1. Introduction
In China, the lead smelting industry mainly relies on the sintering-blast furnace process, followed by the closed-blast furnace smelting process. The smelting flue gas generated by these two processes contains a significant amount of sulfur oxides. In particular, the sintering-blast furnace process can produce approximately 0.5 t of SO
2 gas for every 1 t of crude lead produced [
1]. The specific flue gas composition of some process furnace types is shown in
Table 1 [
2]. In addition to the high emissions of sulfur oxides, lead smelting smoke exhibits a high dust concentration. The main components of lead smelting smoke are lead, elemental zinc and zinc compounds, and small amounts of Cd, Se, Te, Tl, Sb, Sn, In, Al, and other elements and compounds [
3,
4].
The pyrometallurgical processing of lead sulfide ores generates significant sulfur oxide emissions (SO
x), predominantly sulfur dioxide (SO
2) with minor quantities of sulfur trioxide (SO
3) [
5,
6,
7]. These byproducts pose substantial operational and environmental challenges. Below the acid dew point temperature, SO
3 reacts with moisture to form corrosive acid mists and micro-droplets that degrade metal surfaces through low-temperature corrosion. Mitigation strategies include maintaining cold-end temperatures above the dew point or employing acid-resistant construction materials [
8,
9]. A secondary corrosion mechanism involves SO
3 reacting with alkali metals and iron to form aggressive compounds such as alkali metal iron trisulfate, which contributes to furnace wall degradation [
10,
11]. In the environment, unreacted SO
3 escapes as blue plume emissions through flue stacks, primarily through the formation of sulfuric acid (H
2SO
4) aerosols.
The flue gas cooling profile (1100 °C to 300 °C) introduces complex technical constraints for SO
3 mitigation. Effective solutions must achieve three objectives: (1) substantial SO
3 reduction without disrupting smelting operations, (2) compatibility with existing gas treatment infrastructure, and (3) economic viability in construction and operation. Chemical inhibitors present a potential solution but require careful evaluation; they must demonstrate minimal process interference while enabling the recovery of valuable components from heterogeneous flue streams containing gas-phase species, particulate matter, and complex decomposition/oxidation products [
12,
13]. Process simplicity and cost-effectiveness remain critical implementation criteria.
Fundamentally, SO
3 generation in lead smelting originates from high-temperature oxidation reactions during sulfide ore processing [
14,
15]. While SO
2 constitutes the primary sulfur oxide, catalytic conversion mechanisms (particularly in the presence of metal oxides and oxygen) enable partial SO
2 oxidation to SO
3. This conversion intensifies corrosion risks through two pathways: acid condensation below dew points (in the 250–150 °C range) and high-temperature sulfate salt formation. The resulting corrosion products—whether liquid acid films or solid alkali sulfates—progressively degrade refractory linings and metallic components [
16,
17].
The current mitigation approaches present technical tradeoffs. SO
3-specific removal systems could theoretically protect downstream acid plants relying on SO
2 feedstock, but they need to address secondary pollution risks and byproduct management challenges. Process modifications that suppress SO
3 formation at the source (e.g., oxygen control, catalytic inhibitors, etc.) show promise but demand the thorough understanding of reaction mechanisms across the thermal gradient [
18,
19,
20]. Material solutions using advanced ceramics or alloy liners offer localized protection but increase capital costs. This analysis underscores the need for holistic strategies combining the following:
Process optimization to minimize SO3 generation;
Targeted removal technologies preserving SO2 for acid production;
Selection of corrosion-resistant materials;
Continuous monitoring of flue gas composition and thermal profiles.
Further research should quantify SO3 formation kinetics under operational conditions and evaluate inhibitor performance in complex, particulate-laden gas streams. This fundamental understanding will inform the development of cost-effective, integrated solutions for sulfur oxide management in lead pyrometallurgy.
Through the method of thermodynamic simulation and experimental verification, this study reveals the catalytic oxidation mechanism of SO2 in lead dust and proposes the use of PbS as an in situ SO3 inhibitor. This discovery not only provides a theoretical breakthrough for understanding the kinetics of metallurgical multiphase reactions but also can guide smelters to achieve a reduction in SO3 generation by more than 60% (the target value is <0.6%) through the synergy of process parameter optimization and material modification, while avoiding the side effects of traditional technologies. This achievement has direct engineering significance for promoting the transformation of the lead industry towards the direction of low emissions and high energy.
2. Experimental
2.1. Experimental Setup
FactSage 8.0 software was used to simulate the SO2/SO3 thermodynamic equilibrium.
Elemental composition analysis was conducted using X-ray fluorescence spectroscopy [
21,
22].
A dedicated catalytic platform was developed, comprising the following:
A gas distribution system with precision mass flow controllers for gas blending;
A reaction system with a high-temperature tube furnace (±1 °C accuracy) and a customizable quartz reactor (8–12 mm in diameter and 800–1000 mm in length);
An absorption system using isopropanol trapping for SO3 quantification.
The platform supported both homogeneous experiments (with an empty reactor) and heterogeneous tests with lead smelting ash catalysts.
2.2. Experimental Materials
The main reagents used in this experiment were lead glance and isopropanol, which were purchased in high purity forms from China. Furthermore, high-pressure gases such as SO2, O2, and N2 (99.9%) were also utilized in this experiment.
2.3. Experiment
The Equilib module of FactSage 8.0 was used to simulate the SO2/SO3 thermodynamic equilibrium. Using FactSage, multiple multiphase equilibrium conditions can be calculated under multiple constraints, and the results can be output in the form of graphs or tables. This is based on the principle of Gibbs free energy minimization.
With the actual production data from a local lead smelter as a reference, the changes in the remaining components when a group changed in the equilibrium were calculated.
Experimental studies under homogeneous (gas-phase) and heterogeneous (catalytic) conditions were conducted to analyze SO2/SO3 conversion mechanisms. These investigations, combined with catalytic SO3 generation patterns and inhibitor-based suppression strategies, provide actionable insights for SO3 control in non-ferrous smelting operations.
The SO3 measurement method used was as follows: collect the absorption liquid in the absorption bottle of the porous glass plate and the connecting tube between the absorption bottles, and record the volume of the solution in mL. Take 20 mL of the collected absorption solution, put it in a conical flask, and dilute it by adding 40 mL of 80% isopropanol solution. Titrate with a 0.025 mol/L strontium perchlorate titration solution. If the titration is finished, it will not change color, or if the color change effect is not obvious, the titration solution concentration Is halved and the above operation is repeated. Take three titrations and take the average and record the volume of the titrant consumed as b mL , where nSO3 is the number of moles of SO3 produced during the experiment, and c is the concentration of the titrant used.
In this experiment, the effects of varying initial O2 and SO2 contents on PbS decomposition/oxidation behavior and SO3 generation were studied.
3. Results and Discussion
3.1. Generation of SO3 in Simulated Lead Smelting Flue Gas
The Equilib module in FactSage 8.2 was employed to investigate the equilibrium concentration and conversion rate of SO3 in the gas phase under varying temperatures (300–1300 °C). The simulated flue gas composition comprised the following molar fractions: 1% CO, 5% CO2, 15% SO2, 0.5% SO3, 8% O2, 8% H2O, and 62.5% N2.
3.1.1. The Effect of Equilibrium Temperature on SO3 Generation
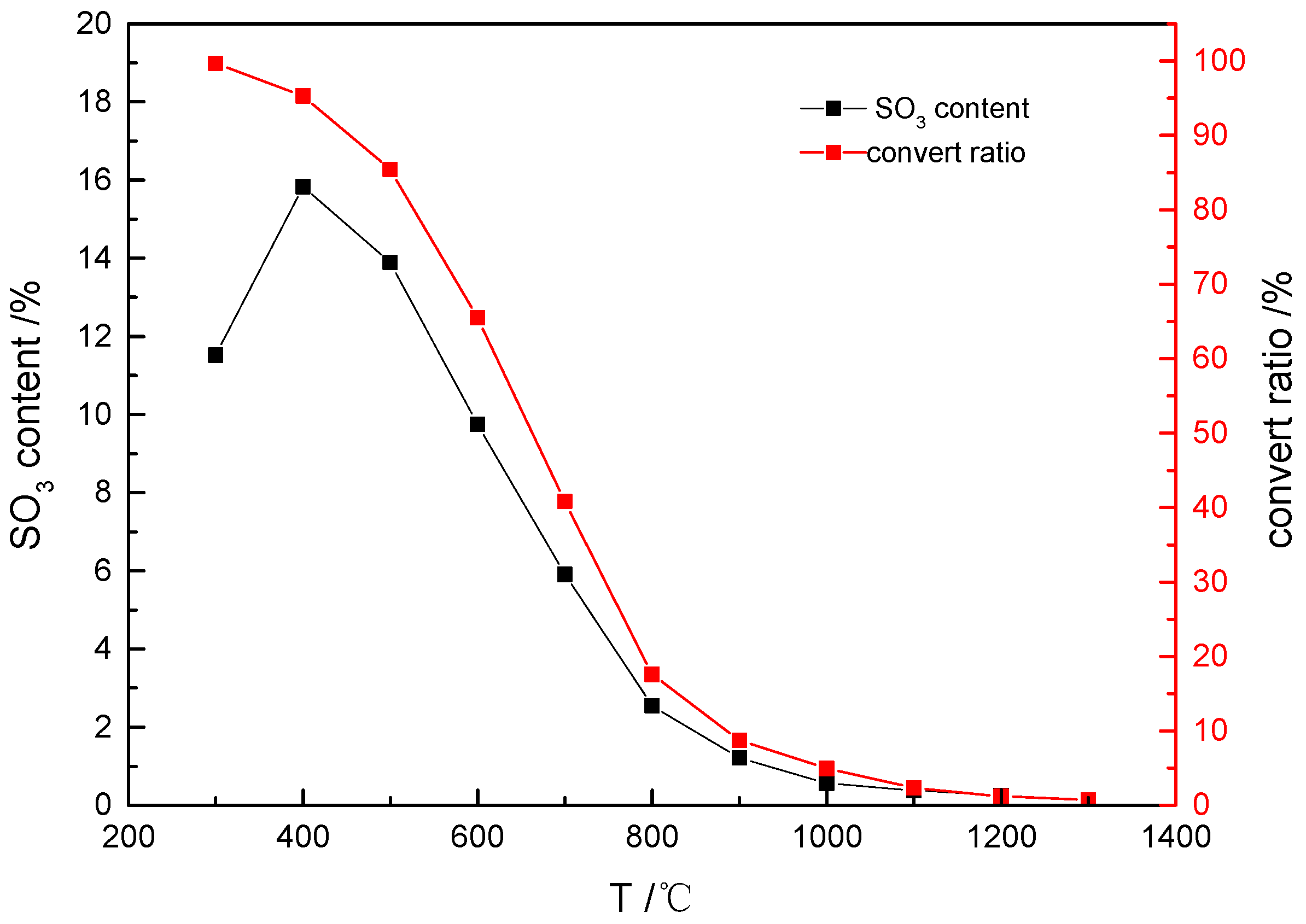
As illustrated in
Figure 1, temperature exerts a significant thermodynamic influence on both the equilibrium SO
3 concentration and its conversion rate. A strong inverse correlation was observed: lower temperatures correspond to higher equilibrium SO
3 concentrations. At 400 °C, the equilibrium SO
3 concentration reached 15.83%, whereas it plummeted to 0.14% at 1300 °C. Notably, an inflection point in SO
3 concentration occurs at 400 °C, attributed to partial SO
3 consumption through sulfuric acid (H
2SO
4) formation within the 300–400 °C range.
The SO3 conversion rate followed a trend similar to that of its concentration profile, with the maximum conversion (99.70%) achieved at 300 °C, indicating the near-complete oxidation of sulfur species to SO3. Conversely, the conversion rate declined sharply to 0.74% at 1300 °C, reflecting the thermodynamic instability of SO3 at elevated temperatures. These results demonstrate that low-temperature regimes (<500 °C) strongly favor SO3 formation and retention in the gas phase, while high-temperature conditions promote SO3 decomposition.
This analysis highlights the critical role of thermal management in controlling SO3 levels during pyrometallurgical processes, with implications for corrosion mitigation and emission control strategies.
An increase in the SO
3 concentration in a flue gas system proportionally increases the ADP, thereby exacerbating the corrosion risk of downstream equipment. SO
3 levels should be kept below 1 vol% through engineered suppression strategies to mitigate the degradation of waste heat boilers and particulate control systems. The complexity of SO
3 formation in lead smelting flue gases stems from its diffuse generation pathways, heterogeneous catalytic oxidation, homogeneous gas-phase reactions, and sulfate decomposition mechanisms. The dominant formation route is as follows:
3.1.2. The Effect of Initial SO2 Content on SO3 Generation
Assuming the fixed temperatures of 200 °C, 400 °C, 900 °C, and 1300 °C, the effects of the initial SO
2 content (0–20% range) on the content of various substances during the final equilibrium of the reaction were studied, as shown in
Figure 2.
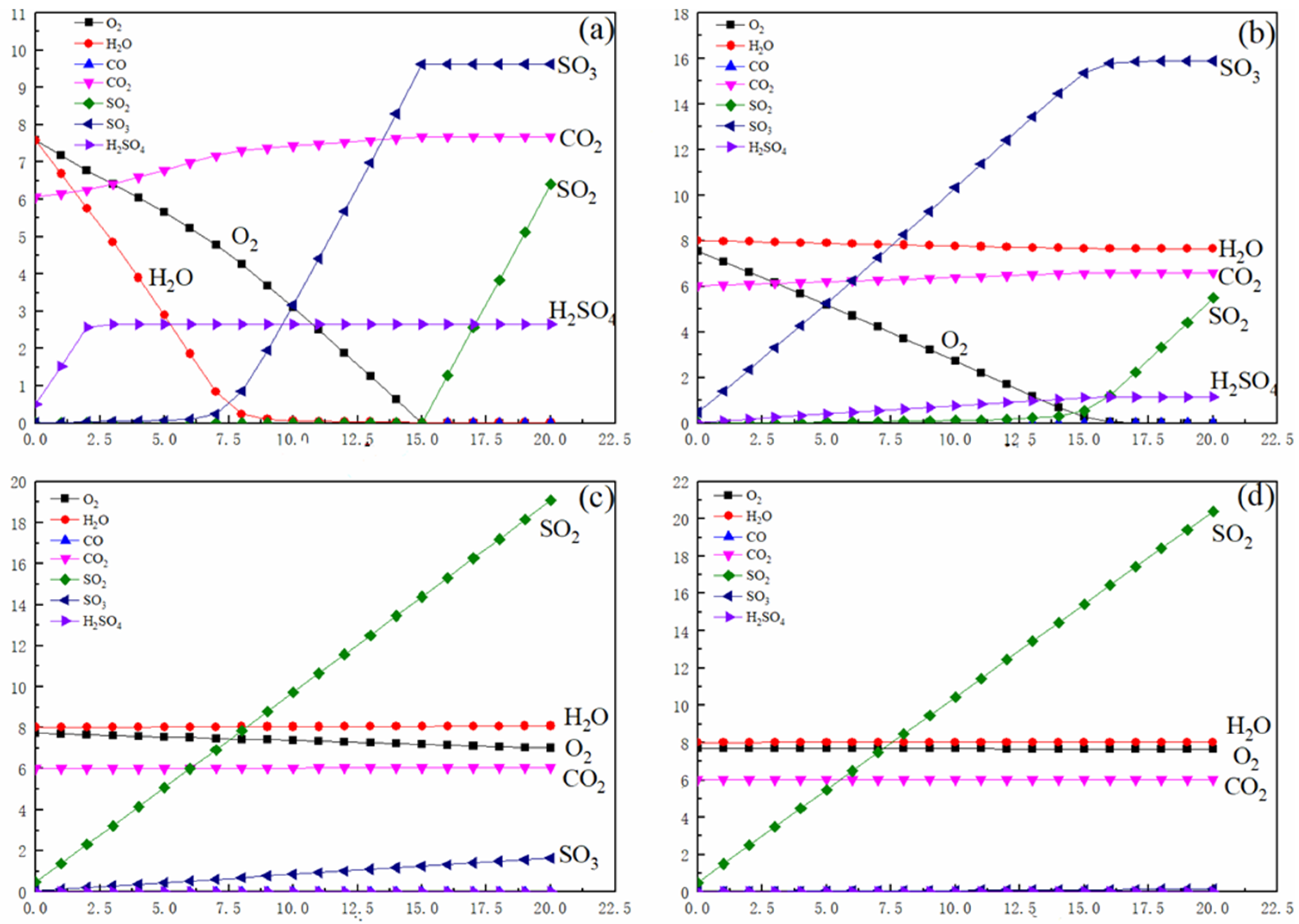
As depicted in
Figure 2a, the equilibrium SO
3 concentration exhibits a three-stage response to increasing initial SO
2 levels: initial stability, followed by a marked increase and eventual stabilization. When the SO
2 concentration exceeds 7%, SO
3 begins accumulating in the gas phase. This phenomenon arises because the limited moisture content at low temperatures becomes insufficient to sustain sulfuric acid (H
2SO
4) formation via SO
3 hydration. At SO
2 concentrations of >15%, SO
3 plateaus at 9.62% due to oxygen depletion, i.e., insufficient O
2 availability, preventing further SO
2-to-SO
3 oxidation and leading to additional inconsequential SO
2 increases.
Figure 2b reveals similar SO
3 trends under 400 °C conditions. Notably, thermodynamic calculations confirm the absence of liquid H
2SO
4 across all SO
2 concentrations, with sulfur species existing exclusively as gaseous SO
3, SO
2, and H
2SO
4 vapor. The initial linear SO
3 increase demonstrates near-complete SO
2 conversion efficiency (up to 15% SO
2), constrained ultimately by O
2 limitations.
Figure 2c,d show analogous SO
3 trends at elevated temperatures. While 1300 °C conditions suppress SO
3 formation due to thermal instability, a weak positive correlation persists between SO
2 and SO
3 concentrations. This subdued response highlights temperature-dependent reaction kinetics overriding stoichiometric drivers.
Key conclusions from the analysis of
Figure 2 are as follows: when the temperature is <500 °C, SO
2 enrichment promotes SO
3 generation until O
2 depletion imposes thermodynamic constraints; the maximum achievable SO
3 concentration is 9.62% (15% SO
2 threshold); and at a temperature >1200 °C, thermal decomposition dominates, minimizing SO
3 yields despite SO
2 availability, and residual SO
3 shows a weak dependence on initial SO
2 concentrations.
3.1.3. The Effect of Initial O2 Content on SO3 Generation
Assuming the fixed temperatures of 200 °C, 400 °C, 900 °C, and 1300 °C, the effects of initial O
2 content (0–10% range) on the content of various substances during the final equilibrium of the reaction were studied, as shown in
Figure 3.
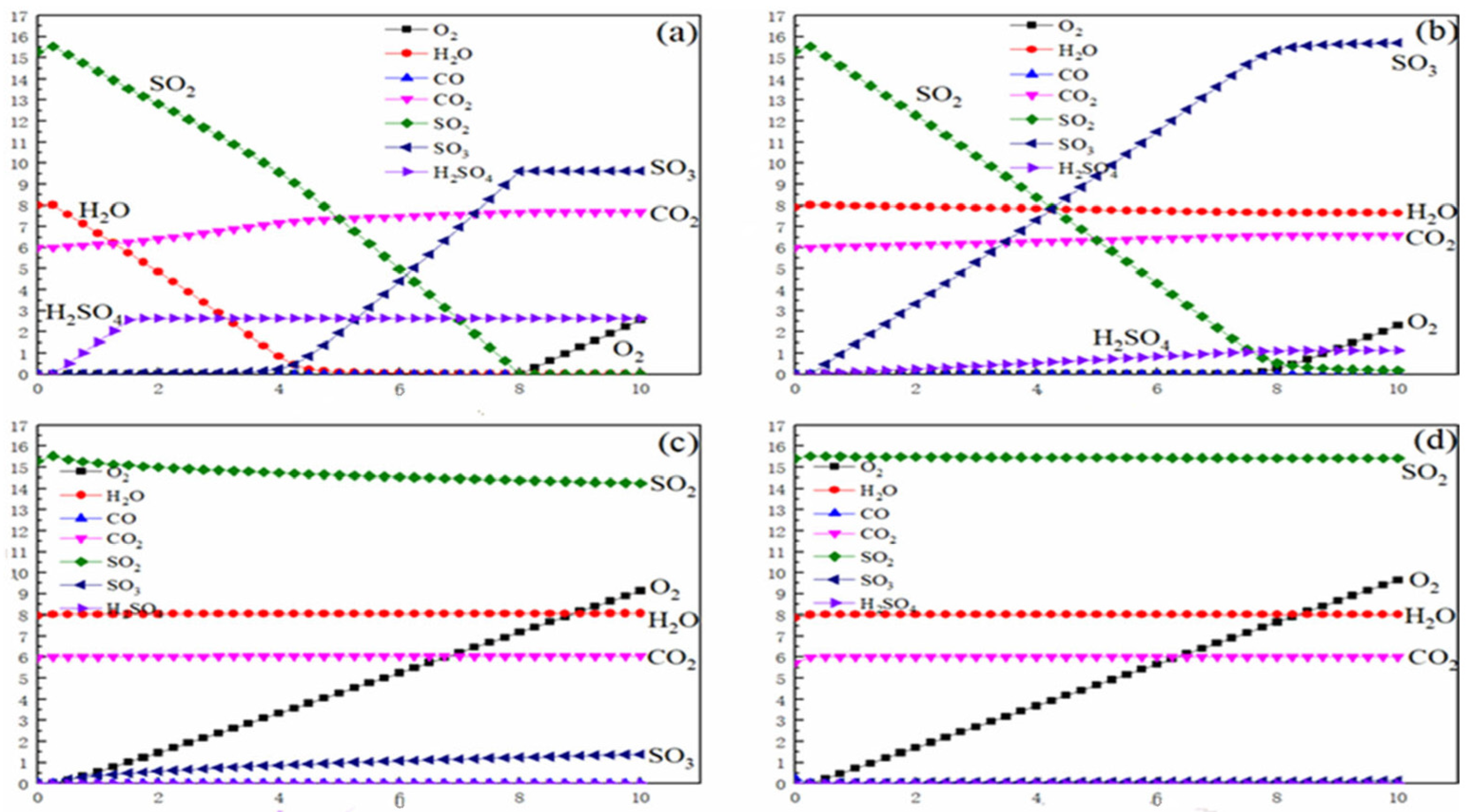
According to the thermodynamic analysis results of
Figure 2a,b, in the low-temperature region below the thermodynamic decomposition temperature of SO
3 (at approximately 783 °C), reducing the oxygen concentration in the reaction atmosphere can significantly inhibit SO
3 formation. When the temperature is 400 °C, and as the volume fraction of O
2 decreases from 5% to 1%, the concentration of SO
3 in the system drops sharply from 8.5% to 1.0%. This phenomenon confirms the crucial regulatory role of oxygen concentration in the oxidation reaction of SO
2→SO
3: this reaction is essentially an oxygen-dependent process, and the absence of O
2 in the system directly leads to the interruption of the formation pathway of SO
3. The thermodynamic calculation results indicate that, in the ultra-low oxygen range of 0–0.5%, sulfur-containing components mainly stably exist in the form of S
2(g), which essentially explains the complete inhibition of SO
2 formation at the molecular level.
By comparing and analyzing the data in the high-temperature region (T > 783 °C) of
Figure 2c,d, it can be found that the influence of oxygen concentration regulation on SO
3 formation exhibits a significant temperature dependence. Above the thermodynamic decomposition temperature, even if the O
2 concentration is decreased from 5% to 1%, the SO
3 concentration decreases from 3.6% to 1.2%, which is mainly attributed to the enhanced effect of the spontaneous decomposition of SO
3 under high-temperature conditions. At this time, the sulfur components in the system mainly follow the dynamic equilibrium of 2SO
3↔2SO
2 + O
2. Although the change in oxygen concentration can still affect the equilibrium shift according to Le Chatelier’s principle, its regulatory efficiency is significantly weakened compared to that in the low-temperature region.
3.1.4. The Effect of Initial CO2, CO, SO3, and H2O Contents on SO3 Generation
Additional equilibrium simulations were conducted at fixed temperatures (200 °C, 400 °C, 900 °C, 1300 °C) to evaluate the effects of initial CO2 (0–15%), CO (0–5%), SO3 (0–5%), and H2O (0–10%) concentrations on final equilibrium compositions. As these parameters demonstrated a limited influence on SO3 generation with minimal graphical variations, the key findings related to these parameters are summarized below:
CO2 Influence: Variations in the initial CO2 concentration showed a negligible impact (<0.5% deviation) on the equilibrium component distributions across all temperatures. The practical implications are that CO2 injection primarily alters flue pressure and SO3 partial pressure rather than chemical equilibria.
CO Effects: CO concentrations >2% significantly suppress SO3 formation via O2 depletion (ΔSO3 ≤ −34% at 200 °C) when temperatures are <400 °C. Minimal SO3 inhibition (ΔSO3 < 3%) is observed due to thermal decomposition dominance at elevated temperatures (>900 °C).
H2O Interactions: A high H2O content (>6%) induces sulfuric acid condensation below 250 °C, exacerbating flue corrosion risks. Moisture control strategies should focus on maintaining a feedstock humidity of <5% and preventing ambient moisture ingress during processing.
SO3 Feedstock Analysis: The initial SO3 concentration is strongly correlated with the final equilibrium SO3 concentration in low-temperature systems (200–400 °C). SO3 decomposes into SO2 and O2 (up to 89% conversion) in high-temperature systems (>900 °C).
This systematic analysis quantifies secondary parameter influences, enabling optimized gas composition control in pyrometallurgical operations [
23,
24,
25].
3.2. Generation of SO3 in Experimentally Simulated Lead Smelting Flue Gas
Based on the simulated SO3 generation data in lead smelting flue gas, the reaction temperature (200–1200 °C), O2 content (3–15%), and SO2 content (5–20%) were identified as dominant factors, with minimal influence from other flue gas components. Experimental studies under homogeneous (gas-phase) and heterogeneous (catalytic) conditions were conducted to analyze SO2/SO3 conversion mechanisms. These investigations, combined with catalytic SO3 generation patterns and inhibitor-based suppression strategies, provide actionable insights for SO3 control in non-ferrous smelting operations. The key findings revealed that catalytic conditions significantly enhance SO3 yields compared to homogeneous reactions, particularly at mid-range temperatures (400–600 °C). These results underscore the importance of temperature management and catalytic material selection in SO3-suppression strategies.
3.2.1. The Effect of Reaction Temperature on SO3 Generation in Experimental Simulations
Assuming a reaction range of 400–1000 °C, a total gas flow rate of 200 mL/min was maintained, and the gas-phase composition was set as follows: an O
2 content of 8%, an O
2 content of 15%, and a N
2 content of 77%. The experimental results are shown in
Figure 4.
Figure 4 demonstrates that gas-phase reactions alone yield minimal SO
3 conversion (<1%) across all temperatures. However, lead dust catalysis substantially enhances SO
3 generation, revealing catalytic activity in smelting particulates. Three thermal regimes govern SO
3 formation in lead smelting flue gas:
High-Temperature Zone (>800 °C): Despite intense molecular collisions, SO3 decomposition dominates due to unfavorable thermodynamics (ΔG > 0). Conversion rates decline sharply above 800 °C.
Medium-Temperature Zone (600–800 °C): Peak SO3 conversion occurs here (exceeding 15%), where thermodynamics (ΔG < 0) and kinetics align. Catalysts reduce the activation energy, enabling efficient SO2 oxidation.
Low-Temperature Zone (<600 °C): While thermodynamically favorable (ΔG < 0), the low molecular kinetic energy restricts conversion to 4–5%.
This behavior stems from competing thermodynamic and kinetic drivers. At high temperatures, SO3 becomes unstable despite rapid molecular motions. Low temperatures maintain thermodynamic favorability but lack the activation energy. The medium-temperature “window” uniquely balances both factors.
Operational variability (±50 °C) in temperature zones may occur due to the furnace design or feedstock differences. Practical mitigation strategies should focus on the following: (1) rapid gas cooling throughout the 600–800 °C range to limit SO3 formation; (2) optimizing catalyst composition at lower effective activation temperatures; and (3) implementing real-time temperature monitoring to account for process fluctuations.
These findings emphasize the critical role of thermal management in controlling SO3 emissions. By targeting the medium-temperature regime through operational adjustments and catalytic optimizations, smelters can significantly reduce SO3 generation while maintaining production efficiency.
3.2.2. The Effect of Initial O2 Content on SO3 Generation in Experimental Simulations
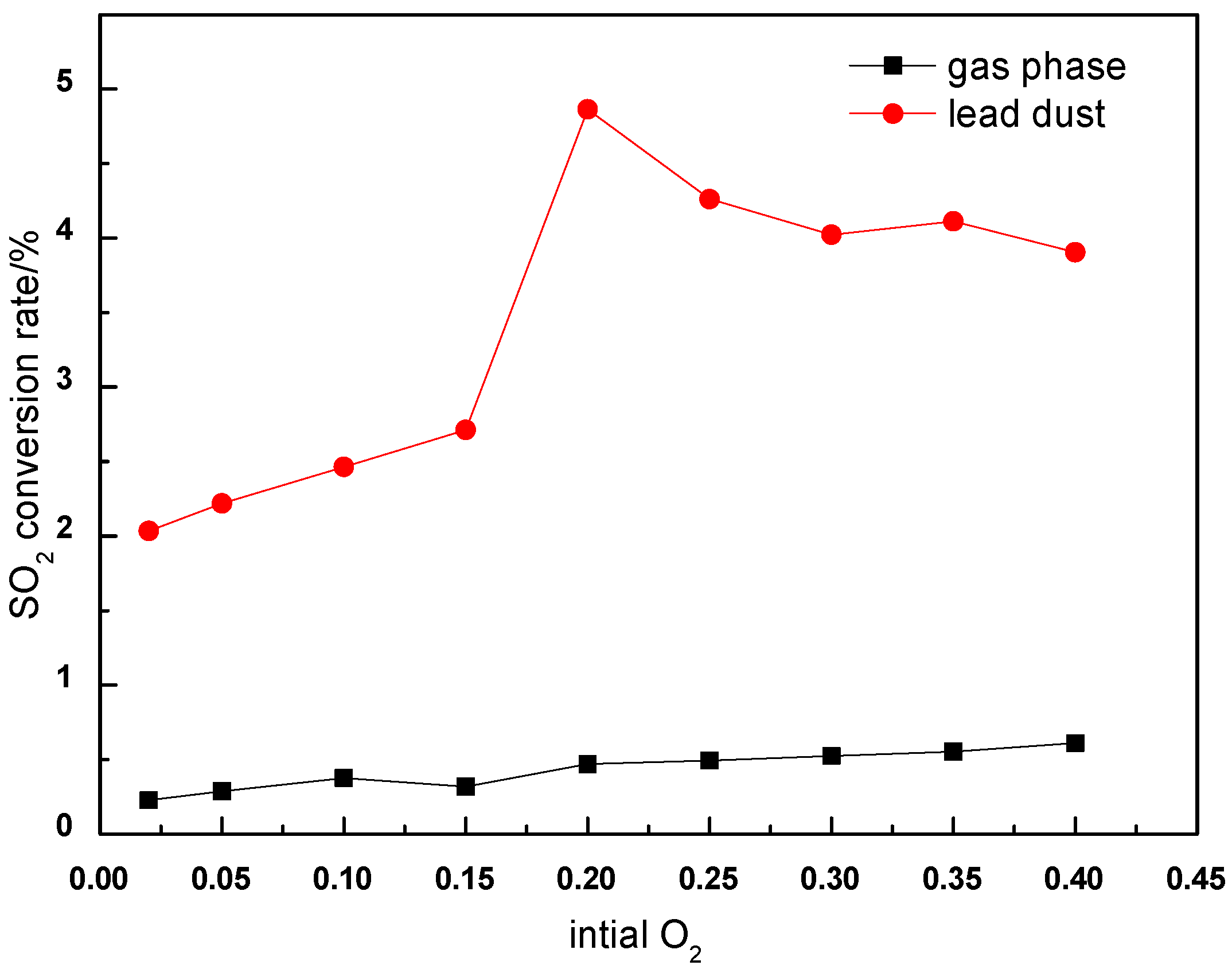
This study examined SO
3 formation at 600 °C with a fixed gas flow rate (200 mL/min) under varying oxygen concentrations (2–40%).
Figure 5 reveals the distinct catalytic impacts: Under homogeneous conditions, SO
3 conversion remains minimal (<1%) despite oxygen concentration increases, confirming the limited gas-phase reactivity. Introducing lead dust catalysts dramatically enhances SO
3 yields. The catalytic effect is amplified with oxygen availability, suggesting O
2 participation in both SO
2 oxidation and catalyst activation [
26,
27].
The two mechanistic regimes that emerge are as follows:
The results highlight oxygen management as critical for optimizing the catalytic SO3 control. While higher O2 concentrations boost conversion, practical implementation requires balancing oxidation efficiency with operational costs and downstream corrosion risks.
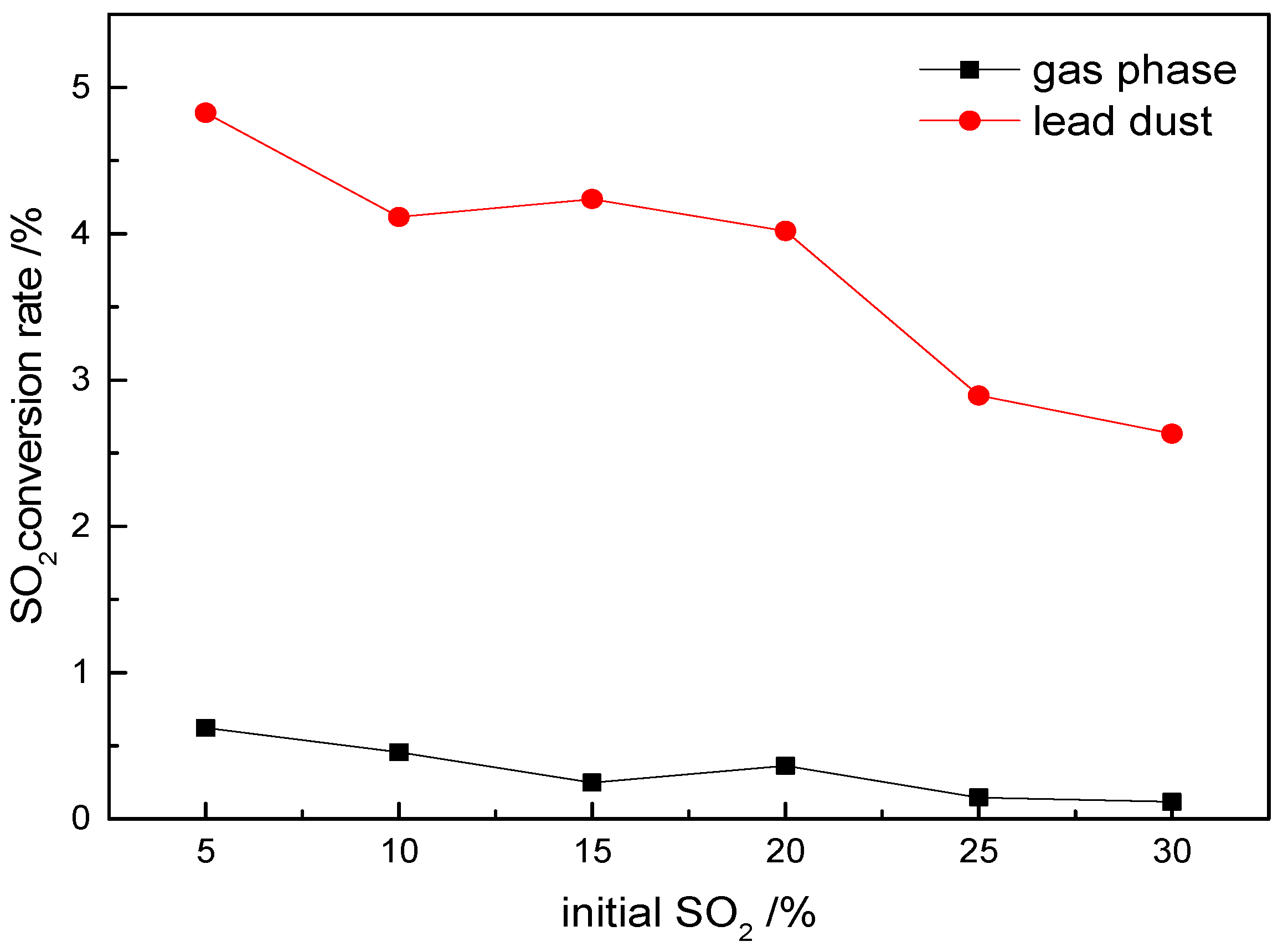
3.2.3. The Effect of Initial SO2 Content on SO3 Generation in Experimental Simulations
Experimental investigations at 600 °C with a fixed gas flow rate (200 mL/min) reveal the limited sensitivity of SO
3 conversion to SO
2 concentration variations (10–30 vol%), as shown in
Figure 6.
Under homogeneous conditions, the SO3 conversion rates remain consistently low (0.5–1.8%) with minimal fluctuations, demonstrating a weak dependence on SO2 levels. In contrast, lead dust catalysis elevates the conversion rates to 12–14%, about an 8-fold enhancement over gas-phase reactions, though still exhibiting limited responsiveness to SO2 increases (<2% change across tested concentrations).
These findings align with thermodynamic simulations showing decreasing SO3 concentrations at elevated temperatures despite the rising SO2/O2 levels. The weak correlation between SO2 content and SO3 generation stems from oxygen availability being the dominant controlling factor: every 1% increase in the initial O2 concentration boosts the conversion rates by 0.8%, whereas SO2 variations show a negligible impact. The experimental conversion rates lag theoretical predictions by 15–22% due to kinetic limitations preventing a full thermodynamic equilibrium.
Lead ash catalysis operates through three synergistic mechanisms: (1) surface-mediated oxidation pathways that lower the activation energy, (2) oxygen activation at metal oxide sites, and (3) the stabilization of intermediate sulfur species. This catalytic system demonstrates remarkable robustness, maintaining a stable performance across wide SO2 ranges (10–30%) and temperature fluctuations (±50 °C), suggesting adaptability to variable smelting feedstocks.
Practical implications emphasize oxygen optimization (>18 vol%) over SO2 adjustments for effective SO3 control. The catalytic approach reduces sensitivity to the feedstock sulfur content while providing consistent and critical advantages for emission mitigation in industrial applications. These insights establish catalytic modification as a superior strategy compared to gas-phase oxidation for SO3 management in lead smelting operations.
3.3. Effect of SO3 Inhibition on the Oxidation Behavior of PbS in a Simulation of Lead Smelting Flue Gas
By investigating the influence of O
2 content on the conversion of SO
2 to SO
3, as well as the thermodynamics and kinetics of PbS decomposition and oxidation behavior under flue gas system conditions, it was found that PbS and its decomposition products exhibit a significantly stronger binding affinity with O
2 compared to SO
2 within the temperature range of 100–1300 °C. Under flue reaction conditions, O
2 is preferentially consumed through chemical reactions with PbS and its decomposition products rather than by reacting with SO
2 to form SO
3. By injecting PbS particles of specific sizes into the flue gas, the decomposition and oxidation reactions of PbS can be utilized to consume oxygen, thereby controlling the reaction direction, extent, and SO
3 partial pressure at equilibrium (2SO
2 + O
2⇋2SO
3). This approach effectively reduces the SO
3 content in lead smelting flue gas [
28]. Experimental verification was conducted using a simulated flue gas platform designed and established by the research team. Under the simulated flue system conditions, the effects of varying initial O
2 and SO
2 contents on PbS decomposition/oxidation behavior and SO
3 generation were studied.
The following flue gas composition was maintained in the laboratory simulation: a baseline gas composition of SO
2 (18%), O
2 (4%), and N
2 (78%). The variable conditions included a fixed initial O
2 content of 4%, with the SO
2 content adjusted to 10%, 14%, 18%, and 22%, while the N
2 content decreased proportionally to maintain a constant total of 96% for SO
2 + N
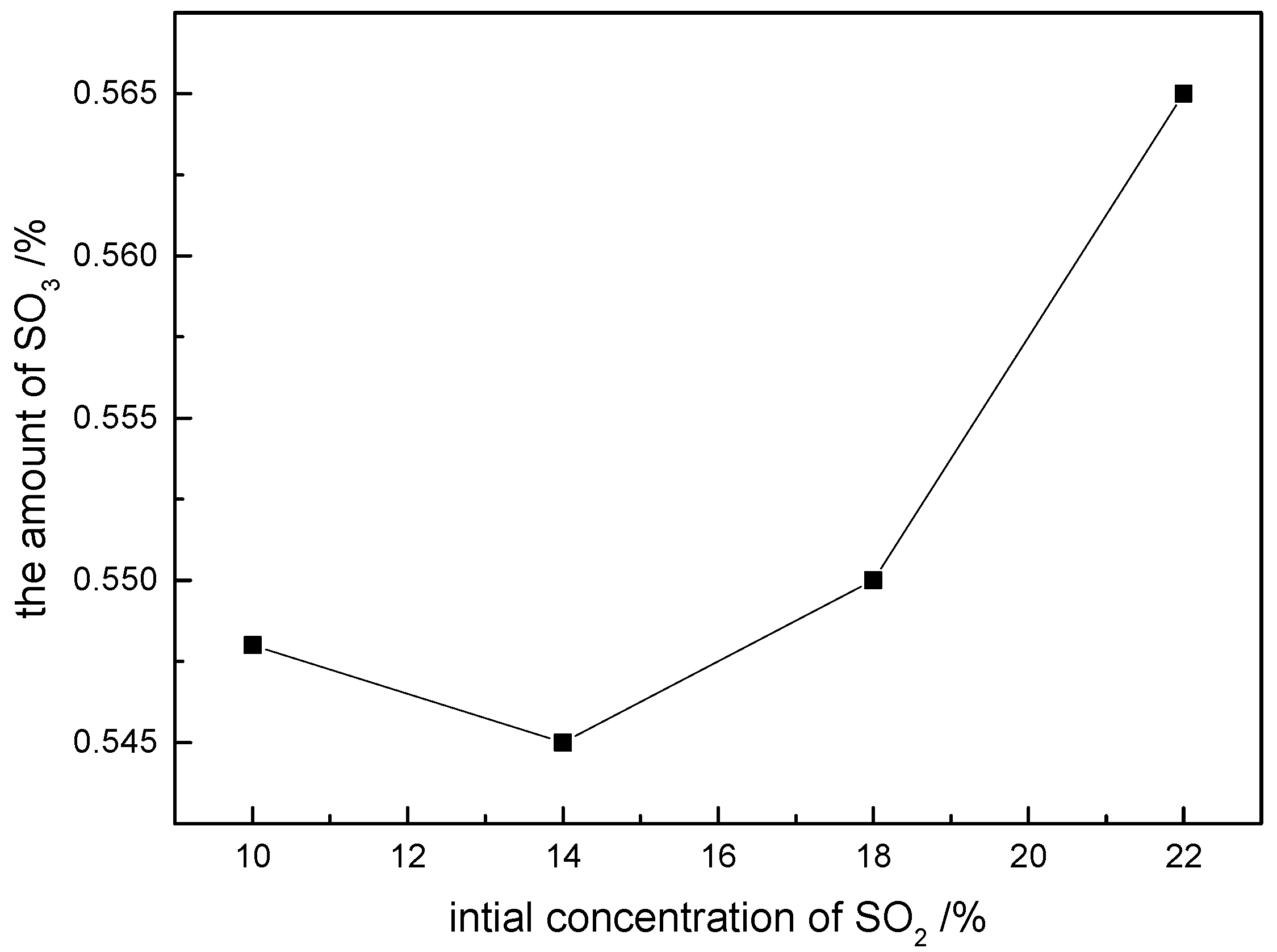
2. The experimental results (
Figure 7) demonstrate the amount of SO
3 formed after PbS addition under these conditions: at the initial SO
2 contents of 10%, 14%, 18%, and 22%, the corresponding SO
3 concentration is below 0.1% after PbS addition.
These values are significantly lower than the theoretical SO3 levels calculated for scenarios without PbS addition. The minimal variation in SO3 generation across different initial SO2 concentrations indicates that the final SO3 content is largely independent of the initial SO2 level under simulated flue conditions at 600 °C, consistent with the theoretical predictions.
Consistently, as the oxygen content increases from 2% to 8%, under varying oxygen concentrations, the gas-phase SO3 concentration is reduced to below 1% after the addition of PbS. In contrast, without an inhibitor, the SO3 content increases with the rising oxygen levels, consistently exceeding 2%.
In summary, the formation behavior of SO3 in the flue gas from lead smelting is regulated by both thermodynamic equilibrium and reaction kinetics. There are significant differences in its homogeneous and heterogeneous reaction mechanisms. In an industrial context, the strategies of thermodynamic inhibition, high-temperature decomposition, and kinetic regulation using catalyst passivation to minimize SO3 emissions should be integrated into designs of flue gas purification systems. The inhibition of SO3 formation by consuming oxygen using PbS is also a feasible and economical method. Utilizing the lead smelting by-product PbS to consume residual oxygen has inherent economic advantages and can realize the recycling of waste. Firstly, it is manifested in the fact that there is no need to purchase reagents externally (such as ammonia or activated carbon), which can save raw materials. Secondly, it can simplify the process, reduce the complexity of the gas treatment system, and lower the investment and operation costs.