Spelt Grass Juice: Phytochemicals and Antiproliferative Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Production Process of Young Spelt Grass Juice
2.2. Sample Preparation
2.3. Chemicals
2.4. Determination of Photosynthetic Pigments in Extracts
2.5. Determination of Individual Phenolic Compounds in Extracts Using HPLC-DAD-MSn Analysis
2.6. Determination of the Antiproliferative Activity of Spelt Grass Juice Extracts
2.6.1. Growth and Culture of the Cell Lines
2.6.2. MTT Assay with Extracts of Spelt Grass Juice
2.7. Statistical Analysis
3. Results and Discussion
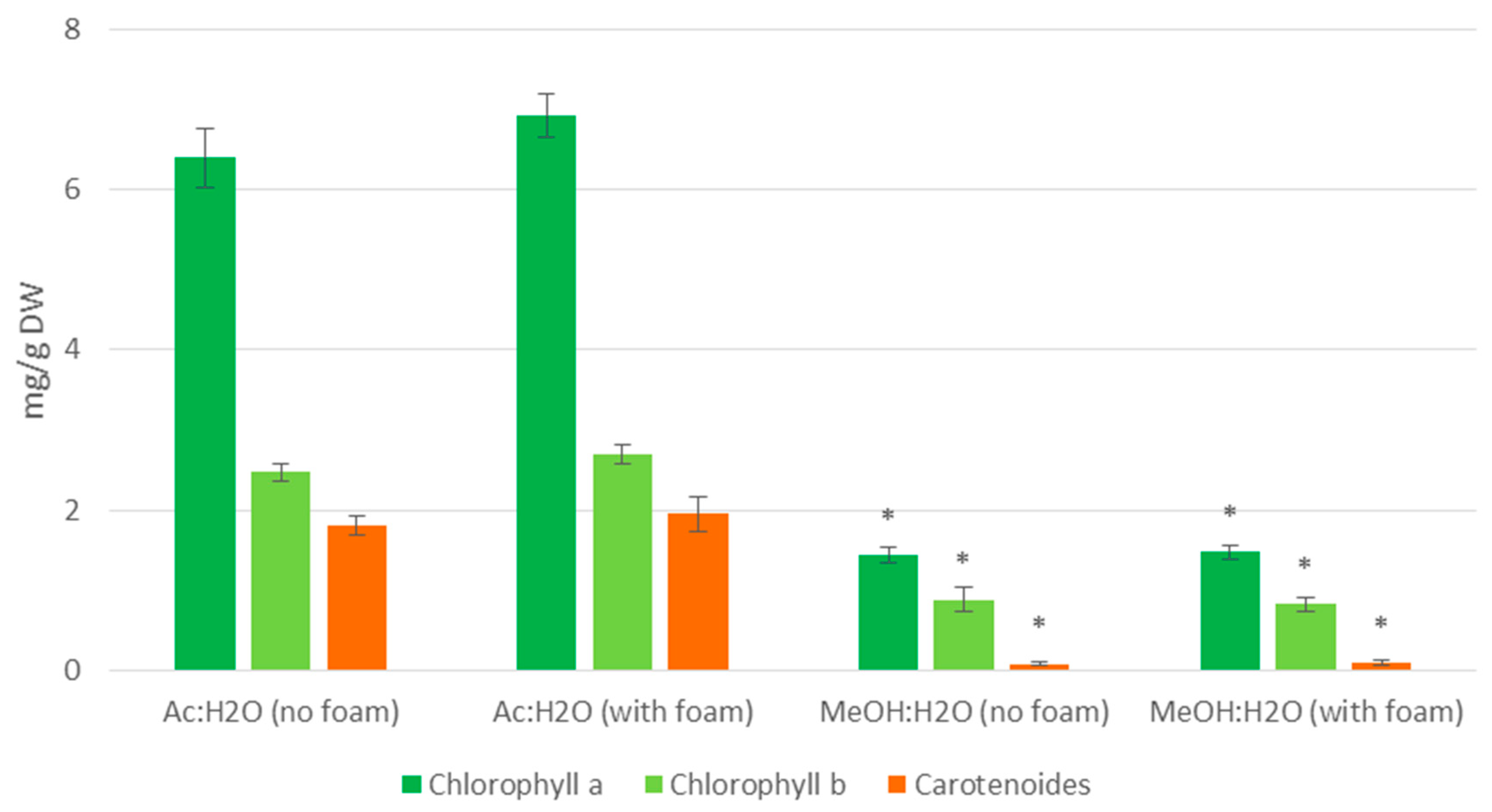
3.1. Photosynthetic Pigments in Extracts
3.2. Phenolic Composition of Extracts
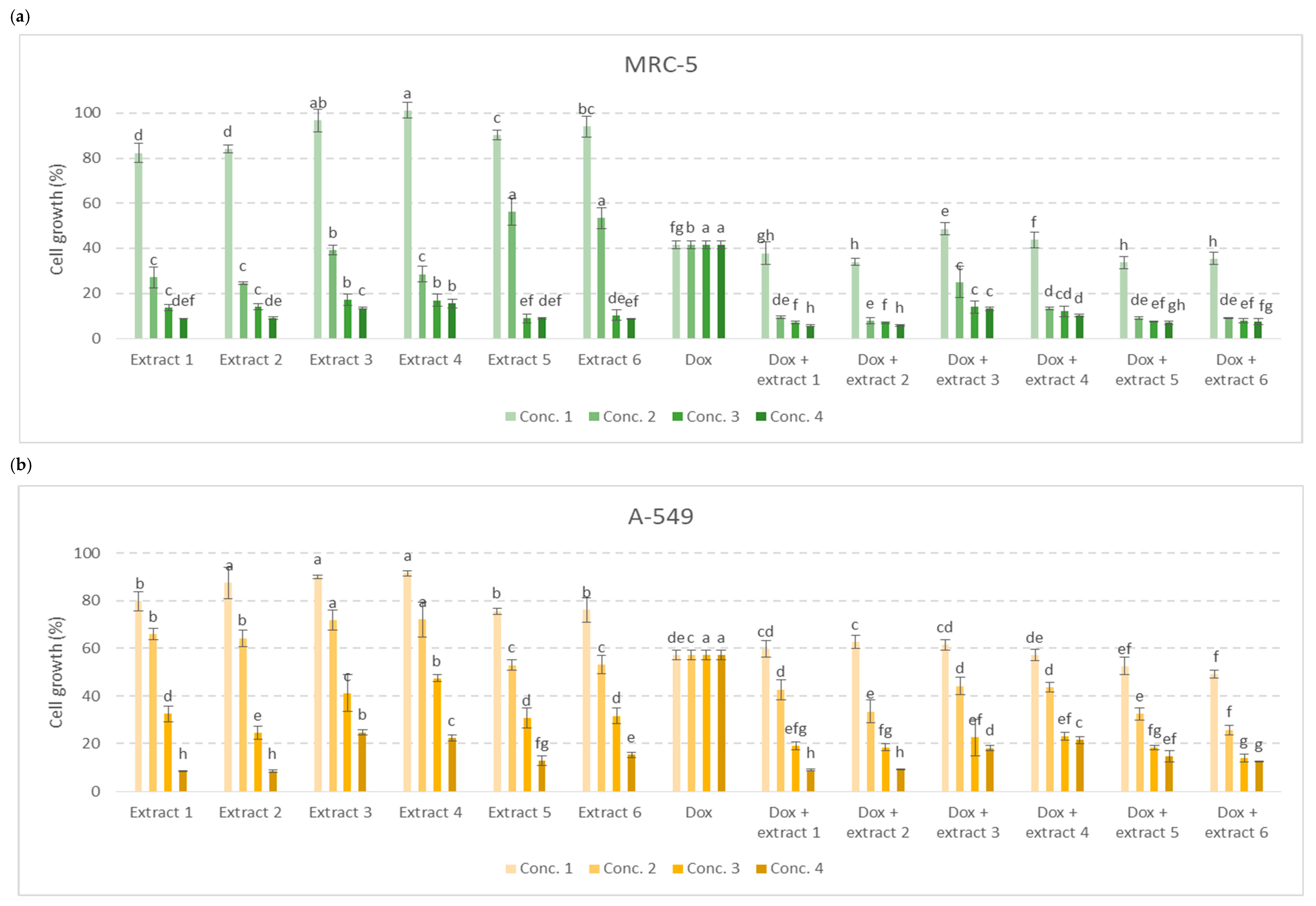
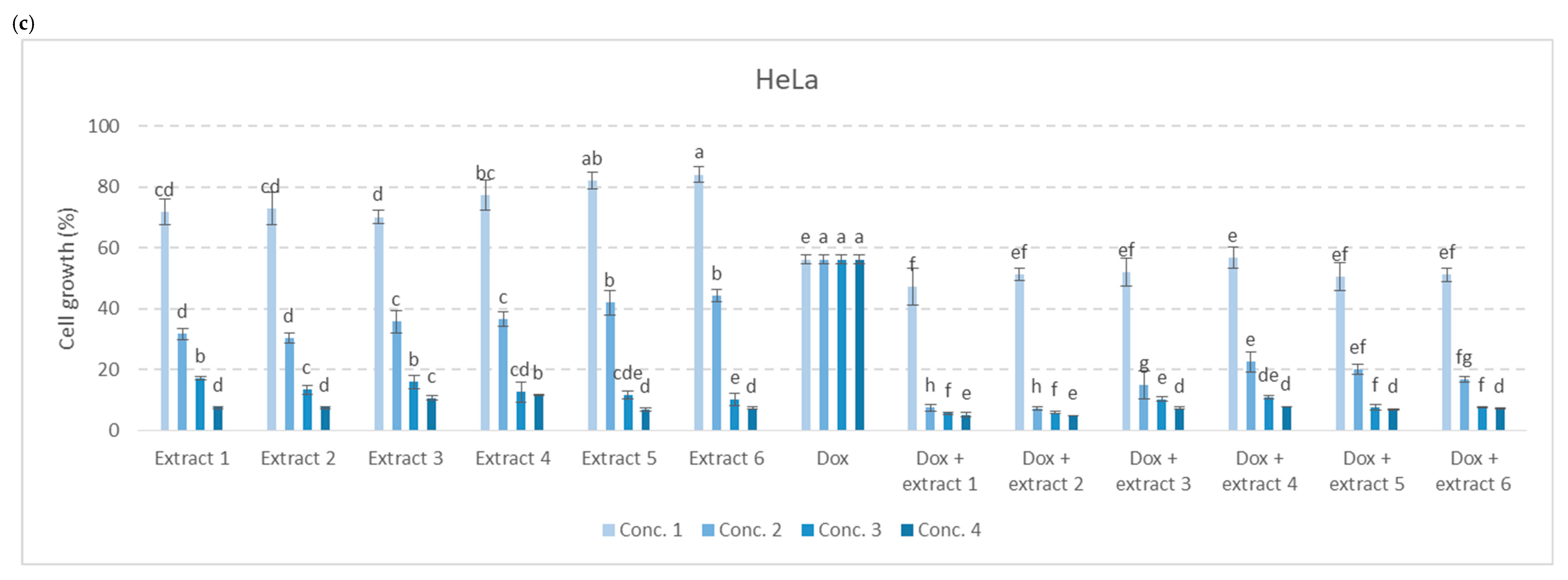
3.3. Antiproliferative Activity of Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golijan, J.; Živanović, L.; Kostić, A.Ž. Nutritivni značaj krupnika (Triticum aestivum ssp. Spelta) u ljudskoj ishrani. Hrana Ishrana 2017, 58, 39–44. [Google Scholar] [CrossRef]
- Jančić, D.; Šuković, D.; Rešetar, J.; Delić, L.; Nikolić, M. Nutritional composition, biologically active substances and antioxidant activity of young spelt grass extract. JSFA Rep. 2022, 2, 385–397. [Google Scholar] [CrossRef]
- Ahmad, G.M.; Abu Serie, M.M.; Abdel-Latif, M.S.; Ghoneem, T.; Ghareeb, D.A.; Yacout, G.A. Potential anti-proliferative activity of Salix mucronata and Triticum spelta plant extracts on liver and colorectal cancer cell lines. Sci. Rep. 2023, 13, 3815. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.Y.; Gaikwad, S.; Srivastava, S.; Srivastava, S.K. Research trend and detailed insights into the molecular mechanisms of food bioactive compounds against cancer: A comprehensive review with special emphasis on probiotics. Cancers 2022, 14, 5482. [Google Scholar] [CrossRef]
- Patel, J.B. Anticancer & cytotoxic potential of aqueous extract of Triticum aestivum on HeLa cell line. J. Drug Deliv. Ther. 2016, 6, 84–89. [Google Scholar]
- Sim, J.H.; Choi, M.-H.; Shin, H.-J.; Lee, J.-E. Wheatgrass extract ameliorates hypoxia-induced mucin gene expression in A549 cells. Pharmacogn. Mag. 2017, 13, 7–12. [Google Scholar]
- Gore, R.D.; Palaskar, S.J.; Bartake, A.R. Wheatgrass: Green blood can help to fight cancer. J. Clin. Diagn. Res. 2017, 11, ZC40–ZC42. [Google Scholar] [CrossRef]
- Skoczylas, Ł.; Korus, A.; Tabaszewska, M.; Gędoś, K.; Szczepańska, E. Evaluation of the quality of fresh and frozen wheatgrass juices depending on the time of grass harvest. J. Food Process. Preserv. 2018, 42, e13401. [Google Scholar] [CrossRef]
- Hebbani, A.V.; Bulle, S.; Kanu, V.R.; Balachandrababu Malini, A.; Reddy, V.D.; Chakravarthula, V.N. Nephro-protective activity of wheatgrass juice against alcohol-induced oxidative damage in rats. Toxicol. Mech. Methods. 2020, 30, 679–686. [Google Scholar] [CrossRef]
- Eissa, H.A.; Mohamed, S.S.; Hussein, A.M.S. Nutritional value and impact of wheatgrass juice (Green Blood Therapy) on increasing fertility in male albino rats. Bull. Natl. Res. Cent. 2020, 44, 30. [Google Scholar] [CrossRef]
- Rodriguez, F.C.; Gallagher, E.; Rai, D.K.; Burgess, C.M. Nutritional and physiochemical properties of wheatgrass juice and preservation strategies. Food Chem. Adv. 2022, 1, 100136. [Google Scholar] [CrossRef]
- Minocha, N.; Saini, S.; Pandey, P. Nutritional prospects of wheatgrass (Triticum aestivum) and its effects in treatment and chemoprevention. Explor. Med. 2022, 3, 432–442. [Google Scholar] [CrossRef]
- Tamraz, M.; Al Ghossaini, N.; Temraz, S. The role of wheatgrass in colorectal cancer: A review of the current evidence. Int. J. Mol. Sci. 2024, 25, 5166. [Google Scholar] [CrossRef] [PubMed]
- Antonic, T.; Stefanovic, A.; Gojkovic, T.; Vladimirov, S.; Spasojevic-Kalimanovska, V.; Kotur-Stevuljevic, J.; Bogavac-Stanojevic, N.; Bajic, B. Antioxidant, anti-inflammatory, and anti-hyperlipidemic properties of the spelt grass juice. Hrana Ishrana 2021, 62, 28–36. [Google Scholar] [CrossRef]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2017, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.R.; Hapidin, H. Synergistic interaction between combination of existing therapy with polyphenols in several human diseases: A review. J. Pharm. 2023, 3, 86–102. [Google Scholar] [CrossRef]
- Hussain, A.; Gheewala, T.M.; Vas, A.J.; Shah, K.; Goala, P.; Khan, S.; Hinduja, S.; Sharma, C. Growth inhibitory and adjuvant therapeutic potential of aqueous extract of Triticum aestivum on MCF-7 and HeLa cells. Exp. Onc. 2014, 36, 9–16. [Google Scholar]
- Marković, S. Biochemical Characterization of Selected Plant Species from the Genera Triticum, Avena and Triticosecale Under Conditions of Heat Stress. Doctoral Dissertation, University of Kragujevac, Kragujevac, Serbia, 2021. [Google Scholar]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 3, pp. 9–12. [Google Scholar]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The impact of food processing on the phenolic content in products made from juneberry Amelanchier lamarckii fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Adnane, F.; Soliman, S.M.A.; ElZayat, E.; Abdelsalam, E.M.; Fahmy, H.M. Evaluation of chlorophyll-loaded mesoporous silica nanoparticles for photodynamic therapy on cancer cell lines. Lasers Med. Sci. 2024, 39, 45. [Google Scholar] [CrossRef] [PubMed]
- Alexeree, S.; ElZorkany, H.E.; Abdel-Salam, Z.; Harith, M.A. A novel synthesis of a chlorophyll b-gold nanoconjugate used for enhancing photodynamic therapy: In vitro study. Photodiagn. Photodyn. Ther. 2021, 35, 102444. [Google Scholar] [CrossRef] [PubMed]
- Siebenhandl, S.; Grausgruber, H.; Pellegrini, N.; Del Rio, D.; Fogliano, V.; Pernice, R.; Berghofer, E. Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J. Agric. Food Chem. 2007, 55, 8541–8547. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D. Comparison of phenolic acids profile and antioxidant potential of six varieties of spelt (Triticum spelta L.). J. Agric. Food Chem. 2012, 60, 4603–4612. [Google Scholar] [CrossRef]
- Barański, M.; Lacko-Bartošová, M.; Rembiałkowska, E.; Lacko-Bartošová, L. The Effect of Species and Cultivation Year on Phenolic Acids Content in Ancient Wheat. Agronomy 2020, 10, 673. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Ouchemoukh, S.; Benchibane, T.; Hernanz, D.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Madani, K.; Luis, J. Valorization of the whole grains of Triticum aestivum L. and Triticum vulgare L. through the investigation of their biochemical composition and in vitro antioxidant, anti-inflammatory, anticancer and anticalpain activities. J. Cereal Sci. 2017, 75, 278–285. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic acid: A natural phenol that inhibits neoplastic events through modulation of oncogenic signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Krzywik, J.; Mozga, W.; Aminpour, M.; Janczak, J.; Maj, E.; Wietrzyk, J.; Tuszyński, J.A.; Huczyński, A. Synthesis, antiproliferative activity and molecular docking studies of novel doubly modified colchicine amides and sulfonamides as anticancer agents. Molecules 2020, 25, 1789. [Google Scholar] [CrossRef]
- Rowles, J.L., 3rd; Erdman, J.W., Jr. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Ye, G.; Wang, L.; Yang, K.; Wang, C. Fucoxanthin may inhibit cervical cancer cell proliferation via downregulation of HIST1H3D. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.K.; Haque, S.; Somvanshi, P.; Bhardwaj, T.; Hussain, A. Epigallocatechin gallate inhibits HeLa cells by modulation of epigenetics and signaling pathways. 3 Biotech 2020, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
| Phenolic Compound | Standard Compound | Phenolic Group | Spelt Grass Juice Extract | |||||
|---|---|---|---|---|---|---|---|---|
| AQUEOUS (No Foam) | AQUEOUS (with Foam) | Ac:H2O (No Foam) | Ac:H2O (with Foam) | MeOH:H2O (No Foam) | MeOH:H2O (with Foam) | |||
| Protocatechuic acid | Gallic acid | HBA | 151.93 ± 8.16 a | 150.16 ± 9.80 a | 12.50 ± 7.14 d | 16.87 ± 6.88 d | 57.07 ± 1.55 b | 40.51 ± 2.36 c |
| Syringic acid | Gallic acid | HBA | 92.23 ± 4.95 a | 91.15 ± 5.95 a | 7.59 ± 4.33 d | 10.24 ± 4.18 d | 34.64 ± 0.94 b | 24.59 ± 1.43 c |
| Salicylic acid | Gallic acid | HBA | 22.04 ± 1.18 a | 21.78 ± 1.42 a | 1.81 ± 1.04 d | 2.45 ± 1.00 d | 8.28 ± 0.22 b | 5.88 ± 0.34 c |
| Ferulic acid derivative 1 | Ferulic acid | HCA | 204.18 ± 14.74 a | 201.02 ± 4.45 a | 98.14 ± 8.83 c | 89.52 ± 14.24 c | 128.85 ± 3.40 b | 121.29 ± 1.95 b |
| Ferulic acid derivative 2 | Ferulic acid | HCA | 52.96 ± 3.72 c | 48.92 ± 5.42 c | 140.28 ± 7.64 a | 137.88 ± 20.26 a | 116.18 ± 2.79 b | 110.01 ± 3.76 b |
| p-coumaric acid | p-coumaric acid | HCA | 1.43 ± 0.11 a | 1.44 ± 0.03 a | 0.64 ± 0.03 b | 0.68 ± 0.14 b | 0.73 ± 0.01 b | 0.73 ± 0.01 b |
| Vanillic acid | Gallic acid | HBA | 0.66 ± 0.05 a | 0.67 ± 0.02 a | 0.30 ± 0.02 b | 0.31 ± 0.06 b | 0.34 ± 0.00 b | 0.34 ± 0.01 b |
| Ferulic acid derivative 3 | Ferulic acid | HCA | 200.41 ± 15.57 a | 202.24 ± 4.77 a | 89.69 ± 4.62 b | 95.03 ± 19.56 b | 102.54 ± 1.47 b | 102.27 ± 1.68 b |
| o-coumaric acid | p-coumaric acid | HCA | 9.35 ± 2.11 c | 7.62 ± 1.66 c | 618.36 ± 20.19 a | 595.05 ± 31.20 a | 297.69 ± 5.17 b | 277.02 ± 9.86 b |
| p-hydroxybenzoic acid | Gallic acid | HBA | 71.78 ± 2.69 a | 68.47 ± 4.07 a | 40.78 ± 5.26 d | 40.85 ± 4.32 d | 61.39 ± 1.36 b | 56.77 ± 0.66 c |
| Ferulic acid | Ferulic acid | HCA | 1177.37 ± 44.12 a | 1122.94 ± 66.78 a | 668.80 ± 86.22 d | 669.93 ± 70.86 d | 1006.91 ± 22.38 b | 931.04 ± 10.81 c |
| 3-feruloylquinic acid | Ferulic acid | HCA | 87.42 ± 6.20 a | 100.78 ± 7.09 a | 65.34 ± 7.06 b | 66.61 ± 15.93 b | 97.06 ± 12.06 a | 92.82 ± 3.29 a |
| Unknown 385 | Ferulic acid | HCA | 195.24 ± 11.32 | 181.07 ± 6.58 | 200.27 ± 40.41 | 182.49 ± 50.41 | 204.08 ± 8.46 | 190.72 ± 12.21 |
| Caffeic acid | Ferulic acid | HCA | 59.95 ± 7.99 c | 51.31 ± 2.14 cd | 92.55 ± 6.83 b | 114.99 ± 16.61 a | 48.79 ± 1.38 cd | 42.86 ± 5.71 d |
| Unknown 579 | Ferulic acid | HCA | 7.86 ± 1.70 c | 8.05 ± 2.30 c | 37.80 ± 1.95 ab | 27.08 ± 16.80 b | 50.44 ± 3.74 a | 47.07 ± 5.76 a |
| Unknown 529 | Ferulic acid | HCA | 165.95 ± 8.93 a | 152.74 ± 10.95 a | 75.34 ± 3.06 b | 64.73 ± 12.75 b | 166.74 ± 11.54 a | 161.41 ± 5.23 a |
| Unknown 563 | Ferulic acid | HCA | 741.56 ± 38.55 b | 783.23 ± 29.76 b | 357.67 ± 12.69 c | 396.78 ± 7.59 c | 865.11 ± 22.77 a | 902.55 ± 19.12 a |
| Unknown 371 | Ferulic acid | HCA | 134.93 ± 7.01 b | 142.52 ± 5.42 b | 65.08 ± 2.31 c | 72.20 ± 1.38 c | 157.41 ± 4.14 a | 164.23 ± 3.48 a |
| 4-feruloylquinic acid | Ferulic acid | HCA | 3.70 ± 0.19 b | 3.90 ± 0.15 b | 1.78 ± 0.06 c | 1.98 ± 0.04 c | 4.31 ± 0.11 a | 4.50 ± 0.10 a |
| Unknown 368 | Ferulic acid | HCA | 18.55 ± 1.72 c | 20.13 ± 3.41 c | 25.95 ± 0.55 b | 29.34 ± 1.31 b | 56.51 ± 1.80 a | 59.95 ± 3.62 a |
| Unknown 469 | Ferulic acid | HB | 76.63 ± 2.41 b | 80.43 ± 1.58 a | 36.90 ± 2.94 e | 34.32 ± 0.75 e | 64.36 ± 1.53 c | 60.16 ± 0.65 d |
| Sinapic acid | Caffeic acid | HB | 23.29 ± 3.44 c | 25.05 ± 3.54 c | 47.00 ± 3.60 b | 50.46 ± 3.33 b | 71.20 ± 4.19 a | 67.49 ± 18.45 a |
| Sum of total analyzed phenolics | 3499 ± 177 a | 3466 ± 166 a | 2685 ± 182 b | 2700 ± 263 b | 3601 ± 83 a | 3464 ± 80 a | ||
| Spelt Grass Juice Extract | IC50 | NT/T | ||||
|---|---|---|---|---|---|---|
| MRC-5 | A-549 | HeLa | A-549 | HeLa | ||
| Extract 1 | Aqueous (no foam) | 1319.30 | 1887.50 | 1162.10 | 0.70 | 1.14 |
| Extract 2 | Aqueous (with foam) | 1405.90 | 2060.12 | 1184.97 | 0.68 | 1.19 |
| Extract 3 | Ac:H2O (no foam) | 331.07 | 530.68 | 182.05 | 0.62 | 1.82 |
| Extract 4 | Ac:H2O (with foam) | 416.84 | 529.20 | 203.07 | 0.79 | 2.05 |
| Extract 5 | MeOH:H2O (no foam) | 271.96 | 453.22 | 221.82 | 0.60 | 1.23 |
| Extract 6 | MeOH:H2O (with foam) | 293.13 | 411.13 | 228.89 | 0.71 | 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topalović, A.; Knežević, M.; Mikulic-Petkovsek, M.; Mrđanović, J. Spelt Grass Juice: Phytochemicals and Antiproliferative Activity. Appl. Sci. 2025, 15, 4917. https://doi.org/10.3390/app15094917
Topalović A, Knežević M, Mikulic-Petkovsek M, Mrđanović J. Spelt Grass Juice: Phytochemicals and Antiproliferative Activity. Applied Sciences. 2025; 15(9):4917. https://doi.org/10.3390/app15094917
Chicago/Turabian StyleTopalović, Ana, Mirko Knežević, Maja Mikulic-Petkovsek, and Jasminka Mrđanović. 2025. "Spelt Grass Juice: Phytochemicals and Antiproliferative Activity" Applied Sciences 15, no. 9: 4917. https://doi.org/10.3390/app15094917
APA StyleTopalović, A., Knežević, M., Mikulic-Petkovsek, M., & Mrđanović, J. (2025). Spelt Grass Juice: Phytochemicals and Antiproliferative Activity. Applied Sciences, 15(9), 4917. https://doi.org/10.3390/app15094917








