Abstract
Background: The open-stance forehand is a fundamental technique in tennis, playing a crucial role in competitive performance. Its execution depends heavily on lower limb coordination and neuromuscular control. Athletes of different skill levels often display distinct muscle activation strategies. This study employs non-negative matrix factorization (NMF) to analyze lower limb muscle synergy patterns during the forehand open stance across skill levels and explores their potential influence on stroke performance. Methods: A total of 30 tennis players, including 15 elite and 15 amateur athletes, participated in this study. Surface electromyography (sEMG) was used to record the activity of major lower limb muscles during the forehand open stance. Muscle synergy patterns were extracted using NMF, and K-means clustering was applied to classify synergy patterns. Independent sample t-tests were conducted to examine differences between muscle synergies. Results: Significant differences (p < 0.05) were observed in the spatial characteristics of each synergy component across different movement phases. However, temporal characteristics showed a significant difference only in Syn2 during the mid-phase of the backswing (BS) (56.2–60.4%) (p = 0.033). Conclusions: Elite athletes exhibited more optimized and stable muscle activation patterns, enabling more efficient coordination of major muscle groups. Based on sEMG decomposition and muscle synergy analysis, these activation patterns may contribute to improved stroke efficiency and energy transfer and potentially reduce the risk of sports-related injuries.
1. Introduction
Tennis, a sport that combines speed, strength, agility, and strategic intelligence, is a key discipline within sports science [1]. It is also a primary focus of research on movement mechanics, training methodologies, and physical conditioning [2]. As sports science advances [3], biomechanics research in tennis has attracted increasing interest [4], particularly in relation to lower limb strength, footwork mechanics, and muscle coordination during stroke execution [5].
In competitive tennis, a player can execute over 1100 strokes in a single match [6]. Among these, the forehand open-stance stroke is widely utilized for its strategic advantage in facilitating swift transitions between offensive and defensive play [7]. Effective execution of this movement relies heavily on lower limb musculature [8]. The performance of a forehand open-stance stroke is primarily influenced by lower limb stability, explosive strength, and coordinated muscle activation [9,10,11]. These elements impose substantial demands on neuromuscular control strategies and energy transfer efficiency [12], both of which are crucial in determining shot precision and effectiveness [13]. Studies indicate that 70–75% of stroke energy originates from the coordinated motion of the lower limbs, primarily involving the ankle–knee–hip joints of the supporting leg to ensure efficient kinetic chain transmission [14]. If the activation timing of lower limb muscles during a forehand open-stance stroke is delayed by more than 60 ms, the peak ground reaction force decreases by 18–22%, directly impairing kinetic energy transfer efficiency [15]. Furthermore, electromyographic (EMG) analysis of professional tennis players reveals that a 50 ms delay in lower limb muscle activation results in a 15% reduction in hip joint angular velocity, significantly lowering stroke speed [16].
One of the key challenges in motor control research is deciphering how the central nervous system (CNS) regulates multi-muscle coordination and adapts neuromuscular strategies accordingly [17,18]. Differences in neuromuscular control strategies reflect the biological foundation of athletic performance [19]. Studies show that elite athletes exhibit a more refined pre-activation mechanism during stroke preparation, establishing a coordinated contraction pattern between the lower limb and core muscles. Notably, the co-activation index (CIC) between the vastus medialis and transversus abdominis in elite players can reach 0.82, whereas it is only 0.51 in amateur players [20]. This timing advantage not only improves energy transmission efficiency (by 23%) but also reduces joint oscillation (by 37%), preserving the integrity of the kinetic chain [15]. However, most existing studies focus either on muscle activation levels or timing patterns individually [21,22], failing to examine their synergistic interactions, which limits a comprehensive understanding of motor skill control mechanisms.
In recent years, non-negative matrix factorization (NMF) has gained significant attention in sports science due to its strong physiological interpretability and stability [21,23]. It is particularly effective in analyzing multi-muscle coordination during complex movement tasks by extracting a limited number of muscle synergy modules and reducing high-dimensional EMG data into a few representative muscle coordination patterns. The non-negativity constraint ensures that extracted synergy patterns align with muscle physiology, where muscle excitations remain non-negative, reflecting realistic activation mechanisms [24]. Moreover, NMF simultaneously quantifies both spatial structures (muscle weight vectors) and temporal activation coefficients of muscle synergies [25,26,27], providing insights into muscle group contributions and their dynamic adaptations over time, overcoming limitations of traditional analytical methods.
Despite increasing interest in tennis biomechanics, most existing studies have primarily focused on single-muscle activation or joint kinematics, overlooking the integrated, time-dependent coordination among multiple muscle groups. Moreover, few studies have explored how muscle synergy structures adapt across different skill levels using physiologically interpretable methods such as NMF. This study addresses this gap by applying synergy-based analysis to reveal neuromuscular control differences that underline skill-dependent variations in stroke execution.
Therefore, this study employs the NMF algorithm to systematically analyze the neuromuscular control strategy variations in lower limb muscle synergy structures between elite and amateur tennis players while executing the forehand open stance stroke. By quantifying the number of synergy modules, activation timing synchronization, and spatial distribution of muscle weights [28], this study seeks to examine variations in muscle coordination and activation characteristics among players of varying skill levels. Focusing on the intrinsic mechanisms of motor skill control, this study further elucidates the role of muscle synergy patterns in movement control and energy transfer. By comparing and analyzing the muscle synergies of players at different skill levels, the findings will not only optimize existing motor skill control theories but also serve as a valuable reference for coaches and sports scientists, promoting neuromuscular-based training approaches to enhance overall athletic performance. Although skill level in tennis lies on a continuum, this study employed a binary classification (elite vs. amateur) to emphasize distinct contrasts in neuromuscular control. This simplification is acknowledged as a methodological limitation, and future studies may consider more nuanced stratification to reflect the spectrum of player abilities.
2. Methods
2.1. Subjects
This was a cross-sectional comparative study conducted under controlled laboratory conditions. The participants were recruited through university tennis programs, local clubs, and professional training centers in Ningbo, China. In this study, a total of 30 male tennis players participated, consisting of 15 elite and 15 amateur players, all of whom were right-handed. The elite group (age: 20 ± 1.05 years; height: 177 ± 2.69 cm; weight: 71 ± 4.15 kg; tennis experience: 11.73 ± 0.96 years; weekly training: 16 ± 3 h) and the amateur group (age: 21 ± 1.94 years; height: 176 ± 2.67 cm; weight: 70 ± 3.65 kg; tennis experience: 6.67 ± 1.29 years; weekly training: 10 ± 2 h) had undergone structured tennis training. However, the elite players received more professional training, achieved rankings in official tournaments, and held sports certifications issued by recognized institutions. They met the Technical Classification Standards for Tennis Players issued by the General Administration of Sport of China in 2021 (www.sport.gov.cn).
All participants had been free of musculoskeletal injuries for at least a year and had no history of neurological disorders [29]. To prevent fatigue from affecting kinematic performance and muscle activity, the participants were required to rest adequately before testing [30]. They were instructed to sleep for at least 8 h and avoid high-intensity training or competition the day before testing. This study received ethical approval from the Ethics Committee of Ningbo University (Approval No. TY2024044), and all participants signed written informed consent forms prior to data collection.
2.2. Experimental Procedures
A 10-camera Vicon motion capture system (Vicon Motion Systems, Oxford, UK) and Kistler force plates (Kistler Instruments, Winterthur, Switzerland) were used to synchronously collect kinematic and kinetic data during the forehand open-stance stroke. The Vicon system recorded lower-limb kinematics at 250 Hz, while the Kistler force plates captured ground reaction forces at 1000 Hz [31,32]. A 49-marker model was used, covering the following anatomical landmarks: thoracic vertebrae 10, scapular-inferior angles, scapula-acromial edge, deltoid tuberosity, humerus-lateral epicondyle, radius–styloid process, ulna–styloid process, the basis of the forefinger, anterior superior iliac spine, iliac crest, posterior superior iliac spine, thigh triad 1–3, lateral epicondyle, medial epicondyle, fibular head, tibial tuberosity, calf triad, lateral prominence of the lateral malleolus, medial prominence of the medial malleolus, root bone, dorsal margin of the first metatarsal head, dorsal aspect of the second metatarsal head, and dorsal margin of the fifth metatarsal head [33,34].
A twelve-channel Delsys surface electromyography (sEMG) system (Delsys Inc., Natick, MA, USA) recorded muscle activity at 1000 Hz. The sEMG signals were synchronized with kinematic data via an A/D converter (Vicon Motion Systems, Oxford, UK) for subsequent analysis. Muscle activity was recorded from 12 muscles, including Rectus femoris (left LRF, right RRF), Biceps femoris (long head) (left LBF, right RBF), Medial gastrocnemius (left LMG, right RMG), Tibialis anterior (left LTA, right RTA), Erector spinae (left LES, right RES), and Gluteus maximus (left LGMAX, right RGMAX). Electrode placement followed the SENIAM (Surface Electromyography for the Non-Invasive Assessment of Muscles) guidelines [35], ensuring proper alignment with the long axis of the muscle fiber [36]. Prior to electrode placement, the skin was prepared by cleansing with alcohol wipes and removing hair to reduce impedance and optimize signal quality [37]. To minimize motion artifacts and enhance data reliability, electrodes were affixed using medical-grade adhesive and secured with elastic bands.
Prior to testing, participants engaged in a 15 min warm-up routine comprising footwork exercises and practice swings to activate key muscle groups. Following this, they executed five high-intensity open-stance forehand strokes, focusing on striking the ball with the racket’s sweet spot to refine force application and movement coordination.
During formal testing, a professional coach tossed balls 1.5 m in front of the participants. The participants were required to hit the ball into a designated target area, a 3 × 3 m2, located 1 m from both the baseline and the singles sideline. A trial was considered successful if the shot landed within the designated area, with participants needing to complete 10 such successful attempts. A 15 s rest was maintained between trials to prevent fatigue-related muscle activity interference [38]. The testing process was supervised by a professional coach to ensure consistency and proper execution. Similar protocols involving coach-supervised skill execution have been used in tennis biomechanics research to ensure the ecological validity and technical accuracy of stroke performance [30]. None of the equipment used in the study interfered with or restricted the participants’ movements (Figure 1a).
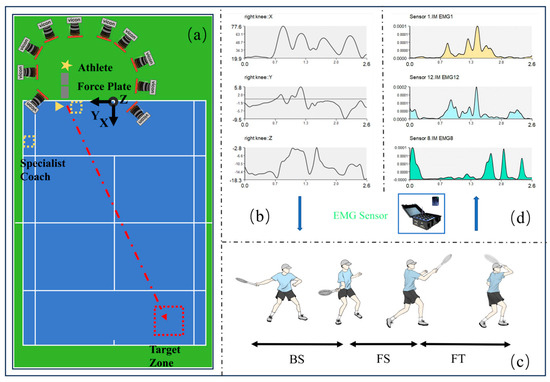
Figure 1.
Overview of the experimental framework. (a) Layout of the experimental setup. (b) Kinematic data processing and analysis using Visual 3D (C-Motion Inc., Germantown, MD, USA). (c) Defined phases of the forehand open-stance stroke. (d) Electromyographic (EMG) data were recorded from 12 muscles throughout the experiment.
2.3. Data Analysis
2.3.1. Kinematic Data Extraction and Phase Segmentation
The kinematic data in this study were processed using Vicon Nexus (v2.6.1, Vicon Motion Systems, Oxford, UK) for standardized analysis. This included marker identification and annotation, interpolation of missing motion trajectories, and data labeling. The complete dataset was then exported in a “*c3d” format to Visual 3D (C-Motion Inc., Germantown, MD, USA) for further computation and analysis (Figure 1b). Based on the extracted kinematic data, the forehand open-stance stroke was divided into three phases and four key time points (Figure 1c). Phase 1: Backward Swing (BS)—From the moment the right foot contacts the ground to the point of maximum knee flexion. Phase 2: Forward Swing (FS)—From the point of maximum knee flexion to the moment of ball impact. Phase 3: Follow-through (FT)—From ball impact to the completion of the follow-through.
2.3.2. Muscle Synergy Extraction
Before extracting muscle synergies, the acquired sEMG data were preprocessed using MATLAB (R2024a) (MathWorks, Natick, MA, USA) (Figure 1d) [39]. First, the baseline of the raw EMG data was adjusted to zero. A fourth-order Butterworth bandpass filter was applied to the raw sEMG signal to remove noise, with a cutoff frequency set at 60 Hz, followed by full-wave rectification [40]. Subsequently, a fourth-order Butterworth low-pass filter was applied to the rectified signal, with a cutoff frequency of 50 Hz, to extract the linear envelope [41]. To eliminate variations in intrinsic muscle activation characteristics, the EMG signal of each muscle was then normalized [42]. NMF was employed to decompose the sEMG signals and extract muscle synergy modules [43], this algorithm was implemented in R (v4.3.2, R Foundation for Statistical Computing, Vienna, Austria). The algorithm decomposes muscle activity (A(t)) into a linear combination of time-invariant synergy vectors (Wi) and time-varying activation coefficients (Bi(t)), computed through an iterative multiplicative update rule. Thus, the sEMG signal can be reconstructed using the following equation:
To determine the number of muscle synergies (Nsyn), we applied the NMF algorithm to each experimental dataset, sequentially extracting between 1 and 12 synergy modules. Since the data were collected from 12 muscles, the maximum number of synergies was set to 12. The optimal number of synergies was defined as the minimum number of synergy modules required to achieve approximately 90% of EMG reconstruction R2 [44]. Based on the standard definition of R2 in NMF [45], the calculation formula is as follows:
The total sum of squares (SST) represents the variance within the dataset, where denotes the sEMG data of the th muscle at the -th time point, and represents the average sEMG value of the th muscle. The sum of squared errors (SSE) quantifies the reconstruction error. To prevent the extracted synergy vectors from converging to suboptimal local minima in an incorrect solution space, each synergy extraction was performed 20 times. The synergy vectors (Wi) and activation coefficients (Bi(t)) were initialized with random values uniformly distributed between zero and the maximum sEMG value. The extraction result with the highest R2 value was selected for further analysis. During each execution of NMF, the iterative update process was terminated when the change in EMG reconstruction R2 was less than 0.001% for 20 consecutive iterations [21]. The K-Means algorithm was then applied to cluster the muscle synergies of the elite and amateur groups, identifying synergy characteristics associated with different skill levels.
2.3.3. Statistical Analysis
All statistical analyses were performed with IBM SPSS Statistics (v27.0, IBM Corp., Armonk, NY, USA) and a custom MATLAB script implementing one-dimensional statistical parametric mapping (spm1d). The Shapiro–Wilk test was applied to verify that the data adhered to a normal distribution. Between-group differences in the muscle synergy matrix (A) were assessed using an independent sample t-test. Additionally, to comprehensively analyze temporal variations in the activation coefficient matrix (B), spm1d approach was employed to assess variations in activation coefficients over distinct time intervals. Statistical significance was determined at p < 0.05.
3. Results
3.1. Muscle Synergy Extraction Using NMF
Figure 2 illustrates the R2 values corresponding to each muscle synergy in both elite and amateur tennis players. The statistical evaluation indicated that the number of synergies did not significantly differ between the two groups, with both groups exhibiting four synergy modules. The R2 values for the elite and amateur groups were 0.96 ± 0.04 and 0.95 ± 0.06, respectively, with no significant difference (p = 0.682).
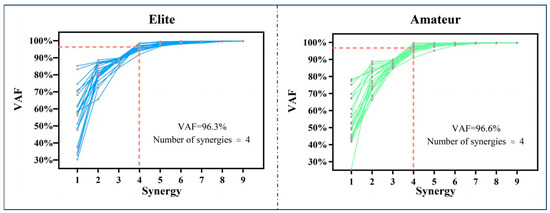
Figure 2.
Illustrations of the muscle synergy count (Nsyn) during the forehand open-stance stroke in elite and amateur tennis players. The red dashed line indicates the threshold applied to identify the extracted muscle synergies.
3.2. Muscle Synergy Characteristics
Notable variations in muscle synergy were identified between the elite and amateur groups across different stages of the open-stance forehand stroke. The muscle activation patterns exhibited distinct characteristics across these phases. Figure 3 illustrates the spatiotemporal structure of muscle synergy in the elite Figure 3a and amateur Figure 3b groups across the three movement phases. The forehand strokes in both groups can be characterized by four muscle synergies. The first (Syn1) and second (Syn2) synergies are primarily activated during the BS phase, the third synergy (Syn3) is dominant in the FS phase, and the fourth synergy (Syn4) is mainly engaged during the FT phase.
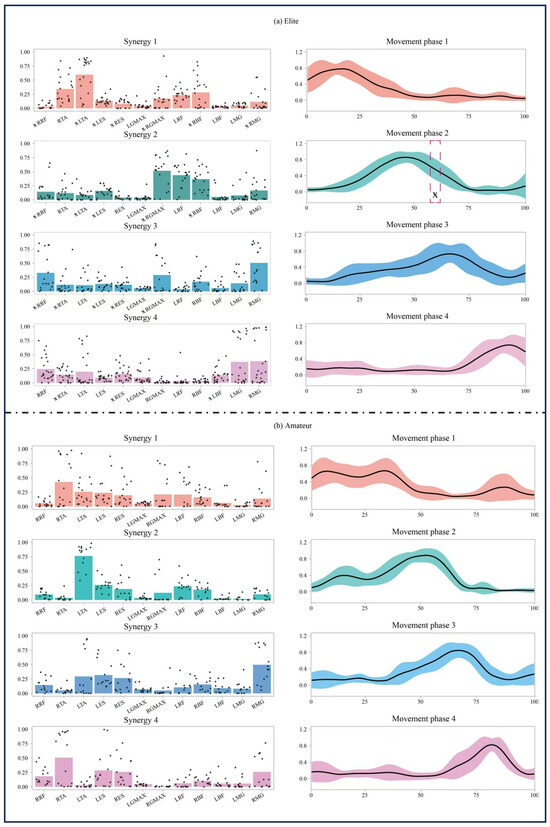
Figure 3.
Illustrations of the muscle synergy patterns observed in the forehand open-stance stroke for both elite and amateur players. The left side of each chart presents bar graphs representing the activation contributions of the four synergy modules (Synergy 1–4). Black dots overlaid on the bars represent individual data points from each participant, indicating inter-subject variability. The right side displays temporal variation curves (Movement Phase 1–4), depicting muscle activation intensity across different time points. The pink dashed box and corresponding symbol in Movement Phase 2 of the elite group indicate a time interval where a statistically significant difference (p < 0.05) was observed.
The muscle synergy activation patterns varied significantly across different phases, with distinct activation differences observed between the elite and amateur groups. Specifically, the activation level of Syn2 in the mid-BS phase (56.2–60.4%) was markedly elevated in the elite group relative to the amateur group (p = 0.033).
In Syn1, the elite group exhibited higher activation levels in LTA (p = 0.019) and RBF (p = 0.001), while RRF (p = 0.002), LES (p = 0.001), RES (p = 0.001), LGMAX (p = 0.033), and RMG (p = 0.003) showed lower activation levels.
In Syn2, the elite group demonstrated increased activation in RRF (p = 0.018), RGMAX (p = 0.001), and RBF (p = 0.001), whereas LTA (p = 0.004) and LES (p = 0.008) exhibited lower activation levels.
In Syn3, the elite group showed significantly higher activation levels in RRF (p = 0.001), RTA (p = 0.022), and RGMAX (p = 0.001), while LES (p = 0.001) and RES (p = 0.005) had lower activation levels.
In Syn4, the elite group displayed increased activation in LBF (p = 0.044) but reduced activation in RTA (p = 0.001) and RES (p = 0.026).
4. Discussion
This study examines the characteristics of lower-limb muscle synergies during the open-stance forehand stroke in tennis players of different skill levels. It also compares the muscle activation patterns of elite and amateur players at various movement phases. The results indicate significant differences in muscle synergy between the two groups. Elite players demonstrate a more stable and optimized activation pattern, whereas amateurs exhibit greater variability in activation timing, intensity, and muscle group distribution.
During the BS phase, the activation patterns of Syn1 and Syn2 in elite players are superior to those of amateurs. Notably, Syn2 activation is significantly higher in the mid-BS phase (56.2–60.4%) (p < 0.05). This suggests that elite players utilize their lower limb muscles more effectively for stable support and power preparation, enhancing stroke efficiency. In Syn1, elite players primarily rely on small distal muscles (LTA) for stability control while adjusting lower-limb posture through RBF. In contrast, amateurs exhibit a more dispersed activation pattern, relying more on core muscles (LES, RES) and larger muscle groups (LGMAX). This indicates that amateur players may require additional muscle adjustments to maintain movement continuity. In Syn2, elite players exhibit stronger quadriceps and gluteus maximus synergy (higher RRF, RGMAX, and RBF activation with lower LTA and LES activation, p < 0.05), whereas amateurs rely more on the lower-leg muscles (LTA) for compensation. This optimized synergy in elite players may reflect precise neuromuscular control of pre-activation within the kinetic chain. According to the “muscle synergy hierarchical control theory” [19], elite players enhance the coupling between the gluteus maximus and quadriceps (RGMAX/RRF contribution Δ = +34%), pre-establishing hip-knee stiffness matching and reducing energy loss during subsequent push-off phases [15]. In contrast, amateurs excessively rely on core muscles in Syn1 (LES/RES activation intensity Δ = +22%) and lower-leg compensation (LTA activation Δ = +18%), aligning with the “compensatory synergy hypothesis” [20], which describes excessive activation of non-dominant muscle groups. While this strategy helps maintain movement continuity to some extent, it reduces overall energy transfer efficiency, compromising stroke explosiveness and stability.
During the FS phase, the activation pattern of Syn3 differs significantly between elite and amateur players. The elite group exhibits higher activation levels of the rectus femoris (RRF), tibialis anterior (RTA), and gluteus maximus (RGMAX), while activation of the lumbar erector spinae (LES) and rectus abdominis (RES) is lower (p < 0.05). This suggests that elite players primarily rely on coordinated activation of the rectus femoris and gluteus maximus to generate stroke power while minimizing excessive core muscle engagement, thereby improving energy transfer efficiency. This pattern aligns with the “proximal-to-distal sequencing” principle, where hip-driven momentum transfer optimizes kinetic energy conversion in the stroke [16]. The elite group’s selective activation of RGMAX/RRF (Δ = +41%, p < 0.01) facilitates smoother energy transfer between the hip and knee joints, while significantly reducing core muscle activation (LES/RES activation Δ = −37%). This neuromuscular strategy likely enhances intersegmental force transmission along the kinetic chain, as suggested in previous studies on multi-joint coordination during tennis strokes [12,13,46]. Moreover, such a proximal-driven activation pattern has been shown to reduce upper-limb loading and improve stroke repeatability [5,16], which may explain the more stable and efficient stroke mechanics observed in elite players. In contrast, the amateur group exhibits a distinct activation pattern, characterized by greater LES and RES activation but lower RRF and RGMAX contributions. This suggests a reliance on core stability to compensate for lower limb force production deficiencies, leading to reduced power output, which affects stroke speed and accuracy. This pattern indicates a potential risk of kinetic chain breakdown and may be biomechanically linked to tennis elbow injuries [47].
During the FT phase, the elite group exhibited higher activation of the LBF and lower activation of the RTA and RES (p < 0.05). This suggests that they predominantly depend on the biceps femoris for stability, promoting post-stroke balance and seamless movement transitions. This activation pattern aligns with the “eccentric-priority strategy”, in which eccentric hamstring control mitigates peak joint reaction forces by 19%, improving movement recovery efficiency by reducing reset time by 230 ms. Conversely, the amateur group displayed greater activation of the RTA and RES alongside lower LBF engagement, indicating a stronger reliance on lower leg muscles for compensation and insufficient biceps femoris control over the lower limb. This lower-leg-dominant pattern corresponds with the “distal compensation hypothesis”, characterized by an ankle-focused stabilization strategy. These findings imply that elite players refine eccentric control of the biceps femoris during the FT phase, enabling more efficient post-stroke body adjustments. In contrast, excessive lower-leg reliance in amateurs may compromise post-stroke stability and delay movement recovery, potentially affecting preparation for the next shot.
The elite group exhibited a more refined muscle synergy pattern throughout all phases of the open-stance forehand stroke. They efficiently engaged larger muscle groups (rectus femoris, biceps femoris, gluteus maximus) to generate power while reducing dependence on smaller muscles (tibialis anterior, plantar flexors). This approach improved movement stability and may help enhance kinetic chain coordination during stroke execution. In contrast, the amateur group displayed a more dispersed muscle activation pattern, lacking clear phase-specific characteristics. Their core stability and lower-limb coordination were comparatively weaker, particularly during the BS and FT phases. These limitations could hinder force generation efficiency and disrupt overall movement stability. Although this study did not directly measure movement stability or force output, previous research has indicated that refined muscle synergy patterns contribute to improved athletic performance [24,28,48]. Therefore, the elite group’s more synchronized muscle activation pattern may reflect a neuromuscular control strategy that facilitates improved stroke coordination and movement efficiency.
Furthermore, the elite group exhibited more refined neuromuscular control throughout the FS and FT phases, which may contribute to more coordinated and technically consistent stroke execution. Conversely, the amateur group displayed inconsistent muscle activation patterns and higher variability in movement adjustments, potentially affecting coordination and energy transfer efficiency. To enhance performance in amateur athletes, targeted training of the gluteus maximus and quadriceps can increase lower limb power contribution during strokes while reducing over-reliance on smaller muscle groups. To address deficiencies in FT control, strengthening the biceps femoris can improve post-stroke balance and center-of-mass adjustments, thereby enhancing stroke fluidity and stability.
This study analyzed muscle synergy patterns in athletes of different skill levels using EMG data, revealing the role of muscle activation patterns in stroke execution. Several limitations related to the study population and design should be acknowledged. The sample size was relatively small and included only male players. Differences in participants’ age, playing experience, body composition, and BMI were not explicitly controlled, which may have introduced variability and limited the statistical power. These factors should be addressed in future studies with larger, more diverse, and stratified samples to improve the precision and generalizability of the findings.
In addition, the testing procedures were conducted in a controlled laboratory environment using a fixed ball toss and stationary stance. This experimental setup does not fully reflect the demands of actual match play, where stroke execution is affected by continuous movement, dynamic ball trajectories, court surface variations, and tactical or psychological stressors. The absence of these variables may constrain the ecological validity of the current results. Furthermore, the binary “in-or-out” classification used to evaluate target accuracy may not adequately capture the technical quality of stroke execution, such as ball speed, power, or racket velocity. This limitation weakens the ability to directly link muscle synergy patterns to stroke performance outcomes. Future research should aim to integrate more realistic testing conditions and include upper body and trunk muscle assessments to better represent the neuromuscular demands of real-world performance.
5. Conclusions
Tennis players at different skill levels exhibit distinct muscle activation patterns. Elite players demonstrate more efficient and stable muscle activation, effectively coordinating major muscle groups such as the quadriceps, gluteus maximus, and gastrocnemius. These activation patterns may help improve stroke coordination and movement stability. In contrast, amateur players show greater variability in muscle activation and lower energy transfer efficiency, particularly during the BS and FT phases. Their reliance on smaller muscle groups for compensation leads to reduced movement stability and coordination. Analyzing these muscle activation patterns can help players optimize their forehand stroke performance. For amateur players, targeted training to strengthen the gluteus maximus and quadriceps can enhance lower-limb contribution during strokes, ultimately improving overall performance.
Author Contributions
Conceptualization, Y.W. and D.S.; methodology, Y.G. and D.W.; software, Z.Z.; validation, D.C., Z.Z., Y.W., and D.W.; investigation, X.C. and Y.S.; resources, F.L.; data curation, D.C.; writing—original draft preparation, Y.W. and D.C.; writing—review and editing, D.S.; visualization, X.C. and Y.S.; supervision, Y.W.; project administration, Y.W. and D.J.; funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by the Zhejiang Province Science Fund for Distinguished Young Scholars (Grant No. LR22A020002), the Zhejiang Provincial Key Project of Education Science Planning (Grant No. 2025SB084), the Ningbo Key Research and Development Program (Grant No. 2022Z196), the Zhejiang Rehabilitation Medical Association Scientific Research Special Fund (Grant No. ZKKY2023001), the Research Academy of Medicine Combining Sports, Ningbo (Grant No. 2023001), the Project of Ningbo Leading Medical & Health Discipline (Grant Nos. 2022-F15, 2022-F22), the Ningbo Natural Science Foundation (Grant No. 2022J065), and K. C. Wong Magna Fund in Ningbo University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Ningbo University (Approval No. TY2024044, Approval Date: 13 December 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data relevant to the current study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks to all the participants in this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernandez-Fernandez, J.; Granacher, U.; Sanz-Rivas, D.; Sarabia Marín, J.M.; Hernandez-Davo, J.L.; Moya, M. Sequencing effects of neuromuscular training on physical fitness in youth elite tennis players. J. Strength Cond. Res. 2018, 32, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcaraz Martínez, B.J. History and evolutation of tennis. Mater. Para La Hist. Del Deporte 2013, 11, 52–56. [Google Scholar]
- Kilit, B.; Arslan, E. Effects of high-intensity interval training vs. on-court tennis training in young tennis players. J. Strength Cond. Res. 2019, 33, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Yevtyfiieva, I.I.; Borysova, O.V.; Boreiko, N.Y.; Yevtyfiiev, A.S.; Donets, Y.G.; Zinchenko, L.V. The relationship between biomechanical indicators of strokes and individual styles of play in tennis on the example of the best Ukrainian tennis players. Slobozhanskyi Herald Sci. Sport 2024, 28, 185–196. [Google Scholar] [CrossRef]
- Elliott, B. Biomechanics and tennis. Br. J. Sports Med. 2006, 40, 392–396. [Google Scholar] [CrossRef]
- Reid, M.; Duffield, R. The development of fatigue during match-play tennis. Br. J. Sports Med. 2014, 48, i7–i11. [Google Scholar] [CrossRef]
- Reid, M.; Morgan, S.; Whiteside, D. Matchplay characteristics of grand slam tennis: Implications for training and conditioning. J. Sports Sci. 2016, 34, 1791–1798. [Google Scholar] [CrossRef]
- Hansen, C.; Teulier, C.; Micallef, J.P.; Millet, G.P.; Girard, O. How does prolonged tennis playing affect lower limb muscles’ activity during first and second tennis serves? Eur. J. Sport Sci. 2024, 24, 1472–1479. [Google Scholar] [CrossRef]
- Knudson, D.; Blackwell, J. Trunk muscle activation in open stance and square stance tennis forehands. Int. J. Sports Med. 2020, 21, 321–324. [Google Scholar] [CrossRef]
- Reid, M.; Elliott, B.; Crespo, M. Mechanics and learning practices associated with the tennis forehand: A review. J. Sports Sci. Med. 2013, 12, 225–231. [Google Scholar]
- Seeley, M.K.; Funk, M.D.; Denning, W.M.; Hager, R.L.; Hopkins, J.T. Tennis forehand kinematics change as post-impact ball speed is altered. Sports Biomech. 2011, 10, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Marsh, T.; Overheu, P. A biomechanical comparison of the multisegment and single unit topspin forehand drives in tennis. Int. J. Sport Biomech. 1989, 5, 350–364. [Google Scholar] [CrossRef][Green Version]
- Kibler, W.B. Biomechanical analysis of the shoulder during tennis activities. Clin. Sports Med. 1995, 14, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Roetert, E.P.; Kovacs, M.; Knudson, D.; Groppel, J.L. Biomechanics of the tennis groundstrokes: Implications for strength training. Strength Cond. J. 2009, 31, 41–49. [Google Scholar] [CrossRef]
- Kibler, W.B.; Press, J.; Sciascia, A. The role of core stability in athletic function. Sports Med. 2006, 36, 189–198. [Google Scholar] [CrossRef]
- Elliott, B.; Fleisig, G.; Nicholls, R.; Escamilia, R. Technique effects on upper limb loading in the tennis serve. J. Sci. Med. Sport 2003, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Biewener, A.A.; Aerts, P.; Ahn, A.N.; Chiel, H.J.; Daley, M.A.; Daniel, T.L.; Full, R.J.; Hale, M.E.; Hedrick, T.L.; et al. Neuromechanics: An integrative approach for understanding motor control. Integr. Comp. Biol. 2007, 47, 16–54. [Google Scholar] [CrossRef]
- Alnajjar, F.; Wojtara, T.; Kimura, H.; Shimoda, S. Muscle synergy space: Learning model to create an optimal muscle synergy. Front. Comput. Neurosci. 2013, 7, 136. [Google Scholar] [CrossRef]
- Tresch, M.C.; Saltiel, P.; Bizzi, E. The construction of movement by the spinal cord. Nat. Neurosci. 1999, 2, 162–167. [Google Scholar] [CrossRef]
- Hodges, P.W.; Richardson, C.A. Contraction of the abdominal muscles associated with movement of the lower limb. Phys. Ther. 1997, 77, 132–142. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Den Otter, A.R.; Geurts, A.C.; Mulder, T.; Duysens, J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin. Neurophysiol. 2006, 117, 4–15. [Google Scholar] [CrossRef]
- Cheung, V.C.; d’Avella, A.; Tresch, M.C.; Bizzi, E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.R.; Nguyen, H.T. Nonnegative matrix factorization for the identification of EMG finger movements: Evaluation using matrix analysis. IEEE J. Biomed. Health Inform. 2015, 19, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Dal Pupo, J.; Detanico, D.; Ache-Dias, J.; Santos, S.G. The fatigue effect of a simulated futsal match protocol on sprint performance and kinematics of the lower limbs. J. Sports Sci. 2017, 35, 81–88. [Google Scholar] [CrossRef]
- Jie, T.; Xu, D.; Zhang, Z.; Teo, E.C.; Baker, J.S.; Zhou, H.; Gu, Y. Structural and organizational strategies of locomotor modules during landing in patients with chronic ankle instability. Bioengineering 2024, 11, 518. [Google Scholar] [CrossRef]
- Tresch, M.C.; Jarc, A. The case for and against muscle synergies. Curr. Opin. Neurobiol. 2009, 19, 601–607. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, J.; Yu, J.; Ying, S.; Liu, Z.; Zhang, Y.; Gu, Y.; Li, J. Comparison and analysis of the biomechanics of the lower limbs of female tennis players of different levels in foot-up serve. Front. Physiol. 2023, 14, 1125240. [Google Scholar] [CrossRef]
- Dong, R.; Su, X.; Li, S.; Ni, X.; Liu, Y. Characteristics, relationships, and differences in muscle activity and impact load attenuation during tennis forehand stroke with different grips. Life 2024, 14, 1433. [Google Scholar] [CrossRef]
- He, Y.; Fekete, G. The effect of cryotherapy on balance recovery at different moments after lower extremity muscle fatigue. Phys. Act. Health 2021, 5, 255–270. [Google Scholar] [CrossRef]
- Chang, H.; Cen, X. Can running technique modification benefit patellofemoral pain improvement in runners? A systematic review and meta-analysis. Int. J. Biomed. Eng. Technol. 2024, 45, 83–101. [Google Scholar] [CrossRef]
- Song, Y.; Cen, X.; Wang, M.; Bálint, K.; Tan, Q.; Sun, D.; Gao, S.; Li, F.; Gu, Y.; Wang, Y.; et al. The influence of simulated worn shoe and foot inversion on heel internal biomechanics during running impact: A subject-specific finite element analysis. J. Biomech. 2025, 180, 112517. [Google Scholar] [CrossRef]
- Cen, X.; Yu, P.; Song, Y.; Sun, D.; Liang, M.; Bíró, I.; Gu, Y. Influence of medial longitudinal arch flexibility on lower limb joint coupling coordination and gait impulse. Gait Posture 2024, 114, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Le Solliec, T.; Hautier, C.; Gassier, R.; Trama, R.; Gilbert, B.; Song, L.; Zhang, Q. Are junior tennis players less exposed to shocks and vibrations than adults? A pilot study. Sensors 2024, 24, 7999. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Chow, J.W.; Knudson, D.V.; Tillman, M.D.; Andrew, D.P. Pre- and post-impact muscle activation in the tennis volley: Effects of ball speed, ball size and side of the body. Br. J. Sports Med. 2007, 41, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.A.; Lee, T.D.; Winstein, C.; Wulf, G.; Zelaznik, H.N. Motor Control and Learning: A Behavioral Emphasis, 6th ed.; Human Kinetics: Champaign, IL, USA, 2018. [Google Scholar]
- Torres-Oviedo, G.; Ting, L.H. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J. Neurophysiol. 2010, 103, 3084–3098. [Google Scholar] [CrossRef]
- Hansen, C.; Teulier, C.; Micallef, J.P.; Millet, G.P.; Girard, O. Lower limb muscle activity during first and second tennis serves: A comparison of three surface electromyography normalisation methods. Sports Biomech. 2023, 1–12. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Janshen, L.; Mersmann, F.; Bohm, S.; Baltzopoulos, V.; Arampatzis, A. Modular control of human movement during running: An open access data set. Front. Physiol. 2018, 9, 1509. [Google Scholar] [CrossRef]
- Ting, L.H.; Macpherson, J.M. A limited set of muscle synergies for force control during a postural task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A. MusclesyneRgies: Factorization of electromyographic data in R with sensible defaults. J. Open Source Softw. 2022, 7, 4439. [Google Scholar] [CrossRef]
- Cheung, V.C.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, K.; Cheung, V.C. On nonnegative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput. 2014, 26, 1128–1168. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Grenier, S.; Kavcic, N.; Cholewicki, J. Coordination of muscle activity to assure stability of the lumbar spine. J. Electromyogr. Kinesiol. 2003, 13, 353–359. [Google Scholar] [CrossRef]
- Roetert, E.P.; Brody, H.; Dillman, C.J.; Groppel, J.L.; Schultheis, J.M. The biomechanics of tennis elbow. An integrated approach. Clin. Sports Med. 1995, 14, 47–57. [Google Scholar] [CrossRef]
- Hug, F.; Turpin, N.A.; Dorel, S.; Guével, A. Smoothing of electromyographic signals can influence the number of extracted muscle synergies. Clin. Neurophysiol. 2012, 123, 1895–1896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).