Mineral Composition of Chelidonium majus L. and Soils in Urban Areas
Abstract
1. Introduction
2. Materials and Methods
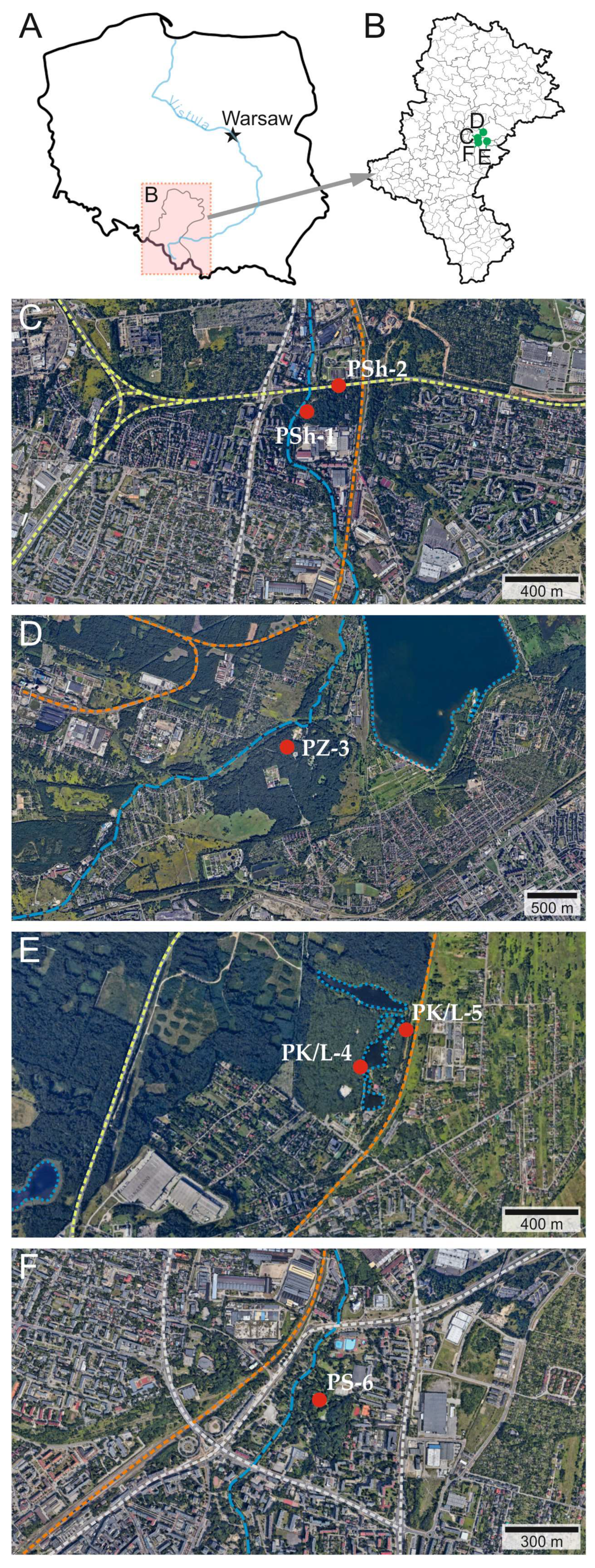
2.1. Study Area
2.2. Plant and Soil Martials Sampling
2.3. Analytical Studies of Soil–Plant Composition by Indices
2.4. Statistical Analysis
3. Results
3.1. The Concentration of Major Elements in Ch. majus and Soil
3.2. Concentration of Microelements in Tissues of Chelidonium majus and Soil
3.3. Environmental Indicators
3.4. Analysis of the Correlation of Major Element Content in the Plant–Soil System
3.5. Analysis of Correlation of Microelement and Rare Earth Metal Content in the Plant–Soil System
4. Discussion
4.1. Environmental Indices
4.2. Bioaccumulation and Translocation
5. Conclusions
- In all analyzed tissues, the dominant element in terms of content was potassium (K), with the highest value recorded in the stems of Ch. majus, while, in the soil, it had low values;
- The highest calcium (Ca) content was found in the leaves in all locations, while, in the rhizomes and stems, the values differed between locations. Compared to K, Ca is characterized by a higher value in the soil;
- The contents of phosphorus, magnesium, and sulfur in tissues and soil are varied, while sodium and aluminum have low values in both the underground and aboveground parts of Ch. majus. The contents of phosphorus, iron, magnesium. and sulfur in tissues and soil are varied. At the same time, sodium and aluminum have low values in both the underground and aboveground parts of Ch. majus, while high contents in the soil characterize Fe and Al;
- In the case of microelements, boron (B) predominates in the leaves, rubidium (Rb) in the stems, and silver (Ag) in the rhizomes and soil;
- The results of the analyzed environmental indicators I-geo, EF, and CF did not show (except for a few cases) significant changes in soil enrichment with major elements from external sources;
- The study found good agreement between the results obtained in this work and the levels of elements in the literature on medicinal plants. However, higher values of some elements (Ba, K) were found in some samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus—An integrative review: Traditional knowledge versus modern findings. Forsch Komplementarmedizin 2010, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Maji, A.K.; Banerji, P. Chelidonium majus L. (Greater celandine)—A review on its phytochemical and therapeutic perspectives. Int. J. Herb. Med. 2015, 3, 10–27. [Google Scholar] [CrossRef]
- Pantano, F.; Mannocchi, G.; Marinelli, E.; Gentili, S.; Graziano, S.; Busardò, F.P.; di Luca, N.M. Hepatotoxicity induced by greater celandine (Chelidonium majus L.): A review of the literature. Eur. Rev. Med. Pharmacol. 2017, 21 (Supp. 1), 46–52. [Google Scholar]
- Zielińska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s Ups and Downs−21 Centuries of Medicinal Uses of Chelidonium majus From the Viewpoint of Today’s. Pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Wang, M.; Wang, Z.; Kuang, H. Alkaloids in Chelidonium majus L.: A review of its phytochemistry, pharmacology and toxicology. Front. Pharmacol. 2024, 15, 1440979. [Google Scholar] [CrossRef]
- Ciornolutchii, V.; Ismaiel, A.; Sabo, C.M.; Al Hajjar, N.; Seicean, A.; Dumitrascu, D.L. A hidden cause of hypertransaminasemia: Liver toxicity caused by Chelidonium majus L.: Report of two cases of herb-induced liver injury and literature review. Am. J. Ther. 2024, 31, e382–e387. [Google Scholar] [CrossRef]
- Borges, A.; Calvo, M.L.M.; Vaz, J.A.; Calhelha, R.C. Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings. Materials 2024, 17, 4199. [Google Scholar] [CrossRef]
- Rahmonov, O.; Środek, D.; Pytel, S.; Kupka, T.; Makieieva, N. Accumulation of heavy metals in soil and Chelidonium majus L. in an urban environment. J. Elem. 2023, 28, 1329–1351. [Google Scholar] [CrossRef]
- Rahmonov, O.; Środek, D.; Pytel, S.; Makieieva, N.; Kupka, T. Relationships between Heavy Metal Concentrations in Greater Celandine (Chelidonium majus L.) Tissues and Soil in Urban Parks. Int. J. Environ. Res. Public Health 2023, 20, 3887. [Google Scholar] [CrossRef]
- Zare, G.; Diker, N.Y.; Arituluk, Z.C.; Tatli Cankaya, I.I. Chelidonium majus L. (Papaveraceae) morphology, anatomy and traditional medicinal uses in Turkey. İstanbul J. Pharm. 2021, 51, 123–132. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA) Committee on Herbal Medicinal Products (HMPC): Public Statement on Chelidonium majus L., Herba EMA/HMPC/743927/2010. Available online: https://www.ema.europa.eu/en/documents/public-statement/final-public-statement-chelidonium-majus-l-herba_en.pdf (accessed on 12 February 2025).
- Moro, P.A.; Cassetti, F.; Giugliano, G.; Falce, M.T.; Mazzanti, G.; Menniti-Ippolito, F.; Raschetti, R.; Santuccio, C. Hepatitis from Greater celandine (Chelidonium majus L.): Review of literature and report of a new case. J. Ethnopharmacol. 2009, 124, 328–332. [Google Scholar] [CrossRef]
- Kokoska, L.; Polensky, Z.; Rada, V.; Nepovim, A.; Vanek, T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002, 82, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Meng, F.; Hao, X.; Zhang, Y.; Wang, G.; Xu, G. Antibacterial alkaloids from Chelidonium majus Linn (Papaveraceae) against clinical isolated of methicillin resistant Staphylococcus aureus. J. Pharm. Pharm. Sci. 2008, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K. The Pharmacology of Chinese Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lee, Y.; Kim, S.; Roh, S.; Choi, H.; Seo, Y. Suppressive effects of Chelidonium majus methanol extract in knee joint, regional lymph nodes, and spleen on collagen-induced arthritis in mice. J. Ethnopharmacol. 2007, 112, 40–48. [Google Scholar] [CrossRef]
- Kedzia, B.; Łozykowska, K.; Gryszczynska, A. Chemical composition and contents of biological active substances in Chelidonium majus L. Postępy Fitoter. 2013, 3, 174–181. [Google Scholar]
- Monavari, S.; Shahrabadi, M.; Keyvani, H.; Bokharaei-Salim, F. Evaluation of in vitro antiviral activity of Chelidonium majus L. against Herpes simplex virus type-1. Afr. J. Microbiol. Res. 2012, 6, 4360–4364. [Google Scholar]
- Hao, D.; Gu, X.; Xiao, P. Medicinal Plants Chemistry, Biology and Omics; Woodhead Publishing: Cambridge, UK, 2015; pp. 171–216. [Google Scholar]
- Makieieva, N.; Kupka, T.; Spaleniak, G.; Rahmonov, O.; Marek, A.; Błażytko, A.; Pentak, D.; Kuś, P.; Kusz, J.; Książek, M. Experimental and theoretical characterization of chelidonic acid structure. Struct. Chem. 2022, 33, 2133–2145. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova-Panovska, T.; Bačeva, K.; Stafilov, T. Metalic trace elements in medicinal plants from Macedonia. MEJSR 2011, 7, 109–114. [Google Scholar]
- Prashanth, L.; Kattapagari, K.; Chitturi, R.; Baddam, V.; Prasad, L.K. A review on role of essential trace elements in health and disease. J. Dr. YSR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar]
- Silva, P.; Francisconi, L.; Gonçalves, R. Evaluation of major and trace elements in medicinal plants. J. Braz. Chem. Soc. 2016, 27, 2273–2289. [Google Scholar] [CrossRef]
- Lovkova, M.; Buzuk, G.; Sokolova, S.; Kliment’eva, N. Chemical features of medicinal plants. Appl. Biochem. Microbiol. 2001, 37, 229–237. [Google Scholar] [CrossRef]
- Soetan, K.; Olaiya, C.; Oyewole, O. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Sárközi, Á.; Then, M.; Szentmihályi, K. Mineral element content of greater celandine (Chelidonium majus L.). Acta Aliment Hung 2005, 34, 113–120. [Google Scholar] [CrossRef]
- Badea, D.N. Determination of potentially toxic heavy metals (Pb, Hg, Cd) in popular medicinal herbs in the coal power plant area. Rev. Chim. 2015, 66, 1132–1136. [Google Scholar]
- Szentmihályi, K.; Szőllősi-Varga, I.; Then, M. Elements, alkaloids and antioxidant value of Chelidonium majus L. and the extracts obtained by different extraction methods. Eur. Chem. Bull. 2021, 10, 58–66. [Google Scholar] [CrossRef]
- Rahmonov, O.; Kowal, A.; Rahmonov, M.; Pytel, S. Variability of Concentrations of Potentially Toxic Metals in the Topsoil of Urban Forest Parks (Southern Poland). Forests 2024, 15, 1020. [Google Scholar] [CrossRef]
- Hantos, M.; Tekes, K.; Szalay, F. Peripheral nociceptin levels in Wilson disease. Acta Pharm. Hung 2002, 72, 260–264. [Google Scholar] [PubMed]
- Hegedus, D.; Ferencz, V.; Lakatos, P.; Mészáros, S.; Lakatos, P.; Horvath, C.; Szalay, F. Decreased bone density, elevated serum osteoprotegerin, and β-cross-laps in Wilson disease. J. Bone Miner. Res. 2002, 17, 1961–1967. [Google Scholar] [CrossRef]
- Chandra, R. Essential and Toxic Trace Elements in Human Health and Disease; Alan R. Liss Inc.: New York, NY, USA, 1988; pp. 337–346. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elementsin Soil and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- De Pasquale, A.; Paino, E.; De Pasquale, R.; Germano, M.P. Contamination by heavy metals in drugs from different commercial sources. Pharmacol. Res. 1993, 27, 9–10. [Google Scholar] [CrossRef]
- Jain, L.; Shahoo, R.; Sondhi, S. Analysis for mineral elements of some medicinal plants. Indian Drugs 1992, 29, 187–190. [Google Scholar]
- Chizzola, R.; Franz, C. Metalic trace elements in medicinal and aromatic plants from Austria. Angew. Bot. 1996, 70, 52–56. [Google Scholar]
- Maday, E.; Szentmihályi, K.; Then, M.; Szőke, É. Mineral element content of chamomile. Acta Aliment. 2000, 29, 51–57. [Google Scholar]
- Leśniewicz, A.; Jaworska, K.; Żyrnicki, W. Macro-and micro-nutrients and their bioavailability in polish herbal medicaments. Food Chem. 2006, 99, 670–679. [Google Scholar] [CrossRef]
- Bello, M.; Ibrahim, A.; Ogunwande, I.; Olawore, N. Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem. 2004, 85, 67–71. [Google Scholar]
- Szentmihályi, K.; Marczal, G.; Then, M. Medicinal plants in view of trace elements. Thaiszia J. Bot. 2006, 16, 99–107. [Google Scholar]
- Ebrahim, A.; Eltayeb, M.; Khalid, H.; Mohamed, H.; Abdalla, W.; Grill, P.; Michalke, B. Study on selected trace elements and heavy metals in some popular medicinal plants from Sudan. J. Nat. Med. 2012, 66, 671–679. [Google Scholar] [CrossRef]
- Aziz, M.A.; Adnan, M.; Begum, S.; Azizullah, A.; Nazir, R.; Iram, S. A review on the elemental contents of Pakistani medicinal plants: Implications for folk medicines. J. Ethnopharmacol. 2016, 188, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lemberkovics, É.; Czinner, E.; Szentmihályi, K.; Balázs, A.; Szőke, É. Comparative evaluation of Helichrysi flos herbal extracts as dietary sources of plant polyphenols, and macro-and microelements. Food Chem. 2002, 78, 119–127. [Google Scholar] [CrossRef]
- Karahan, F. Evaluation of trace element and heavy metal levels of some ethnobotanically important medicinal plants used as remedies in Southern Turkey in terms of human health risk. Biol. Trace Elem. Res. 2023, 201, 493–513. [Google Scholar] [CrossRef]
- Then, M.; Szentmihalyi, K.; Sárközi, Á.; Illés, V.; Forgacs, E. Effect of sample handling on alkaloid and mineral content of aqueous extracts of greater celandine (Chelidonium majus L.). J. Chromatogr. A 2000, 889, 69–74. [Google Scholar] [CrossRef]
- MacNaeidhe, F. Procedures and Precautions Used in Sampling Techniques and Analysis of Trace Elements in Plant Matrices. Sci. Total Environ. 1995, 176, 25–31. [Google Scholar] [CrossRef]
- Markert, B. Sample Preparation (Cleaning, Drying, Homogenization) for Trace Element Analysis in Plant Matrices. Sci. Total Environ. 1995, 176, 45–61. [Google Scholar] [CrossRef]
- Wedepohl, K. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Bern, C.R.; Walton-Day, K.; Naftz, D.L. Improved enrichment factor calculations through principal component analysis: Examples from soils near breccia pipe uranium mines, Arizona, USA. Environ. Pollut. 2019, 248, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Okedeyi, O.; Dube, S.; Awofolu, O.; Nindi, M. Assessing the Enrichment of Heavy Metals in Surface Soil and Plant (Digitaria eriantha) around Coal-Fired Power Plants in South Africa. Environ. Sci. Pollut. Res. 2014, 21, 4686–4696. [Google Scholar] [CrossRef]
- Baker, A.; McGrath, S.; Reeves, J.; Smith, J. Metal Hyberaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation od Metal-Polluted Soils. In Phytoremediation of Conatminated Soil and Water; Terry, N., Banuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–23. [Google Scholar]
- Sekabira, K.; Oryem-Origa, H.; Mutumba, G.M.; Kakudidi, E.; Basamba, T.A. Heavy Metal Phytoremediation by Commelina benghalensis (L.) and Cynodon dactylon (L.) Growing in Urban Stream Sediments. Int. J. Plant Physiol. Biochem. 2011, 3, 133–142. [Google Scholar]
- Álvarez-Mateos, P.; Alés-Álvarez, F.; García-Martín, J. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef]
- Dinu, C.; Vasile, G.G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Runge, J. Research Methods In Socio-Economic Geography—Elementary Methodology, Selected Research Tools; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2007. (In Polish) [Google Scholar]
- White, P.; Broadley, M. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press, Harcourt Press and Co.: London, UK, 1995; p. 889. [Google Scholar]
- Tölgyesi, G. A Növények Mikroelem-Tartalma és Ennek Mezőgazdasági Vonatkozásai; Mezőgazdasági Kiadó: Budapest, Hungary, 1969. [Google Scholar]
- Obiajunwa, E.; Adebajo, A.; Omobuwajo, O. Essential and trace element contents of some Nigerian medicinal plants. J. Radioanal. Nucl. Chem. 2002, 252, 473–476. [Google Scholar] [CrossRef]
- Sheded, M.; Pulford, I.; Hamed, A. Presence of major and trace elements in seven medicinal plants growing in the South-Eastern Desert, Egypt. J. Arid. Environ. 2006, 66, 210–217. [Google Scholar] [CrossRef]
- Jabeen, S.; Shah, M.; Khan, S.; Hayat, M. Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin, Pakistan. J. Med. Plants Res. 2010, 4, 559–566. [Google Scholar]
- Sen, I.; Peucker-Ehrenbrink, B. Anthropogenic disturbance of element cycles at the Earth’s surface. Environ. Sci. Technol. 2012, 46, 8601–8609. [Google Scholar] [CrossRef]
- Badri, M.; Hamed, A. Nutrient value of plants in an extremely arid environment (Wadi Allaqi biosphere reserve, Egypt). J. Arid. Environ. 2000, 44, 347–356. [Google Scholar] [CrossRef]
- Özcan, M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2008, 106, 852–858. [Google Scholar] [CrossRef]
- Ghani, A.; Ali, Z.; Ishtiaq, M.; Maqbool, M.; Parveen, S. Estimation of macro and micro nutrients in some important medicinal plants of Soon Valley, District Khushab, Pakistan. Afr. J. Biotechnol. 2012, 11, 14386–14391. [Google Scholar] [CrossRef]
- Raghothama, K. Phosphorus and plant nutrition: An overview. In Phosphorus: Agriculture and the Environment; Sims, T., Sharpley, A., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 2005; Volume 46, pp. 353–378. [Google Scholar]
- Narayan, O.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2023, 18, 2030082. [Google Scholar] [CrossRef]
- Hulisz, P.; Charzyński, P.; Greinert, A. Urban soil resources of medium-sized cities in Poland: A comparative case study of Toruń and Zielona Góra. J. Soils Sediments 2018, 18, 358–372. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, G.; Liu, Y.; Wan, K.; Zhang, R.; Chen, F. Soil nutrient assessment for urban ecosystems in Hubei, China. PLoS ONE 2013, 8, e75856. [Google Scholar] [CrossRef]
- Anal, J.; Chase, P. Trace elements analysis in some medicinal plants using graphite furnace-atomic absorption spectroscopy. Environ. Eng. Res. 2016, 21, 247–255. [Google Scholar] [CrossRef]
- Hammel, W.; Debus, R.; Steubing, L. Mobility of antimony in soil and its availability to plants. Chemosphere 2000, 41, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Beek, B.; Böhling, S.; Bruckmann, U.; Franke, C.; Jöhncke, U.; Studinger, G. The assessment of bioaccumulation. Bioaccumulation–New aspects and developments. In The Book of Environmental Chemistry; Part J, Bioaccumulation, Beek, B., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2000; Volume 2, pp. 235–276. [Google Scholar]
| Fe | Ca | P | Mg | Al | Na | K | S | ||
|---|---|---|---|---|---|---|---|---|---|
| [mg·kg−1] | |||||||||
| Reference Values | 4.32 * | 3.85 * | 0.08 * | 2.20 * | 7.96 * | 2.36 * | 2.14 * | 0.07 * | |
| PSh-1 | Leaves | 1700 | 29,900 | 5600 | 4400 | 500 | 100 | 45200 | 3200 |
| Stems | 600 | 9700 | 3500 | 1700 | 200 | 100 | >100,000 | 800 | |
| Rhizomes | 1800 | 7200 | 4500 | 3000 | 700 | 100 | 26,500 | 1700 | |
| Soil | 25,700 | 10,600 | 800 | 1900 | 8500 | 200 | 1200 | 800 | |
| PSh-2 | Leaves | 700 | 34,400 | 3100 | 4100 | 100 | 100 | 43,900 | 3300 |
| Stems | 400 | 8400 | 1800 | 1500 | n.d. | 100 | >100,000 | 700 | |
| Rhizomes | 1500 | 8900 | 3700 | 2600 | 500 | 200 | 24,400 | 2000 | |
| Soil | 27,600 | 12,600 | 600 | 2100 | 9400 | 200 | 1200 | 900 | |
| PZ-3 | Leaves | 700 | 24,700 | 3600 | 3500 | 200 | n.d. | 50,000 | 2200 |
| Stems | 300 | 8200 | 2100 | 1600 | n.d. | 100 | >100,000 | 700 | |
| Rhizomes | 800 | 8500 | 3600 | 2900 | 300 | 100 | 26,600 | 1800 | |
| Soil | 14,400 | 6500 | 500 | 1300 | 5300 | 100 | 700 | 800 | |
| PK\L-4 | Leaves | 1400 | 32,600 | 4800 | 4400 | 400 | 100 | 37,700 | 2600 |
| Stems | 500 | 8100 | 4500 | 2000 | 200 | 100 | 53,100 | 800 | |
| Rhizomes | 1300 | 5400 | 4700 | 3600 | 600 | 100 | 25,900 | 1700 | |
| Soil | 20,200 | 21,400 | 1000 | 4500 | 9800 | 900 | 1200 | 1800 | |
| PK\L-5 | Leaves | 800 | 25,100 | 4400 | 4500 | 200 | 100 | 46,700 | 2700 |
| Stems | 500 | 6500 | 3000 | 1500 | 200 | 100 | 63,000 | 700 | |
| Rhizomes | 700 | 7200 | 4300 | 3800 | 300 | 100 | 22,800 | 1500 | |
| Soil | 10,700 | 15,000 | 1100 | 3200 | 6000 | 100 | 1200 | 1100 | |
| PS-6 | Leaves | 800 | 40,700 | 5100 | 4300 | 100 | n.d. | 35,400 | 2700 |
| Stems | 600 | 6300 | 3100 | 1000 | 100 | n.d. | 59,200 | 700 | |
| Rhizomes | 1100 | 5400 | 4800 | 2400 | 300 | 100 | 29,900 | 1900 | |
| Soil | 21,000 | 11,000 | 900 | 1700 | 5100 | 100 | 1100 | 2100 | |
| Mo | Ag | Co | U | Au | Sr | Sb | Bi | V | La | Ba | Ti | B | W | Sc | Tl | Se | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg·kg−1] | ||||||||||||||||||
| Reference Value * | 1.1 * | 70 * | 24 * | 1.7 * | 2.5 * | 333 * | 0.3 * | 0.09 * | 98 * | 30 * | 584 * | 4010 * | 11 * | 1 * | 16 * | 0.53 * | 0.12 * | |
| 1.3 ** | 5.5 ** | 87 ** | 67 ** | 330 ** | 26,000 | 4.4 ** | 5 ** | 0.25 ** | ||||||||||
| PSh-1 | Leaves | 4.74 | 51.00 | 0.48 | 0.06 | 0.80 | 68.80 | 0.31 | 0.03 | n.d. | 0.56 | 29.10 | 18.00 | 55.00 | 0.10 | 0.10 | 0.12 | 0.10 |
| Stems | 0.47 | 28.00 | 0.20 | 0.02 | 0.20 | 40.80 | 0.10 | n.d. | n.d. | 0.21 | 31.80 | 8.00 | 26.00 | n.d. | n.d. | 0.23 | n.d. | |
| Rhizomes | 0.76 | 100.00 | 0.75 | 0.11 | 0.20 | 29.60 | 0.35 | 0.03 | 3.00 | 0.88 | 32.90 | 23.00 | 25.00 | 0.10 | 0.20 | 0.38 | 0.20 | |
| Soil | 2.02 | 619.00 | 10.20 | 1.30 | 1.90 | 54.90 | 9.92 | 0.45 | 28.00 | 11.20 | 278.80 | 0.01 | n.d. | 1.70 | 1.90 | 0.80 | 1.60 | |
| PSh-2 | Leaves | 3.79 | 51.00 | 0.34 | 0.01 | 0.40 | 96.50 | 0.35 | 0.03 | n.d. | 0.13 | 84.30 | 6.00 | 83.00 | n.d. | n.d. | 0.13 | 0.20 |
| Stems | 0.28 | 19.00 | 0.12 | 0.02 | n.d. | 40.20 | 0.10 | n.d. | n.d. | 0.10 | 53.70 | 5.00 | 25.00 | n.d. | n.d. | 0.25 | n.d. | |
| Rhizomes | 0.62 | 65.00 | 0.59 | 0.10 | n.d. | 39.80 | 0.41 | 0.03 | 2.00 | 0.55 | 57.60 | 15.00 | 30.00 | 0.10 | 0.10 | 0.30 | 0.20 | |
| Soil | 2.75 | 583.00 | 12.20 | 1.70 | 1.10 | 61.20 | 2.41 | 0.34 | 52.00 | 11.60 | 215.20 | 0.02 | n.d. | 0.90 | 2.80 | 0.65 | 1.60 | |
| PZ-3 | Leaves | 1.87 | 25.00 | 0.19 | 0.02 | 0.30 | 52.20 | 0.09 | n.d. | n.d. | 0.19 | 50.70 | 7.00 | 42.00 | n.d. | n.d. | 0.11 | n.d. |
| Stems | 0.42 | 11.00 | 0.10 | n.d. | n.d. | 27.70 | 0.02 | n.d. | n.d. | 0.08 | 38.80 | 4.00 | 25.00 | n.d. | n.d. | 0.21 | n.d. | |
| Rhizomes | 0.54 | 29.00 | 0.32 | 0.05 | n.d. | 30.00 | 0.10 | n.d. | n.d. | 0.38 | 45.80 | 11.00 | 31.00 | n.d. | n.d. | 0.21 | n.d. | |
| Soil | 0.87 | 179.00 | 4.00 | 0.80 | 0.80 | 18.30 | 0.94 | 0.21 | 15.00 | 7.50 | 71.90 | 0.01 | n.d. | 0.50 | 0.80 | 0.42 | 0.70 | |
| PK\L-4 | Leaves | 5.42 | 44.00 | 0.48 | 0.05 | n.d. | 64.40 | 0.20 | 0.04 | n.d. | 0.48 | 39.80 | 19.00 | 56.00 | n.d. | 0.10 | 0.41 | n.d. |
| Stems | 0.60 | 17.00 | 0.19 | 0.03 | n.d. | 24.10 | 0.07 | n.d. | n.d. | 0.16 | 21.30 | 10.00 | 23.00 | n.d. | n.d. | 0.64 | n.d. | |
| Rhizomes | 0.58 | 36.00 | 0.59 | 0.08 | n.d. | 20.30 | 0.17 | n.d. | n.d. | 0.63 | 22.40 | 22.00 | 25.00 | n.d. | 0.10 | 0.31 | n.d. | |
| Soil | 1.57 | 421.00 | 12.00 | 1.40 | 1.60 | 98.20 | 2.84 | 0.33 | 27.00 | 12.30 | 232.50 | 0.03 | 69.00 | 0.80 | 2.10 | 0.73 | 1.60 | |
| PK\L-5 | Leaves | 1.87 | 28.00 | 0.25 | 0.03 | n.d. | 46.50 | 0.15 | 0.04 | n.d. | 0.26 | 22.20 | 9.00 | 75.00 | n.d. | n.d. | 0.05 | n.d. |
| Stems | 0.32 | 12.00 | 0.18 | 0.02 | n.d. | 22.10 | 0.06 | n.d. | n.d. | 0.27 | 20.70 | 8.00 | 23.00 | n.d. | n.d. | 0.07 | n.d. | |
| Rhizomes | 0.27 | 18.00 | 0.28 | 0.05 | n.d. | 26.70 | 0.09 | n.d. | n.d. | 0.37 | 25.30 | 10.00 | 27.00 | n.d. | n.d. | 0.09 | n.d. | |
| Soil | 0.62 | 264.00 | 4.90 | 0.90 | 1.20 | 35.80 | 1.09 | 0.26 | 16.00 | 9.90 | 115.20 | 0.01 | n.d. | 0.60 | 0.80 | 0.46 | 1.00 | |
| PS-6 | Leaves | 5.03 | 29.00 | 0.19 | 0.01 | 0.30 | 68.00 | 0.32 | 0.02 | n.d. | 0.12 | 33.60 | 7.00 | 86.00 | n.d. | n.d. | 0.44 | n.d. |
| Stems | 0.52 | 13.00 | 0.15 | 0.01 | n.d. | 19.20 | 0.06 | n.d. | n.d. | 0.13 | 22.80 | 7.00 | 21.00 | n.d. | n.d. | 0.99 | n.d. | |
| Rhizomes | 1.16 | 49.00 | 0.30 | 0.04 | n.d. | 18.20 | 0.15 | n.d. | n.d. | 0.32 | 22.70 | 12.00 | 26.00 | 0.10 | n.d. | 1.07 | n.d. | |
| Soil | 1.49 | 515.00 | 5.30 | 0.70 | 1.40 | 35.70 | 2.02 | 0.30 | 16.00 | 6.60 | 197.50 | 0.01 | n.d. | 0.70 | 0.90 | 0.86 | 1.70 | |
| Te | Ga | Cs | Ge | Hf | Nb | Rb | Sn | Zr | Y | Ce | In | Re | Be | Li | Pd | |||
| [mg·kg−1] | ||||||||||||||||||
| Reference value * | 0.005 | 15 * | 3.4 * | 1.4 * | 4.9 * | 19 * | 78 * | 2.3 * | 203 * | 24 * | 60 * | 0.05 * | 0.4 * | 2.4 * | 18 * | 0.4 * | ||
| 22 ** | ||||||||||||||||||
| PSh-1 | Leaves | n.d. | 0.20 | 0.07 | 0.15 | 0.01 | 0.07 | 18.40 | 0.68 | 0.41 | 0.51 | 1.20 | n.d. | 4.00 | 0.10 | 0.54 | n.d. | |
| Stems | n.d. | n.d. | 0.03 | 0.08 | n.d. | 0.02 | 30.60 | 0.19 | 0.19 | 0.18 | 0.40 | n.d. | n.d. | n.d. | 0.52 | n.d. | ||
| Rhizomes | n.d. | 0.30 | 0.09 | 0.15 | 0.01 | 0.09 | 19.00 | 0.36 | 0.34 | 0.73 | 1.70 | n.d. | n.d. | 0.20 | 1.33 | n.d. | ||
| Soil | 0.04 | 4.10 | 1.11 | n.d. | n.d. | 0.57 | 8.90 | 33.50 | 0.70 | 10.12 | 22.40 | 0.04 | 2.00 | 3.30 | 8.90 | n.d. | ||
| PSh-2 | Leaves | n.d. | n.d. | 0.05 | 0.06 | n.d. | 0.03 | 19.70 | 0.31 | 0.17 | 0.11 | 0.30 | n.d. | 6.00 | n.d. | 0.25 | 2.00 | |
| Stems | n.d. | n.d. | 0.04 | 0.07 | n.d. | 0.02 | 31.50 | 0.11 | 0.12 | 0.09 | 0.20 | n.d. | n.d. | n.d. | 0.33 | n.d. | ||
| Rhizomes | n.d. | 0.20 | 0.07 | 0.19 | 0.01 | 0.07 | 14.50 | 0.47 | 0.41 | 0.51 | 1.10 | n.d. | n.d. | 0.10 | 0.63 | n.d. | ||
| Soil | 0.04 | 5.50 | 1.21 | 0.30 | n.d. | 0.57 | 9.20 | 12.70 | 1.00 | 11.11 | 23.90 | 0.05 | 12.00 | 5.80 | 9.90 | 14.00 | ||
| PZ-3 | Leaves | n.d. | n.d. | 0.04 | 0.03 | n.d. | 0.03 | 19.70 | 0.14 | 0.16 | 0.15 | 0.40 | n.d. | 2.00 | n.d. | 0.28 | n.d. | |
| Stems | n.d. | n.d. | n.d. | 0.04 | n.d. | n.d. | 24.20 | 0.04 | 0.08 | 0.06 | 0.20 | n.d. | n.d. | n.d. | 0.15 | n.d. | ||
| Rhizomes | n.d. | 0.10 | 0.06 | 0.05 | 0.01 | 0.04 | 12.40 | 0.11 | 0.23 | 0.30 | 0.70 | n.d. | n.d. | n.d. | 0.55 | n.d. | ||
| Soil | 0.03 | 1.90 | 1.11 | n.d. | n.d. | 0.54 | 7.50 | 2.10 | 0.50 | 3.83 | 15.30 | 0.03 | n.d. | 0.50 | 5.80 | n.d. | ||
| PK\L-4 | Leaves | n.d. | 0.10 | 0.13 | 0.05 | 0.01 | 0.09 | 38.20 | 0.24 | 0.41 | 0.38 | 0.90 | n.d. | n.d. | n.d. | 0.95 | n.d. | |
| Stems | n.d. | n.d. | 0.06 | 0.04 | n.d. | 0.03 | 50.90 | 0.06 | 0.18 | 0.13 | 0.30 | n.d. | n.d. | n.d. | 0.67 | n.d. | ||
| Rhizomes | n.d. | 0.20 | 0.08 | 0.06 | 0.01 | 0.07 | 26.00 | 0.15 | 0.35 | 0.52 | 1.20 | n.d. | n.d. | 0.10 | 1.35 | n.d. | ||
| Soil | 0.05 | 3.90 | 0.96 | n.d. | 0.03 | 0.95 | 9.20 | 2.30 | 1.60 | 10.12 | 23.10 | 0.04 | n.d. | 2.40 | 13.60 | 13.00 | ||
| PK\L-5 | Leaves | n.d. | n.d. | 0.05 | 0.04 | 0.01 | 0.05 | 30.80 | 0.22 | 0.24 | 0.21 | 0.50 | n.d. | 3.00 | n.d. | 0.26 | n.d. | |
| Stems | n.d. | n.d. | 0.04 | 0.04 | 0.01 | 0.03 | 40.00 | 0.10 | 0.19 | 0.20 | 0.50 | n.d. | n.d. | n.d. | 0.23 | n.d. | ||
| Rhizomes | n.d. | n.d. | 0.05 | 0.04 | 0.01 | 0.03 | 17.70 | 0.13 | 0.22 | 0.30 | 0.70 | n.d. | n.d. | n.d. | 0.31 | n.d. | ||
| Soil | 0.04 | 2.30 | 1.19 | n.d. | n.d. | 0.41 | 8.60 | 2.60 | 0.50 | 8.37 | 19.10 | 0.03 | n.d. | 0.90 | 6.00 | n.d. | ||
| PS-6 | Leaves | n.d. | n.d. | 0.15 | 0.04 | 0.00 | 0.01 | 29.60 | 0.15 | 0.12 | 0.10 | 0.20 | n.d. | 2.00 | n.d. | 0.34 | n.d. | |
| Stems | n.d. | n.d. | 0.11 | 0.05 | 0.00 | 0.01 | 51.10 | 0.08 | 0.13 | 0.12 | 0.30 | n.d. | n.d. | n.d. | 0.34 | n.d. | ||
| Rhizomes | n.d. | 0.10 | 0.14 | 0.13 | 0.01 | 0.04 | 32.40 | 0.12 | 0.25 | 0.25 | 0.60 | n.d. | n.d. | n.d. | 0.58 | n.d. | ||
| Soil | 0.04 | 2.30 | 1.25 | n.d. | n.d. | 0.44 | 9.30 | 3.10 | 0.30 | 5.32 | 13.60 | 0.03 | n.d. | 1.30 | 5.10 | 10.00 | ||
| Fe | Ca | P | Mg | Al | Na | K | S | ||
|---|---|---|---|---|---|---|---|---|---|
| The geo-accumulation index (I-geo) | PSh-1 | −1.14 | −2.01 | −0.13 | −3.58 | −4.51 | −8.59 | −5.29 | 0.55 |
| PSh-2 | −0.75 | −1.81 | −0.76 | −3.27 | −3.63 | −7.91 | −5.16 | −0.67 | |
| PZ-3 | −1.69 | −2.76 | −0.91 | −3.96 | −4.45 | −9.33 | −5.94 | −0.84 | |
| PK\L-4 | −1.20 | −1.05 | 0.06 | −2.17 | −3.57 | −5.47 | −5.16 | 0.33 | |
| PK\L-5 | −2.11 | −1.56 | 0.07 | −2.66 | −4.28 | −8.74 | −5.16 | −0.38 | |
| PS-6 | −1.14 | −2.01 | −0.13 | −3.58 | −4.51 | −8.59 | −5.29 | 0.55 | |
| The enrichment factor (EF) | PSh-1 | 1.00 | 0.43 | 1.37 | 0.17 | 0.13 | 0.01 | 0.05 | 1.01 |
| PSh-2 | n.d | 0.48 | 0.99 | 0.17 | 0.14 | 0.01 | 0.05 | 1.06 | |
| PZ-3 | n.d | 0.47 | 1.71 | 0.21 | 0.15 | 0.01 | 0.05 | 1.80 | |
| PK\L-4 | n.d | 1.11 | 2.39 | 0.51 | 0.19 | 0.05 | 0.06 | 2.89 | |
| PK\L-5 | n.d | 1.47 | 4.56 | 0.69 | 0.22 | 0.01 | 0.12 | 3.33 | |
| PS-6 | n.d | 0.55 | 2.01 | 0.19 | 0.10 | 0.01 | 0.06 | 2.20 | |
| The contamination factors (CF) | PSh-1 | 0.83 | 0.36 | 1.14 | 0.14 | 0.11 | 0.01 | 0.04 | 0.84 |
| PSh-2 | 0.89 | 0.43 | 0.89 | 0.16 | 0.12 | 0.01 | 0.04 | 0.94 | |
| PZ-3 | 0.47 | 0.22 | 0.80 | 0.10 | 0.07 | 0.00 | 0.02 | 0.84 | |
| PK\L-4 | 0.65 | 0.73 | 1.56 | 0.33 | 0.13 | 0.03 | 0.04 | 1.89 | |
| PK\L-5 | 0.35 | 0.51 | 1.58 | 0.24 | 0.08 | 0.00 | 0.04 | 1.15 | |
| PS-6 | 0.68 | 0.37 | 1.37 | 0.13 | 0.07 | 0.00 | 0.06 | 2.20 |
| Fe | Ca | P | Mg | Al | Na | K | S | |
|---|---|---|---|---|---|---|---|---|
| Bioaccumulation | 0.14 | 3.60 | 14.30 | 3.59 | 0.11 | 0.99 | 89.44 | 4.23 |
| Translocation | 1.23 | 5.51 | 1.74 | 1.90 | 0.81 | 0.95 | 2.78 | 1.99 |
| Soil | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ca | P | Mg | Al | Na | K | ||
| Leaves | Ca | 0.6116 | ||||||
| P | 0.0735 | 0.2043 | ||||||
| Mg | 0.1384 | 0.6672 | 0.8662 * | |||||
| Al | 0.1713 | 0.3012 | 0.2364 | 0.3632 | ||||
| Na | −0.2675 | −0.6312 | −0.3901 | −0.6186 | −0.7831 | |||
| K | −0.3501 | −0.5222 | −0.4674 | −0.3624 | −0.1839 | −0.468 | ||
| S | 0.7768 | 0.102 | 0.0035 | −0.0475 | 0.5973 | −0.0834 | 0.7371 | |
| Stems | Ca | 0.5777 | ||||||
| P | −0.0263 | 0.7141 | ||||||
| Mg | 0.0189 | 0.5 | 0.0396 | |||||
| Al | 0.1278 | −0.508 | −0.8916 * | −0.4883 | ||||
| Na | 0.0808 | −0.1801 | 0.1762 | −0.3089 | −0.5183 | |||
| K | 0.3843 | −0.7005 | −0.8444 * | −0.6824 | 0.1098 | −0.4636 | ||
| S | 0.3613 | 0.4849 | 0.2786 | 0.4884 | 0.6556 | 0.6987 | 0.3873 | |
| Rhizomes | Ca | 0.0717 | ||||||
| P | 0.0649 | 0.5189 | ||||||
| Mg | −0.5832 | 0.6278 | 0.6352 | |||||
| Al | 0.6885 | 0.3646 | 0.0245 | 0.3104 | ||||
| Na | 0.5808 | −0.0243 | −0.4582 | −0.1441 | 0.4722 | |||
| K | 0.2711 | −0.3237 | −0.163 | −0.417 | −0.3394 | −0.055 | ||
| S | 0.6723 | −0.3427 | −0.6738 | −0.4993 | 0.1235 | −0.1333 | −0.1713 | |
| Major Elements | |
| Leaves | K>Ca>P>Mg>S>Fe>Al>Na |
| Stems | K>Ca>P>Mg>S>Fe>Al>Na |
| Rhizomes | K>Ca>P>Mg>S>Fe>Al>Na |
| Soil | Fe>Ca>Al>Mg>S>K>P>Na |
| Microelements and rare earth metals | |
| Leaves | B>Sr>Ba>Ag>Rb>Ti>Mo>Ce>Li>Co>La>Sn>Zr>Y>Sb>Tl>Cs>Ge>Nb>U>Hf>Ta |
| Stems | Rb>Ba>Sr>B>Ag>Ti>Mo>Tl>Li>Ce>La>Co>Zr>Y>Sn>Sb>Ge>Hf>Ta |
| Rhizomes | Ag>Ba>Sr>B>Rb>Ti>Ce>Li>Mo>La>Co>Y>Tl>Zr>Sn>Sb>Ge>Cs>U>Nb>Hf>Ta |
| Soil | Ag>Ba>Sr>V>Ce>La>Sn>Rb>Li>Y>Co>Ga>Sb>Be>Mo>Sc>Se>Au>Cs>U>W>Zr>Tl>Th>Nb>Bi>Te>In>Ti |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmonov, O.; Środek, D.; Pytel, S.; Kupka, T.; Makieieva, N. Mineral Composition of Chelidonium majus L. and Soils in Urban Areas. Appl. Sci. 2025, 15, 4718. https://doi.org/10.3390/app15094718
Rahmonov O, Środek D, Pytel S, Kupka T, Makieieva N. Mineral Composition of Chelidonium majus L. and Soils in Urban Areas. Applied Sciences. 2025; 15(9):4718. https://doi.org/10.3390/app15094718
Chicago/Turabian StyleRahmonov, Oimahmad, Dorota Środek, Sławomir Pytel, Teobald Kupka, and Natalina Makieieva. 2025. "Mineral Composition of Chelidonium majus L. and Soils in Urban Areas" Applied Sciences 15, no. 9: 4718. https://doi.org/10.3390/app15094718
APA StyleRahmonov, O., Środek, D., Pytel, S., Kupka, T., & Makieieva, N. (2025). Mineral Composition of Chelidonium majus L. and Soils in Urban Areas. Applied Sciences, 15(9), 4718. https://doi.org/10.3390/app15094718







