Production. Chem. Rev. 2007, 107, 4048–4077. [Google Scholar] [PubMed]
- Palumbo, R.; Diver, R.B.; Larson, C.; Coker, E.N.; Miller, J.E.; Guertin, J.; Schoer, J.; Meyer, M.; Siegel, N.P. Solar Thermal Decoupled Water Electrolysis Process I: Proof of Concept. Chem. Eng. Sci. 2012, 84, 372–380. [Google Scholar] [CrossRef]
- Young, D.J.; Watson, S. High-Temperature Corrosion in Mixed Gas Environments. Oxid. Met. 1995, 44, 239–264. [Google Scholar] [CrossRef]
- Svoboda, K.; Slowinski, G.; Rogut, J.; Baxter, D. Thermodynamic Possibilities and Constraints for Pure Hydrogen Production by Iron Based Chemical Looping Process at Lower Temperatures. Energy Convers. Manag. 2007, 48, 3063–3073. [Google Scholar] [CrossRef]
- Michel, R.; Ammar, M.R.; Véron, E.; Simon, P.; Poirier, J. Investigating the Mechanism of Phase Transformations and Migration in Olivine at High Temperature. RSC Adv. 2014, 4, 26645–26652. [Google Scholar] [CrossRef]
- van Genuchten, C.M.; Hamaekers, H.; Fraiquin, D.; Hollanders, S.; Ahmad, A. Heavy Metal Removal Potential of Olivine. Water Res. 2023, 245, 120583. [Google Scholar] [CrossRef] [PubMed]
- Freund, F.; Oberheuser, G. Water Dissolved in Olivine: A Single-crystal Infrared Study. J. Geophys. Res. Solid Earth 1986, 91, 745–761. [Google Scholar]
- McCollom, T.M.; Bach, W. Thermodynamic Constraints on Hydrogen Generation during Serpentinization of Ultramafic Rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar] [CrossRef]
- Olsson, J.; Bovet, N.; Makovicky, E.; Bechgaard, K.; Balogh, Z.; Stipp, S.L.S. Olivine Reactivity with CO2 and H2O on a Microscale: Implications for Carbon Sequestration. Geochim. Cosmochim. Acta 2012, 77, 86–97. [Google Scholar] [CrossRef]
- Wang, J.; Watanabe, N.; Okamoto, A.; Nakamura, K.; Komai, T. Acceleration of Hydrogen Production during Water-Olivine-CO2 Reactions via High-Temperature-Facilitated Fe(II) Release. Int. J. Hydrogen Energy 2019, 44, 11514–11524. [Google Scholar] [CrossRef]
- Shestopalov, V.M.; Koliabina, I.L.; Ponomarenko, O.M.; Lukin, A.Y.; Rud, A.D. Thermodynamic Assessment of the Possibility of Olivine Interaction with Deep-Seated Hydrogen. Int. J. Hydrogen Energy 2022, 47, 7062–7071. [Google Scholar] [CrossRef]
- Opila, E.J.; Hann Jr, R.E. Paralinear Oxidation of CVD SiC in Water Vapor. J. Am. Ceram. Soc. 1997, 80, 197–205. [Google Scholar]
- Opila, E.J. Variation of the Oxidation Rate of Silicon Carbide with Water-vapor Pressure. J. Am. Ceram. Soc. 1999, 82, 625–636. [Google Scholar]
- Kendelewicz, T.; Doyle, C.S.; Carrier, X.; Brown Jr, G.E. Reaction of Water with Clean Surfaces of MnO (100). Surf. Rev. Lett. 1999, 6, 1255–1263. [Google Scholar]
- Charvin, P.; Abanades, S.; Lemort, F.; Flamant, G. Hydrogen Production by Three-Step Solar Thermochemical Cycles Using Hydroxides and Metal Oxide Systems. Energy Fuels 2007, 21, 2919–2928. [Google Scholar]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Marugán, J.; Sanz, R. Thermochemical Hydrogen Production Using Manganese Cobalt Spinels as Redox Materials. Int. J. Hydrogen Energy 2017, 42, 13532–13543. [Google Scholar] [CrossRef]
- Bilgen, E.; Ducarroir, M.; Foex, M.; Sibieude, F.; Trombe, F. Use of Solar Energy for Direct and Two-Step Water Decomposition Cycles. Int. J. Hydrogen Energy 1977, 2, 251–257. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar Hydrogen Production via a Two-Step Water-Splitting Thermochemical Cycle Based on Zn/ZnO Redox Reactions. Int. J. Hydrogen Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Steinfeld, A. Solar Syngas Production from CO2 and H2O in a Two-Step Thermochemical Cycle via Zn/ZnO Redox Reactions: Thermodynamic Cycle Analysis. Int. J. Hydrogen Energy 2011, 36, 12141–12147. [Google Scholar] [CrossRef]
- Bhosale, R.R. H2 Producing Hybrid Solar Thermochemical ZnSO4/ZnO Water Splitting Cycle: Thermodynamic Efficiency Analysis. Int. J. Hydrogen Energy 2024, 49, 1584–1592. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Chen, Z.; Peng, C.; Zhu, X.; Zhu, M. Hydrogen Production via Hydrolysis of Mg-Oxide Composites. Int. J. Hydrogen Energy 2017, 42, 22305–22311. [Google Scholar] [CrossRef]
- Nissen, D.A. The Low-Temperature Oxidation of Calcium by Water Vapor. Oxid. Met. 1977, 11, 241–261. [Google Scholar] [CrossRef]
- Bhosale, R.R. H2 Generation via Solar Assisted CaO/Ca Thermochemical H2O Splitting Cycle. Int. J. Hydrogen Energy 2021, 46, 12095–12104. [Google Scholar] [CrossRef]
- Whitney, W.R. The Corrosion of Iron. J. Am. Chem. Soc. 1903, 25, 394–406. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K. and 1 Bar. (105 Pascals) Pressure and at Higher Temperatures (No. 2131); U.S. G.P.O.: Washington, DC, USA, 1995.
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite Oxidation: A Review. Earth Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Conner, K.; Anderson, C. Enargite Treatments and Pressure Oxidation of Concentrates. J. Metall. Eng. 2013, 2, 115–123. [Google Scholar]
- Zhou, S.; Li, T.; Wang, C.; Tan, Z.; Guan, X.; Li, H.; Zhang, W. Tennantite and Enargite Rejection in the Copper Flotation—A Mini-Review. J. Miner. Mater. Charact. Eng. 2023, 11, 63–68. [Google Scholar] [CrossRef]
- Véliz, M.; Aracena, A.; Jerez, O.; Pérez-Tello, M.; Balladares, E. Copper Sulfation from Enargite Roasting Using Coal and Fayalite Slag Mixture. Minerals 2023, 13, 1489. [Google Scholar] [CrossRef]
- Baken, S.; Delbeke, K.; Rodriguez, P.H. Copper Concentrates: Environmental and Human. Health Hazard. Classification. 2023. Available online: https://internationalcopper.org/wp-content/uploads/2024/02/ICA-Copper-con-classification-guidance-1.pdf (accessed on 13 January 2025).
- Charitos, A.; Wrobel, M.; Runkel, M.; Hammerschmidt, J.; Demopoulos, G.P. Partial Roasting Combined with Scorodite Technology for Efficient Arsenic Removal and Stabilization from Cu-Concentrates. In Proceedings of the Conference of Metallurgists, Halifax, NS, Canada, 19 August 2024; Springer: Berlin, Germany, 2024; pp. 327–335. [Google Scholar]
- Manca, P.P.; Massacci, G.; Pintus, D.; Sogos, G. The Flotation of Sphalerite Mine Tailings as a Remediation Method. Min. Eng. 2021, 165, 106862. [Google Scholar] [CrossRef]
- Liang, Q.; Jiang, L.; Zheng, J.; Duan, N. Mechanism Study of the Effect of Copper Ions on the Stability of As(III) Sulfuration Precipitation in Acidic Copper Smelting Wastewater. Env. Monit. Assess. 2024, 197, 36. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]

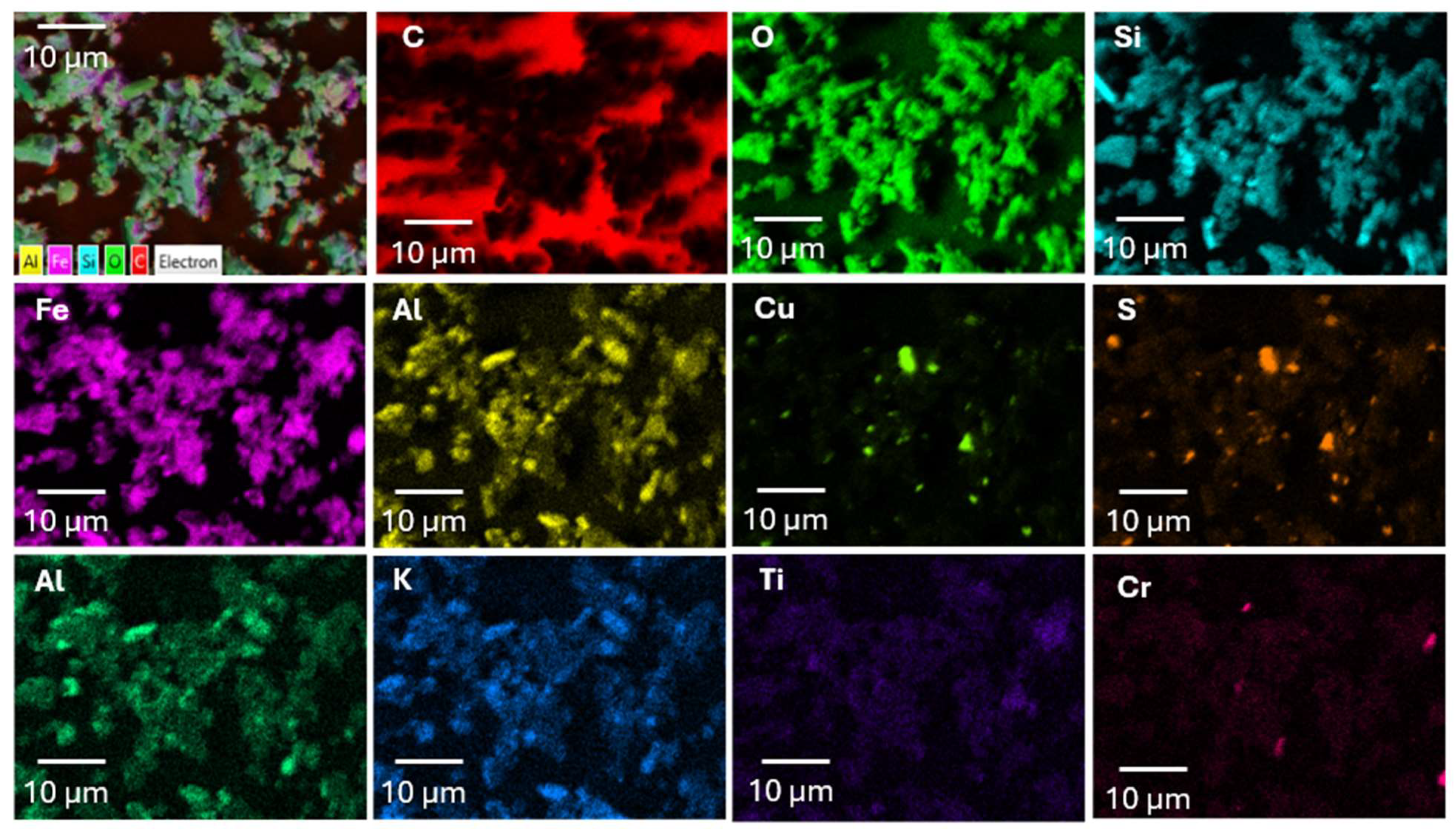
| Property | Value |
|---|---|
| Unit weight (T/m3) | 2.8–3.8 |
| Absorption | 0.13 |
| Bulk density (T/m3) | 2.3–2.6 |
| Conductivity (µs/cm) | 500 |
| Specific gravity | 2.8–3.8 |
| Hardness (Moh) | 6–7 |
| Moisture (ppm) | <5 |
| Abrasion loss (%) | 2.4–10 |
| Internal friction angle | 40–53 |
| Property | Description | Ref. |
|---|---|---|
| Particle shape | Angular | [32,33,34] |
| Irregular | [31,35,36] | |
| Multifaceted | [37] | |
| Surface texture | Glassy | [24,32,34,36] |
| Smooth | [6,9,31,33,38,39] | |
| Granular rough | [34,40,41] | |
| Color | Black | [32,42] |
| Blackish gray | [43,44] | |
| Brown with green, red, or black tint | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, M.; Leiva-Guajardo, S.; Sagade, A.; Sepúlveda, F.; Soliz, A.; Toro, N.; Cobos Murcia, J.Á.; Reyes Cruz, V.E.; Toledo, M.; Fuentealba, E.; et al. The Use of Copper Slag in the Thermolysis Process for Solar Hydrogen Production—A Novel Alternative for the Circular Economy. Appl. Sci. 2025, 15, 4988. https://doi.org/10.3390/app15094988
Fuentes M, Leiva-Guajardo S, Sagade A, Sepúlveda F, Soliz A, Toro N, Cobos Murcia JÁ, Reyes Cruz VE, Toledo M, Fuentealba E, et al. The Use of Copper Slag in the Thermolysis Process for Solar Hydrogen Production—A Novel Alternative for the Circular Economy. Applied Sciences. 2025; 15(9):4988. https://doi.org/10.3390/app15094988
Chicago/Turabian StyleFuentes, Manuel, Susana Leiva-Guajardo, Atul Sagade, Felipe Sepúlveda, Alvaro Soliz, Norman Toro, José Ángel Cobos Murcia, V. E. Reyes Cruz, Mario Toledo, Edward Fuentealba, and et al. 2025. "The Use of Copper Slag in the Thermolysis Process for Solar Hydrogen Production—A Novel Alternative for the Circular Economy" Applied Sciences 15, no. 9: 4988. https://doi.org/10.3390/app15094988
APA StyleFuentes, M., Leiva-Guajardo, S., Sagade, A., Sepúlveda, F., Soliz, A., Toro, N., Cobos Murcia, J. Á., Reyes Cruz, V. E., Toledo, M., Fuentealba, E., & Galleguillos Madrid, F. M. (2025). The Use of Copper Slag in the Thermolysis Process for Solar Hydrogen Production—A Novel Alternative for the Circular Economy. Applied Sciences, 15(9), 4988. https://doi.org/10.3390/app15094988










