Detection of Undeclared Meat Species and Fatty Acid Variations in Industrial and Traditional Beef Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sampling
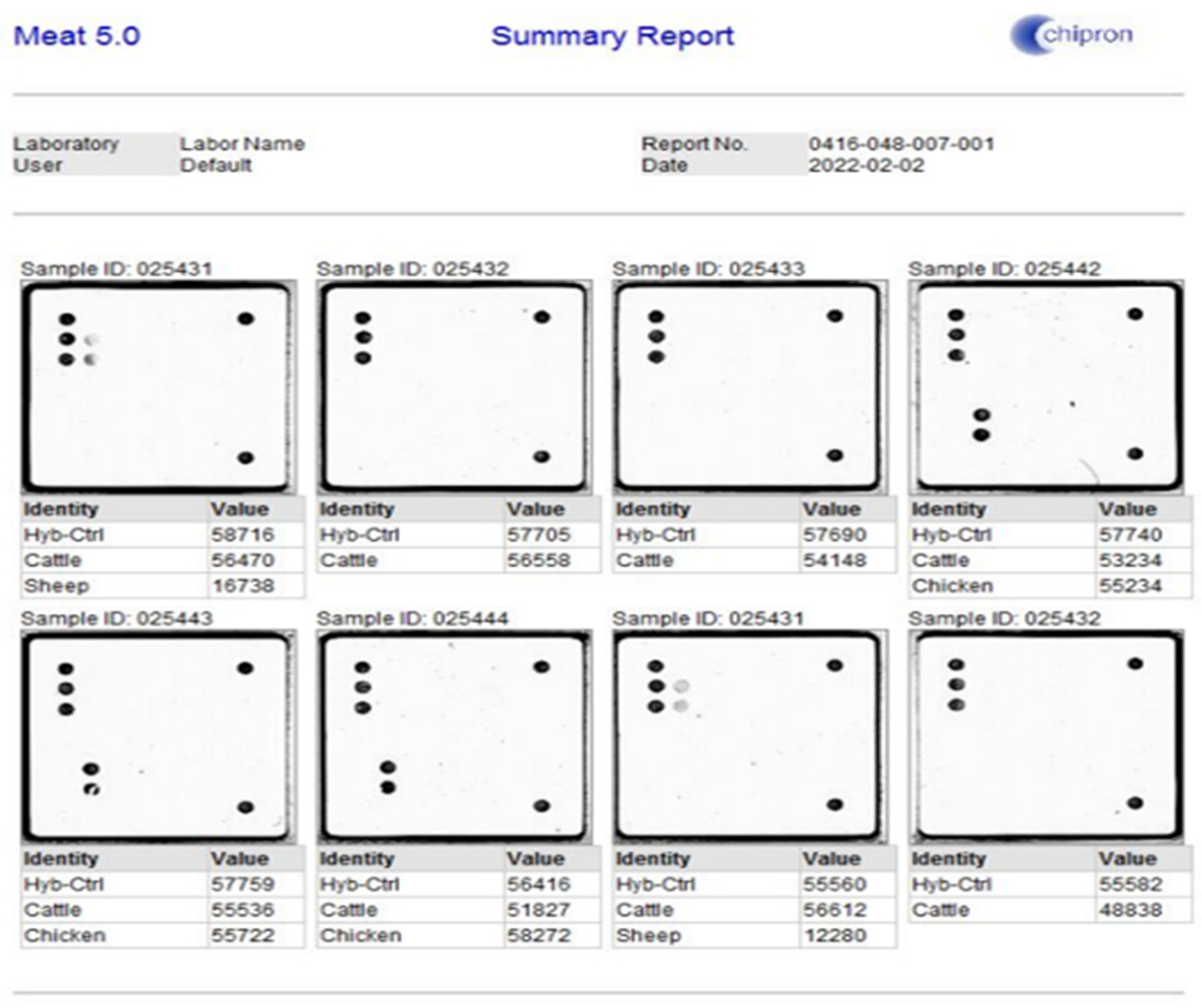
2.2. Chipron LCD Array Analysis System, Meat 5.0—A DNA-Based Identification of 24 Animal Species in Meat Products
2.2.1. Extraction of Sample
2.2.2. LCD-Array
2.3. Gas Chromatography–Flame Ionization Detector (GC/FID)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Detection of DNA of Species in Beef Sausages
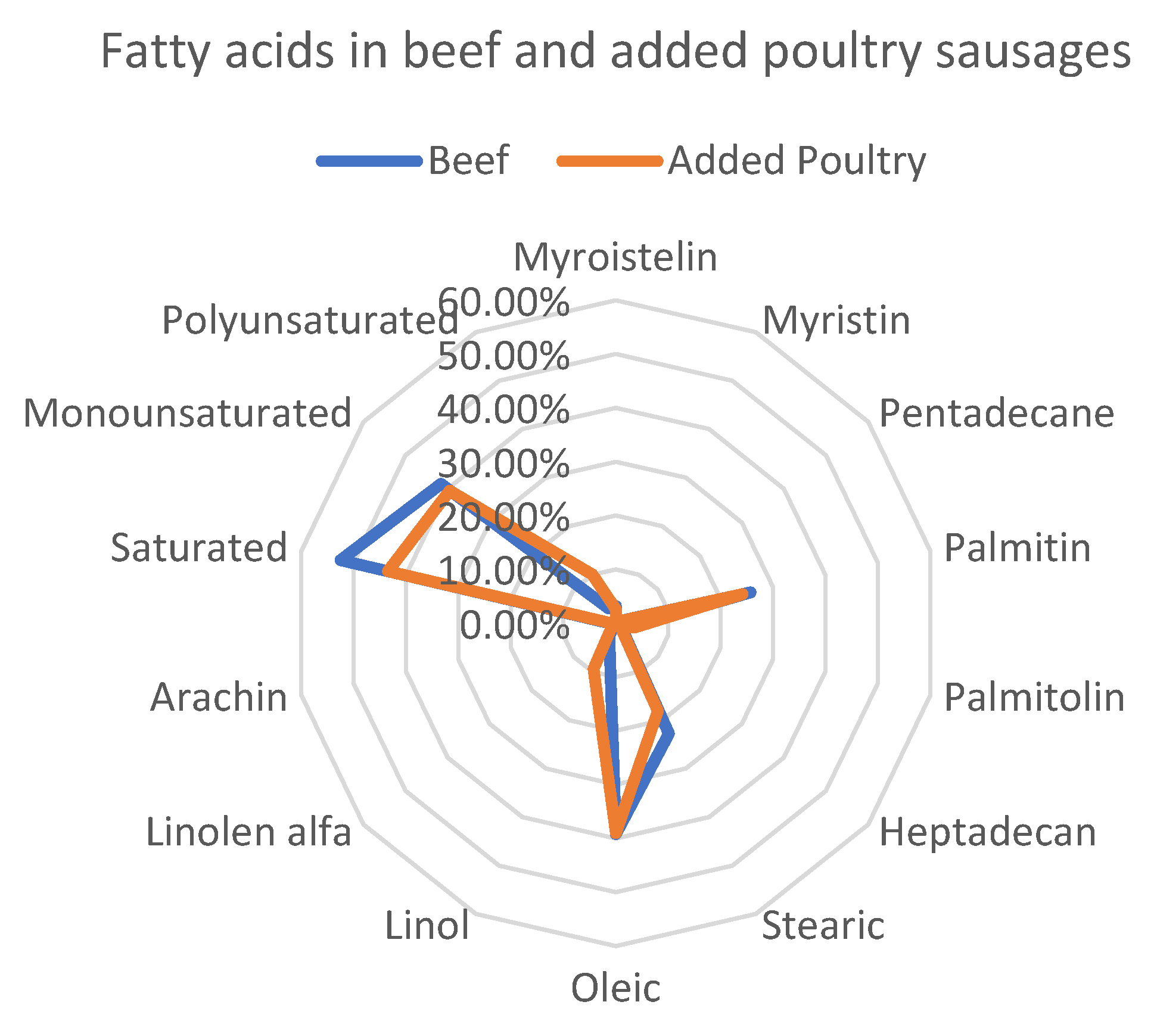
3.2. Prevalence of the Fatty Acid in Sausages Made with 100% Beef and Added Poultry Meat
3.3. The Fatty Acid Profile Depends on the % of Chicken Meat Addition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kavanaugh, M.; Rodgers, D.; Rodriguez, N.; Leroy, F. Considering the nutritional benefits and health implications of red meat in the era of meatless initiatives. Front. Nutr. 2025, 12, 1525011. [Google Scholar] [CrossRef] [PubMed]
- Ajomiwe, N.; Boland, M.; Phongthai, S.; Bagiyal, M.; Singh, J.; Kaur, L. Protein Nutrition: Understanding Structure, Digestibility, and Bioavailability for Optimal Health. Foods 2024, 13, 1771. [Google Scholar] [CrossRef] [PubMed]
- Carballo, J. Sausages Nutrition, Safety, Processing and Quality Improvement. Foods 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Akpan, I.P. Trends in Sausage Production. Afr. J. Food Sci. Technol. 2017, 8, 5. [Google Scholar]
- Dooley, J.; Paine, K.; Garrett, S.; Brown, H. Detection of meat species using TaqMan real-time PCR assays. Meat Sci. 2024, 68, 431–438. [Google Scholar] [CrossRef]
- Özlü, H.; Çevik, B.; Atasever, M.; Sarıalioğlu, M. Investigation of meat species adulteration in beef-based meat products via real-time PCR in Türkiye. Qual. Assur. Saf. Crops Foods 2023, 15, 42–48. [Google Scholar] [CrossRef]
- Amaral, J.S.; Soares, S.; Mafra, I.; Oliveira, B. Assessing the variability of the fatty acid profile and cholesterol content of meat sausages. Riv. Ital. Delle Sostanze Grasse 2014, 91, 261–272. [Google Scholar]
- Berisha, K.; Gashi, A.; Mednyánszky, Z.; Bytyqi, H.; Sarkadi, S. Nutritional characterization of homemade beef sausage based on amino acid, biogenic amines, and fatty acid composition. Acta Aliment. 2023, 52, 439–448. [Google Scholar] [CrossRef]
- Almeida, C.; Perassolo, M.; Camargo, J.; Bragagnolo, N.; Gross, J. Fatty acid composition and cholesterol content of beef and chicken meat in Southern Brazil. Rev. Bras. Cienc. Farm. 2006, 42, 109–117. [Google Scholar] [CrossRef]
- Belichovska, D.; Pejkovski, Z.; Silovska-Nikolova, A.; Belichovski, K. Chemical and fatty acid composition of poultry meat and pork fatback as a raw material for the production of frankfurters. J. Anim. Sci. 2020, 10, 23–28. [Google Scholar] [CrossRef]
- Hassoun, A.; Måge, I.; Schmidt, W.; Temiz, H.; Li, L.; Kim, H.Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods over the past Five Years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, M.; Bubli, S.; Khan, M. Mechanisms and Health Aspects of Food Adulteration: A Comprehensive Review. Foods 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Vatin, G.; Théolier, J.; Dominguez, S.; Godefroy, S. Quantification of beef in products sold in Canada declaring multiple meat species—Regulatory and consumer implications related to accurate labeling. Food Humanit. 2024, 3, 100375. [Google Scholar] [CrossRef]
- Adams, R. Food Safety Regulations and Consumer Confidence. Int. J. Livest. Policy 2024, 2, 15–25. [Google Scholar] [CrossRef]
- Tonkin, E.; Wilson, A.; Coveney, J.; Webb, T. Trust in and through labelling—A systematic review and critique. Br. Food J. 2015, 117, 318–338. [Google Scholar] [CrossRef]
- Regulation No. 09/2013 on Labelling, Presentation and Advertising and Food Products; Ministry of Trade and Industry, Government: Prishtina, Republic of Korea, 2013.
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Official Journal of the European Union, L 304, 22. 2011, 18–63. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R1169 (accessed on 30 March 2025).
- Du, J.; Gan, M.; Xie, Z.; Li, C.; Wang, M.; Dai, H.; Huang, Z.; Chen, L.; Zhao, Y.; Niu, L. Corrigendum to “Current progress on meat food authenticity detection methods” [Food Control 152 (2023) 109842]. Food Control 2024, 155, 110055. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.R.; Sharma, B.D.; Gokulakrishnan, P.; Mendiratta, S.K.; Sharma, D. Identification of species origin of meat and meat products on the DNA basis: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1340–1351. [Google Scholar] [CrossRef]
- Jonker, K.J.; Tilburg, J.I.H.C.; Hagele, G.H.H.; de Boer, E. Species identification in meat products using real-time PCR. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2008, 25, 527–533. [Google Scholar] [CrossRef]
- Rojas, M.; González, I.; Pavón, M.G.; Pegels, N.; Lago, A.; Hernández, P.E.; García, T.; Martín, R. Novel TaqMan real-time polymerase chain reaction assay for verifying the authenticity of meat and commercial meat products from game birds. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2010, 27, 749–763. [Google Scholar] [CrossRef]
- Hossain, A.; Hossain, M.S.; Munshi, M.K.; Huque, R. Detection of species adulteration in meat products and Mozzarella-type cheeses using duplex PCR of mitochondrial cyt b gene: A food safety concern in Bangladesh. Food Chem. Mol. Sci. 2021, 2, 100017. [Google Scholar]
- Asensio, L.; González, I.; Pavón, M.A.; García, T.; Martín, R. An indirect ELISA and a PCR technique for the detection of Grouper (Epinephelus marginatus) mislabeling. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2008, 25, 677–683. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Guidance for Industry, Q2B Validation of Analytical Procedures: Methodology; ICH: Rockville, MD, USA, 1996. [Google Scholar]
- Szyłak, A.; Kostrzewa, W.; Bania, J.; Tabiś, A. Do You Know What You Eat? Kebab Adulteration in Poland. Foods 2023, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.O.; He, B.X.; Effarizah, M.S.; Syahariza, Z.A.; Shamila-Syuhada, K.; Rusul, G. Mislabelling of beef and poultry products sold in Malaysia. Food Control 2016, 62, 157–164. [Google Scholar] [CrossRef]
- Tembe, D.; Mukaratirwa, S.; Zishiri, O. Undeclared Meat Species in Processed Meat Products from Retail Franchises in the Durban Metropole, KwaZulu-Natal Province, South Africa, Using Species-specific DNA Primers. Food Prot. Trends 2018, 38, 440–449. [Google Scholar]
- Keyvan, E.; Çil, G.I.; Kul, B.Ç.; Bilgen, N.; Şireli, U.Ş. Identification of meat species in different types of meat products by PCR. Ank. Üniv. Vet. Fak. Derg. 2017, 64, 261–266. [Google Scholar]
- Sreenivasan, S.; Viljoen, C.D. Determining the presence of undeclared animal species using Real-time PCR in canned and ready-to-eat meat products in South Africa. J. Food Sci. Technol. 2020, 58, 2699–2704. [Google Scholar] [CrossRef]
- Beltramo, C.; Riina, M.V.; Colussi, S.; Campia, V.; Maniaci, M.G.; Biolatti, C.; Trisorio, S.; Modesto, P.; Peletto, S.; Acutis, P.L. Validation of a DNA biochip for species identification in food forensic science. Food Control 2017, 78, 366–373. [Google Scholar] [CrossRef]
- Golian, J.; Drdolová, Z.; Martišová, P.; Semjon, B.; Benešová, L. Molecular diagnostic test systems for meat identification: A comparison study of the MEAT 5.0 LCD-Array and innuDETECT Assay detection methods. Acta Vet. Brno 2020, 89, 89–96. [Google Scholar] [CrossRef]
- Simsek, Y.O.; Isıklı, M. Fatty acid composition and quality characteristics of low-fat cooked sausages made with beef and chicken meat, tomato juice, and sunflower oil. Meat Sci. 2002, 62, 253–258. [Google Scholar]
- Pereira, N.R.; Tarley, C.; Matsushita, M.; de Souza, N. Proximate Composition and Fatty Acid Profile in Brazilian Poultry Sausages. J. Food Compos. Anal. 2000, 13, 915–920. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Aladejana, E.B. Effect of Cooking Oil on the Fatty Acid Profile of Beef Sausage Fortified with Edible Deboned Meat Waste. Int. J. Food Sci. 2021, 2021, 5592554. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.A.; de Souza, X.R.; dos Santos Garcia, V.A.; Rodrigues, E.C.; Faria, P.B.; de Faria, R.A.P.G. Quality and fatty acid profile of chicken sausage added canola oil as partial replacement for animal fat. Braz. J. Dev. 2022, 8, 26161–26181. [Google Scholar] [CrossRef]
- Mehmetukaj, D.; Bytyçi, X.; Cana, A.; Gashi-Zogejani, V.; Shandro-Zeqiri, M.; Bajraktari, D.; Jankuloski, D.; Hajrulai-Musliu, Z. Presence of Soya in Industrial and Homemade Sausage Production in Kosovo and Its Reflection on Fatty Acid Profile. Separations 2024, 11, 457. [Google Scholar] [CrossRef]
- Valsta, L.M.; Tapanainen, H.; Männistö, S. Meat fats in nutrition. Meat Sci. 2005, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Guntarti, A.; Ahda, M.; Kusbandari, A. Determining fatty acids and halal authentication of sausage. Food Res. 2020, 4, 495–499. [Google Scholar] [CrossRef]
- Morales-Barrera, J.E.; Gonzalez-Alcorta, M.J.; Castillo-Dominguez, R.M.; Prado-Rebolledo, O.F.; Hernandez-Velasco, X.; Anita Menconi, A.; Tellez, G.; Hargis, B.M.; Carrillo-Dominguez, S. Fatty Acid Deposition on Broiler Meat in Chickens Supplemented with Tuna Oil. Food Nutr. Sci. 2013, 4, 16–20. [Google Scholar] [CrossRef]
- Araujo de Vizcarrondo, C.; Carrillo de Padilla, F.; Martín, E. Fatty acid composition of beef, pork, and poultry fresh cuts, and some of their processed products. Agricultural and Food Sciences. Arch. Latinoam. Nutr. 1998, 48, 354–358. [Google Scholar]
| GC System | 8890 GC |
|---|---|
| S/SL inlet | 250 °C, split ration 50:1 |
| Liner | Split, ultra inert, glass wool, low-pressure drops (p/n 5190-295) |
| Oven ramp program | 50 °C (0.5 min) |
| 30 °C/min to 194 °C (3.5 min) | |
| 5 °C/min to 240 °C (3 min) | |
| Carrier gas | Nitrogen, 13 psi, constant pressure mode |
| Column | DB-Fast FAME 30 m × 0.250 mm × 0.25 µm 40 °C to 250/260 °C |
| Detector-fid | 260 °C, |
| H2: 40 mL/min | |
| Air: 400 mL/min | |
| Makeup gas: 25 mL/min | |
| Injection volume | 1 µL |
| Chicken Meat (%) | Beef Sausage (%) | C14:0 | C14:1 | C15:0 | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | SFA | MUFA | PUFA | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 0 | 0.08 | 0.33 | 0.06 | 18.28 | 3.44 | 0.13 | 6.57 | 29.18 | 37.59 | 4.28 | 0.05 | 25.17 | 32.95 | 41.87 | 99.99 |
| 0 | 100 | 3.58 | 0.44 | 0.55 | 29.05 | 2.98 | 1.88 | 24.84 | 33.34 | 2.76 | 0.35 | 0.14 | 60.04 | 36.76 | 3.11 | 99.91 |
| 99 | 1 | 0.46 | 0.16 | 0.08 | 19.4 | 3.37 | 0.17 | 8.31 | 29.37 | 35.33 | 3.29 | 0.06 | 28.48 | 32.9 | 38.62 | 100 |
| 90 | 10 | 0.66 | 0.17 | 0.11 | 20.2 | 3.32 | 0.29 | 9.84 | 16.5 | 32.18 | 3.14 | 0.07 | 31.17 | 19.99 | 35.32 | 86.48 |
| 80 | 20 | 1.23 | 0.27 | 0.19 | 22.23 | 3.02 | 0.58 | 14.18 | 31.26 | 24.64 | 2.29 | 0.08 | 38.49 | 34.55 | 26.93 | 99.97 |
| 70 | 30 | 1.57 | 0.32 | 0.26 | 23.91 | 2.96 | 0.85 | 15.46 | 31.85 | 20.72 | 2.04 | 0.04 | 42.09 | 35.13 | 22.76 | 99.98 |
| 60 | 40 | 2.05 | 0.43 | 0.31 | 25.3 | 3.03 | 0.95 | 17.5 | 32.12 | 16.66 | 1.59 | 0 | 46.11 | 35.58 | 18.25 | 99.94 |
| 50 | 50 | 2.12 | 0.42 | 0.32 | 25.73 | 2.93 | 1.09 | 19.39 | 32.46 | 14.03 | 1.33 | 0.1 | 48.75 | 35.81 | 15.36 | 99.92 |
| 40 | 60 | 2.28 | 0.43 | 0.34 | 26.43 | 3.06 | 1.11 | 18.37 | 32.76 | 13.84 | 1.37 | 0 | 48.53 | 36.25 | 15.21 | 99.99 |
| 30 | 70 | 2.84 | 0.55 | 0.44 | 28.35 | 2.85 | 1.41 | 21.65 | 33.24 | 7.77 | 0 | 0.07 | 54.76 | 36.64 | 7.77 | 99.17 |
| 20 | 80 | 2.77 | 0.37 | 0.44 | 28.85 | 2.79 | 1.6 | 22.78 | 34.25 | 5.5 | 0.56 | 0.07 | 56.51 | 37.41 | 6.06 | 99.98 |
| 10 | 90 | 3.22 | 0.61 | 0.49 | 28.64 | 2.81 | 1.58 | 24.24 | 34.27 | 3.53 | 0.37 | 0.12 | 58.29 | 37.69 | 3.9 | 99.88 |
| 7 | 93 | 3.04 | 0.27 | 0.46 | 29.55 | 2.68 | 1.59 | 23.85 | 34.9 | 3.11 | 0.32 | 0.11 | 58.6 | 37.85 | 3.43 | 99.88 |
| 2 | 98 | 3.57 | 0.66 | 0.56 | 31.06 | 2.9 | 1.87 | 23.7 | 33.15 | 2.05 | 0.27 | 0.11 | 60.87 | 36.71 | 2.32 | 99.9 |
| Category | Industrial | Traditional | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sample distribution | 43 | 63.23% | 25 | 36.76% | 68 | 100% |
| Samples with only beef DNA | 15 | 34.88% | 17 | 68.00% | 32 | 47.05% |
| Samples with DNA of other species | 28 | 65.12% | 8 | 32.00% | 36 | 52.94% |
| Containing chicken DNA | 23 | 82.14% * | 7 | 87.50% * | 31 | 45.55% |
| Containing mutton DNA | 3 | 10.71% * | 1 | 12.50% * | 3 | 4.41% |
| Containing turkey DNA | 2 | 7.14% * | 0 | 0.00% | 2 | 2.94% |
| Containing DNA of other species ** | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% |
| Declared the added species meat | 15 | 41.60% | 0 | 100.00% | 15 | 41.46% |
| Did not declare the added species meat | 13 | 46.42% | 8 | 100.00% | 21 | 58.33% |
| Fatty Acids | Beef Sausage | Beef Sausage with Poultry DNA | p-Value |
|---|---|---|---|
| Myroistelin | 3.17 ± 12.28 | 2.56 ± 35.82 | 0.000893 * |
| Myristin | 0.39 ± 58.87 | 0.30 ± 87.46 | 0.156974 |
| Pentadecane | 0.57 ± 24.95 | 0.43 ± 42.26 | 0.000555 * |
| Palmitin | 25.66 ± 5.45 | 24.14 ± 7.33 | 0.000238 |
| Palmitolin | 3.17 ± 26.05 | 3.55 ± 23.26 | 0.066677 |
| Heptadecan | 1.34 ± 17.90 | 1.09 ± 38.45 | 0.003113 * |
| Stearic | 22.76 ± 16.73 | 18.11 ± 32.46 | 0.000305 * |
| Oleic | 39.18 ± 9.79 | 39.00 ± 7.24 | 0.828574 |
| Linol | 2.72 ± 23.96 | 9.46 ± 80.19 | 3.28 × 10−6 * |
| Linolen alfa | 0.41 ± 43.26 | 0.81 ± 56.59 | 1.15 × 10−5 * |
| Arachin | 0.58 ± 35.28 | 0.51 ± 59.36 | 0.250436 |
| Saturated | 54.12 ± 7.53 | 46.59 ± 18.58 | 0.002973 * |
| Monosaturated | 42.75 ± 10.38 | 41.70 ± 17.43 | 0.425882 |
| Polyunsaturated | 3.13 ± 25.26 | 10.27 ± 77.80 | 7.64 × 10−6 * |
| Added Meat Poultry % | Samples (No) | C14:0 | C14:1 | C15:0 | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | SFA | MUFA | PUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.95 | 0.21 | 0.43 | 23.77 | 1.94 | 1.24 | 28.63 | 36.73 | 3.29 | 0.34 | 0.47 | 57.49 | 38.88 | 3.63 | |
| 2 | 2.91 | 0.81 | 0.47 | 24.97 | 3.32 | 1.08 | 24 | 38.3 | 3.1 | 0.39 | 0.67 | 54.10 | 42.43 | 3.49 | |
| 3 | 3.35 | 0.41 | 0.71 | 26.56 | 2.37 | 1.52 | 28.25 | 31.75 | 3.94 | 0.61 | 0.53 | 60.92 | 34.53 | 4.55 | |
| 10% | 4 | 3.08 | 0.24 | 0.59 | 26.14 | 3.3 | 1.26 | 21.65 | 39.29 | 3.47 | 0.48 | 0.51 | 53.23 | 42.83 | 3.95 |
| 5 | 3.96 | 0.23 | 0.7 | 27.68 | 3.46 | 1.41 | 21.21 | 37.42 | 3.13 | 0.35 | 0.43 | 55.39 | 41.11 | 3.48 | |
| 6 | 3.17 | 0.32 | 0.58 | 24.4 | 2.69 | 1.38 | 22.62 | 40 | 3.6 | 0.57 | 0.67 | 52.82 | 43.01 | 4.17 | |
| 7 | 3.02 | 0.28 | 0.49 | 24.19 | 2.95 | 1.24 | 22.65 | 40.01 | 3.73 | 0.71 | 0.73 | 52.32 | 43.24 | 4.44 | |
| 8 | 2.72 | 0.24 | 0.5 | 24.27 | 3.67 | 1.26 | 17.58 | 44.99 | 3.74 | 0.39 | 0.63 | 46.96 | 48.9 | 4.13 | |
| MEAN ± SD * | 3.15 ± 11.25 | 0.34 ± 54.50 | 0.56 ± 17.55 | 25.25 ± 5.14 | 2.96 ± 18.72 | 1.30 ± 9.65 | 23.32 ± 14.70 | 38.56 ± 9.04 | 3.50 ± 8.18 | 0.48 ± 26.62 | 0.57 ± 18.12 | 54.16 ± 7.52 | 41.79 ± 9.78 | 4.05 ± 9.02 | |
| 1 | 3.24 | 1.19 | 0.56 | 24.32 | 5.05 | 1.24 | 15.33 | 43.73 | 3.61 | 0.5 | 1.23 | 45.92 | 49.97 | 4.11 | |
| 2 | 3.06 | 0.64 | 0.51 | 26.26 | 3.94 | 1.15 | 19.73 | 40.03 | 3.68 | 0.47 | 0.52 | 51.23 | 44.61 | 4.15 | |
| 3 | 2.72 | 0.4 | 0.43 | 27.06 | 2.7 | 1.39 | 24.25 | 37.76 | 2.6 | 0.37 | 0.33 | 56.18 | 40.86 | 2.97 | |
| 4 | 2.92 | 0.47 | 0.56 | 25.4 | 2.56 | 1.65 | 24.34 | 37.97 | 3.22 | 0.45 | 0.47 | 55.34 | 41 | 3.67 | |
| 20% | 5 | 3.04 | 0.35 | 0.53 | 25.5 | 3.73 | 1.28 | 19.81 | 38.59 | 5.78 | 0.68 | 0.71 | 50.87 | 42.67 | 6.46 |
| 6 | 2.88 | 0.22 | 0.6 | 23.99 | 2.97 | 1.31 | 23.72 | 38.12 | 4.79 | 0.55 | 0.86 | 53.36 | 41.31 | 5.34 | |
| 7 | 3.39 | 0.2 | 0.57 | 26.01 | 2.43 | 1.36 | 24.52 | 35.7 | 4.88 | 0.43 | 0.51 | 56.36 | 38.33 | 5.31 | |
| 8 | 4.09 | 0 | 0.48 | 24.07 | 4.22 | 1.29 | 15.29 | 43.4 | 5.9 | 0.53 | 0.7 | 45.92 | 47.62 | 6.43 | |
| 9 | 3.19 | 0.26 | 0.5 | 23.24 | 3.13 | 1.22 | 21.14 | 41.37 | 4.12 | 0.41 | 1.43 | 50.72 | 44.76 | 4.53 | |
| 10 | 3.02 | 0.12 | 0.5 | 25.52 | 3.87 | 1.56 | 17.13 | 43.62 | 3.82 | 0.4 | 0.45 | 48.18 | 47.61 | 4.22 | |
| MEAN ± SD | 3.15 ± 11.42 | 0.39 ± 82.84 | 0.52 ± 9.08 | 25.14 ± 4.50 | 3.46 ± 23.08 | 1.34 ± 10.89 | 20.53 ± 17.02 | 40.03 ± 6.78 | 4.24 ± 24.13 | 0.48 ± 18.04 | 0.72 ± 47.15 | 51.41 ± 7.23 | 43.87 ± 7.98 | 4.72 ± 23.05 | |
| 1 | 3.34 | 1.03 | 0.53 | 25.53 | 4.69 | 1.02 | 13.27 | 41.18 | 7.66 | 0.95 | 0.77 | 44.46 | 46.9 | 8.61 | |
| 2 | 2.62 | 0.28 | 0.5 | 23.9 | 3.92 | 1.16 | 16.35 | 40.84 | 8.61 | 1.1 | 0.71 | 45.24 | 45.04 | 9.71 | |
| 30% | 3 | 2.36 | 0.2 | 0.46 | 24.43 | 2.72 | 1.24 | 22.7 | 34.81 | 9.67 | 0.98 | 0.43 | 51.62 | 37.33 | 10.65 |
| 4 | 2.77 | 0.37 | 0.59 | 25.19 | 2.66 | 1.61 | 23.03 | 35.65 | 7.07 | 0.52 | 0.55 | 53.74 | 38.68 | 7.59 | |
| 5 | 2.57 | 0.22 | 0.48 | 22.38 | 3.71 | 1.34 | 14.77 | 44.24 | 8.68 | 0.89 | 0.72 | 42.26 | 48.17 | 9.57 | |
| MEAN ± SD | 2.73 ± 12.12 | 0.42 ± 73.97 | 0.51 ± 8.86 | 24.29 ± 4.57 | 3.54 ± 21.67 | 1.27 ± 15.54 | 18.02 ± 22.59 | 39.34 ± 9.08 | 8.34 ± 10.77 | 0.89 ± 22.11 | 0.64 ± 19.93 | 47.46 ± 9.31 | 43.22 ± 10.17 | 9.23 ± 11.30 | |
| 50% | 1 | 2.42 | 0.16 | 0.21 | 22.57 | 3.43 | 0.71 | 15.16 | 37.87 | 15.86 | 1.35 | 0.26 | 41.33 | 41.46 | 17.21 |
| 1 | 1.52 | 0.47 | 0.26 | 21.03 | 3.78 | 0.63 | 11.4 | 40.83 | 17.99 | 1.58 | 0.51 | 35.35 | 45.08 | 19.57 | |
| 60% | 2 | 1.51 | 0.11 | 0.23 | 23.09 | 4.13 | 0.57 | 14.84 | 38.85 | 15.28 | 1.2 | 0.18 | 40.42 | 43.09 | 16.48 |
| MEAN ± SD | 1.52 ± 0.33 | 0.29 ± 62.07 | 0.25 ± 6.12 | 22.06 ± 4.67 | 3.96 ± 4.42 | 0.60 ± 5.00 | 13.12 ± 13.11 | 39.84 ± 2.48 | 16.64 ± 8.15 | 1.39 ± 13.67 | 0.35 ± 47.83 | 37.89 ± 6.69 | 44.09 ± 2.26 | 18.03 ± 8.57 | |
| 1 | 1.37 | 0 | 0 | 23.32 | 4.13 | 1.41 | 10.16 | 38.52 | 19.74 | 1.35 | 0 | 36.26 | 42.65 | 21.09 | |
| 70% | 2 | 1.68 | 0.14 | 0.31 | 23.2 | 3.26 | 0.81 | 13.43 | 36.62 | 19.07 | 1.18 | 0.3 | 39.73 | 40.02 | 20.25 |
| AV | 1.53 ± 10.16 | 0.07 ± 100.00 | 0.16 ± 100.00 | 23.26 ± 0.26 | 3.70 ± 11.77 | 1.11 ± 27.03 | 11.80 ± 13.86 | 37.57 ± 2.53 | 19.41 ± 1.73 | 1.27 ± 6.72 | 0.15 ± 100.00 | 38.00 ± 4.57 | 41.34 ± 3.18 | 20.67 ± 2.03 | |
| 1 | 1.16 | 0.15 | 0.18 | 20.93 | 4.24 | 0.42 | 13.35 | 36.64 | 21.81 | 0.99 | 0.14 | 36.18 | 41.03 | 22.8 | |
| 80% | 2 | 1.93 | 0.04 | 0.17 | 22.39 | 4.77 | 0.4 | 11.16 | 36.95 | 20.51 | 1.57 | 0.11 | 36.16 | 41.76 | 22.08 |
| MEAN ± SD | 1.55 ± 24.92 | 0.10 ± 57.89 | 0.18 ± 2.86 | 21.66 ± 3.37 | 4.51 ± 5.88 | 0.41 ± 2.44 | 12.26 ± 8.94 | 36.80 ± 0.42 | 21.16 ± 3.07 | 1.28 ± 22.66 | 0.13 ± 12.00 | 36.17 ± 0.03 | 41.40 ± 0.88 | 22.44 ± 1.60 | |
| 1 | 0.67 | 0.15 | 0.21 | 22.04 | 4.53 | 0.26 | 7.77 | 37.12 | 24.49 | 1.6 | 0.16 | 31.11 | 41.8 | 26.09 | |
| 90% | 2 | 0.51 | 0.01 | 0.09 | 20.46 | 4.61 | 0.16 | 7.25 | 39.01 | 26.45 | 1.02 | 0.1 | 28.57 | 4.63 | 27.47 |
| 3 | 0.54 | 0.16 | 0.08 | 21.54 | 5.22 | 0.13 | 6.57 | 39.26 | 24.19 | 2.19 | 0.12 | 28.98 | 44.64 | 26.38 | |
| MEAN ± SD | 0.57 ± 12.11 | 0.11 ± 64.20 | 0.13 ± 46.63 | 21.35 ± 3.09 | 4.79 ± 6.44 | 0.18 ± 30.32 | 7.20 ± 6.83 | 38.46 ± 2.48 | 25.04 ± 4.00 | 1.60 ± 29.79 | 0.13 ± 19.69 | 29.55 ± 3.77 | 30.36 ± 60.05 | 26.65 ± 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmetukaj, D.; Cana, A.; Gashi-Zogëjani, V.; Shandro-Zeqiri, M.; Bajraktari, D.; Jankuloski, D.; Hajrulai-Musliu, Z.; Bytyçi, X. Detection of Undeclared Meat Species and Fatty Acid Variations in Industrial and Traditional Beef Sausages. Appl. Sci. 2025, 15, 4440. https://doi.org/10.3390/app15084440
Mehmetukaj D, Cana A, Gashi-Zogëjani V, Shandro-Zeqiri M, Bajraktari D, Jankuloski D, Hajrulai-Musliu Z, Bytyçi X. Detection of Undeclared Meat Species and Fatty Acid Variations in Industrial and Traditional Beef Sausages. Applied Sciences. 2025; 15(8):4440. https://doi.org/10.3390/app15084440
Chicago/Turabian StyleMehmetukaj, Dafina, Armend Cana, Vlora Gashi-Zogëjani, Malbora Shandro-Zeqiri, Drita Bajraktari, Dean Jankuloski, Zehra Hajrulai-Musliu, and Xhavit Bytyçi. 2025. "Detection of Undeclared Meat Species and Fatty Acid Variations in Industrial and Traditional Beef Sausages" Applied Sciences 15, no. 8: 4440. https://doi.org/10.3390/app15084440
APA StyleMehmetukaj, D., Cana, A., Gashi-Zogëjani, V., Shandro-Zeqiri, M., Bajraktari, D., Jankuloski, D., Hajrulai-Musliu, Z., & Bytyçi, X. (2025). Detection of Undeclared Meat Species and Fatty Acid Variations in Industrial and Traditional Beef Sausages. Applied Sciences, 15(8), 4440. https://doi.org/10.3390/app15084440







