Featured Application
Building on the ability of current innovations to improve patient, staff, and intervention outcomes in open, laparoscopic, computerized (robotic), and image-guided robotic surgeries, this paper proposes, among others, a potential application related to the exploitation of a suitable digital background for planning, predicting, prospecting, training, and execution, with staff in the loop, of surgical activities in general.
Abstract
This review aims to place open, laparoscopic, computerized (robotic), and image-guided robotic surgical interventions in the context of complex medical surgeries, taking into account patient well-being, staff effort, and task reliability. It deduces the specificities of each technique and subsequently focuses on image-guided interventions and their practice in staff training, preparation, and implementation of a possible autonomous intervention. These complex interventions are intended to be minimally invasive (MI), precise, and safe therapies. The accuracy of robotic positioning could be improved by reductions in complexity and uncertainty involved in the intervention procedure. These can be achieved by matching the real controlled procedure and its virtual replica. The contribution discusses considerations for staff training and/or the planning of surgical interventions using real and virtual phantoms, and the use of augmented matched digital twins (DTs) for real interventions. This paper successively approaches open, laparoscopic and robotic surgeries, image-guided robotic interventions, the control and DT monitoring of MRI-assisted interventions, MRI field ruling equations and MRI compatibility, DT monitoring involvements in surgical interventions, and it ends with a discussion and main conclusions. The different topics presented in this article, although explicit, are reinforced by examples from the literature to facilitate a deeper understanding. The outcome of this review highlights the importance of robotic imaging-assisted procedures involving MI, nonionizing, and precise interventions. It also illustrates the potential of DTs combined with digital tools to offer an effective solution for the management of these interventions. The exploitation of such a suitable digital environment allows the planning, forecasting, prospecting, training, and execution, with staff in the loop, of surgical activities in general. This methodology allows for the precise consideration of specific anatomies, particularly in microsurgery and neurosurgery.
1. Introduction
Throughout history, the need for progress has been constant. Long before recorded history, health-related methodologies concentrated on human well-being have used surgery to achieve better therapeutic outcomes. The potential of surgery has been explored throughout history, and early surgeons mastered the basics of human anatomy and organ structures. However, evidence of healing behaviors and surgical practices remains a difficult scientific frontier to explore in paleopathology, but exploring such a frontier can help understand medical and healthcare practices in general [1,2,3,4]. Nowadays, many repetitive practices and skills contribute to a new well-being through health-related approaches that emphasize safety, comfort, and therapeutic results. Recent advances in medicine have made it possible to discover the origins of various diseases and establish approaches to treat them, mainly through surgical interventions. The effectiveness of these operations is directly related to the aforementioned factors related to the patient’s health, which largely depend on the visual and tactile abilities of the therapeutic team. These surgeries aim to be minimally invasive (MI) and precisely monitored, thus preserving healthy tissues surrounding the affected areas.
In recent decades, surgical procedures have developed quick-progressing disciplines, where innovative skills are quickly initiated and spread out through different surgical areas of expertise. Additionally, surgical strategy has progressed considerably and is continuously expanding across open, laparoscopic and robotic surgeries. Advances in experienced surgical practices have brought many benefits to patients. On the other hand, methodological complexity has increased, and the tasks of surgeons have changed significantly.
Since the beginning of early surgery practices, the open technique has been commonly used to “see well” and is still used in situations related to complex anatomy and problematic processes. Laparoscopic surgery has significantly transformed the long-established open surgical methodology, due to the various advantages that it presents to patients through a MI procedure [5,6]. Such a MI procedure has changed the line of attack to particular body areas by providing an expanded view through a microscopic camera and an illuminated tip of a mini-sized tool. In addition, laparoscopic intervention is concomitant with reduced postoperative distress and faster healing, allowing the management of small clinical episodes [7,8]. In addition, it allows important returns, such as improved wound aesthetics and decreased risk of obstruction [7]. However, laparoscopic procedures using elongated tools as well as 2-D vision exposures could create several surgical ergonomic risks [5,9,10]. Robotic MI surgery avoids such limitations, and its use is increasing in different situations such as for hernia surgery. Thus, surgical dexterity and ergonomics are improved thanks to an increased robotic degree of freedom and 3-D vision as well as a general rise in the practice of MI surgeries. Surgical robotic intervention has evolved from simple passive actions for instrument retraction, or holding devices such as rail-mounted tools or camera routing, to active robotic organizations whose motion span of instruments allows elevated performing and improved strictness for splitting up and stitching while expending a MI approach [11]. In addition, it removes tactile tremors and laparoscopic surgery fulcrum effects. Robotic wrist instruments offer multiple degrees of freedom to surmount the restriction of laparoscopic tools that often do not allow its tip to attain the anterior of the tissue [12] and permit suturing in difficult ergonomic positions.
From the above discussion, the safety, comfort, and therapeutic outcomes related to the patient’s health, which are the main objectives of different surgical technologies, depend on different factors related to the degree of tissue invasiveness, accuracy of tracking, duration of the procedure, speed of healing, etc. These factors are related not only to the patient but also to the medical staff regarding surgical dexterity, ergonomics, and ease of execution. In open surgery, almost all outcomes depend largely on the visual and tactile abilities of medical staff. These abilities are, in short, advantageously replaced by less invasive laparoscopic or robotic technologies. The difference between the latter two lies mainly in the way of replacing open surgery and the corresponding ease of execution by staff. These abilities or their substitutes are relatively situation-specific considering the patient, the staff, the operational complexity, and the cost. Thus, despite the clear superiority of robotic technology, laparoscopy and even open surgery have their places in many specific situations.
The prospect of safe, autonomous MI surgery with technology-substituted tactile and visual skills, all under the control of staff (staff in the loop), was a dream of the past and became achievable with recent digital intelligence. This ensures the well-being of patients as well as ease and added value for staff. As abovementioned, surgery interventions aim at large to be MI and accurately targeted, thus preserving healthful tissues adjacent to the suffering spots. In addition, staff are expected to provide individualized treatment to patients, taking into account factors such as genetic characteristics, lifestyle, environment and response to medical procedures. In addition to the benefits of MI intervention, the precise position and visual ability could be substituted by laparoscopic technology [7,8,13], robotics [9,10,11], or advantageously reliable image-assisted robotics [14,15,16,17]. In fact, image-assisted robotic surgery seems to be a logical descendant of laparoscopic and robotic surgeries with an obvious enhancement in skill as well as in surgeon resources, adopting a more comfortable position for the whole procedure interval. In addition, such robotically image-assisted intervention could be managed as autonomous surgery as mentioned earlier. Moreover, such an autonomous procedure could be employed for the planning and/or training of staff by using phantoms representing the real living tissue body portion of concern. In fact, such an autonomous procedure is mainly more advocated for further complex surgeries. Additionally, such intricacy that can be faced in foremost surgical interventions [18,19,20,21] or the restricted delivery of drugs [22,23,24] necessitates activities in a constrained zone, to preserve healthful tissues contiguous the disturbed region. In such autonomous robotic-controlled interventions, several challenges may be met, associated with the supervision of uncertainty, complexity, and unforeseen hazardous occurrences. Such difficulties can be treated by exploiting methods of control centered on paired real–virtual matching [17,25]. Moreover, the personalized treatment as well as the interventional specifications mentioned above could be predetermined by a pair of corresponding real and virtual phantoms, allowing the confirmation of the intervention outcome. In general, such adapted management could be autonomous [26], assisted by staff arbitration. Moreover, in perspective, it can include the real patient with the staff in the loop.
This contribution aims to highlight the role of innovative concepts of the moment in improving the conditions of patients, staff, and interventional results in the surgical practices of open, laparoscopic, robotic, and image-guided robotic surgeries. It also aims to illustrate the roles and needs of each of these types of surgery today. These analyses take into account the precision of the intervention, the safety and the degrees of invasive and autonomous characteristics of the intervention, as well as the comfort of the staff and healing conditions. The exploitation hence orients and focuses on image-guided interventions and their practice in staff training, preparation, and implementation of a possible autonomous intervention. The accuracy of robotic positioning could be improved by reductions in complexity and uncertainty. These can be achieved by matching the actual controlled procedures involved and their virtual procedures. The contribution examines considerations for staff training and/or the planning of surgical interventions using real and virtual phantoms, and the use of augmented matched digital twins (DTs) for real interventions.
The contents of this article are summarized as follows: In the second section, open, laparoscopic, and robotic surgeries are discussed and analyzed. The third section is dedicated to the details of image-guided robotic interventions and their performances in the case of nonionizing scanners and particularly magnetic resonance imaging (MRI). The fourth section introduces the possibility of a self-sufficient scheme for controlling MRI-assisted interventions and its monitoring for managing complexities and uncertainties via DT tools. Section 5 presents MRI radiofrequency field ruling equations and their implication in the scanner as well as the verification of MRI compatibility of inserted robotic tools in scanner scaffold. Section 6 details DT monitoring involvements in surgical interventions including projecting, tutoring, and implementation with human involvement in increased DT. Section 7 discusses some notions revealed in the preceding analyses. The last section summarizes the main conclusions of this paper.
2. Open, Laparoscopic, and Robotic Surgeries
2.1. Open vs. MI Surgeries
There are two commonly performed open and MI surgery techniques that have different characteristics and benefits. The open procedure, also termed long-established surgery, is the customary method that has been practiced for many years. In this case, generally one sizable cut is fashioned to access the surgical spot. This opening permits surgeons to directly see and enter the organ tissue that is worked on. The open technique permits some profitable situations, such as a distinct and straight sight of the surgical zone, allowing accurate handling and skill, and an enhanced entrance to intricate or outsized configurations that might be challenging to attain by means of MI practices. Conversely, open intervention as well presents a number of weaknesses. The big cut can steer to further suffering, bigger bleeding, and an elongated healing period compared to MI procedures. Moreover, there is a greater risk of infectivity and a further perceptible big cut trace.
2.2. MI Laparoscopic and Robotic Surgeries
Laparoscopic surgery uses some slight cuts on the body surface, routinely of length in the range of cm. Across these little openings, dedicated surgical mechanisms and a miniature camera called the laparoscope are introduced. This technology allows, as mentioned before, different benefits related to MI incisions and enlarged laparoscope vision of the operation zone. They, respectively, help reduce pain, bleeding, healing time, and visible scars, as well as precise and manageable engagements. Laparoscopy is very commonly used in abdominal surgeries and also as a diagnostic tool [27]. Conversely, a laparoscopy procedure might be incompatible with all cases concerning patients or surgery types [28] that need, in general, dedicated tuition and instrumentation in specialized healthcare services. Actually, specific procedures that are as intricate as neurosurgery or wide-ranging might yet necessitate open technique or more sophisticated robot-assisted laparoscopic techniques [29]. Note that the difference between laparoscopy and the robotized option pertain mainly to the medical staff regarding surgical dexterity, ergonomics, and ease of execution.
When it comes to robotic surgery, the goal is generally not to take the place of the surgeon but to increase their ability to care for the patient. The robot is therefore a surgical device, which is managed by computer, where its control is commonly mutual between the surgeon and the computer. Medical robots are therefore often referred to as surgical aides [30]. Surgical robots involve different practiced or prospective categories depending on their concept and corresponding appropriate types of surgery [31,32]. The first brand is planned remotely by the surgeon and involves laparoscopic operation, robotic-supported suturing, and tissue handling [29]. The second is controlled through staff supervision, which reflects better abilities contrasted to the last one and can be used for surgical removals (e.g., hysterectomy, prostatectomy, or cholecystectomy) [33,34]. The third category is the joint control of surgeon skills and robot capability and can be used in surgeries such as retinal, sinus, or orthopedic [35,36]. The fourth involves more autonomous robotics administered by staff and could be used, e.g., for microsurgery, neurosurgery, tumor ablation, anastomosis [37,38]. The last category involves an entirely autonomous surgical robot executing complex surgeries devoid of direct staff interaction (but still surveyed) and could be used, e.g., for biopsies, transplantation, intricate reconstructive surgeries, or microsurgery in sensitive bodily zones [39,40].
3. Image-Guided Interventions
It is clear from the Discussion in the last Section that both open and laparoscopic surgery options each have a number of advantages and disadvantages. In summary, the open strategy allows for direct entry and improved panorama but is accompanied by a larger incision and slower healing, while laparoscopic option allows for smaller incisions, faster healing, and less visible scars but may not be appropriate in all circumstances. As mentioned earlier, the choice of surgical strategy depends on several factors, including the patient’s disease, the complexity of the procedure, and the expertise of the staff. Only after a comprehensive assessment can the most appropriate choice be offered to the patient, through evidence-based decision making [41]. We have also seen that robot-assisted laparoscopic and robotic techniques allow for more complex problems to be performed with improved surgical dexterity, ergonomics, and staff ease. An advanced extended strategy of such assistance going further towards task enhancement could be image-guided robotic surgery, thus exploiting the precision of robotic positioning and high-resolution 3-D vision thanks to scanner imaging. In fact, if we only consider vision and positioning skills in surgery, open surgery uses external light projection and the surgeon’s hands, laparoscopic surgery replaces external light projection with internal light projection, robotic surgery uses pre-images and computer positioning, and image-guided surgery uses instantaneous imaging aiding in immediate positioning.
The MI image-guided robotic interventions, in general, improve patient comfort and security along with precision in execution and therapeutic effectiveness. Moreover, such approaches can work at practically all body zones, involving surgery or implanted restricted drug release. The scanner type plays an important role in the procedure. Several imagers can be employed in image-assisted interventions. Still, even though the imaging processes are each adapted to specific cases [42], those employing ionizing emissions as positron or X-ray seem inappropriate for medical treatments of wide intervals as in the case of complex image-guided surgeries. Thus, scanners fitting such elongated surgeries are those reflecting nonionizing features, specifically ultrasound and magnetic resonance [43,44,45]. The scanner is supposed to deliver 3-D high-resolution panorama of tissue details alongside interventional robotic tools. Thus, the robotic support performs inside the scaffold of the image together with tissue partly involved, permitting action administration in a closed-loop manner, pursuing tissue topology, locating tools, and monitoring their actions. In such a way, the scanner capacities and the robot skills are fused in an effectual task. Note that the dimensions and positional accuracy of tools should be in compliance with the resolution of the scanner.
The two nonionizing scanners can achieve the abovementioned duty with specific restrictions for each. The ultrasound scanner can only perform in airless and boneless windows [16,17], while MRI requires a scaffold with a free electromagnetic field (EMF) environment, and different examples of metal-artifact evaluation in MRI and corresponding reduction techniques could be found in the studies of [46,47,48,49,50,51], but it can work universally in all organs of the body. In addition, ultrasound has appropriate flexibility and cost, while MRI, which allows excellent soft tissue images, has a more expensive and complex use. The choice between the two scanners therefore depends on the circumstances. However, when patient comfort and safety are important, procedures, such as brain surgery, should use MRI-guided robotics. Note that MRI offers an unequaled contrast permitting the imaging of tumors, besides other anomalies, imperceptible by other imaging scanners. It reflects exact 3-D vision competency, comprising multimodal imaging of, for instance, the blood stream, temperature, and tracking of biomarkers. Thus, under such conditions, the administration by MRI assistance of robots would permit an outstanding intervention.
Considering the universal (all body parts) use of MRI-assisted surgery, to fruitfully conduct an intervention, the MRI faces important implementation issues mainly related to the involvement of three different EMFs in its functioning. These EMFs exhibit dissimilar natures (strength and frequency), displaying sensitive responses to external electromagnetic (EM) noise and forcing a limited position zone within the imaging configuration.
4. Control and Monitoring of MRI-Assisted Interventions
Intraoperative image-guided surgery that meets safety demands related to scanners and surgical procedures, in general, uses MRI and ultrasound scanners [16,17,19]. MRI scanners are increasingly being used in surgery, mainly because of their greater ability to distinguish tumors from healthy tissue in procedures involving tumor removal [52,53,54,55]. As abovementioned, the MRI scanner is EM-sensitive. In addition to its possible disruption by external EMFs, the introduction of external substances could disrupt its operation; thus, robotic components introduced into the scaffold, near tissue parts, must be MRI-compatible, i.e., without magnetic or conductive constituents. However, robotic engagements typically require high-performance actuation actions, while a small number of actuator types behave in an MRI-compatible manner. One potential class of actuation mechanisms that are generally compatible with MRI exploits piezoelectric materials, which come in different types. Further detailed information about these actuators, their configurations, ingredients, fabrication, research, and uses are presented elsewhere, for example, in the studies of [56,57,58,59,60,61,62,63,64,65], which correspond to different applications unconnected to the topic of this work but illustrate parameters such as positioning accuracy that are related to the topic. These tools are devoid of magnetic substances and made of dielectric piezoelectric materials but are equipped with thin conductive electrodes necessary for their excitation. In fact, piezoelectric materials are presumed to be compatible with MRI, while the compatibility of conductive electrodes depends on their structural configuration and therefore needs to be verified. This issue will be addressed in Section 5.2.
4.1. Closed-Loop Control of Image-Guided Robotic Actions
As mentioned before, patient safety is associated with the restriction quality of therapy to the distressed zone in the course of surgery. Such correctness hinges on the actuation precision of the surgical tool and its localization in the space. Consequently, the requirement for such high-quality spatial pursuing indicates an image-controlled position detection. Such clauses entail a joint configuration functioning autonomously, as illustrated by the schematics of a self-controlled surgical setting in Figure 1. Such organization consists of a scanner, tissue-touched zone, surgical tool, position and action processing, and robotic control and actuation [16,17]. The precision involved in such a closed-loop control procedure, related to position localization and actuation quality, would be shaped by various confounding features, including the degree of complexity of the combined constituents of the procedure, the associated uncertainties, and various unexpected peripheral risk occurrences. Only a reduction in such perturbing features permits a consistent control.
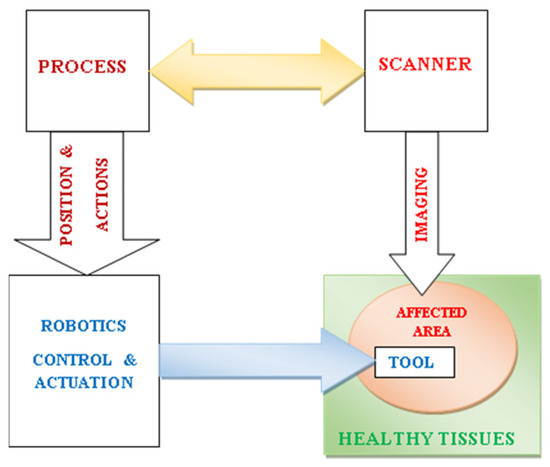
Figure 1.
Schematics of a self-controlled image-guided robotic surgical setting including scanner, tissue-touched zone, surgical tool, position and action processing, and robotic control and actuation.
4.2. Compatibility and Control Perturbations
As mentioned above, MRI scanners are subject to disturbances due to exposures to external EMFs or the introduction of substances that disturb their own fields. On the other hand, the control of their robotic assistance may involve disturbances due to the complexity and uncertainties as well as unexpected external risk incidences. This section aims to analyze the reduction in these various disturbances.
4.2.1. MRI-EMFs and Compatibility
This section aims to illustrate the problem of MRI compatibility of inserted materials related to robotic tools in the scanner scaffold. Such compatibility is associated with image fidelity and accuracy. An MRI-compatible material is not supposed to alter the image.
An image generated in an MRI scanner is shaped by signals occasioned by the connections of magnetic fields with living tissues. Thus, three different fields are exploited to construct 3-D images. A high static field engenders a vector of magnetization in tissues aligning their protons and quantifying their intensity. Three low-frequency repeated gradient field space-positioning aligned tissue protons fashioned a 3-D spatial reconstruction. Finally, a radiofrequency (RF) field exciting the tissue vector of magnetization allowed its reveal by the scanner, which can be processed and converted into images [16].
Actually, MRI shows the hydrogen atom nuclei that are detained within the body. A hydrogen nucleus is a proton, which is a mass of positive charge rotating on itself around an axis. Protons are arbitrarily oriented in tissues and spin individually. Therefore, they display zero magnetic field performing out of phase. Considering the MRI theory, tissue protons require three arrangements inside the concerned tissue: aligning protons in a settled direction, rotating them together, and localizing their space distinct origin. These three arrangements could be, respectively, realized by the following: the static magnetic field B0, excitation by wave RF field B1 having a frequency corresponding to the natural frequency of protons’ rotation fL (Larmor frequency) allowing a resonance action, and 3-D space gradient G(x, y, z) applied to the field B0, permitting the position distinct values of B0d, given by the following:
B0d (x, y, z) = B0 + G(x, y, z)
Note that the value of fL is a function of B0 and equal to 42.5 MHz per tesla, and the corresponding position distinct values fLd (x, y, z) will be a function of B0d (x, y, z) and given by the following:
fLd (x, y, z) = 42.5 × 106 × B0d (x, y, z)
As mentioned before, the three fields used in MRI, static, gradient and RF, differ in strength, frequency, and presence while operating. Thus, B0: 0.2–10 T, 0 Hz, permanently present; gradient: 0–50 mT/m, 0–10 kHz, pulses of few ms; and B1: 0–50 μT, 8–300 MHz (amp. mod. pulses) of few ms. A representation of the three MRI field components is shown in Figure 2.
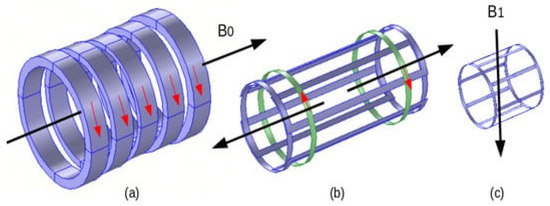
Figure 2.
MRI field components: (a) electromagnet B0, (b) gradient coils (one couple for one axis), and (c) RF coil (birdcage) B1.
The components producing the three fields are normally protected. Those of static and gradient fields are compensated and regulated. The arrangement of the RF field (often birdcage form, see Figure 2c), which is directly related to images, is the most exposed to perturbations mainly due to inserted external matters. Thus, the MRI compatibility of a matter inserted in the scanner scaffold (in the birdcage) is determined by its effect on the distribution of the field B1; i.e., for an MRI-compatible matter, the field distributions without or in the presence of the matter are identical. Such compatibility could be checked from EMF computations of RF field B1 distributions in the birdcage (see Section 5.2).
4.2.2. Complexity and Uncertainty Handlings
As stated previously, the precision implicated in the image-assisted robotic control associated with actuation and window positioning is conditional on troubling dynamics, including the complexity degree of different involved blended components, the related identification uncertainties, and unusual outer threat occurrences. The reduction in such potential troubles as well as the consideration of specific individual process information are indispensable for the correct functioning of the controlled image-guided procedure. Such objectives could be accomplished by monitoring the involved parameters in a matched real–virtual pair by means of a digital twin (DT) tool [66]. A DT is characterized by an integration of data within a pair of a physical event and its digital replica in a two-way manner. Such an approach is practiced for the managing of complexity in controlled procedures [67] and structured as a physical–virtual pair allowing self-adjusting behavior. Thus, the physical side of the pair delivers processed sensed data to the virtual side, while the last transmits control commands to the physical side. This self-adjustment matching helps reduce pair uncertainties and unexpected randomness in process control. It thus clearly appears that the management of complexity, uncertainties, and randomness by DT responds well to the troubling dynamics of image-assisted robotic control mentioned above. Note that, generally, DTs can be planned at the echelons of a structure, a substructure, or a particular component and employed for diagnostics, prediction, procedure condition monitoring, optimization, and risk evaluation [68].
Recently, DT has been gradually introduced into the health sector, which has allowed its use in different treatments to be gradually explored, thus enabling the renovation of daily life, health, and the connected monitoring and prolonged management of disorders. Many cases have been studied for this purpose; see, for example, the studies of [69,70,71,72,73,74,75,76,77,78,79], which are mainly general reviews illustrating the abovementioned treatments, monitoring, and managements.
4.3. DT Supervision of MRI-Assisted Interventions
The detailed DT supervision organization of an image-assisted robotic control is fashioned in this section. In general, concerning the abovementioned communications between the DT sides, the processed data of the physical side deliver detected information compared and adjusted by external Internet of Things “IoT” data and the learnt historical one. The outcome, once trained as data analysis, is conveyed, with an appropriate model reduction indication, to the DT virtual side. Actually, a swift communication (matching) between the sides of DT necessitates a faithful virtual model but with undersized execution time. Thus, the comprehensive coupled model, which realistically represents the physical procedure, would be reduced, allowing moderate computation time but preserving the picture of the real procedure. The management of an image-guided robotic control employing a DT pair fulfills an operational adaptive control procedure involving the real procedure matched with a reduced virtual model [80]. Figure 3 schematically illustrates the features of a DT monitoring an image-assisted robotic surgery. Such procedure monitoring could be used for staff training, predictions using physical phantoms and their digital replicas, or a real patient–virtual replica model, involving self-ruling matching with staff in the loop.
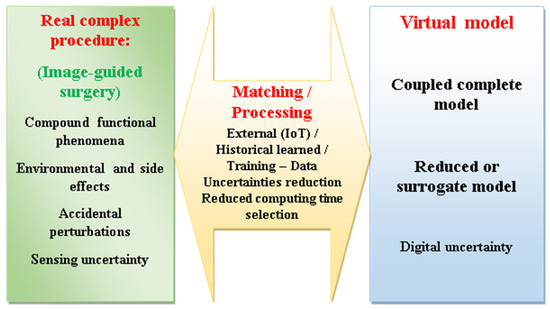
Figure 3.
Schematic illustration of the features of a DT monitoring an image-assisted robotic surgery.
The coupled model includes all components and actions of the image-guided robotic surgery procedure illustrated in Figure 1, namely, the MRI scanner, tissue-affected area, surgical tool, position and action processing, robotic control, and actuation. A significant part of the procedure is related to the MRI imaging involving the tissues and the surgical robotic tool. As mentioned earlier, the two components of the MRI magnetic fields related to the static and gradient fields are compensated and regulated, while the RF field arrangement is directly related to the images. This occurs through the processing and transformation of the field signals following the excitation and restoration of the B1-wave RF field. Thus, the 3-D RF field distribution in the birdcage comprising the tissues and the tool enables the outcome of the imaging process as well as the control of the MRI compatibility.
5. MRI RF Field Ruling Equations
As abovementioned, the excitation and restoration of the RF wave field in the MRI scanner produce a 3-D field distribution permitting after signal processing and image conversion to obtain a 3-D image of tissue details including the robotic surgical tool. The mathematical equations governing such RF EMF phenomena are based on Maxwell’s local comportment differential form of the general EMF equations [81]. In the situation of RF EMF excitation of living tissues, we can use the next formulation of EMF harmonic fields:
∇ × H = J
J = Je + σ E + j ω D
E = −∇ V − j ω A
B = ∇ × A with ∇ · A = 0
In the above Equations (3)–(6), H and E are the vectors of the magnetic and electric fields in A/m and V/m, B and D are the vectors of the magnetic and electric inductions in T and C/m2, and A and V are the magnetic vector and electric scalar potentials in Wb/m and volt. J and Je are the vectors of the total and source current densities in A/m2, σ is the electric conductivity in S/m, ω is the angular frequency = 2 π f, and f is the frequency in Hz of the exciting EMF. The symbol ∇ is a vector of partial derivative operators. The magnetic and electric comportment laws, respectively, between B/H and D/E are represented by the permeability μ and the permittivity ε in H/m and F/m.
The solution of the above EMF equations should count for the particular features of involved structures such as geometrical complexity, matter inhomogeneity, and variable nonlinear behaviors, which indicate advanced computational approaches. Fulfilling such characteristics imposes the matter of local answers, insinuating the employ of discretized 3-D methods as the finite element method (FEM) or comparable approaches (BEM, FDTD, etc.) [82,83,84,85,86,87,88,89].
Such a solution could be used to verify the MRI compatibility of the different objects introduced into the scanner, using EM compatibility (EMC) routines [16].
5.1. Models in DT
In the present contribution, the term model is employed to designate several issues. These involve the physical tissue representation model (physical phantom), digital tissue virtual model (virtual phantom), different procedure components models, compound coupled procedure model, and the reduced procedure model. For example, a DT procedure such as this given in Figure 3 used for a prediction (pre-interventional) task would involve a physical and virtual phantom in addition to a procedure coupled model [90,91] and its reduced or surrogate [92,93] model version.
Virtual numerical models (digital phantoms) can personify the living tissue body part involved in the surgical procedure. Important features of these models should correspond to the nature of material and organic properties, geometric form, and consistency with the computational approach used. Diverse body copies with living tissue features exist in the literature [94,95,96,97,98,99]. Figure 4 displays a structural body with its different body parts and tissues [100].
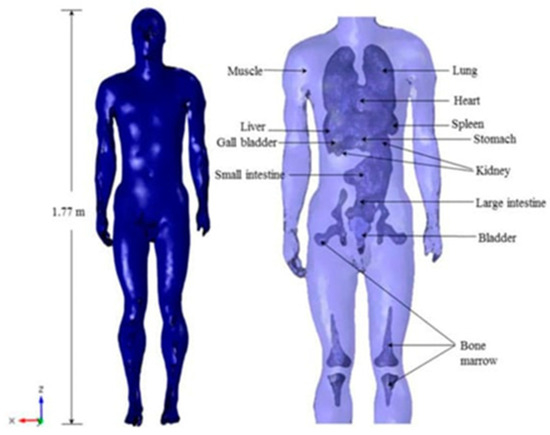
Figure 4.
High-resolution human body model including different organs and tissues [100].
5.2. EMC Analysis in MRI Setting
As mentioned in Section 4, the MRI scanner displays sensitive responses to EM noise due to exposure to external EMFs or the insertion of EM-sensitive materials into the scanner scaffold, both of which can disrupt the scanner’s own EMFs. The MRI EM-sensitive matters are mainly ferromagnetic and conductive materials.
Considering that the static and gradient fields are compensated and regulated as mentioned in Section 4.2.1, the interaction of a material introduced (such as those constituting the robotic structure) into the MRI scaffold with the RF B1 field can be examined. Via such an analysis, the compatibility of the material with MRI can be verified or established. Thus, the EMC analysis allows to identify the impact of the insertion of external materials into the MRI atmosphere on the 3-D distribution of the B1 field obtained from the solution of Equations (3)–(6) that is image-correlated. The equations are solved for a given field excitation source amplitude and frequency with the inserted stuff behavior laws parameters σ, μ, and ε.
As mentioned in Section 4, typical MRI-compatible actuation mechanisms are those using piezoelectric materials, which are free of magnetic substances and made of dielectric piezoelectric materials (assumed to be MRI-compatible) but equipped with thin conductive electrodes (their MRI compatibility is subject to their structural configuration) necessary for their excitation. These conductive electrodes could be responsible for the disturbance of the 3-D distribution of the RF B1 field, due to their eddy currents induced by this field. These currents mainly depend on the conductive surface perpendicular to the field. Such occurrence could be used in the actuation perspective to reduce disturbances in the distribution of RF field [17]. Perturbation control could be achieved by matching the field distributions with and without the introduced structure. Figure 5 and Figure 6 show such a demonstration (see the study of [17] for more details). Figure 5 shows the RF field B1 distribution (vertically directed) in the section of an empty birdcage within the tunnel of the scanner. Figure 6 displays a case of a cubic piezoelectric covered by skinny electrodes on two opposite surfaces of the cube typifying a simple piezoelectric actuator (Figure 6a). Figure 6b,c show the distributions of the field in the two situations with the electrodes perpendicular and parallel to the field direction, respectively.
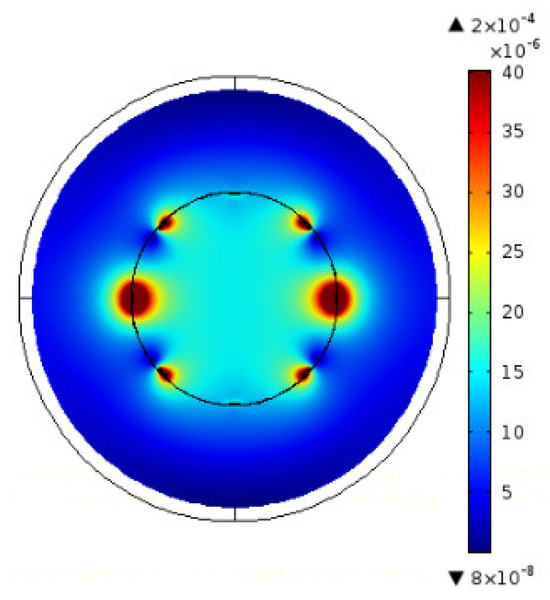
Figure 5.
MRI RF B1—vertically directed—(at 63.87 MHz, B0 1.5 T) distribution in the situation of no material [17].
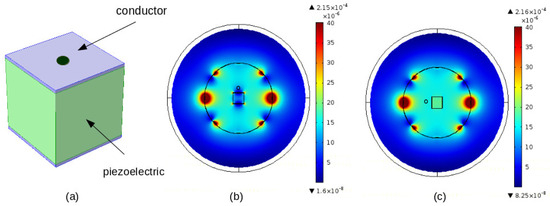
Figure 6.
MRI RF B1 (vertically directed) field distribution in the case of insertion of a piezoelectric covered by two electrodes, (a) material framework (b) distribution of B1 field—electrodes perpendicular to the field, and (c) distribution of B1 field—electrodes parallel to the field [17].
6. DT Monitoring Involvements
6.1. Projecting of Medical Arrangements
Following the DT supervision of MRI-assisted surgery discussed in Section 4.3, such supervision requires planning to adjust and verify the various medical pre-arrangements. This would be accomplished by using physical phantoms and their digital replicas in the DT tool. These pre-arrangements include patient and surgery specific data, corresponding adjustments of MRI scanner parameters, the MRI compatibility of objects inserted into the scaffold, and the related actuation and control of robotics specific to the surgery involved. Such pre-arrangements are illustrated in Figure 7.
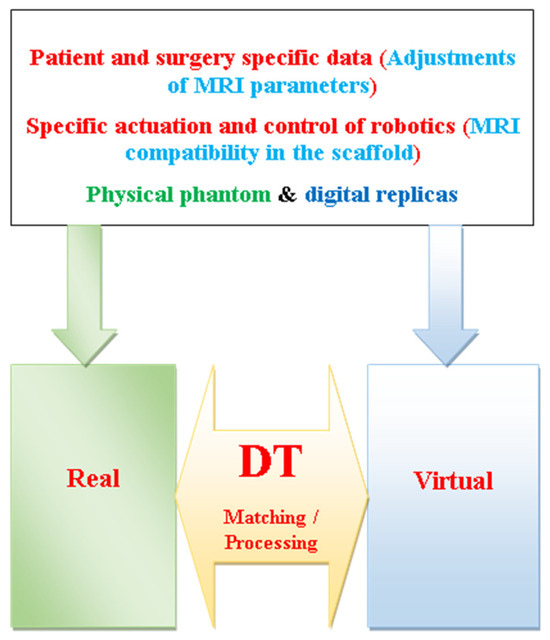
Figure 7.
Schematics of projection of medical arrangements in DT supervision of MRI-assisted surgery.
6.2. Perspectives for Implementation and Tutoring
The planning mentioned in the last section allows to regulate the smooth handling of the intervention and the expected adjustments involved in the therapeutic procedure carried out. Thus, the upcoming surgery involving the patient could be performed in a decent imaging-assisted joint atmosphere, under staff supervision, or possibly as a stand-alone DT monitoring intervention involving staff in the loop. In addition, such preparation permits staff to forecast the disturbances that patients might undergo through the intervention. Such disorders might be associated with robotic surgical tools, implementations, or drugs. Moreover, a DT patient-personalized planning task permits staff, in a harmless manner without the patient, to perform processes, arbitrate decisions, and be aware of likely mistakes of evaluation [17,101,102,103].
Regarding living tissues of different parts of the body, they are represented in the two wings of the DT, as mentioned before, by physical phantoms and their digital replicas (see Figure 7). The tissue behaviors in these models (physical and digital) are of static character, which might be acceptable for some body organs in the context of specific procedures. However, these models are supposed to take into account the biological belongings of real tissues in general [104]. A typical example is the case of soft tissues moistened by fluids that support and manage them. The behavior of soft tissues corresponds to a complex active dynamic behavior corresponding to the mechanics of displacement and deformation of living soft tissues [105,106,107,108,109]. In fact, dealing with such sophisticated mechanical behavior requires adapted behavior laws and numerical approaches, which can only address such a problem in an estimated manner [94,95,96,97,98,99].
6.2.1. Human Involvement and Increased DT
The human–robotic association materialized by the concept of DT with personnel in the loop allows the advanced image-assisted supervision of surgical interventions, thus reducing the risk to the patient and ensuring a reliable end for the personnel [110,111,112]. Moreover, artificial intelligence (AI) exercises in such therapies help to reduce the complexity of data acquisition and post-processing and to perform recurring planned training tasks [113,114]. Furthermore, the intervention can be significantly improved through expanded human–robot connections, advancing the entire arrangement through augmented reality (AR)-assisted robotic actions. Thus, AR combined with an MRI scanner can minimize risks in complex surgical procedures, such as tissue damage, hemorrhage, and post-interventional trauma. In addition, DTs can perform an important job in AR-assisted robotic interventions. Thus, the possible cause of the disorder and its remedy action could be accurately identified via individual patient testing through deep learning databases. Moreover, several additional benefits of AR-DT combined are related to improved accuracy in suturing, gluing, and fixation compared to manual tasks [115,116,117,118,119].
6.2.2. Training Potentials
DT has the potential to transform patient healthcare by progressively bringing personalized and data-driven medical care. Its use is part of digital care and is generally used in individualized medical treatments, which can be disease monitoring and identification, training, or interventions. DTs are often associated, as mentioned earlier, with AI and AR tools, as well as virtual reality (VR). Similarly, VR tutoring improves the ability to imitate everyday training situations with the ability to properly measure execution. Tutoring mainly involves planning a personalized situation or researching new treatments as part of general training.
The basic DT training of commencing staff is connected to the establishment of virtual anatomy and body models to permit performing, skill augmenting, and polishing their perception of tissues of the body. DT common staff training comprises performing scenarios, attainment of vital characters, administrating drugs, and fronting urgent circumstances. This can be associated with patient interests, e.g., cardiac attacks, or environmental situations, e.g., fires. In addition, DT allows training on the use and protection of therapeutic tools and infection management procedures [69,70]. In addition, the training of DTs allows staff to observe the progression of disorders and adapt treatment policies, selecting the most relevant therapy. Such training in individualized planning helps to advance premature diagnosis and explore new treatments or interventions [120,121].
7. Discussion
In a general analysis of different surgical techniques, the results of this review highlighted the importance of minimally invasive, non-ionizing, and precise interventions. In addition, taking advantage of an appropriate digital environment allows staff to plan, predict, prospect, train, and execute with staff in the loop and surgical activities in general. Following the above analyses, some points deserve further discussions:
- The analyses carried out in this contribution focused on open, laparoscopic, robotic, and image-guided surgeries. The only hierarchical classification of these techniques is related to their chronological entry into medicine. The chronological classification is often related to technical innovations such as optical fibers, robotics, numerical control, DT, etc., and mainly to their use in medicine (see for example the studies of [122,123]). Today, each of these surgical techniques has its own specificity and impact depending on the operation concerned and the organ involved. This article in addition has highlighted some potentialities offered by the option of image-assisted surgery and by DT monitoring of the controlled procedure involved. Following the analyses accomplished in this paper relative to the different intervention procedures, Table 1 summarizes an indicative comparison of the different types of interventions regarding invasive class, postoperative scar trace, patient healing time, visualization mode, the control of medical tools, surgical dexterity, the ease of execution, as well as staff ergonomics.
 Table 1. Summarized indicative comparison of the different types of interventions.
Table 1. Summarized indicative comparison of the different types of interventions.
- Regarding the technical innovations mentioned in the previous point, these are often presented as innovative because they find a new modern application even if their notion existed before, for example, the association of earlier neural networks and recent AI. In the context of this article, the recent concept of DT does not escape this observation. The action of DT is based on observation and iterative deduction by imitation introduced by Grieves in 2002 [67], which uses a strategy identical to the oldest method of survival, namely, camouflage described by Bates in 1862 [124], which allows creatures to blend into their environment thanks to adaptive matching.
- All the mentioned types of interventions in this paper are assisted by offline, computerized, or real-time imaging strategies. In open surgery, a pre-made image is exploited offline by the staff. In laparoscopic and robotic surgeries, a pre-made computerized image is involved, and real-time imaging is concerned in image-guided robotic surgery. As for autonomous closed-loop procedures including imaging, they can be used generally in computerized or image-assisted robotic surgeries.
- The concept of DT mentioned and practiced in this article corresponds to different brands of DTs. Indeed, these emphasize the progression of the concept from simple to more sophisticated by adding more capabilities in between. Initially, DT was defined as a static twin, which was followed by twins labeled as mirror (functional or dynamic), shadow (self-adaptive or real-time), and intelligent (self-adaptive augmented by AI). Each of these categories is well suited for specific uses, for example, dynamic for intervention planning, self-adaptive for biomarkers and drug improvement, and intelligent for strategy and care alignment and for individualized treatment.
- In the analysis of DT monitoring (tracking), the notions of complexity, fast matching, coupled models, and reduced models have been addressed at different places. In fact, these four notions are related. A correct matching of a real complex procedure with its virtual replica implies taking into account the real complexity in the virtual model. Thus, the interdependent compound phenomena involved in the complex procedure must be mathematically modeled in a coupled manner [125]. The problem of such an exact full coupled model is that its huge execution time is antagonistic to a supposedly fast real-time matching. One must then find a reduced coupled model behaving exactly physically but with a reduced execution time. In summary, the physical complexity introduces a mathematical complexity, and, for a fast matching, the latter must be reduced but can still represent the former. Thus, reducing a model comprises hurrying its execution while degrading its precision as slim as possible [126]. The dilemma is then often to attain the boundary among saving time and deteriorating correctness. A reduced (surrogate) model is therefore substituted [92,93] for the superior model to obtain a pre-sizing. In addition, one can exercise non-intrusive stochastic methods (e.g., kriging and polynomial chaos) [127,128] that spend 3-D FEM computations with a contained set of realizations (training trials), thus offering efficient (reduction) metamodels.
- Regarding the potential and challenges of using DTs, in addition to studies indicating that their use in the healthcare sector is comprehensive and offers a wide variety of content, other analyses highlighted the challenges and difficulties related to technical fragility as well as data security and confidentiality, which are all obstacles to overcome. DTs in the healthcare sector illustrate a promising fusion of cutting-edge technologies such as AI, IoT, Big Data, and VR to design treatments that improve the efficiency of care. They have transformative potential at various levels of healthcare, from treatment planning to clinical trial design. Their intrinsic ability to provide personalized, prognostic, and dynamic models of specific patients could significantly improve health outcomes, and their ability to replicate complex organizations and leverage available data offers the potential to generate the performance sought by both healthcare providers and technology manufacturers. Nevertheless, DTs are not without challenges. From a technical perspective, the integration of composite technologies, initially not designed to work together, and the need for task synchronization pose a significant challenge. The quality of data shaping DT policy, including its likely direction, the boldness of models, and the diversity of data origins, are just some of the real weaknesses of DTs that need to be seriously assessed to ensure their accuracy and consistency. Moreover, the socio-ethical inferences of DTs are complex. Issues of security and privacy, trust in DT systems, and availability disparities require careful consideration and tailored policy responses [129,130].
- The three different MRI fields B0, B1, and G are assumed to be protected and secure. In fact, the use of MRI scanners is normally safe for patients and medical personnel in the case of using common scanners with moderate efficiency (static field strength and gradient output). For recent high-performance scanners, some traumatic concerns could be observed. Actually, in such circumstances, the EMFs of the scanner can trigger possible uncomfortable side effects for patients or nearby personnel. The RF B1 field reflects a trivial intensity for a negligible duration, and the corresponding tissues that induced currents are insignificant. The pulsed gradient field G produces a variable electric field, which can create unpleasant peripheral nerve stimulation (PNS), especially in modern gradient coils with high output (intensity and scanning speed) [131,132]. In fact, efficient gradient coils produce shorter cycles with higher resolutions, thus amplifying their output, which leads to a shorter imaging duration. These PNSs can trigger sensations of muscle compression, irritation, or numbness. The static B0 field is normally safe for common field strengths around 1.5 T. Recently, due to improved performance, ultra-high-field (UHF) MRI scanners (above 7 T) have been introduced [133]. These UHF scanners induce low-frequency currents in the conductive tissues of the moving body inside or near the scanner [134]. These induced currents and their fields trigger uncomfortable sensations such as falling sensation, light flashes, loss of balance, or muscle tremors (PNS). In addition, the interaction between a strong B0 field and living tissues creates magnetic induction effects due not only to the induced currents but also to Lorenz forces [135]. These forces correspond to charged particles moving through the static field and experiencing forces in a direction perpendicular to the motion. These forces depend on the speed of movement and field intensity and could therefore be important in tissues for UHF scanners. Different disorders could arise due to forces such as, for example, magnetic vestibular stimulation (MVS) [136]. A common reported significant side effect caused by these forces is dizziness, which can eventually lead to nausea [137]. They can also cause involuntary eye motion and other effects [138].
- Regarding MRI compatibility issues, conventionally, an MRI is shielded regarding external field exposure. We can largely typify an external object as MRI compatible if it behaves in an MRI-safe manner, not affecting image quality, and working as expected. An incompatible matter can perturb mainly the RF field as abovementioned, which alters the image. The image quality can be deteriorated for diverse reasons related to the scanner fine tuning, living tissue susceptibility discrepancies, body-embedded matters, and medical tools. In the last two cases, metallic materials, which present susceptibility variations, depend on the size, the shape, and the orientation, which are responsible for image alteration. The electrically conducted materials can behave between dielectric and electric conducting functions of EMF wave frequency. Magnetic, dielectric, or conductor materials are characterized, respectively, by the permeability μ (or the susceptibility χ), by the permittivity ε, or by the conductivity σ. In highly magnetic material, μr >> 1 and μr ≈ χ note that μ = μ0 · μr and χ = μr − 1. For non-magnetic material, μr = 1 and χ = 0. The relative values of σ and ω · ε (ω = 2 π f) characterize dielectric vs. electrically conducting behaviors of electrically conducted materials. For low f, σ >> ω · ε ≈ σ and for high f, ω · ε >> σ ≈ ω · ε and σ ≈ 0. A fully MRI-compatible material has zero values for both χ and σ. The dielectric nature of matters does not affect the compatibility. Regarding the RF field distribution, the eventually introduced matters should have μr = 1 and χ = 0, with high ω · ε, or conductors with a trivial cross-section perpendicular to the RF field B1. With such features, the RF field distribution would not be altered. The MRI compatibility check can be accomplished for existing image-guided MRI systems by using experimental means. This can be carried out by measuring the perturbations of the field resulting in the insertion of checked objects within or near the scaffold according to the case. This is generally accomplished via sensors positioned in specific points in the system. Such techniques in the case of MRI are relatively complex due to the necessity of special shielded expensive chambers and the self-perturbation effects of the measuring sensors. Additionally, the characteristics of a tested object could prove dangerous, leading to the degradation of imagery components. Furthermore, such a compatibility check is only possible for existing systems and cannot be used for the design of unbuilt systems. In these circumstances, a more advantageous solution could be a compatibility check with numerical modeling techniques via an EMC analysis for the different inserted objects. In fact, disturbances in the distribution of EMFs in a given structure caused by the introduction of an external material are related to the EMFs produced in that material. In this case, if the EMF noise is reduced or removed, the field distribution of the target structure will be marginal or not affected.
- Interventional robotic operations are difficult to perform and risky for the patient. It is therefore necessary to be aware of possible adverse events in robotic interventions and healthcare robotic safety standards. Indeed, the use of robotic systems for MI intervention has recently experienced rapid growth. Despite their widespread adoption, numerous technical problems and challenges persist during procedures. Understanding the origin of potential adverse events (death, injury, device malfunctions, etc.) and their consequences for patients will help improve systems and operational procedures to prevent future incidents. Adopting advanced practices in the design and operation of robotic interventional devices and the utilization of strengthened tools for recording adverse events could reduce these avoidable incidents [139]. The involved healthcare robotic safety standards can be found in the study of [140].
- Regarding the closed-loop control of image-guided robotic procedures discussed in Section 4.1, different strategies could be exploited. For example, a shape tracking and feedback control method could be used for operative tools during MRI-guided procedures. Information from sensors and position tracking devices can be integrated to enable shape estimation of the tool under MRI and then integrated into an MRI-compatible robotic tool system. A machine learning modeling method can be used for the robotic tool, with shape tracking used for system characterization (see for example the study of [141]).
- Positioning accuracy (or repeatability) was mentioned at various points in the analyses conducted. A robot’s accuracy is its ability to reach a specific target in its workspace. It ensures that it can follow programmed instructions to perform tasks requiring precise positioning. Accuracy depends on the sensors, the control system, and environmental conditions (temperature, vibration, etc.). The robot’s repeatability (consistency) measures how reliably it returns to the same location. It is affected by joint stability (degree of actuation drift), calibration, and durability [142].
- Perturbations in MRI-guided robotic interventions could be reciprocal. Thus, the MRI scanner is sensitive to EM interference caused by the robot, and the robot’s control system may also be disturbed by the scanner’s field [143].
- MRI-compatible robots could be equipped with piezoelectric actuators as mentioned in Section 5.2. The inherent motion range of these actuators is relatively small. Indeed, this range can be extended by an external means of displacement amplification, directly or indirectly, by converting piezoelectric deformation into displacement. The latter can use elaborate structures, repetition, and stepping strategies. Thus, augmented piezoelectric actuators can achieve larger strokes and responses with more degrees of freedom [144].
- Regarding the applicability of MRI and ultrasound to specific image-guided surgical scenarios, these scanners have common features as well as differentiated attributes, including the intended anatomical application [145,146]. Both enable minimally invasive, non-ionizing, and precise strategies, thus improving patient comfort, safety during execution, and therapeutic efficacy. They can operate in real-time closed-loop control, tracking tissue topology, localizing tools, and monitoring their actions. The scanner’s capabilities and robot skills are thus merged into an efficient task. They can also work with technologies associated with AI, VR, and AR in a digital context well suited for planning, prediction, prospecting, and training in surgical activities in general. The main structural difference between these scanners lies in their limitations. Ultrasound can only operate in airless and boneless windows, while MRI requires support with an environment free of EMFs. This difference is related to their complexity, flexibility of use, portability, cost, and, last but not least, their target anatomy. Thus, MRI can work almost in all organs of the body, unlike ultrasound. The latter offers appropriate flexibility, portability, and cost, while MRI, which provides excellent soft tissue images, is more expensive and complex to use. The anatomical interventions targeted by ultrasound are mainly abdominal, obstetric, vascular, gastric, breast, etc. Examples of corresponding surgical scenarios can be found in the studies of [147,148,149,150,151]. Regarding MRI, the anatomies concerned can have a complex background including prostatic, neurosurgical, breast, orthopedic, cardiac, and oral interventions. Examples of concerned surgical scenarios can be found in different sections and references of [19].
- Regarding the applicability of robot-assisted interventions in terms of feasibility, safety, accuracy, immediate clinical success, and short-term local tumor control, different applications can be found in the literature. For example, for the use of robots in medical thermal ablation procedures, see, e.g., the studies of [152,153,154,155].
8. Conclusions
This contribution analyzed and paralleled open, laparoscopic, robotic, and image-guided robotic surgical interventions with regard to patient well-being, staff efforts, and assignment reliability. Thus, surgeries targeted to be minimally invasive, precise, and safe. The specificities of each type of surgeries have be highlighted. Each of these surgical techniques has its own impact and worth today depending on the operation concerned and the organ involved. Considering the general targets and different advantages of these surgery types, we hence focused on image-guided interventions and their practice in staff training, preparation, and the implementation of a possible autonomous intervention. In order to enhance the accuracy of robotic positioning, the reductions in complexity and uncertainty were managed by matching the actual controlled procedures involved and their virtual replicas through digital twins. Thus, the staff training and/or planning of interventions using real and virtual phantoms were analyzed. As well, the use of augmented matched digital twins for real interventions was discussed.
Like open, laparoscopic, and robotic surgeries, robotic image-assisted surgery has a clear potential in complex and restricted positioning procedures, which could be augmented by its monitoring via digital twins in a computerized environment. In addition, such a digital background is well adapted for the planning, predicting, prospecting, and training of surgical activities in general. The incorporation of robots into MRI-guided interventional procedures advances significant potential. This methodology makes it likely to accurately pursue specific anatomies, such as in microsurgery and neurosurgery, that are effortlessly visible on MRI and otherwise undistinguishable. However, this prospect arrives with many challenges and complexities. Precautious attention to the work stream and other necessities supports attaining the fitting design and compromises to advance high-performance MRI-compatible robotic schemes. In most circumstances, robots are compact, managing specific devices planned to function within the scanner scaffold during imaging. Actuation methodologies must be judiciously appraised and incorporated into designs, and, if required, sensing methods allow robot and instrument tracking during the procedure. The capacity to achieve actions more swiftly and exactly within intraoperative feedback offers MRI-compatible robotics substantial potential to transform the prospect of image-guided interventions. Furthermore, regarding potential adverse events in robotic interventions, future efforts could focus on adopting advanced practices in the design and operation of interventional devices as well as the use of reinforced devices for recording these events, which could reduce these incidents. Moreover, regarding the use of DTs, future efforts will need to focus on developing the technology, promoting its understanding among key stakeholders, and establishing strict rules to reconcile the exploitation of DTs’ broad capabilities with the resolution of associated challenges. A comprehensive and tailored approach, integrating technical progress, supervisory compliance, and ethical concerns, will be essential to harness DTs’ considerable potential for overall healthcare improvement.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Tondini, T.; Isidro, A.; Camarós, E. Case report: Boundaries of oncological and traumatological medical care in ancient Egypt: New palaeopathological insights from two human skulls. Front. Med. 2024, 11, 1371645. [Google Scholar] [CrossRef]
- Bestetti, R.B.; Daniel, R.F.; Geleilete, T.M.; Almeida, A.L.N. From Shamans to Priests of Sekhmet: A Review of the Literature in Search for the Origins of Doctors in Ancient Egypt. Cureus 2024, 16, e67195. [Google Scholar] [CrossRef]
- Hernigou, P.; Hosny, G.A.; Scarlat, M. Evolution of orthopaedic diseases through four thousand three hundred years: From ancient Egypt with virtual examinations of mummies to the twenty-first century. Int. Orthop. 2024, 48, 865–884. [Google Scholar] [CrossRef]
- Whitlock, J. The Evolution of Surgery: A Historical Timeline. A History of Surgery. Verywell Health. Available online: https://www.verywellhealth.com/the-history-of-surgery-timeline-3157332 (accessed on 3 January 2025).
- Tetteh, E.; Wang, T.; Kim, J.Y.; Smith, T.; Norasi, H.; Van Straaten, M.G.; Lal, G.; Chrouser, K.L.; Shao, J.M.; Hallbeck, M.S. Optimizing ergonomics during open, laparoscopic, and robotic-assisted surgery: A review of surgical ergonomics literature and development of educational illustrations. Am. J. Surg. 2024, 235, 115551. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Mechler, U.; Mettler, L.; Pape, J.; Maass, N.; Biebl, M.; Gitas, G.; Laganà, A.S.; Freytag, D. The Development of Laparoscopy-A Historical Overview. Front. Surg. 2021, 8, 799442. [Google Scholar] [CrossRef] [PubMed]
- Barrios, E.L.; Polcz, V.E.; Hensley, S.E.; Sarosi, G.A., Jr.; Mohr, A.M.; Loftus, T.J.; Upchurch, G.R., Jr.; Sumfest, J.M.; Efron, P.A.; Dunleavy, K.; et al. A narrative review of ergonomic problems, principles, and potential solutions in surgical operations. Surgery 2023, 174, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bittner, R. Laparoscopic surgery-15 years after clinical introduction. World J. Surg. 2006, 30, 1190–1203. [Google Scholar] [CrossRef]
- Pérez-Salazar, M.J.; Caballero, D.; Sánchez-Margallo, J.A.; Sánchez-Margallo, F.M. Comparative Study of Ergonomics in Conventional and Robotic-Assisted Laparoscopic Surgery. Sensors 2024, 24, 3840. [Google Scholar] [CrossRef]
- Heemskerk, J.; Zandbergen, R.; Maessen, J.G.; Greve, J.W.; Bouvy, N.D. Advantages of advanced laparoscopic systems. Surg. Endosc. 2006, 20, 730–733. [Google Scholar] [CrossRef]
- Bittner, J.G.; Alrefai, S.; Vy, M.; Mabe, M.; Del Prado, P.A.R.; Clingempeel, N.L. Comparative Analysis of Open and Robotic Transversus Abdominis Release for Ventral Hernia Repair. Surg. Endosc. 2017, 32, 727–734. [Google Scholar] [CrossRef]
- Misiakos, E.P.; Patapis, P.; Zavras, N.; Tzanetis, P.; Machairas, A. Current Trends in Laparoscopic Ventral Hernia Repair. JSLS 2015, 19, e2015.00048. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Piccioni, S.A.; Carbone, L.; Petrioli, R.; Costantini, M.; Malagnino, V.; Bagnacci, G.; Rizzoli, G.; Calomino, N.; Piagnerelli, R.; et al. Posterior and Para-Aortic (D2plus) Lymphadenectomy after Neoadjuvant/Conversion Therapy for Locally Advanced/Oligometastatic Gastric Cancer. Cancers 2024, 16, 1376. [Google Scholar] [CrossRef]
- Chinzei, K.; Hata, N.; Jolesz, F.A.; Kikinis, R. Surgical Assist Robot for the Active Navigation in the Intraoperative MRI: Hardware Design Issues. In Proceedings of the 2000 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2000) (Cat. No.00CH37113), Takamatsu, Japan, 31 October–5 November 2000; pp. 727–732. [Google Scholar] [CrossRef]
- Tsekos, N.V.; Khanicheh, A.; Christoforou, E.; Mavroidis, C. Magnetic resonance-compatible robotic and mechatronics systems for image-guided interventions and rehabilitation: A review study. Annu. Rev. Biomed. Eng. 2007, 9, 351–387. [Google Scholar] [CrossRef]
- Razek, A. Towards an image-guided restricted drug release in friendly implanted therapeutics. Eur. Phys. J. Appl. Phys. 2018, 82, 31401. [Google Scholar] [CrossRef]
- Razek, A. Image-guided surgical and pharmacotherapeutic routines as part of diligent medical treatment. Appl. Sci. 2023, 13, 13039. [Google Scholar] [CrossRef]
- Faoro, G.; Maglio, S.; Pane, S.; Iacovacci, V.; Menciassi, A. An artificial intelligence-aided robotic platform for ultrasound-guided transcarotid revascularization. IEEE Robot. Autom. Lett. 2023, 8, 2349–2356. [Google Scholar] [CrossRef]
- Su, H.; Kwok, K.W.; Cleary, K.; Iordachita, I.I.; Çavuşoğlu, M.C.; Desai, J.P.; Fischer, G.S. State of the art and future opportunities in MRI-guided robot-assisted surgery and interventions. Proc. IEEE Inst. Electr. Electron. Eng. 2022, 110, 968–992. [Google Scholar] [CrossRef] [PubMed]
- Padhan, J.; Tsekos, N.; Al-Ansari, A.; Abinahed, J.; Deng, Z.; Navkar, N.V. Dynamic Guidance Virtual Fixtures for Guiding Robotic Interventions: Intraoperative MRI-guided Transapical Cardiac Intervention Paradigm. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; pp. 265–270. [Google Scholar] [CrossRef]
- Singh, S.; Torrealdea, F.; Bandula, S. MR imaging-guided intervention: Evaluation of MR conditional biopsy and ablation needle tip artifacts at 3T using a balanced fast field echo sequence. J. Vasc. Interv. Radiol. 2021, 32, 1068–1074. [Google Scholar] [CrossRef]
- Xu, L.; Pacia, C.P.; Gong, Y.; Hu, Z.; Chien, C.Y.; Yang, L.; Gach, H.M.; Hao, Y.; Comron, H.; Huang, J.; et al. Characterization of the targeting accuracy of a neuronavigation-guided transcranial FUS system in vitro, in vivo, and in silico. IEEE Trans. Biomed. Eng. 2023, 70, 1528–1538. [Google Scholar] [CrossRef]
- Navarro-Becerra, J.A.; Borden, M.A. Targeted microbubbles for drug, gene, and cell delivery in therapy and immunotherapy. Pharmaceutics 2023, 15, 1625. [Google Scholar] [CrossRef]
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040. [Google Scholar] [CrossRef] [PubMed]
- Razek, A. Augmented therapeutic tutoring in diligent image-assisted robotic interventions. AIMS Med. Sci. 2024, 11, 210–219. [Google Scholar] [CrossRef]
- Razek, A. Planning and performing image-assisted robotic interventions using personalized, minimally invasive, safe, and precise therapeutics. INNOSC Theranostics Pharmacol. Sci. 2024, 8, 4567. [Google Scholar] [CrossRef]
- Bizzarri, N.; Pedone Anchora, L.; Teodorico, E.; Certelli, C.; Galati, G.; Carbone, V.; Gallotta, V.; Naldini, A.; Costantini, B.; Querleu, D.; et al. The role of diagnostic laparoscopy in locally advanced cervical cancer staging. Eur. J. Surg. Oncol. 2024, 50, 108645. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, Y.; Xin, C.; Ji, L.Q.; Li, S.H.; Jiang, W.D.; Zhang, C.M.; Zhang, W.; Lou, Z. Laparoscopic surgery is associated with increased risk of postoperative peritoneal metastases in T4 colon cancer: A propensity score analysis. Int. J. Colorectal. Dis. 2025, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, K.; Glenisson, M.; Loiselet, K.; Fiorenza, V.; Cornet, M.; Capito, C.; Vinit, N.; Pire, A.; Sarnacki, S.; Blanc, T. Robot-assisted laparoscopic adrenalectomy: Extended application in children. Eur. J. Surg. Oncol. 2024, 50, 108627. [Google Scholar] [CrossRef]
- Taylor, R.H.; Menciassi, A.; Fichtinger, G.; Fiorini, P.; Dario, P. Medical Robotics and Computer-Integrated Surgery. In Springer Handbook of Robotics; Siciliano, B., Khatib, O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1657–1684. [Google Scholar] [CrossRef]
- Wan, Q.; Shi, Y.; Xiao, X.; Li, X.; Mo, H. Review of Human–Robot Collaboration in Robotic Surgery. Adv. Intell. Syst. 2024, 2400319. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Wong, S.; Razjigaev, A.; Beier, S.; Peng, S.; Do, T.N.; Song, S.; Chu, D.; Wang, C.H.; et al. A Review on the Form and Complexity of Human–Robot Interaction in the Evolution of Autonomous Surgery. Adv. Intell. Syst. 2024, 6, 2400197. [Google Scholar] [CrossRef]
- Schreiter, J.; Schott, D.; Schwenderling, L.; Hansen, C.; Heinrich, F.; Joeres, F. AR-Supported Supervision of Conditional Autonomous Robots: Considerations for Pedicle Screw Placement in the Future. J. Imaging 2022, 8, 255. [Google Scholar] [CrossRef]
- Williamson, T.; Song, S.E. Robotic Surgery Techniques to Improve Traditional Laparoscopy. JSLS 2022, 26, e2022.00002. [Google Scholar] [CrossRef]
- Rivero-Moreno, Y.; Echevarria, S.; Vidal-Valderrama, C.; Pianetti, L.; Cordova-Guilarte, J.; Navarro-Gonzalez, J.; Acevedo-Rodríguez, J.; Dorado-Avila, G.; Osorio-Romero, L.; Chavez-Campos, C.; et al. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus 2023, 15, e42370. [Google Scholar] [CrossRef]
- Lima, V.L.; de Almeida, R.C.; Neto, T.R.; Rosa, A.A.M. Chapter 72—Robotic ophthalmologic surgery. In Handbook of Robotic Surgery; Zequi, S.C., Ren, H., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 701–704. [Google Scholar] [CrossRef]
- Rivero-Moreno, Y.; Rodriguez, M.; Losada-Muñoz, P.; Redden, S.; Lopez-Lezama, S.; Vidal-Gallardo, A.; Machado-Paled, D.; Cordova Guilarte, J.; Teran-Quintero, S. Autonomous Robotic Surgery: Has the Future Arrived? Cureus 2024, 16, e52243. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Davids, J.; Ashrafian, H.; Darzi, A.; Elson, D.S.; Sodergren, M. A systematic review of robotic surgery: From supervised paradigms to fully autonomous robotic approaches. Int. J. Med. Robot. 2022, 18, e2358. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Baker, T.S.; Bederson, J.B.; Rapoport, B.I. Levels of autonomy in FDA-cleared surgical robots: A systematic review. NPJ Digit. Med. 2024, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Haidegger, T. Autonomy for surgical robots: Concepts and paradigms. IEEE Trans. Med. Robot. Bionics 2019, 1, 65–76. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Meng, D.; Kue, C.S. Medical imaging technology: Principles and systems. INNOSC Theranostics Pharmacol. Sci. 2024, 7, 3360. [Google Scholar] [CrossRef]
- Kraus, M.S.; Coblentz, A.C.; Deshpande, V.S.; Peeters, J.M.; Itriago-Leon, P.M.; Chavhan, G.B. State-of-the-art magnetic resonance imaging sequences for pediatric body imaging. Pediatr. Radiol. 2023, 53, 1285–1299. [Google Scholar] [CrossRef]
- Sennimalai, K.; Selvaraj, M.; Kharbanda, O.P.; Kandasamy, D.; Mohaideen, K. MRI-based cephalometrics: A scoping review of current insights and future perspectives. Dentomaxillofac. Radiol. 2023, 52, 20230024. [Google Scholar] [CrossRef]
- Chianca, V.; Vincenzo, B.; Cuocolo, R.; Zappia, M.; Guarino, S.; Di Pietto, F.; Del Grande, F. MRI Quantitative Evaluation of Muscle Fatty Infiltration. Magnetochemistry 2023, 9, 111. [Google Scholar] [CrossRef]
- Sato, Y.; Takeuchi, T.; Fuju, A.; Takahashi, M.; Hashimoto, M.; Okawa, R.; Hayashi, R. MRI safety for leave-on powdered hair thickeners under 1.5-T and 3.0-T MRI: Measurement of deflection force, MRI artifact, and evaluation of preexamination screening. Phys. Eng. Sci. Med. 2023, 46, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, G.; Istanbullu, O. Analysing the effects of metallic biomaterial design and imaging sequences on MRI interpretation challenges due to image artefacts. Phys. Eng. Sci. Med. 2022, 45, 1163–1174. [Google Scholar] [CrossRef]
- Germann, C.; Nanz, D.; Sutter, R. Magnetic resonance imaging around metal at 1.5 Tesla: Techniques from basic to advanced and clinical impact. Investig. Radiol. 2021, 56, 734–748. [Google Scholar] [CrossRef]
- Germann, C.; Falkowski, A.L.; von Deuster, C.; Nanz, D.; Sutter, R. Basic and advanced metal-artifact reduction techniques at ultra- high field 7-T magnetic resonance imaging-phantom study investigating feasibility and efficacy. Investig. Radiol. 2022, 57, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, T.; Kitamura, N.; Sugeta, M.; Nakatsuka, T.; Ishikawa, R.; Kasuya, S.; Sugiura, Y.; Nakajima, A.; Nakagawa, K.; Terada, H. Diagnostic value of advanced metal artifact reduction magnetic resonance imaging for periprosthetic joint infection. J. Comput. Assist. Tomogr. 2022, 46, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.W.; Nielsen, J.F.; Noll, D.C. Off-resonance artifact correction for MRI: A review. NMR Biomed. 2023, 36, e4867. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Du, H.; Yu, Y. Experimental study of double cable-conduit driving device for MRI compatible biopsy robots. J. Mech. Med. Biol. 2021, 21, 2140014. [Google Scholar] [CrossRef]
- Li, X.; Young, A.S.; Raman, S.S.; Lu, D.S.; Lee, Y.H.; Tsao, T.C.; Wu, H.H. Automatic needle tracking using Mask R-CNN for MRI-guided percutaneous interventions. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1673–1684. [Google Scholar] [CrossRef]
- Bernardes, M.C.; Moreira, P.; Lezcano, D.; Foley, L.; Tuncali, K.; Tempany, C.; Kim, J.S.; Hata, N.; Iordachita, I.; Tokuda, J. In Vivo Feasibility Study: Evaluating Autonomous Data-Driven Robotic Needle Trajectory Correction in MRI-Guided Transperineal Procedures. IEEE Robot. Autom. Lett. 2024, 9, 8975–8982. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.; Patel, N.; Yan, J.; Monfaredi, R.; Cleary, K.; Iordachita, I. Remotely Actuated Needle Driving Device for MRI-Guided Percutaneous Interventions: Force and Accuracy Evaluation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar] [CrossRef]
- Mohith, S.; Upadhya, A.R.; Navin, K.P.; Kulkarni, S.M.; Rao, M. Recent trends in piezoelectric actuators for precision motion and their applications: A review. Smart Mater. Struct. 2020, 30, 013002. [Google Scholar] [CrossRef]
- Gao, X.; Yang, J.; Wu, J.; Xin, X.; Li, Z.; Yuan, X.; Shen, X.; Dong, S. Piezoelectric actuators and motors: Materials, designs, and applications. Adv. Mater. Technol. 2020, 5, 1900716. [Google Scholar] [CrossRef]
- Qiao, G.; Li, H.; Lu, X.; Wen, J.; Cheng, T. Piezoelectric stick-slip actuators with flexure hinge mechanisms: A review. J. Intell. Mater. Syst. Struct. 2022, 33, 1879–1901. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Jin, H.; Ren, K.; Guo, J.; Qiao, L.; Qiu, C.; Chen, W.; He, Y.; Dong, S.; et al. Miniaturized electromechanical devices with multi-vibration modes achieved by orderly stacked structure with piezoelectric strain units. Nat. Commun. 2022, 13, 6567. [Google Scholar] [CrossRef]
- Fu, D.K.; Fan, P.Q.; Yuan, T.; Wang, Y.S. A novel hybrid mode linear ultrasonic motor with double driving feet. Rev. Sci. Instrum. 2022, 93, 025003. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Han, H.; Su, Z.; Sun, H. Design and characteristic analysis of multi-degree-of-freedom ultrasonic motor based on spherical stator. Rev. Sci. Instrum. 2022, 93, 025004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, S.; Zhang, X.; Xu, P.; Zhang, Z.; Ren, L. Bionic stepping motors driven by piezoelectric materials. J. Bionic. Eng. 2023, 20, 858–872. [Google Scholar] [CrossRef]
- Hernandez, C.; Bernard, Y.; Razek, A. Design and manufacturing of a piezoelectric traveling-wave pumping device. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Deng, J.; Gao, X.; Li, J.; Wang, W.; Xun, M.; Ma, X.; Chang, Q.; Liu, J.; et al. Piezo robotic hand for motion manipulation from micro to macro. Nat. Commun. 2023, 14, 500. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Tang, J.; Huang, H.; Zhao, H.; Cheng, Y.; Liu, S.; Li, C.; Xiong, M. A bionic stick-slip piezo-driven positioning platform designed by imitating the structure and movement of the crab. J. Bionic. Eng. 2023, 20, 2590–2600. [Google Scholar] [CrossRef]
- Tao, F.; Sui, F.; Liu, A.; Qi, Q.; Zhang, M.; Song, B.; Guo, Z.; Lu, S.C.Y.; Nee, A.Y.C. Digital twin-driven product design framework. Int. J. Prod. Res. 2019, 57, 3935–3953. [Google Scholar] [CrossRef]
- Grieves, M.; Vickers, J. Digital twin: Mitigating unpredictable, undesirable emergent behavior in complex systems. In Trans-Disciplinary Perspectives on Complex Systems; Springer: Cham, Switzerland, 2017; pp. 85–113. [Google Scholar] [CrossRef]
- Fuller, A.; Fan, Z.; Day, C.; Barlow, C. Digital twin: Enabling technologies, challenges and open research. IEEE Access 2020, 8, 108952–108971. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit. Health 2023, 9, 20552076221149651. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; He, X.; Song, X.; Shu, L.; Li, Z. The digital twin in medicine: A key to the future of healthcare? Front. Med. 2022, 9, 907066. [Google Scholar] [CrossRef]
- De Benedictis, A.; Mazzocca, N.; Somma, A.; Strigaroet, C. Digital twins in healthcare: An architectural proposal and its application in a social distancing case study. IEEE J. Biomed. Health Inform. 2022, 27, 5143–5154. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Exploring the revolution in healthcare systems through the applications of digital twin technology. Biomed. Technol. 2023, 4, 28–38. [Google Scholar] [CrossRef]
- Mohamed, N.; Al-Jaroodi, J.; Jawhar, I.; Kesserwan, N. Leveraging digital twins for healthcare systems engineering. IEEE Access 2023, 11, 69841–69853. [Google Scholar] [CrossRef]
- Al-Jaroodi, J.; Mohamed, N. Enhancing the Efficiency of Healthcare Facilities Management with Digital Twins. In Proceedings of the 2024 International Conference on Smart Applications, Communications and Networking (SmartNets), Harrisonburg, VA, USA, 28–30 May 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Ricci, A.; Croatti, A.; Montagna, S. Pervasive and connected digital twins-a vision for digital health. IEEE Internet Comput. 2022, 26, 26–32. [Google Scholar] [CrossRef]
- Okegbile, S.D.; Cai, J. Edge-Assisted Human-to-Virtual Twin Connectivity Scheme for Human Digital Twin Frameworks. In Proceedings of the 2022 IEEE 95th Vehicular Technology Conference: (VTC2022-Spring), Helsinki, Finland, 19–22 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Das, C.; Mumu, A.A.; Ali, M.F.; Sarker, S.K.; Mmuyeen, S.; Das, S.K.; Das, P.; Hasan, M.; Tasneem, Z.; Islam, M.; et al. Toward IoRT collaborative digital twin technology enabled future surgical sector: Technical innovations, opportunities and challenges. IEEE Access 2022, 10, 129079–129104. [Google Scholar] [CrossRef]
- Carbonaro, A.; Marfoglia, A.; Nardini, F.; Mellone, S. CONNECTED: Leveraging digital twins and personal knowledge graphs in healthcare digitalization. Front. Digit. Health 2023, 5, 1322428. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Ulapane, N.; Sloane, E.B.; Gehlot, V. Digital Twins for More Precise and Personalized Treatment. Stud. Health Technol. Inform. 2024, 310, 229–233. [Google Scholar] [CrossRef]
- Razek, A. Monitoring complexity in clean energy systems applications. Clean Energy Sustain. 2024, 2, 10007. [Google Scholar] [CrossRef]
- Maxwell, J.C., VIII. A dynamical theory of the electromagnetic field. Philos. Trans. R. Soc. 1865, 155, 459–512. [Google Scholar] [CrossRef]
- Nunes, A.S.; Chadebec, O.; Kuo-Peng, P.; Dular, P.; Meunier, G. A Coupling between the Facet Finite Element and Reluctance Network Methods in 3-D. IEEE Trans. Magn. 2017, 53, 2723576. [Google Scholar] [CrossRef]
- Henrotte, F.; Geuzaine, C. Electromagnetic forces and their finite element computation. Int. J. Numer. Model. 2024, 37, e3290. [Google Scholar] [CrossRef]
- Antunes, O.J.; Bastos, J.P.A.; Sadowski, N.; Razek, A.; Santandrea, L.; Bouillault, F.; Rapetti, F. Comparison between nonconforming movement methods. IEEE Trans. Magn. 2006, 42, 599–602. [Google Scholar] [CrossRef]
- Ren, Z.; Razek, A. Comparison of some 3D eddy current formulations in dual systems. IEEE Trans. Magn. 2000, 36, 751–755. [Google Scholar] [CrossRef]
- Gürbüz, I.T.; Martin, F.; Rasilo, P.; Billah, M.M.; Belahcen, A. A new methodology for incorporating the cutting deterioration of electrical sheets into electromagnetic finite-element simulation. J. Magn. Magn. Mater. 2024, 593, 171843. [Google Scholar] [CrossRef]
- da Silva, I.P.C.; de Miranda, R.A.; Sadowski, N.; Batistela, N.J.; Bernard, L.; Bastos, J.P. Modeling of Electrical Machines Hysteresis Losses under Mechanical Stresses. J. Microw. Optoelectron. Electromagn. Appl. 2024, 23, e2024283838. [Google Scholar] [CrossRef]
- Urdaneta-Calzadilla, A.; Chadebec, O.; Galopin, N.; Niyonzima, I.; Meunier, G.; Bannwarth, B. Modeling of Magnetoelectric Effects in Composite Structures by FEM–BEM Coupling. IEEE Trans. Magn. 2023, 59, 3235211. [Google Scholar] [CrossRef]
- Pohlmann, A.; Lessmann, M.; Finocchiaro, T.; Schmitz-Rode, T.; Hameyer, K. Numerical Computation Can Save Life: FEM Simulations for the Development of Artificial Hearts. IEEE Trans. Magn. 2011, 47, 1166–1169. [Google Scholar] [CrossRef]
- Gu, B.; Li, H.; Li, B. An internal ballistic model of electromagnetic railgun based on PFN coupled with multi-physical field and experimental validation. Def. Technol. 2024, 32, 254–261. [Google Scholar] [CrossRef]
- Razek, A. Coupled Models in Electromagnetic and Energy Conversion Systems from Smart Theories Paradigm to That of Complex Events: A Review. Appl. Sci. 2022, 12, 4675. [Google Scholar] [CrossRef]
- Kudela, J.; Matousek, R. Recent advances and applications of surrogate models for finite element method computations: A review. Soft. Comput. 2022, 26, 13709–13733. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, X.; Dhimish, M.; Qiu, W.; Niu, S. A Review of Data-driven Surrogate Models for Design Optimization of Electric Motors. IEEE Trans. Transp. Electrif. 2024, 10, 8413–84311. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Barchanski, A.; Steiner, T.; De Gersem, H.; Clemens, M.; Weiland, T. Local grid refinement for low-frequency current computations in 3-D human anatomy models. IEEE Trans. Magn. 2006, 42, 1371–1374. [Google Scholar] [CrossRef]
- Hasgall, P.A.; Neufeld, E. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, Version 4.1. 2022. Available online: https://itis.swiss/virtual-population/tissue-properties/database/dielectric-properties (accessed on 22 February 2022).
- Makarov, S.N.; Noetscher, G.M.; Yanamadala, J.; Piazza, M.W.; Louie, S.; Prokop, A.; Nazarian, A.; Nummenmaa, A. Virtual Human Models for Electromagnetic Studies and Their Applications. IEEE Rev. Biomed. Eng. 2017, 10, 95–121. [Google Scholar] [CrossRef]
- Harris, L.R.; Zhadobov, M.; Chahat, N.; Sauleau, R. Electromagnetic dosimetry for adult and child models within a car: Multi-exposure scenarios. Int. J. Microw. Wirel. Technol. 2011, 3, 707–715. [Google Scholar] [CrossRef]
- Gjonaj, E.; Bartsch, M.; Clemens, M.; Schupp, S.; Weiland, T. High-resolution human anatomy models for advanced electromagnetic field computations. IEEE Trans. Magn. 2002, 38, 357–360. [Google Scholar] [CrossRef]
- Razek, A. Biological and Medical Disturbances Due to Exposure to Fields Emitted by Electromagnetic Energy Devices—A Review. Energies 2022, 15, 4455. [Google Scholar] [CrossRef]
- Sears, V.A.; Morris, J.M. Establishing a point-of-care virtual planning and 3D printing program. Semin. Plast. Surg. 2022, 36, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Elkefi, S.; Asan, O. Digital twins for managing health care systems: Rapid literature review. J. Med. Internet Res. 2022, 24, e37641. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Alì, M.; Irmici, G.; Ibba, S.; Caloro, E.; Fazzini, D.; Oliva, G.; Papa, S. Digital Twins: The New Frontier for Personalized Medicine? Appl. Sci. 2023, 13, 7940. [Google Scholar] [CrossRef]
- Kangasmaa, O.; Laakso, I.; Schmid, G. Estimating Human Fat and Muscle Conductivity From 100 Hz to 1 MHz Using Measurements and Modelling. Bioelectromagnetics 2025, 46, e22541. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D. Biological soft tissues. In Springer Handbook of Experimental Solid Mechanics; Sharpe, W., Ed.; Springer: Boston, MA, USA, 2008. [Google Scholar] [CrossRef]
- Kallin, S. Deformation of Human Soft Tissues: Experimental and Numerical Aspects. Licentiate Thesis, Jönköping University, School of Health and Welfare, HHJ, Department of Rehabilitation, Jönköping, Sweden, 2019. Available online: https://hj.diva-portal.org/smash/get/diva2:1344790/FULLTEXT01.pdf (accessed on 17 January 2025).
- Al-Dirini, R.M.A.; Reed, M.P.; Hu, J.W.; Thewlis, D. Development and validation of a high anatomical fidelity FE model for the buttock and thigh of a seated individual. Ann. Biomed. Eng. 2016, 44, 2805–2816. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer: New York, NY, USA; Berlin, Germany, 1993. [Google Scholar] [CrossRef]
- Henninger, H.B.; Reese, S.P.; Anderson, A.E.; Weiss, J.A. Validation of computational models in biomechanics. Proc. Inst. Mech. Eng. H 2010, 224, 801–881. [Google Scholar] [CrossRef]
- Song, Y. Human digital twin, the development and impact on design. J. Comput. Inf. Sci. Eng. 2023, 23, 060819. [Google Scholar] [CrossRef]
- Burattini, S.; Montagna, S.; Croatti, A.; Gentili, N.; Ricci, A.; Leonardi, L.; Pandolfini, S.; Tosi, S. An Ecosystem of Digital Twins for Operating Room Management. In Proceedings of the 2023 IEEE 36th International Symposium on Computer-Based Medical Systems (CBMS), L’Aquila, Italy, 22–24 June 2023; pp. 770–775. [Google Scholar] [CrossRef]
- Hagmann, K.; Hellings-Kuß, A.; Klodmann, J.; Richter, R.; Stulp, F.; Leidner, D. A digital twin approach for contextual assistance for surgeons during surgical robotics training. Front. Robot. AI 2021, 8, 735566. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef]
- Cusumano, D.; Boldrini, L.; Dhont, J.; Fiorino, C.; Green, O.; Güngör, G.; Jornet, N.; Klüter, S.; Landry, G.; Mattiucci, G.C.; et al. Artificial intelligence in magnetic resonance guided radiotherapy: Medical and physical considerations on state of art and future perspectives. Phys. Med. 2021, 85, 175–191. [Google Scholar] [CrossRef]
- Seetohul, J.; Shafiee, M.; Sirlantzis, K. Augmented reality (AR) for surgical robotic and autonomous systems: State of the art, challenges, and solutions. Sensors 2023, 23, 6202. [Google Scholar] [CrossRef] [PubMed]
- Avrumova, F.; Lebl, D.R. Augmented reality for minimally invasive spinal surgery. Front. Surg. 2023, 9, 1086988. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, J.; Deguet, A.; Taylor, R.H.; Dou, Q. Integrating Artificial Intelligence and Augmented Reality in Robotic Surgery: An Initial dVRK Study Using a Surgical Education Scenario. In Proceedings of the International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 13–15 April 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Fu, J.; Rota, A.; Li, S.; Zhao, J.; Liu, Q.; Iovene, E.; Ferrigno, G.; De Momi, E. Recent Advancements in Augmented Reality for Robotic Applications: A Survey. Actuators 2023, 12, 323. [Google Scholar] [CrossRef]
- Qian, L.; Wu, J.Y.; DiMaio, S.P.; Navab, N.; Kazanzides, P. A review of augmented reality in robotic-assisted surgery. IEEE Trans. Med. Robot. Bionics 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Kukushkin, K.; Ryabov, Y.; Borovkov, A. Digital twins: A systematic literature review based on data analysis and topic modeling. Data 2022, 7, 173. [Google Scholar] [CrossRef]
- Armeni, P.; Polat, I.; De Rossi, L.M.; Diaferia, L.; Meregalli, S.; Gatti, A. Digital twins in healthcare: Is it the beginning of a new era of evidence-based medicine? A critical review. J. Pers. Med. 2022, 12, 1255. [Google Scholar] [CrossRef]
- Tortorici, S.; Difalco, P.; Caradonna, L.; Tetè, S. Traditional endodontic surgery versus modern technique: A 5-year controlled clinical trial. J. Craniofac. Surg. 2014, 25, 804–807. [Google Scholar] [CrossRef]
- You, C.; Lu, H.; Zhao, J.; Qin, B.; Liu, W. The Comparison Between Traditional Versus 3D Printing Combined with Computer Navigation Technique in the Management of Orbital Blowout Fractures. J. Craniofac. Surg. 2025, 36, 201–205. [Google Scholar] [CrossRef]
- Bates, H.W. Contributions to an insect fauna of the amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 1862, 23, 495–566. [Google Scholar] [CrossRef]
- Razek, A. Matching of an observed event and its virtual model in relation to smart theories, coupled models and supervision of complex procedures—A review. Comptes Rendus Phys. 2024, 25, 141–156. [Google Scholar] [CrossRef]
- Razek, A. Strategies for managing models regarding environmental confidence and complexity involved in intelligent control of energy systems—A review. Adv. Environ. Energies 2023, 2, aee020104. [Google Scholar] [CrossRef]
- Lebensztajn, L.; Marretto, C.A.R.; Caldora Costa, M.; Coulomb, J.L. Kriging: A useful tool for electromagnetic device optimization. IEEE Trans. Magn. 2004, 40, 1196–1199. [Google Scholar] [CrossRef]
- Gaignaire, R.; Scorretti, R.; Sabariego, R.V.; Geuzaine, C. Stochastic uncertainty quantification of eddy currents in the human body by polynomial chaos decomposition. IEEE Trans. Magn. 2012, 48, 451–454. [Google Scholar] [CrossRef]
- Sheng, B.; Wang, Z.; Qiao, Y.; Xie, S.Q.; Tao, J.; Duan, C. Detecting latent topics and trends of digital twins in healthcare: A structural topic model-based systematic review. Digit. Health 2023, 12, 20552076231203672. [Google Scholar] [CrossRef]
- Armeni, P.; Polat, I.; Maria De Rossi, L.; Diaferia, L.; Visioli, G.; Meregalli, S.; Gatti, A. Perspective Chapter: Digital Twins for Health–Opportunities, Barriers and a Path Forward. In Digital Twin Technology—Fundamentals and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Bourland, J.D.; Nyenhuis, J.A.; Ham, C.L.; Engels, J.M.; Hebrank, F.X.; Frese, G.; Schaefer, D.J. Comparison of the threshold for peripheral nerve stimulation during gradient switching in whole body MR systems. J. Magn. Reson. Imaging 2002, 15, 520–525. [Google Scholar] [CrossRef]
- Davids, M.; Guerin, B.; Klein, V.; Wald, L.L. Optimization of MRI Gradient Coils with Explicit Peripheral Nerve Stimulation Constraints. IEEE Trans. Med. Imaging 2021, 40, 129–142. [Google Scholar] [CrossRef]
- Thylur, D.S.; Jacobs, R.E.; Go, J.L.; Toga, A.W.; Niparko, J.K. Ultra-High-Field Magnetic Resonance Imaging of the Human Inner Ear at 11.7 Tesla. Otol. Neurotol. 2017, 38, 133–138. [Google Scholar] [CrossRef]
- Crozier, S.; Liu, F. Numerical evaluation of the fields induced by body motion in or near high-field MRI scanners. Prog. Biophys. Mol. Biol. 2005, 87, 267–278. [Google Scholar] [CrossRef]
- van Osch, M.J.P.; Webb, A.G. Safety of Ultra-High Field MRI: What are the Specific Risks? Curr. Radiol. Rep. 2014, 2, 61. [Google Scholar] [CrossRef][Green Version]
- Bouisset, N.; Laakso, I. Induced electric fields in MRI settings and electric vestibular stimulations: Same vestibular effects? Exp. Brain Res. 2024, 242, 2493–2507. [Google Scholar] [CrossRef]
- Mian, O.S.; Li, Y.; Antunes, A.; Glover, P.M.; Day, B.L. On the Vertigo Due to Static Magnetic Fields. PLoS ONE 2013, 8, e78748. [Google Scholar] [CrossRef] [PubMed]
- Boegle, R.; Dieterich, M.; Kirsch, V. Comment on: Modulatory Effects of Magnetic Vestibular Stimulation on Resting-State Networks Can be Explained by Subject-Specific Orientation of Inner Ear Anatomy in the MR Static Magnetic Field. J. Exp. Neurol. 2020, 1, 109–114. [Google Scholar] [CrossRef]
- Alemzadeh, H.; Raman, J.; Leveson, N.; Kalbarczyk, Z.; Iyer, R.K. Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data. PLoS ONE 2016, 11, e0151470. [Google Scholar] [CrossRef] [PubMed]
- IEC 80601-2-77; Medical Electrical Equipment–Part 2-77: Particular Requirements for the Basic Safety and Essential Performance of Robotically Assisted Surgical Equipment. Edition 1.0 2023-11. International Organization for Standardization: Geneva, Switzerland, 2023. Available online: https://cdn.standards.iteh.ai/samples/105816/bbc0798be8804b099e40cde2ca1988f6/IEC-80601-2-77-2019-AMD1-2023.pdf (accessed on 1 March 2025).
- Dong, Z.; Wang, X.; Fang, G.; He, Z.; Ho, J.D.L.; Cheung, C.L.; Tang, W.L.; Xie, X.; Liang, L.; Chang, H.C.; et al. Shape Tracking and Feedback Control of Cardiac Catheter Using MRI-Guided Robotic Platform-Validation with Pulmonary Vein Isolation Simulator in MRI. IEEE Trans. Robot. 2022, 38, 2781–2798. [Google Scholar] [CrossRef]
- Li, G.; Patel, N.A.; Melzer, A.; Sharma, K.; Iordachita, I.; Cleary, K. MRI-guided lumbar spinal injections with body-mounted robotic system: Cadaver studies. Minim. Invasive Ther. Allied Technol. 2022, 31, 297–305. [Google Scholar] [CrossRef]
- Yu, N.; Gassert, R.; Riener, R. Mutual interferences and design principles for mechatronic devices in magnetic resonance imaging. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 473–488. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Wang, X.; Wang, Z.; Zhu, Q.; Sun, J.; Huang, P.; Wang, X.; Huang, W.; Lu, Q. Review on piezoelectric actuators: Materials, classifications, applications, and recent trends. Front. Mech. Eng. 2024, 19, 6. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhao, B.; Xie, D.; Du, H.; Duan, X.; Hu, Y.; Zhang, L. Advances of surgical robotics: Image-guided classification and application. Natl. Sci. Rev. 2024, 11, nwae186. [Google Scholar] [CrossRef]
- Biswas, P.; Sikander, S.; Kulkarni, P. Recent advances in robot-assisted surgical systems. Biomed. Eng. Adv. 2023, 6, 100109. [Google Scholar] [CrossRef]
- Binte Gazali, N.A.; Bakar, R.A.; Tan, C.L.C.; Bo, J.; Lee, S.H.E.; Ho, H.P.; Lim, S.Y.; Cheong, W.K.; Liaw, S.C.J.; Ooi, C.C. Comparing conventional abdominal ultrasound scanning versus a ROBotic assisted UltraSonography sysTem (ROBUST). WFUMB Ultrasound Open 2024, 2, 100040. [Google Scholar] [CrossRef]
- Okuzaki, K.; Koizumi, N.; Yoshinaka, K.; Nishiyama, Y.; Zhou, J.; Tsumura, R. Rib region detection for scanning path planning for fully automated robotic abdominal ultrasonography. Int. J. CARS 2024, 19, 449–457. [Google Scholar] [CrossRef]
- Kapuria, S.; Bonyun, J.; Kulkarni, Y.; Ikoma, N.; Chinchali, S.; Alambeigi, F. Robot-Enabled Machine Learning-Based Diagnosis of Gastric Cancer Polyps Using Partial Surface Tactile Imaging. In Proceedings of the 2024 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2024), Abu Dhabi, United Arab Emirates, 14–18 October 2024. [Google Scholar] [CrossRef]
- Tan, J.; Li, J.; Li, Y.; Li, B.; Leng, Y.; Rong, Y.; Fu, C. Autonomous Trajectory Planning for Ultrasound-Guided Real-Time Tracking of Suspicious Breast Tumor Targets. IEEE Trans. Autom. Sci. Eng. 2024, 21, 2478–2493. [Google Scholar] [CrossRef]
- von Haxthausen, F.; Böttger, S.; Wulff, D.; Hagenah, J.; García-Vázquez, V.; Ipsen, S. Medical Robotics for Ultrasound Imaging: Current Systems and Future Trends. Curr. Robot. Rep. 2021, 2, 55–71. [Google Scholar] [CrossRef]
- Bonnet, B.; de Baère, T.; Beunon, P.; Feddal, A.; Tselikas, L.; Deschamps, F. Robotic-assisted CT-guided percutaneous thermal ablation of abdominal tumors: An analysis of 41 patients. Diagn. Interv. Imaging 2024, 105, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Shen, N.; Feng, L.; Ran, Z.; Deng, Z. A practical pretreatment planning method of multiple puncturing for thermal ablation surgery. Biocybern. Biomed. Eng. 2020, 40, 1469–1485. [Google Scholar] [CrossRef]
- Miaskowski, A.; Gas, P. Numerical Estimation of SAR and Temperature Distributions inside Differently Shaped Female Breast Tumors during Radio-Frequency Ablation. Materials 2023, 16, 223. [Google Scholar] [CrossRef]
- Gas, P.; Miaskowski, A. Influence of the Radiofrequency Applicators Arrangement on the Sizes of Ablative Zones inside Hepatic Tumor. Arch. Electr. Eng. 2024, 73, 557–571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).