Wrinkle Improvement in HanDam (Twist) on Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice
Abstract
1. Introduction
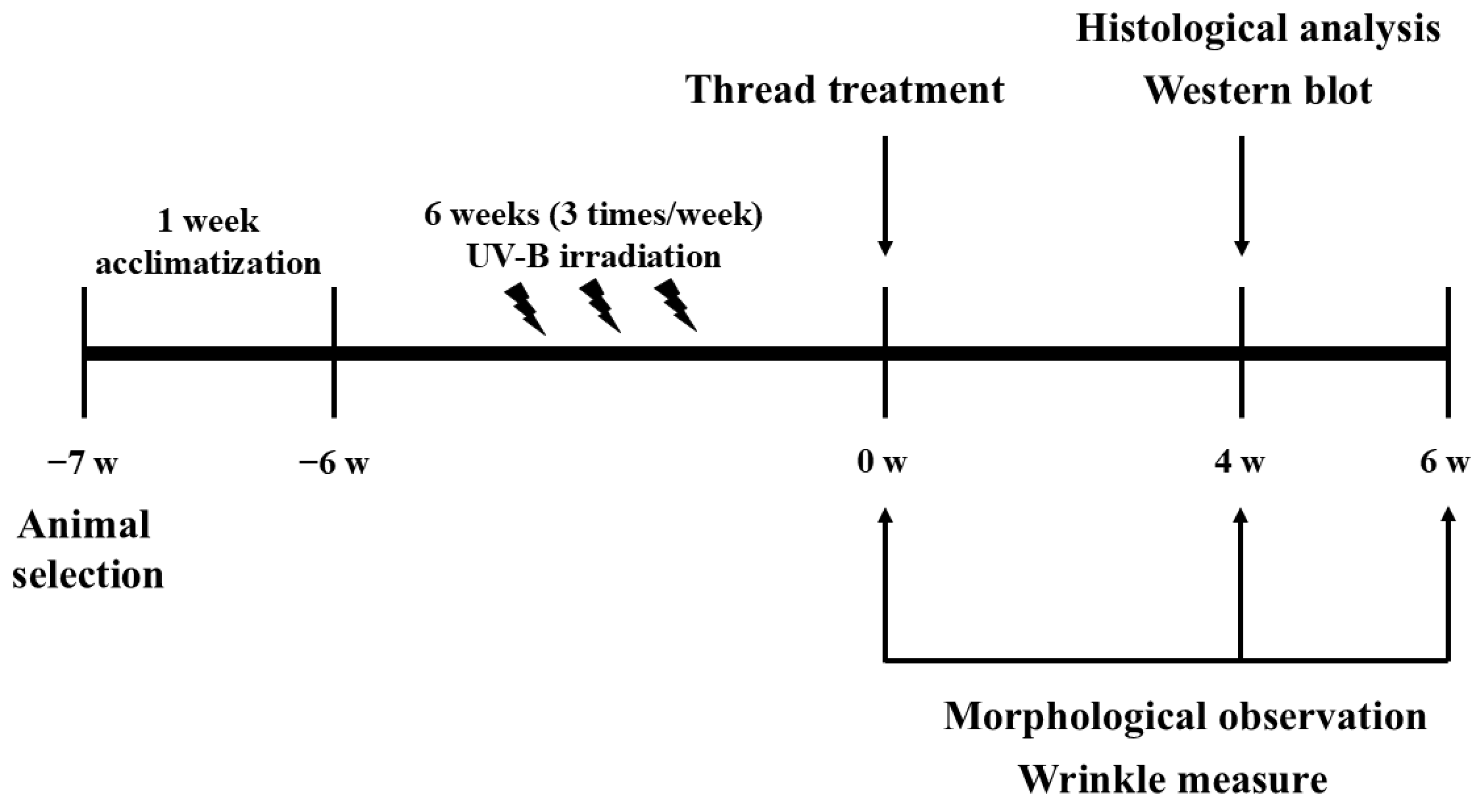
2. Materials and Methods
2.1. Animals and Treatment
2.2. UV-B Irradiation
2.3. Thread Treatment
2.4. Wrinkle Measurement
2.5. Histological Analysis
2.6. Western Blotting Analysis
2.7. Statistical Analysis
3. Results
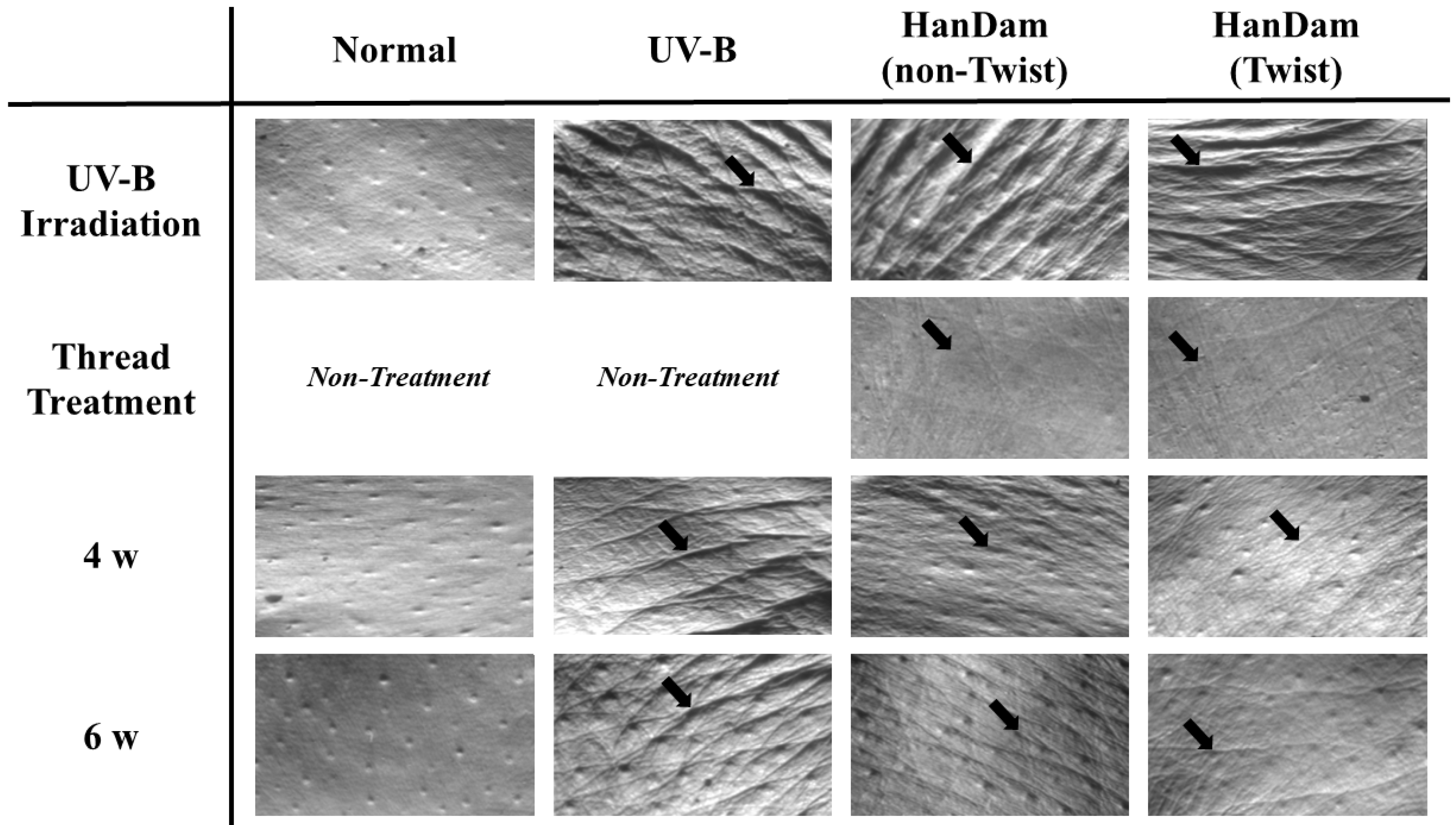
3.1. Effects of HanDam (Twist) on Wrinkle Formation in UV-B-Irradiated Hairless Mice
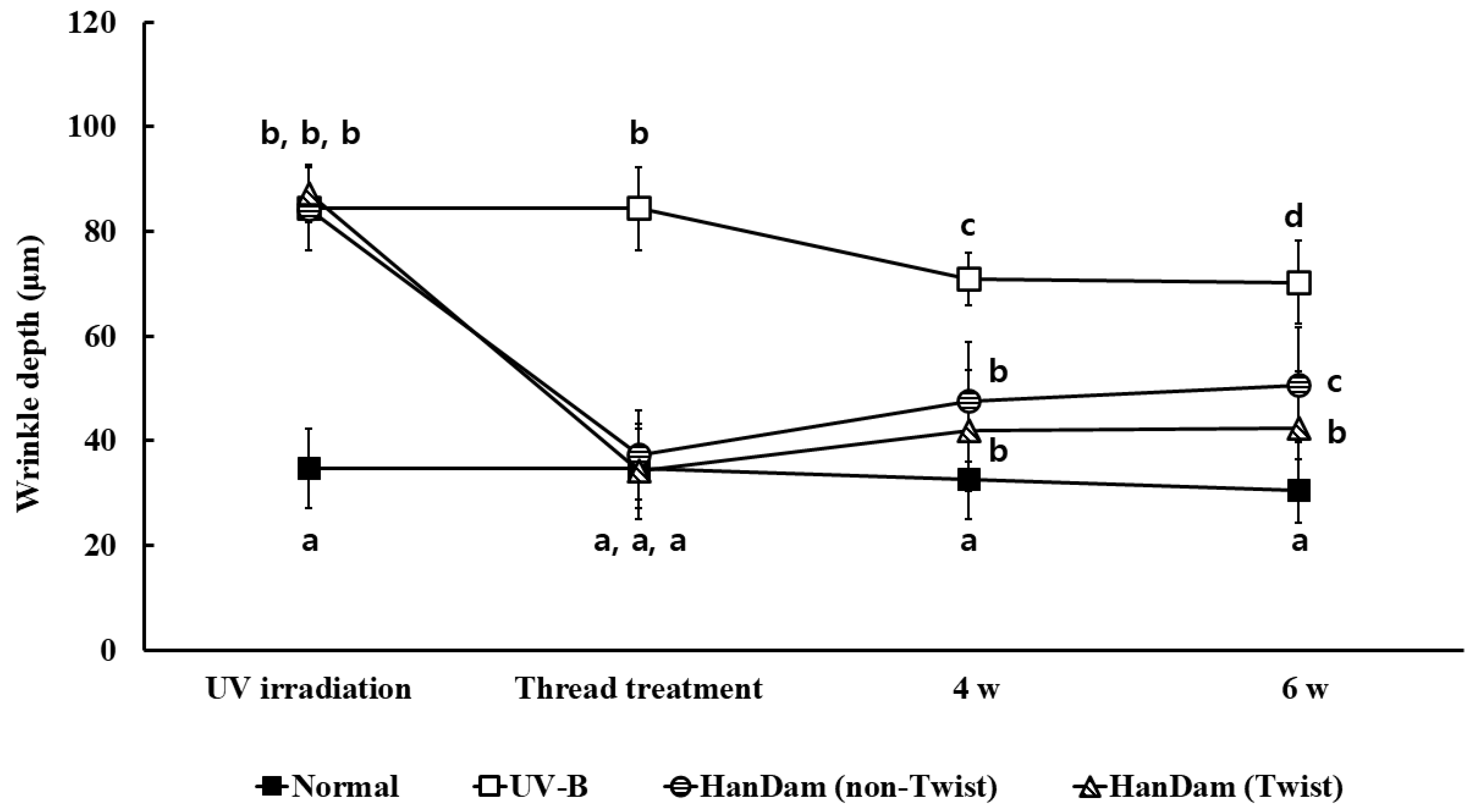
3.2. Effects of HanDam (Twist) on Wrinkle Depth in UV-B-Irradiated Hairless Mice
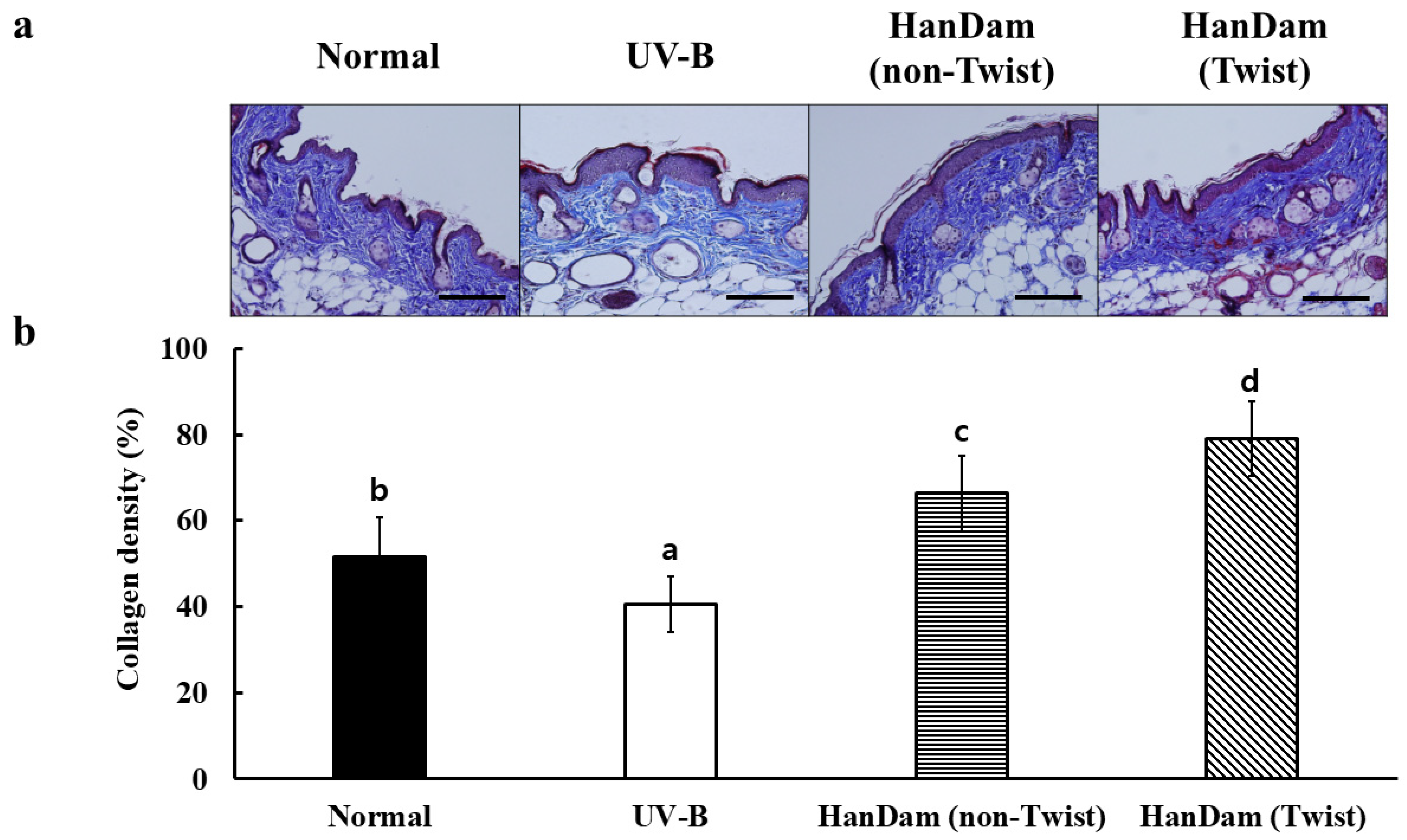
3.3. HanDam (Twist) Ameliorated Collagen Loss in UV-B-Irradiated Hairless Mice
3.4. HanDam (Twist)-Induced Collagen Synthesis Protein Expression in UV-B-Irradiated Hairless Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV-B | Ultraviolet B |
| PDO | Polydioxanone |
| TGF-β | Transforming growth factor beta |
| COL1 | Collagen type 1 |
| MMP-1 | Matrix metalloproteinase-1 |
References
- Choi, S.I.; Han, H.S.; Kim, J.M.; Park, G.; Jang, Y.P.; Shin, Y.K.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Bruls, W.A.; Slaper, H.; van der Leun, J.C.; Berrens, L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem. Photobiol. 1984, 40, 485–494. [Google Scholar] [CrossRef]
- Meunier, L. Ultraviolet light and dendritic cells. Eur. J. Dermatol. 1999, 9, 269–275. [Google Scholar]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, T.H.; Park, S.Y.; Yi, T.H.; Kim, S.Y. Enzyme-modified Panax ginseng inhibits UVB-induced skin aging through the regulation of procollagen type I and MMP-1 expression. Food Funct. 2014, 5, 265–274. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.N.; Shin, M.S.; Choi, B.T. Thread Embedding Acupuncture Inhibits Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 539172. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef]
- Varani, J.; Perone, P.; Fligiel, S.E.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen production in photodamage: Correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J. Investig. Dermatol. 2002, 119, 122–129. [Google Scholar] [CrossRef][Green Version]
- Fisher, G.J.; Choi, H.C.; Bata-Csorgo, Z.; Shao, Y.; Datta, S.; Wang, Z.Q.; Kang, S.; Voorhees, J.J. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J. Investig. Dermatol. 2001, 117, 219–226. [Google Scholar] [CrossRef]
- Papadavid, E.; Katsambas, A. Lasers for facial rejuvenation: A review. Int. J. Dermatol. 2003, 42, 480–487. [Google Scholar] [CrossRef]
- Romagnoli, M.; Belmontesi, M. Hyaluronic acid-based fillers: Theory and practice. Clin. Dermatol. 2008, 26, 123–159. [Google Scholar] [CrossRef]
- Sundaram, H.; Signorini, M.; Liew, S.; Trindade de Almeida, A.R.; Wu, Y.; Vieira Braz, A.; Fagien, S.; Goodman, G.J.; Monheit, G.; Raspaldo, H. Global Aesthetics Consensus: Botulinum Toxin Type A-Evidence-Based Review, Emerging Concepts, and Consensus Recommendations for Aesthetic Use, Including Updates on Complications. Plast. Reconstr. Surg. 2016, 137, 518–529. [Google Scholar] [CrossRef]
- Kim, J.; Zheng, Z.; Kim, H.; Nam, K.A.; Chung, K.Y. Investigation on the Cutaneous Change Induced by Face-Lifting Monodirectional Barbed Polydioxanone Thread. Dermatol. Surg. 2017, 43, 74–80. [Google Scholar] [CrossRef]
- Dulguerov, N.; D’Souza, A. Update on treatment rationale and options for the ageing face. Curr. Opin. Otolaryngol. Head. Neck Surg. 2011, 19, 269–275. [Google Scholar] [CrossRef]
- Siddharth, M.; Abhijit, D.; Vandana, P.; Hans, C.; Alexander, R.; Günther, W. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Sulamanidze, M.; Sulamanidze, G. APTOS suture lifting methods: 10 years of experience. Clin. Plast. Surg. 2009, 36, 281–306. [Google Scholar] [CrossRef]
- Hong, G.W.; Kim, S.B.; Park, S.Y.; Wan, J.; Yi, K.H. SMAS repositioning technique utilizing cog thread: Anatomical perspectives. Skin. Res. Technol. 2024, 30, 13650. [Google Scholar] [CrossRef]
- Kapicioğlu, Y.; Gül, M.; Saraç, G.; Yiğitcan, B.; Gözükara, H. Comparison of Antiaging Effects on Rat Skin of Cog Thread and Poly-L-Lactic Acid Thread. Dermatol. Surg. 2019, 45, 438–445. [Google Scholar] [CrossRef]
- Hong, G.W.; Hu, H.W.; Park, S.Y.; Wan, J.; Yi, K.H. What Are the Factors That Enable Thread Lifting to Last Longer? Cosmetics 2024, 11, 42. [Google Scholar] [CrossRef]
- Cho, S.W.; Shin, B.H.; Heo, C.Y.; Shim, J.H. Efficacy study of the new polycaprolactone thread compared with other commercialized threads in a murine model. J. Cosmet. Dermatol. 2021, 20, 2743–2749. [Google Scholar] [CrossRef]
- Junye, L.; Liang, Z.; Xin, X.; Yuhan, Z.; Baoping, J.; Feng, Z. The effects and mechanism of collagen peptide and elastin peptide on photoaging of skin cells induced by ultraviolet radiation. Food Biosci. 2025, 68, 2212–4292. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways. Antioxidants 2024, 13, 294. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1323–1332. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, D.Y.; Oh, H.; Kim, S.I.; Oh, S.Y.; Na, W.; Park, S.H.; Park, K.; Kim, J.I.; Kim, A.H.; et al. Dietary Collagen Hydrolysates Ameliorate Furrowed and Parched Skin Caused by Photoaging in Hairless Mice. Int. J. Mol. Sci. 2021, 22, 6137. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Wang, F.; Shao, Y.; Rittié, L.; Xia, W.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J. Investig. Dermatol. 2013, 133, 658–667. [Google Scholar] [CrossRef]
- El-Domyati, M.; El-Ammawi, T.S.; Medhat, W.; Moawad, O.; Mahoney, M.G.; Uitto, J. Expression of transforming growth factor-β after different non-invasive facial rejuvenation modalities. Int. J. Dermatol. 2015, 54, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.J.; Park, T.J.; Kim, B.Y.; Kim, C.M.; Suh, D.H.; Lee, S.J.; Moon, H.R.; Ryu, H.J. Comparative effects of various absorbable threads in a rat model. J. Cosmet. Laser Ther. 2019, 21, 158–162. [Google Scholar] [CrossRef]
- Benito, J.; Pizzamiglio, R.; Theodorou, D.; Arvas, L. Facial rejuvenation and improvement of malar projection using sutures with absorbable cones: Surgical technique and case series. Aesthetic Plast. Surg. 2011, 35, 248–253. [Google Scholar] [CrossRef]
- Langevin, H.M.; Bouffard, N.A.; Badger, G.J.; Churchill, D.L.; Howe, A.K. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction-based mechanism. J. Cell Physiol. 2006, 207, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Churchill, D.L.; Cipolla, M.J. Mechanical signaling through connective tissue: A mechanism for the therapeutic effect of acupuncture. FASEB J. 2001, 15, 2275–2282. [Google Scholar] [CrossRef]
- JuncosaMelvin, N.; Matlin, K.S.; Holdcraft, R.W.; Nirmalanandhan, V.S.; Butler, D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007, 13, 1219–1226. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, I.; Song, J.; Jang, I.; Noh, D.; Lee, C.; Kwon, J. Wrinkle Improvement in HanDam (Twist) on Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice. Appl. Sci. 2025, 15, 4879. https://doi.org/10.3390/app15094879
Song I, Song J, Jang I, Noh D, Lee C, Kwon J. Wrinkle Improvement in HanDam (Twist) on Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice. Applied Sciences. 2025; 15(9):4879. https://doi.org/10.3390/app15094879
Chicago/Turabian StyleSong, Inbong, Judong Song, Ilseok Jang, Dayoung Noh, Chaemyeong Lee, and Jungkee Kwon. 2025. "Wrinkle Improvement in HanDam (Twist) on Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice" Applied Sciences 15, no. 9: 4879. https://doi.org/10.3390/app15094879
APA StyleSong, I., Song, J., Jang, I., Noh, D., Lee, C., & Kwon, J. (2025). Wrinkle Improvement in HanDam (Twist) on Ultraviolet B Irradiation-Induced Skin Photoaging in Hairless Mice. Applied Sciences, 15(9), 4879. https://doi.org/10.3390/app15094879





