Lactoferrin Production: A Systematic Review of the Latest Analytical Methods
Abstract
1. Introduction
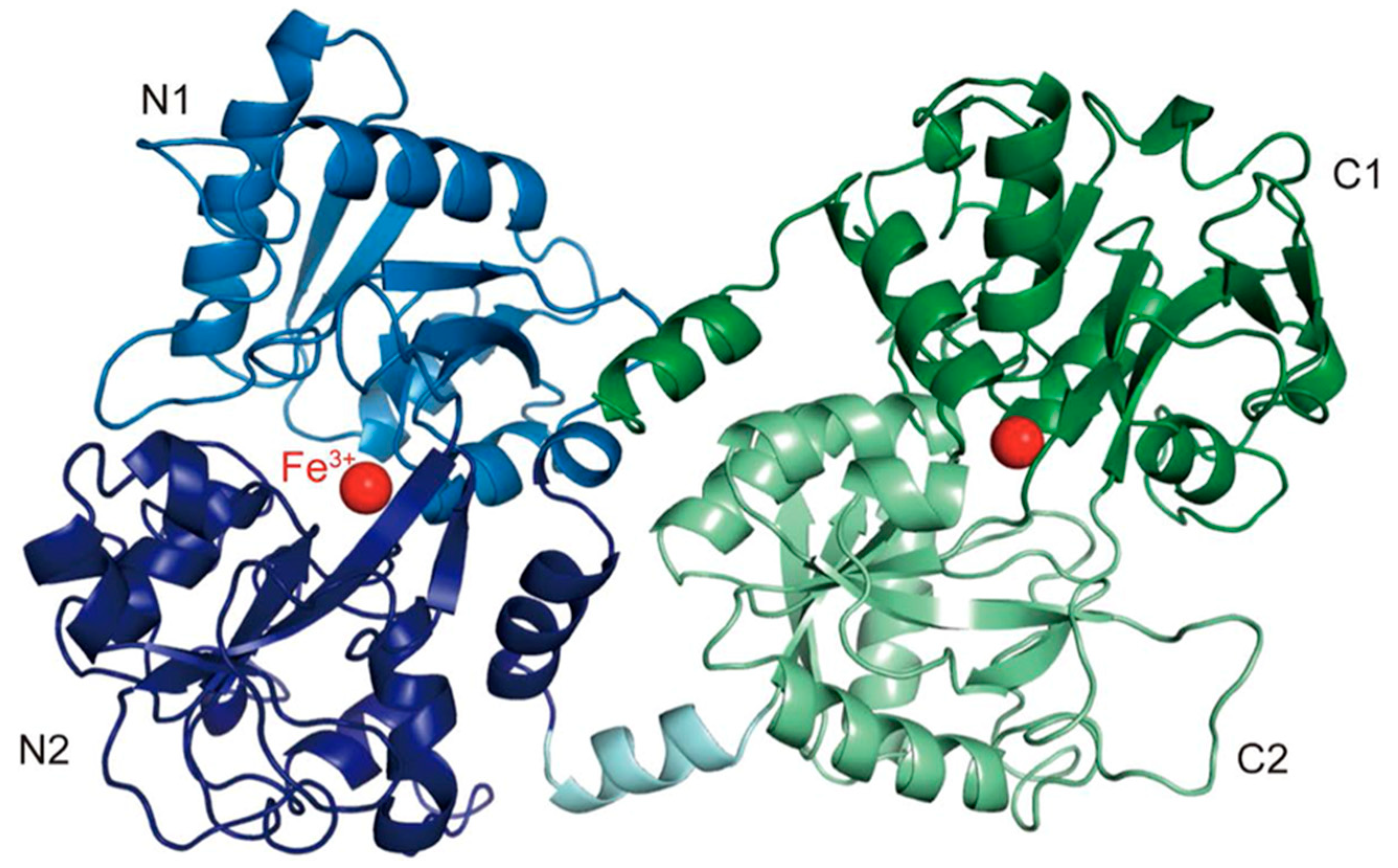
1.1. Lactoferrin Structure
1.2. The Effects of Bovine Lactoferrin on the Human Body
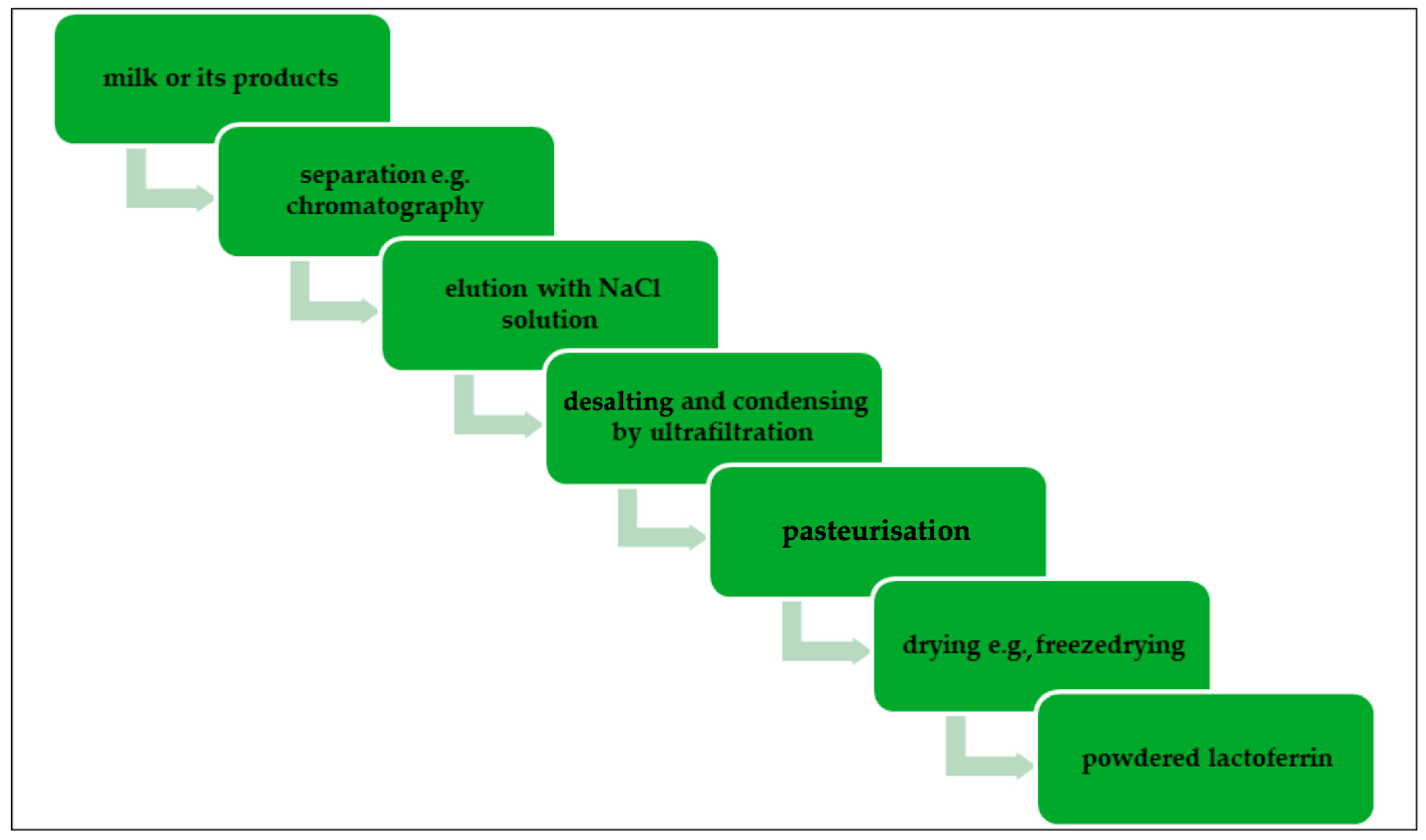
1.3. Lactoferrin Production
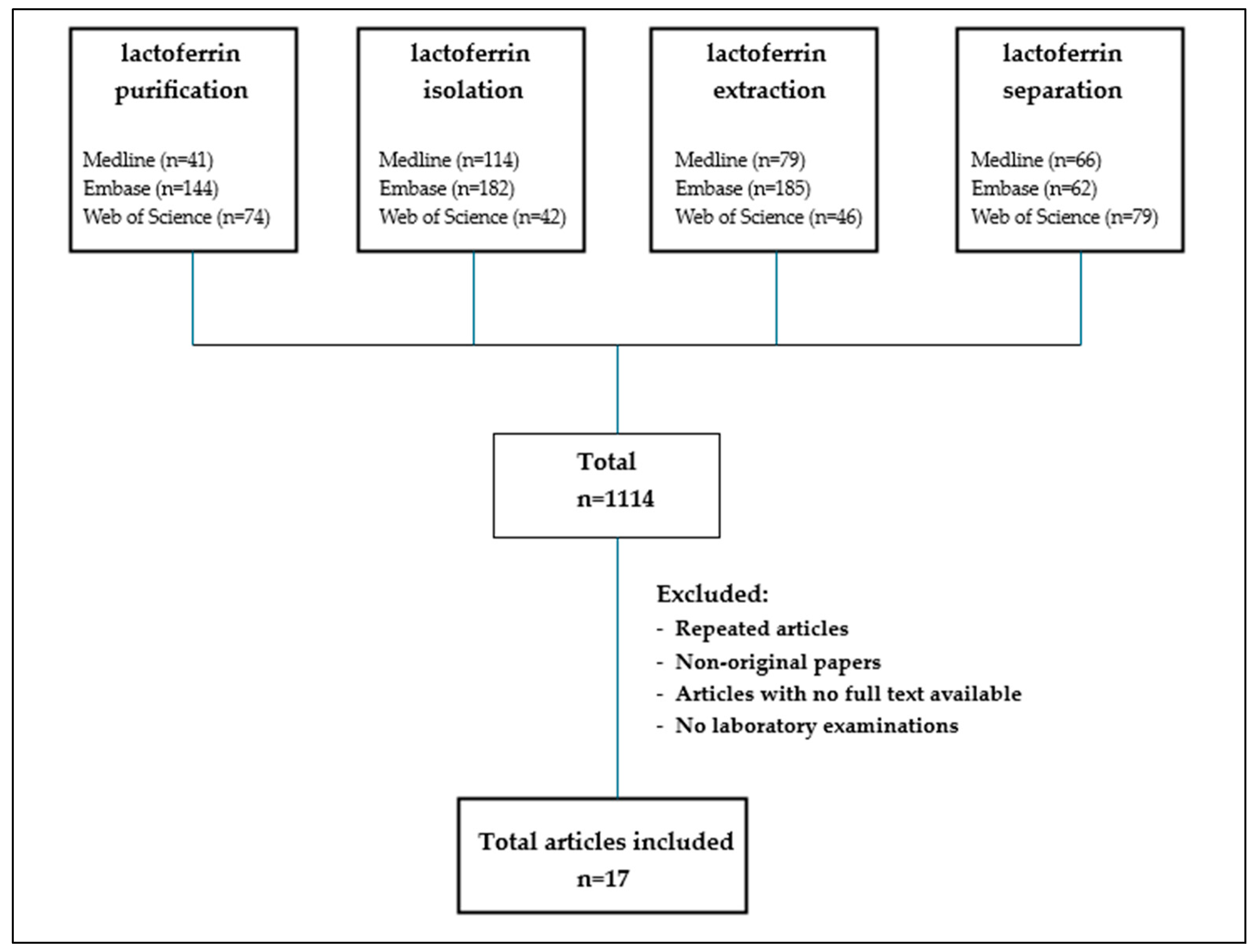
2. Materials and Methods
3. Results and Discussion
3.1. Conclusion Remarks
3.1.1. Conclusion Remarks
3.1.2. Determination and Identification of Lactoferrin
- -
- optical biosensor immunoassay [98] with an immobilised anti-Lf antibody, which offers the advantages of simpler sample preparation and estimation of intact physiologically active protein. It has been implemented in the New Zealand dairy industry for 18 years.
- -
- Image J software to determine the intensity of the protein bands after SD-PAGE [107].
- -
- capillary electrophoresis, which is a good alternative for determining the content of obtained Lf, but is affected by the system pH, surfactant, organic solvent, buffer system, and auxiliary air pressure [103].
- -
- Fourier transform infrared spectroscopy (FTIR) with an attenuated total reflectance (ATR) attachment [109]. Infrared spectroscopy measures the absorption of infrared radiation by each bond in a molecule and results in a spectrum that is commonly defined as % transmittance per wavenumber (cm). The ATR mode is simple compared to the conventional transmission model [117].
- -
- spectrofluorimetry to determine the Lf content of pharmaceutical preparations (capsules, sachets) and milk powder, obtaining a low LOD: 0.00002 mg/mL and LOQ: 0.000082 mg/mL. The authors indicate that the availability, sensitivity, durability, and affordability of this method make it better than routine Lf analysis [108].
- -
- the latex agglutination assay based on the agglutination that occurs when shiny latex beads come into contact with antigens or filters. It is a very quick and simple method for determining Lf with very good validation parameters [110].
- -
- electroanalysis, electrochemical quartz crystal microbalance (EQCM), and single radial immunodiffusion (SRID). The EIS results are within a linear range with an LOD of 125 nM to 3250 μM and 65.2 nM, respectively [118]. The application of impedance and single-frequency capacitance spectroscopy was successfully demonstrated for Lf determination and shortened analysis time.
3.1.3. Industrial-Scale Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F. Lactoferrin in Milk from Different Species. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1971, 39, 119–129, IN11–IN13. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Sorensen, S.P.L. The Proteins in Whey. Comptes Rendus Des. Trav. Du. Lab. Carlsberg 1939, 23, 55–99. [Google Scholar]
- Johanson, B.; Virtanen, A.I.; Tweit, R.C.; Dodson, R.M. Isolation of an Iron-Containing Red Protein from Human Milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of Human Lactoferrin: Crystallographic Structure Analysis and Refinement at 2·8 Å Resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef]
- Anghel, L.; Radulescu, A.; Erhan, R.V. Structural Aspects of Human Lactoferrin in the Iron-Binding Process Studied by Molecular Dynamics and Small-Angle Neutron Scattering. Eur. Phys. J. E 2018, 41, 109. [Google Scholar] [CrossRef]
- Spik, G.; Coddeville, B.; Montreuil, J. Comparative Study of the Primary Structures of Sero-, Lacto- and Ovotransferrin Glycans from Different Species. Biochimie 1988, 70, 1459–1469. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent Advances and Prospects in Purification and Heterologous Expression of Lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B.; et al. Concentration of Lactoferrin in Human Milk and Its Variation during Lactation in Different Chinese Populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Li, Y.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E.W. Effects of Bovine Lactoferrin on the Immature Porcine Intestine. Br. J. Nutr. 2014, 111, 321–331. [Google Scholar] [CrossRef]
- Marshall, K. Therapeutic Applications of Whey Protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar] [PubMed]
- Hiss, S.; Meyer, T.; Sauerwein, H. Lactoferrin Concentrations in Goat Milk throughout Lactation. Small Rumin. Res. 2008, 80, 87–90. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Levieux, D. Lactoferrin and Immunoglobulin Contents in Camel’s Milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J. Dairy Sci. 2007, 90, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, G.; Basiricò, L.; Ronchi, B.; Bernabucci, U. Lactoferrin Concentration in Buffalo Milk. Ital. J. Anim. Sci. 2013, 12, e23. [Google Scholar] [CrossRef]
- Satué-Gracia, M.T.; Frankel, E.N.; Rangavajhyala, N.; German, J.B. Lactoferrin in Infant Formulas: Effect on Oxidation. J. Agric. Food Chem. 2000, 48, 4984–4990. [Google Scholar] [CrossRef]
- Arnold, D.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Valenti, P.; Seganti, L.; Superti, F. Antiadenovirus Activity of Milk Proteins: Lactoferrin Prevents Viral Infection. Antivir. Res. 2002, 53, 153–158. [Google Scholar] [CrossRef]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal Activity of Human Lactoferrin: Sensitivity of a Variety of Microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef]
- Sortino, O.; Hullsiek, K.H.; Richards, E.; Rupert, A.; Schminke, A.; Tetekpor, N.; Quinones, M.; Prosser, R.; Schacker, T.; Sereti, I.; et al. The Effects of Recombinant Human Lactoferrin on Immune Activation and the Intestinal Microbiome Among Persons Living with Human Immunodeficiency Virus and Receiving Antiretroviral Therapy. J. Infect. Dis. 2019, 219, 1963–1968. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-Pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and Tolerability of Oral Bovine Lactoferrin Compared to Ferrous Sulfate in Pregnant Women with Iron Deficiency Anemia: A Prospective Controlled Randomized Study. Acta Obs. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral Administration of Lactoferrin Increases Hemoglobin and Total Serum Iron in Pregnant womenThis Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 7th International Conference on Lactoferrin: Structure, Function, and Applications, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and Efficacy of Lactoferrin versus Ferrous Sulphate in Curing Iron Deficiency and Iron Deficiency Anaemia in Hereditary Thrombophilia Pregnant Women: An Interventional Study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin Efficacy versus Ferrous Sulfate in Curing Iron Disorders in Pregnant and Non-Pregnant Women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.; Dawood, R.; Abo-Elnasr, M.; Al Halaby, A.; Marawan, H. RETRACTED ARTICLE: Lactoferrin versus Ferrous Sulphate for the Treatment of Iron Deficiency Anemia during Pregnancy: A Randomized Clinical Trial. J. Matern. -Fetal Neonatal Med. 2016, 29, 1387–1390. [Google Scholar] [CrossRef]
- El-Asheer, O.M.; Ahmed, A.G.; Hafez, Z.A.A.; Dahpy, M.A.; Soliman, A.A. Lactoferrin Efficacy versus Ferrous Sulfate in Treatment of Children with Iron Deficiency Anemia. J. Child. Sci. 2021, 11, e199–e204. [Google Scholar] [CrossRef]
- El-Hawy, M.A.; Abd Al-Salam, S.A.; Bahbah, W.A. Comparing Oral Iron Bisglycinate Chelate, Lactoferrin, Lactoferrin with Iron and Iron Polymaltose Complex in the Treatment of Children with Iron Deficiency Anemia. Clin. Nutr. ESPEN 2021, 46, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Lan, Z.; Hua, L.; Ying, Z.; Humina, X.; Jia, S.; Tian, W.; Yang, P.; Chai, L.; Mao, M. Iron Metabolism in Infants: Influence of Bovine Lactoferrin from Iron-Fortified Formula. Nutrition 2015, 31, 304–309. [Google Scholar] [CrossRef]
- El-Khawaga, A.; Abdelmaksoud, H. Effect of Lactoferrin Supplementation on Iron Deficiency Anemia in Primary School Children. Int. J. Med. Art 2019, 1, 48–52. [Google Scholar] [CrossRef]
- Omar, O.M.; Assem, H.; Ahmed, D.; Abd Elmaksoud, M.S. Lactoferrin versus Iron Hydroxide Polymaltose Complex for the Treatment of Iron Deficiency Anemia in Children with Cerebral Palsy: A Randomized Controlled Trial. Eur. J. Pediatr. 2021, 180, 2609–2618. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for Iron-Deficiency Anemia in Children with Inflammatory Bowel Disease: A Clinical Trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef]
- El-Nasr, I.A.S.; Mahmou, S.A.; Elnaddar, E.M.; Ammar, H.A. Ferrous Sulphate Alone Versus Combination of Ferrous Sulphate and Lactoferrin for The Treatment of Iron Deficiency Anemia during Pregnancy and Their Effect on Neonatal Iron Store: A Randomized Clinical Trial. Egypt. J. Hosp. Med. 2021, 84, 1955–1960. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Sanna, E.; Mantovani, G. Efficacy and Safety of Oral Lactoferrin Supplementation in Combination with rHuEPO-β for the Treatment of Anemia in Advanced Cancer Patients Undergoing Chemotherapy: Open-Label, Randomized Controlled Study. Oncologist 2010, 15, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.M.A.; Mohammed, A. Lactoferrin: A Promising New Player in Treatment of Iron Deficiency Anemia in Patients on Regular Hemodialysis: A Randomized Controlled Trial. Saudi J. Kidney Dis. Transplant. 2023, 34, 235–241. [Google Scholar] [CrossRef]
- Wander, K.; Shell-Duncan, B.; McDade, T.W. Evaluation of Iron Deficiency as a Nutritional Adaptation to Infectious Disease: An Evolutionary Medicine Perspective. Am. J. Hum. Biol. 2009, 21, 172–179. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The Clinical Efficacy of a Bovine Lactoferrin/Whey Protein Ig-Rich Fraction (Lf/IgF) for the Common Cold: A Double Blind Randomized Study. Complement. Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; De Waard, R. A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Gathwala, G. Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-Onset Sepsis in Low Birth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Sugita, C.; Yoshida, H.; Abe, F.; Sonoda, T.; Kurokawa, M. Effects of Lactoferrin on Infectious Diseases in Japanese Summer: A Randomized, Double-Blinded, Placebo-Controlled Trial. J. Microbiol. Immunol. Infect. 2021, 54, 566–574. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates: A Randomized Trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- Akin, I.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.; Ikinciogullari, A.; Turmen, T. Oral Lactoferrin to Prevent Nosocomial Sepsis and Necrotizing Enterocolitis of Premature Neonates and Effect on T-Regulatory Cells. Amer J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef]
- Kaito, M.; Iwasa, M.; Fujita, N.; Kobayashi, Y.; Kojima, Y.; Ikoma, J.; Imoto, I.; Adachi, Y.; Hamano, H.; Yamauchi, K. Effect of Lactoferrin in Patients with Chronic Hepatitis C: Combination Therapy with Interferon and Ribavirin. J. Gastro Hepatol. 2007, 22, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Ikeda, M.; Saito, S.; Matsumoto, S.; Numata, K.; Kato, N.; Tanaka, K.; Sekihara, H. Lactoferrin Inhibits Hepatitis B Virus Infection in Cultured Human Hepatocytes. Hepatol. Res. 2002, 24, 228. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Huang, G.; Liu, N. Inhibition of HBV Infection by Bovine Lactoferrin and Iron-, Zinc-Saturated Lactoferrin. Med. Microbiol. Immunol. 2009, 198, 19–25. [Google Scholar] [CrossRef]
- Drobni, P.; Naslund, J.; Evander, M. Lactoferrin Inhibits Human Papillomavirus Binding and Uptake in Vitro. Antivir. Res. 2004, 64, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Drobni, P.; Näslund, J.; Sunkari, V.G.; Jenssen, H.; Evander, M. The Anti-Papillomavirus Activity of Human and Bovine Lactoferricin. Antivir. Res. 2007, 75, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Groot, F.; Geijtenbeek, T.B.H.; Sanders, R.W.; Baldwin, C.E.; Sanchez-Hernandez, M.; Floris, R.; Van Kooyk, Y.; De Jong, E.C.; Berkhout, B. Lactoferrin Prevents Dendritic Cell-Mediated Human Immunodeficiency Virus Type 1 Transmission by Blocking the DC-SIGN—Gp120 Interaction. J. Virol. 2005, 79, 3009–3015. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wong, J.H.; Ip, D.T.M.; Wan, D.C.C.; Cheung, R.C.; Ng, T.B. Bovine Lactoferrampin, Human Lactoferricin, and Lactoferrin 1-11 Inhibit Nuclear Translocation of HIV Integrase. Appl. Biochem. Biotechnol. 2016, 179, 1202–1212. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmi, Y.S.; Kondapi, A.K. Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: An Effective Nano First-Line Regimen for HIV Therapy. Pharm. Res. 2017, 34, 257–268. [Google Scholar] [CrossRef]
- Senapathi, J.; Bommakanti, A.; Mallepalli, S.; Mukhopadhyay, S.; Kondapi, A.K. Sulfonate Modified Lactoferrin Nanoparticles as Drug Carriers with Dual Activity against HIV-1. Colloids Surf. B Biointerfaces 2020, 191, 110979. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. JCM 2021, 10, 4276. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 08–15. [Google Scholar] [CrossRef]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of Microbicidal Activity and Increased Formation of Biofilm Due to Decreased Lactoferrin Activity in Patients with Cystic Fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef]
- Superti, F.; Pietrantoni, A.; Di Biase, A.M.; Longhi, C.; Valenti, P.; Tinari, A. Inv-Mediated Apoptosis of Epithelial Cells Infected with Enteropathogenic Yersinia: A Protective Effect of Lactoferrin. Res. Microbiol. 2005, 156, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Ajello, M.; Bosso, P.; Morea, C.; Andrea, P.; Giovanni, A.; Piera, V. Both Lactoferrin and Iron Influence Aggregation and Biofilm Formation in Streptococcus Mutans. Biometals 2004, 17, 271–278. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J. Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; He, T.; Lu, Y.; Szeto, I.M.-Y.; Duan, S.; Zhang, Y.; Liu, B.; Zhang, Y.; Zhang, W.; et al. Synergistic Effect of Lactoferrin and Osteopontin on Intestinal Barrier Injury. Int. J. Biol. Macromol. 2023, 253, 127416. [Google Scholar] [CrossRef]
- Ochoa, T.; Cleary, T. Effect of Lactoferrin on Enteric Pathogens. Biochimie 2009, 91, 30–34. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, K.; Yang, N.; Hao, Y.; Mao, R.; Teng, D.; Wang, J. Treatment of Lactoferrin and Antimicrobial Peptide N6 on Bacterial Enteritis Caused by Escherichia Coli in Mice. Biochem. Cell Biol. 2025, 103, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, H.; Wang, W.; Li, Y.; Wu, R.; Wei, L.; Su, S.; Wang, X.; Wang, X.; Wang, X.; et al. Investigating the Modulatory Effects of Lactoferrin on Depressed Rats through 16S rDNA Gene Sequencing and LC–MS Metabolomics Analysis. Sci. Rep. 2024, 14, 22111. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Martínez, L.E.; Canizalez-Román, A.; Angulo-Zamudio, U.A.; Flores-Villaseñor, H.; Velázquez-Román, J.; Bolscher, J.G.M.; Nazmi, K.; León-Sicairos, N. Bovine Lactoferrin and Chimera Lactoferrin Prevent and Destroy Salmonella Typhimurium Biofilms in Caco-2 Cells. Biochem. Cell Biol. 2024, 102, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, R.I.; Milner, J.A. Inhibition of 7,12-Dimethylbenz[a]Anthracene-Induced Mammary Tumors and DNA Adducts by Garlic Powder. Carcinogenesis 1992, 13, 1847–1851. [Google Scholar] [CrossRef]
- Risiglione, P.; Leggio, L.; Cubisino, S.A.M.; Reina, S.; Paternò, G.; Marchetti, B.; Magrì, A.; Iraci, N.; Messina, A. High-Resolution Respirometry Reveals MPP+ Mitochondrial Toxicity Mechanism in a Cellular Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 7809. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A Pilot Study on the Effect of Lactoferrin on Alzheimer’s Disease Pathological Sequelae: Impact of the p-Akt/PTEN Pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef]
- Wang, J.; Bi, M.; Liu, H.; Song, N.; Xie, J. The Protective Effect of Lactoferrin on Ventral Mesencephalon Neurons against MPP+ Is Not Connected with Its Iron Binding Ability. Sci. Rep. 2015, 5, 10729. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Moon, J.-H.; Park, S.-Y. Lactoferrin from Bovine Colostrum Regulates Prolyl Hydroxylase 2 Activity and Prevents Prion Protein-Mediated Neuronal Cell Damage via Cellular Prion Protein. Neuroscience 2014, 274, 187–197. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Sanches, E.; Van De Looij, Y.; Sow, S.; Toulotte, A.; Da Silva, A.; Modernell, L.; Sizonenko, S. Dose-Dependent Neuroprotective Effects of Bovine Lactoferrin Following Neonatal Hypoxia–Ischemia in the Immature Rat Brain. Nutrients 2021, 13, 3880. [Google Scholar] [CrossRef]
- Han, N.; Li, H.; Li, G.; Shen, Y.; Fei, M.; Nan, Y. Effect of Bovine Lactoferrin as a Novel Therapeutic Agent in a Rat Model of Sepsis-Induced Acute Lung Injury. AMB Expr. 2019, 9, 177. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Dong, Y.; Wang, C.; Cai, Z. Bovine Lactoferrin Inhibits Inflammatory Response and Apoptosis in Lipopolysaccharide-Induced Acute Lung Injury by Targeting the PPAR-γ Pathway. Mol. Biol. Rep. 2024, 51, 492. [Google Scholar] [CrossRef]
- Madkour, A.H.; Helal, M.G.; Said, E.; Salem, H.A. Dose-Dependent Renoprotective Impact of Lactoferrin against Glycerol-Induced Rhabdomyolysis and Acute Kidney Injury. Life Sci. 2022, 302, 120646. [Google Scholar] [CrossRef]
- Salama, R.M.; Darwish, S.F.; Yehia, R.; Sallam, A.A.; Elmongy, N.F.; Abd-Elgalil, M.M.; El Wakeel, S.A. Lactoferrin Alleviates Gentamicin-Induced Acute Kidney Injury in Rats by Suppressing Ferroptosis: Highlight on ACSL4, SLC7A11, NCOA4, FSP1 Pathways and miR-378a-3p, LINC00618 Expression. Food Chem. Toxicol. 2024, 193, 115027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, X.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; Wang, J. Three in One with Dual-Functional Hydrogel of Lactoferrin/NZ2114/LMSH Promoting Staphylococcus Aureus-Infected Wound Healing. Antibiotics 2024, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, J.; Lu, W.; Ma, Y.; Wang, X.; An, X.; Fan, Z. The Preparation of Lactoferrin/Magnesium Silicate Lithium Injectable Hydrogel and Application in Promoting Wound Healing. Int. J. Biol. Macromol. 2022, 220, 1501–1511. [Google Scholar] [CrossRef]
- Tang, L.; Wu, J.J.; Ma, Q.; Cui, T.; Andreopoulos, F.M.; Gil, J.; Valdes, J.; Davis, S.C.; Li, J. Human Lactoferrin Stimulates Skin Keratinocyte Function and Wound Re-Epithelialization: hLF Stimulates Wound Re-Epithelialization. Br. J. Dermatol. 2010, 163, 38–47. [Google Scholar] [CrossRef]
- Corridore, D.; Vozza, I.; Di Carlo, G.; Guerra, F.; Santucci, F.; Mercuri, P.; Saccucci, M. Home Treatment Protocol for Dentin Hypersensitivity with Hydroxyapatite-Based Biodynamic Toothpaste and Lactoferrin: A Pilot Study. Minerva Dent. Oral. Sci. 2025, 73, 312–318. [Google Scholar] [CrossRef]
- Yamada, S.; Chea, C.; Furusho, H.; Oda, K.; Shiba, F.; Tanimoto, K.; Tate, S.; Miyauchi, M.; Takata, T. Effects of Novel Lactoferrin Peptides on LPS -induced Alveolar Bone Destruction in a Rat Model. Chem. Biol. Drug Des. 2024, 104, e14574. [Google Scholar] [CrossRef]
- Tomita, M.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H. Bovine Lactoferrin and Lactoferricin Derived from Milk: Production and Applications. Biochem. Cell Biol. 2002, 80, 109–112. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Krolitzki, E.; Schwaminger, S.P.; Pagel, M.; Ostertag, F.; Hinrichs, J.; Berensmeier, S. Current Practices with Commercial Scale Bovine Lactoferrin Production and Alternative Approaches. Int. Dairy J. 2022, 126, 105263. [Google Scholar] [CrossRef]
- Hancocks, N. Lactoferrin Market Set to Surpass €265 Million by 2027. 2021. Available online: https://www.nutraingredients.com/Article/2021/04/09/Lactoferrin-market-set-to-surpass-265-million-by-2027/ (accessed on 15 February 2025).
- Dairy Reporter 2020. Available online: https://www.dairyreporter.com/Article/2020/10/29/Morinaga-Milk-expanding-lactoferrin-production-capacity-through-German-subsidiary/ (accessed on 18 March 2025).
- Choi, B.-K.; Actor, J.K.; Rios, S.; d’Anjou, M.; Stadheim, T.A.; Warburton, S.; Giaccone, E.; Cukan, M.; Li, H.; Kull, A.; et al. Recombinant Human Lactoferrin Expressed in Glycoengineered Pichia Pastoris: Effect of Terminal N-Acetylneuraminic Acid on in Vitro Secondary Humoral Immune Response. Glycoconj. J. 2008, 25, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, J.; Gong, G.; Sun, X.; Zhang, R.; Du, Z.; Liu, Y.; Li, R.; Ding, F.; Tang, B.; et al. Cattle Mammary Bioreactor Generated by a Novel Procedure of Transgenic Cloning for Large-Scale Production of Functional Human Lactoferrin. PLoS ONE 2008, 3, e3453. [Google Scholar] [CrossRef]
- Min, S.R.; Woo, J.W.; Jeong, W.J.; Han, S.K.; Lee, Y.B.; Liu, J.R. Production of Human Lactoferrin in Transgenic Cell Suspension Cultures of Sweet Potato. Biol. Plant 2006, 50, 131–134. [Google Scholar] [CrossRef]
- Stefanova, G.; Slavov, S.; Gecheff, K.; Vlahova, M.; Atanassov, A. Expression of Recombinant Human Lactoferrin in Transgenic Alfalfa Plants. Biol. Plant 2013, 57, 457–464. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Behnam, B.; Balouch Zehi, Z.; Tavassoli, M.; Sadeghi, E.; Assadpour, E.; Zhang, F.; Jafari, S.M. Detection and Quantification of Lactoferrin: Innovations, Applications, and Challenges. Food Chem. 2025, 466, 142204. [Google Scholar] [CrossRef]
- Conesa, C.; Calvo, M.; Sánchez, L. Recombinant Human Lactoferrin: A Valuable Protein for Pharmaceutical Products and Functional Foods. Biotechnol. Adv. 2010, 28, 831–838. [Google Scholar] [CrossRef]
- Wang, S.-H.; Yang, T.-S.; Lin, S.-M.; Tsai, M.-S.; Wu, S.-C.; Mao, S.J.T. Expression, Characterization, and Purification of Recombinant Porcine Lactoferrin in Pichia Pastoris. Protein Expr. Purif. 2002, 25, 41–49. [Google Scholar] [CrossRef]
- Yen, C.-C.; Wu, P.-Y.; Ou-Yang, H.; Chen, H.-L.; Chong, K.-Y.; Chang, R.-L.; Chen, C.-M. Production of Bioactive Porcine Lactoferrin through a Novel Glucose-Inducible Expression System in Pichia Pastoris: Unveiling Antimicrobial and Anticancer Functionalities. Int. J. Mol. Sci. 2024, 25, 1818. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Natarajan, S.; Mandal, S.; Mitra, A. Lactoferrin-Derived Resistance against Plant Pathogens in Transgenic Plants. J. Agric. Food Chem. 2013, 61, 11730–11735. [Google Scholar] [CrossRef]
- Hu, C.; Shen, W.; Xia, Y.; Yang, H.; Chen, X. Lactoferrin: Current Situation and Future Prospects. Food Biosci. 2024, 62, 105183. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Abe, F. Quality Control of Commercial Bovine Lactoferrin. Biometals 2018, 31, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/media/124472/download (accessed on 12 April 2025).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Bovine Lactoferrin. EFSA J. 2012, 10, 2701. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Tang, Y.; Mujahid Ali, M.; Shen, Z.; Atta Debrah, A.; Yuan, Q.; Du, Z. Boron-Doped Titania for Separation and Purification of Lactoferrin in Dairy Products. J. Chromatogr. B 2022, 1212, 123501. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.D.; Kobayashi, T.; Wood, J.E.; Indyk, H.E. Analysis of Bovine Lactoferrin in Infant Formula and Adult Nutritional Products by Optical Biosensor Immunoassay: First Action 2021.07. J. AOAC Int. 2022, 105, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xiao, Q.; Yan, H.; Cao, Y.; Miao, J.; Wang, S.; Li, X.; Li, H.; Cheng, Z. Bovine Lactoferrin Quantification in Dairy Products by a Simple Immunoaffinity Magnetic Purification Method Coupled with High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2020, 68, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jiang, X.; Xu, X.; Liu, Y.; Liu, L.; Lu, A.; Lu, J.; Luan, Y. An Aptamer Affinity Column for Purification and Enrichment of Lactoferrin in Milk. J. Chromatogr. B 2021, 1178, 122724. [Google Scholar] [CrossRef]
- Gerstweiler, L.; Schad, P.; Trunzer, T.; Enghauser, L.; Mayr, M.; Billakanti, J. Model Based Process Optimization of an Industrial Chromatographic Process for Separation of Lactoferrin from Bovine Milk. J. Chromatogr. A 2023, 1710, 464428. [Google Scholar] [CrossRef]
- Cecconello, A.; Tonolo, F.; Rilievo, G.; Molinari, S.; Talpe, A.; Cozza, G.; Venerando, A.; Kariyawasam, I.D.H.; Govardhan, G.T.; Arusei, R.J.; et al. Highly Specific Colloidal Ɣ-Fe2O3-DNA Hybrids: From Bioinspired Recognition to Large-Scale Lactoferrin Purification. Colloids Surf. B: Biointerfaces 2024, 234, 113700. [Google Scholar] [CrossRef]
- Magdy, G.; Elmansi, H.; Shabana, R.A.; El-Enany, N. A Sustainable Sensitive Spectrofluorimetric Approach for the Determination of the Multipotent Protein Lactoferrin in Different Pharmaceuticals and Infant Milk Formula: Compliance with Greenness Metrics. Luminescence 2024, 39, e4772. [Google Scholar] [CrossRef]
- Ellingson, D.J.; Shippar, J.J.; Vennard, T.R.; Moloney, C.; O’Connor, D.; O’Regan, J.; McMahon, A.; Affolter, M. Analytical Method for Lactoferrin in Milk-Based Infant Formulas by Signature Peptide Quantification with Ultra-High Performance LC-Tandem Mass Spectrometry. J. AOAC Int. 2019, 102, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Frueh, J.L.; Shu, P.; Vennard, T.R.; Gray, M.A.; Phillips, S.C. Determination of Bovine Lactoferrin in Powdered Infant Formula and Adult Nutritionals by Heparin Affinity Extraction and Reverse-Phase High-Performance Liquid Chromatography/Ultraviolet Detection (HPLC/UV): Single-Laboratory Validation, First Action 2021.10. J. AOAC Int. 2024, 107, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, F.; Schmidt, C.M.; Berensmeier, S.; Hinrichs, J. Development and Validation of an RP-HPLC DAD Method for the Simultaneous Quantification of Minor and Major Whey Proteins. Food Chem. 2021, 342, 128176. [Google Scholar] [CrossRef]
- Wu, X.; Peng, Q.; Chai, Y.; Wang, M.; Li, H.; Li, H.; Li, C.; Yu, J. Isolation and Purification of Bovine N-glycans from Whey Protein Concentrate (WPC 70). Int. J. Dairy. Tech. 2024, 77, 1225–1234. [Google Scholar] [CrossRef]
- Parra-Saavedra, K.J.; Macias-Lamas, A.M.; Silva-Jara, J.M.; Solís-Pacheco, J.R.; Ortiz-Lazareno, P.C.; Aguilar-Uscanga, B.R. Human Lactoferrin from Breast Milk: Characterization by HPLC and Its in Vitro Antibiofilm Performance. J. Food Sci. Technol. 2022, 59, 4907–4914. [Google Scholar] [CrossRef]
- Tanaka, M.; Date, M.; Mizuno, K. New Latex Agglutination Assay for the Determination of Lactoferrin in Human Milk. Int. Breastfeed. J. 2024, 19, 74. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Amoli, H.S.; Mozaffari, S.A. Impedimetric and Single-Frequency Capacitance Spectroscopy Strategy in Label-Free Rapid Screening of Lactoferrin. Sens. Actuators B Chem. 2022, 354, 131107. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Xu, E.; Chen, L.; Feng, H.; Chen, L.; Deng, L.; Guo, D. Determination of Lactoferrin in Camel Milk by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry Using an Isotope-Labeled Winged Peptide as Internal Standard. Molecules 2019, 24, 4199. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Fan, F.; Shi, P.; Xu, X.; Du, M.; Wang, C. Analysis Method of Lactoferrin Based on Uncoated Capillary Electrophoresis. eFood 2021, 2, 147–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]
- Kussendrager, K.D.; Kivits, M.G.C.; Verver, A.B. Process for Isolating Lactoferrin and Lactoperoxidase from Milk and Milk Products, and Products Obtained by Such Process (1997). Patent No. US5596082A, 14 January 1993. [Google Scholar]
- Wang, W.; Liu, Z.; Liu, Y.; Su, Z.; Liu, Y. Plant Polypeptides: A Review on Extraction, Isolation, Bioactivities and Prospects. Int. J. Biol. Macromol. 2022, 207, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; John, H. Historical Perspective and Modern Applications of Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR). Drug Test. Anal. 2012, 4, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Khan, S.B.; Khan, L.U.; Farooq, A.; Akhtar, K.; Asiri, A.M. Fourier Transform Infrared Spectroscopy: Fundamentals and Application in Functional Groups and Nanomaterials Characterization. In Handbook of Materials Characterization; Sharma, S.K., Ed.; Springer: Cham, Switzerland, 2018; pp. 317–344. ISBN 978-3-319-92954-5. [Google Scholar]
- Zhang, J.L.; Di, W.; Gong, P.M.; Lin, K.; Lyu, L.Z.; Zhang, L.W.; Han, X.; Ma, Y. Direct and Fast Capture Lactoferrin from Cheese Whey on Nanoparticles of Fe3O4 Combined with Concanavalin A. Food Chem. 2019, 274, 314–318. [Google Scholar] [CrossRef] [PubMed]
| Product | Lf Concentration | Reference |
|---|---|---|
| Human colostrum | 7 mg/mL | [11] |
| Human mature milk | 2 mg/mL | [11] |
| Bovine colostrum | 1.5 mg/mL | [10] |
| Bovine mature milk | 0.2 mg/mL | [10] |
| Goat colostrum | 0.39 mg/mL | [12] |
| Goat mature milk | 0.06 mg/mL | [12] |
| Camel milk | 0.229 ± 0.135 mg/mL | [13] |
| Buffalo milk | 0.332 ± 0.165 mg/mL | [14] |
| Infant formula (cow’s milk-based) | 0.1 mg/mL | [15] |
| Matrix | Methods | Result | Characteristic of Analytical Method | References | |
|---|---|---|---|---|---|
| Sample Preparation | Conditions and Validation Parameters | ||||
| Liquid milk, Fermented milk, Infant formula | UHPLC-UV | Developed a cheap and efficient method for detecting Lf in dairy products | 4 mL of liquid milk or 4 g of fermented milk and infant formula mixed with 800 μL of dichloromethane and 400 μL of water with 5% acetic acid; centrifuged; supernatant mixed with 8 mL ammonium chloride buffer; added 5 mg of B-doped TiO2; sonicated for 5 min; shaken for 75 min; 200 μL of water containing 5% formic acid added and shaken to elute the bound Lf; filtered with a 0.22 μm polyethersulfone filter membrane | Detector: UV, column: Waters Acquity TM Protein BEH C4 column (2.1 mm × 100 mm, 1.7 μm). Eluent A: 0.1% formic acid in water, B: acetonitrile/water/ formic acid (71.4:28.6:0.075, v/v/v), gradient elution Flow rate: 0.3 mL/min LOD: 0.0002–0.0012 mg/g LOQ: 0.0006–0.0029 mg/g | Wang M et al. (2022) [97] |
| Infant formula | LC-MS/MS | Developed and validated a method for quantifying LF in powder infant formulae | 1 g powder infant formula or 10 g liquid (ready-to-feed) infant formula + 30 mL extraction buffer and diluted to volume (50 mL) with extraction buffer samples that contained between 1000 and 2000 mg/100 g: diluted by transferring 50 μL to a new well and adding 100 μL of 85 + 15 water–acetonitrile (0.15% formic acid) samples that contained < 1000 mg/100 g: diluted by transferring 50 μL to a new well and adding 50 μL of 80 + 20 water–acetonitrile (0.2% formic acid) final dilutions: added 150 μL internal standard working solution IS-SAMP (for samples containing 1000–2000 mg/100 g) or 100 μL IS-SAMP for samples containing < 1000 mg/100 g) | Column: Acquity UPLC BEH C18 (2.1 × 50 mm, 1.7 μm), mobile phase A: 0.1% formic acid in water, B: 0.1% formic acid in acetonitrile, gradient elution, flow rate 0.6 mL/min. MS/MS: 4000 Q-Trap with Electrospray Ionisation, IonSpray voltage: 4000 V LOQ: 0.25 mg/g LOD: no data | Ellingson DJ et al. (2019) [104] |
| Infant formula, milk powder, powder reconstituted ready-to-feed basis | Optical Biosensor Immunoassay | Developed and evaluated a rapid, sensitive, precise, and simple method for detecting Lf in nutritional formula powder | Infant formula powder or milk powder diluted 1:2000 w/v. 0.5 g of sample + 8 mL buffer HPS-EP (2.044 g sodium chloride in 100 mL), stored in the dark ≥ 15 min. 990 µL buffer + 10 µL, cap and vortex Powder reconstituted ready-to-feed basis diluted 1:2000 w/v. 25 g powder + 200 g water, warm to 25 °C for 10 min and mix. 4.5 g aliquot of sample solution and make up to 10 mL with HPS-EP, cap and vortex. 990 µL solution buffer + 10 µL of the diluted sample, cap and vortex | Biacore T200. HPS-EP flow rate: 10 µL/min, contact time 300 s. LOD: 0.008 mg/g LOQ: 0.025 mg/g | Gill BD et al. (2022) [98] |
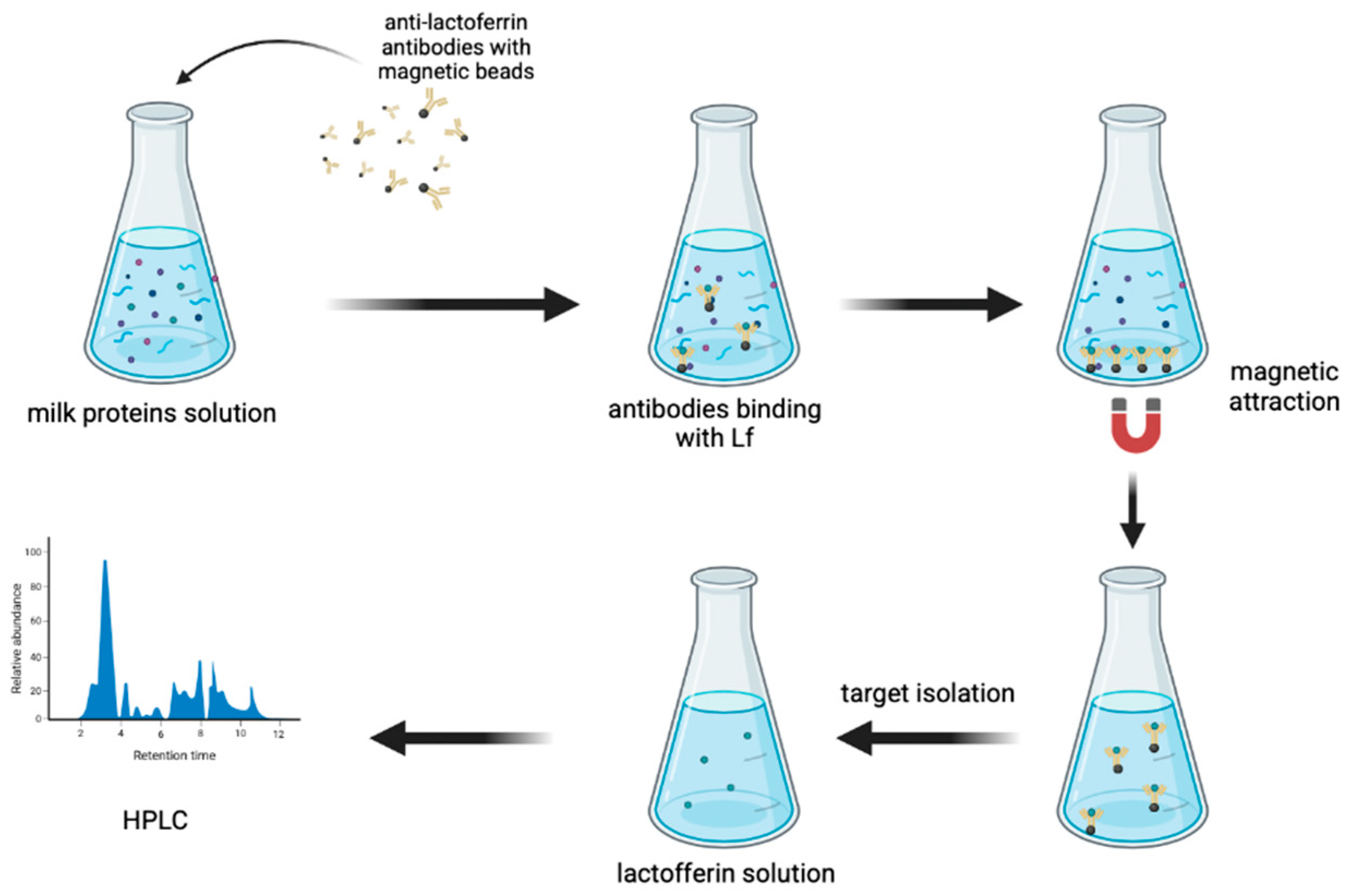
| Pasteurisation milk, infant formula, whey protein concentrate | HPLC-FL | Developed a method of Lf purification by immunoaffinity magnetic beads. Determined the Lf by HPLC-FL, which is characterised as more sensitive than HPLC-UV | Liquid samples: 200 μL of milk mixed with 800 μL of PBSN (phosphate buffered saline containing 0.1% Tween 20, pH = 7.4), centrifuged at 10,000 rpm/5 min at 4 °C to remove the fat, 500 μL of supernatant incubated with 5.0 mg of antibody-coated beads at 37 °C/2 h Beads washed (immobilise anti-Lf antibody with magnetic beads) using 1.0 mL of PBSN three times and attracted with a magnet: supernatant removed. The bead-bound target protein was eluted using 1.5 mL of citrate buffer (0.1 M, pH = 3.0) containing 0.1% Tween (CBN) Lf saturated with iron at room temp. with a freshly prepared FeNTA solution [9.9 mM ferric nitrate and 8.5 mM nitrilotriacetic acid (disodium salt) in water, adjusted to pH = 7.0 by adding solid NaHCO3]/1 h Solid samples: 35 mg of samples dissolved in 1.0 mL of PBSN, fat removed by centrifuging, 500 μL of the supernatant incubated with 10 mg of antibody-coated beads at 37 °C/2 h, and treated like a liquid sample | Detector: FL, 280 nm (excitation), 344 nm (emission), Column: Innoval Neo XD C18 column (150 × 4.6 mm, 5 μm), Mobile phase: A—water + 0.1% trifluoroacetic acid, B-acetonitrile + 0.1% trifluoroacetic acid, gradient elution, flow rate 1 mL/min LOD: 0.00025 mg/mL LOQ: 0.0008 mg/mL | Pang J et al. (2020) [99] |
| Raw milk | HPLC-UVD | Improved Lf purification using an aptamer column combined with HPLC-UVD | 10 mL milk + 40 mL phosphate buffer (0.2 mol/L Na2HPO4·12H2O, pH = 7.5), centrifuged at 4 °C and 10,000 rpm for 15 min. Supernatant was purified using the AAC (activated with 5 mL of binding buffer) 10 mL of the supernatant (10 mL milk + 40 mL buffer) loaded on the column. AAC washed with 10 mL of washing buffer (0.01 mol/L Na2HPO4·12H2O, pH = 7.5) and flushed with 2–3 mL of air. Lf eluted from the AAC with 2 mL of elution buffer (0.05 mol/L Na2HPO4·12H2O, 1 mol/L NaCl, pH = 8.0). Eluent filtered through a 0.2 μm syringe filter | Detector: UVD, column: Xbridge Protein BEH C4 300 Å (250 × 4.6 mm, 5 μm), mobile phase: A—0.1% trifluoroacetic acid, B—acetonitrile, gradient elution, flow rate: 0.5 mL/min LOD: 0.000003 mg/mL LOQ: 0.00001 mg/mL | Wang N et al. (2021) [100] |
| Skim milk | IEC RP-HPLC SDS-PAGE | Optimised an industrial chromatographic process for separating Lf from skim milk | Experiments for model development and validation were conducted on an Akta avant 150 (Cytiva®, Sweden). The column was packed with SP Sepharose Big Beads Food Grade strong cation exchanger. The developed model was used for in-silico process optimisation of a two-step elution process, which is typically used in commercial Lf manufacturing | IEC—Column exchange chromatography: SOURCE 15STricorn 5/50 filled with 1 mL of SOURCE 15S resin, buffer A: 50 mM Tris, pH = 7.5, B: 1 M NaCl, 50 mM Tris, pH = 7.5 RP-HPLC—buffer A: MilliQ water, 0.1% TFA, B: Acetonitrile, 0.1% TFA, gradient elution, flow rate 1 mL/min SDS-PAGE—Sodium dodecylsulphate–polyacrylamide gel electrophoresis under reducing conditions on pre-cast BoltTM 12% Bis-Tri Plus 1.00 mm X 12 well gels as per manufacturer recommendations. LOD: no data LOQ: no data | Gerstweiler L et al. (2023) [101] |
| Milk | Spectrophotometry | Used hybrids constituted of nanomaterials, surface active maghemite nanoparticles, and DNA to bind Lf | Milk samples skimmed by centrifugation at 4000 g for 15 min at 4 °C and then ultra-centrifuged. Bovine whey incubated in the presence of SAMN@DNA (0.5 g/L) and mixed for 2 h at room temperature, leading to the formation of a SAMN@DNA@LF complex. SAMN@DNA@LF hybrid released by incubation in a high ionic strength solution (4 M NaCl) for 1 h at 4 °C. The eluted Lf solutions concentrated using Vivaspin tubes | Spectrophotometry at 280 nm, Lf in the 0.05–0.6 mg/mL concentration range. LOD: no data LOQ: no data | Cecconello A et al. (2024) [102] |
| Camel milk | UHPLC-MS/MS | Determined Lf from camel milk | Camel milk adjusted to pH = 4.6 with acetic acid, centrifuged; aliquots of 100 μL of whey spiked with 100 μL of 1 μM stable isotope-labelled internal standard and mixed with 180 μL of 50 mM NH4CO3. reduction by adding 15 μL of 500 mM TCEP solution at 50 °C /30 min; alkylation in the presence of 45 μL of 500 mM IAA solution; 10 μL of 100 mM CaCl2 solution and trypsin added, incubated at 37 °C overnight; terminated by adding 10 μL of formic acid; filtered through a 0.22 mm nylon filter | Column: UPLC BEH C18 (100 mm×2.1 mm, 1.7 μm, 300 A), Mobile phases A: Milli-Q water + 0.1% ammonia, B: acetonitrile, gradient elution, and flow rate of 0.3 mL/min Electrospray ion source of the MS, capillary voltage: 3.50 kV, cone voltage: 15 V, desolvation gas: nitrogen, flow rate of 850 L/h LOD: 0.0038 mg/g LOQ:0.011 mg/g | Li X et al. (2019) [111] |
| Formula powder, paediatric/adult nutritional powders | UHPLC/UV | Developed and validated a method for detecting Lf in nutritional formula powder. | 0.5–1.50 g sample + 11.5 mL warm (40 °C) 0.2 M sodium phosphate (pH = 8.0), shaken for 60 min, centrifuged for at least 20 min at 8000 g at 4 °C | Column: BEH C4 column (4.6 × 150 mm, 3.5 µm), mobile phase A: 0.1% trifluoroacetic acid solution, B: 0.1% trifluoroacetic acid in acetonitrile solution, flow rate: 0.5 mL/min, gradient elution LOD: 0.002 mg/g LOQ: 0.04 mg/g | Frueh JL et al. (2024) [105] |
| Whey | RP-HPLC-DAD | Developed and validated a method for detecting Lf in whey | Whey samples centrifuged at 15,000× g for 10 min (at room temperature) to remove aggregates and micelles | RP-HPLC. Detector: DAD, column: BioResolve RP mAb 450 A Polyphenyl column (2.7 µm, 150 × 4.6 mm), mobile phase A: 99% double distilled water + 1% Acetonitrile + 0.1% of the ion-pairing agent trifluoroacetic acid, B: 99% ACN and 1% AqDD added with 0.072% TFA, gradient elution flow rate: 0.8 mL/min LOD: 0.006 mg/mL LOQ: 0.019 mg/mL | Ostertag F et al. (2021) [106] |
| Whey protein concentrate | Anion exchange chromatography | Improved isolation and purification of Lf (N-glycans) from whey protein concentrate by anion exchange chromatography | SDS-PAGE: the protein concentration was diluted and mixed with 5× loading buffer to reach a concentration of 4 mg/mL, heated at 95 °C for 5 min, and then loaded onto the gel, next run with 5% stacking gel, and 12% separating gel. The running buffer contains 0.05 M Tris, 0.19 M glycine, and 0.1% (w/v) SDS. Pre-stained protein marker was used for the quantification and molecular weight determination of the protein bands The electrophoresis was run through stacking gels and separating gels at a constant voltage of 80 V and 120 V, respectively. After that, the gels were stained with Coomassie Brilliant Blue R250 for 0.5 h and decoloured overnight | DEAE (diethylaminoethyl) Sepharose Fast Flow column (90 μm particle size) with Advanced Kinetic and Transport Analysis pure system LOD: no data LOQ: no data | Wu X et al. (2024) [107] |
| Lactoferrin powder | Capillary electrophoresis | Optimised the parameters of uncoated capillary electrophoresis as a perspective for separating Lf | Sample dissolved in buffer and filtered through a 0.22 μm filter | Different tests. Capillary electrophoresis apparatus: uncoated fused capillary, inner diameter of 50 μm, outer diameter of 75 μm, length of 61.2 cm, actual length of 51.2 cm. The best buffer system was determined to be 50 mmol/L phosphates with 6 mol/L urea. The auxiliary pressure of 0.5 psi LOQ: 0.04 mg/mL LOD: 0.01 mg/mL | Chen H. et al. (2021) [112] |
| Capsules, sachets, milk powder | Spectrofluorimetry | Determined Lf by spectrofluorimetry without using toxic reagents | Lacto-Apex® capsules. 10.0 mg of powder. Seventy millilitres of methanol were added, and the flask contents underwent sonication for 10 min, filtered into a 100 mL measuring flask, and further serial dilutions were performed to get the range of study. The steps for constructing the calibration curve. For sachets, Lineal® sachets labelled to contain 100 mg Lf/3 g were used, where a weight of the sachet powder corresponding to 10.0 mg Lf was transferred into a 100 mL measuring flask, and then the procedure mentioned above was followed. 0.5 g of milk powder + methanol to 5.0 mL, sonicated/1 h, followed by 10 min of centrifugation at 8000 rpm. 1 mL of the supernatant transferred to a 5 mL measuring flask + completed to the volume with methanol to obtain the final concentrations of LTF (2.0, 4.0, 6.0, 8.0, and 10.0 μg/mL) | Spectrofluorimeter: Xenon flash lamp operated at 800 V, slit width = 5 nm, smoothing factor of 20, excitation: 230 nm, emission: 337 nm LOD: 0.00002 mg/mL LOQ: 0.000082 mg/mL | Magdy G et al. (2024) [103] |
| Human milk (lactoferrin lyophilisate) | HPLC Infrared spectroscopy (FTIR) SDS-PAGE electrophoresis | Obtained a lyophilisate of purified Lf by using a heparin affinity column and determined by the HPLC method | Pre-treatment of the milk: Centrifuged at 4.000 rpm/20 min. Sample extraction: 5 mL of sample + 5 mL of buffer 0.2 M Na2HPO4 (pH = 8), mixed for 30 s, centrifuged at 4.000 rpm/20 min at 4 °C Lf purification: 1 mL HiTrap Heparin affinity column (conditioned with 5 mL of 0.2 M Na2HPO4 buffer (pH = 8), addition rate of 1 mL/min. The extraction solution: injected into the column, followed by a wash with 10 mL of buffer 0.2 M Na2HPO4 (pH = 8). Lf eluted with 3 mL of buffer 0.05 M Na2HPO4 and 1.0 M NaCl (pH = 8) Freeze-drying: eluted fractions taken to an ultrafiltration cell, passed through a 30 kDa membrane pressure of 60 psi, performing two washes with 0.05 M NaCl Lyophiliser at 0.147 mBar/6 h | Detector: DAD Column: Kinetex XB C18 (15 × 4.6 mm, 5 μm) Mobile phase: 0.1% trifluoride acetic acid in water, acetonitrile, gradient elution, flow rate 0.6 mL/min Infrared Spectroscopy: Cary 630 IR spectrophotometer analysed with a resolution of 2 cm−1, 32 scans, range of 400 to 4000 cm−1. SDS-PAGE electrophoresis: denaturing and reducing conditions 40 µL of protein in solution in 10% polyacrylamide gel wells. Run at 150 V in a Hoeffer Mighty Small 250 chamber LOQ: 0.004 mg/mL LOD: 0.001 mg/mL | Parra-Saavedra et al. (2022) [108] |
| Human milk | Latex agglutination assay ELISA | Developed a cheap and fast method for determining Lf in human milk | Milk samples diluted 100-fold (samples with values exceeding 16 µg/mL diluted 200-fold); 4 µL of diluted milk + 100 µL of buffer solution + 100 µL of polystyrene latex particles coated with anti-human LF mouse monoclonal antibody ELISA, milk samples centrifuged 1/50,000 sample dilution preparation: 5 µL of sample + 495 µL of diluent solution; 2 µL of the 1/100 sample + 998 µL of diluent solution | Absorbance measurement: 600 nm, 30 s, and 5 min after adding latex particles The concentration of Lf was measured using a Human Lf ELISA Kit LOD: 0.0002 mg/mL LOQ: 0.02 mg/g | Tanaka M et al. (2024) [109] |
| Colostrum | Electroanalysis electrochemical quartz crystal microbalance (EQCM) Single radial immunodiffusion (SRID) | Determined Lf in colostrum | - Samples centrifuged (3000 rpm/5 min) - Sample diluted (1:100) in 0.1 M PBS (phosphate buffer solution) containing 3.0 mM [Fe(CN)6]3−/4− electroanalysis - Samples diluted | Electroanalysis: Autolab electrochemical analyser. LBP as a working electrode, Ag/AgCl reference electrode, and platinum rod counter electrode are used for all electrochemical investigations. 0.1 M PBS (pH = 7.0) solution containing 3.0 mM [Fe(CN)6]3-/4-redox probe. CV: range of −0.2 to +0.6 V; scan rate of 100 mVs−1. DPV: range of −0.1 to +0.4 V; scan rate of 10 mV s−1. The impedance spectra were recorded at an open-circuit voltage in the frequency range of 100 kHz to 1 Hz and an alternating voltage amplitude of 10 mV Electrochemical quartz crystal microbalance: The module is connected to a PGSTAT 302N potentiostat/galvanostat, and the working electrode is a gold-coated AT-cut quartz crystal sensor with a fundamental resonant frequency of 6 MHz. A gold wire counter electrode and a KCl-saturated gel electrolyte Ag/AgCl reference electrode were used to complete the electrochemical circuit Single radial immunodiffusion: Specific antibody and 1.2% agar in 0.005 M barbital buffer (pH = 7.3), incubation: 37 °C for 15–20 h LOD: 65.2 nM LOQ: no data | Ebrahimi F et al. (2022) [110] |
| Matrix | Method | Yield/Recovery | Study |
|---|---|---|---|
| Liquid milk, fermented milk, infant formula | UHPLC-UV | Recovery: 83.6–90.8% | Wang M et al. (2022) [97] |
| Infant formula, milk powder, powder reconstituted ready-to-feed basis | Optical Biosensor Immunoassay | Recovery: 96.1–109.2% | Gill BD et al. (2022) [98] |
| Milk, infant formula, whey protein concentrates (WPC) | HPLC-FL | Recovery: 96.3% for milk, 95.8% for infant formula, 93.3% for WPC | Pang J et al. (2020) [99] |
| Raw milk | HPLC-UVD | Recovery: 103.44% | Wang N et al. (2021) [100] |
| Skim milk | IEC, RP-HPLC, SDS-PAGE | Recovery: up to 100% | Gerstweiler L et al. (2023) [101] |
| Milk | Spectrophotometry | Yield: ≥98% | Cecconello A et al. (2024) [102] |
| Capsules, sachets, milk powder | Spectrofluorimetry | Recovery: 96.45–104.92% | Magdy G et al. (2024) [103] |
| Infant formula | LC-MS/MS | Recovery: 91.7–96.4% | Ellingson DJ et al. (2019) [104] |
| Formula powder, paediatric/adult nutritional powders | UHPLC/UV | Recovery: 92.1–97.7% | Frueh JL et al. (2024) [105] |
| Whey | RP-HPLC-DAD | Recovery: 96–102% | Ostertag F et al. (2021) [106] |
| Human milk | HPLC, Infrared Spectroscopy (FTIR), SDS-PAGE electrophoresis | Recovery: 70% | Parra-Saavedra et al. (2022) [108] |
| Human milk | Latex agglutination assay, ELISA | Recovery: 90–120% | Tanaka M et al. (2024) [109] |
| Camel milk | UHPLC-MS/MS | Recovery: 74.5–103.6% | Li X et al. (2019) [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, K.A.; Kosewski, G.; Dobrzyńska, M.; Drzymała-Czyż, S. Lactoferrin Production: A Systematic Review of the Latest Analytical Methods. Appl. Sci. 2025, 15, 4540. https://doi.org/10.3390/app15084540
Kaczmarek KA, Kosewski G, Dobrzyńska M, Drzymała-Czyż S. Lactoferrin Production: A Systematic Review of the Latest Analytical Methods. Applied Sciences. 2025; 15(8):4540. https://doi.org/10.3390/app15084540
Chicago/Turabian StyleKaczmarek, Katarzyna A., Grzegorz Kosewski, Małgorzata Dobrzyńska, and Sławomira Drzymała-Czyż. 2025. "Lactoferrin Production: A Systematic Review of the Latest Analytical Methods" Applied Sciences 15, no. 8: 4540. https://doi.org/10.3390/app15084540
APA StyleKaczmarek, K. A., Kosewski, G., Dobrzyńska, M., & Drzymała-Czyż, S. (2025). Lactoferrin Production: A Systematic Review of the Latest Analytical Methods. Applied Sciences, 15(8), 4540. https://doi.org/10.3390/app15084540






