Abstract
Au and Ag nanoparticles (NPs) exhibit distinct pharmacological activities, cytotoxicity profiles, and catalytic properties. This study sought to maximize the advantages of both metals while reducing the production of toxic byproducts and promoting the rapid synthesis of Au-Ag alloy NPs. For this, we used Melaleuca quinquenervia leaf extract (MQLE) as a reducing and capping agent alongside microwave-assisted green synthesis techniques. The physicochemical properties and biological activities of the synthesized Au-Ag alloy NPs were systematically evaluated. Our findings confirmed successful synthesis of nearly spherical Au-Ag alloy NPs with an average diameter of 37 nm, achieved within a 60 s irradiation period. Energy-dispersive spectroscopy (EDS) revealed a nearly uniform elemental composition, with Au and Ag constituting 43.56% and 40.21%, respectively, of the alloy. Thermogravimetric (TG) analysis confirmed the complete coating of the NPs with MQLE. Owing to these characteristics, the Au-Ag alloy NPs exhibited low cytotoxicity (half-maximal inhibitory concentration [IC50] > 110 mg/L), strong antioxidant activity (IC50 < 15 mg/L), reasonable antimicrobial efficacy (minimum inhibitory concentration: 2.5–10 mg/L), considerable anti-inflammatory potential (IC50: 9.45–35.41 mg/L), promising wound healing capacity (72.5% in 24 h), and excellent catalytic performance (apparent rate constant: 0.254–0.654 min−1). In conclusion, the rapid, efficient, and environmentally friendly synthesis of Au-Ag alloy NPs demonstrated in this study holds promise for various industrial applications, particularly in pharmaceutical and therapeutic development.
1. Introduction
Compared with bulk metals, metal nanoparticles (NPs) have a higher surface area–volume ratio, which enhances their bioavailability and confers antioxidant, antibacterial, anti-inflammatory, and anticancer properties [1]. Consequently, metal NPs, particularly Au NPs, Ag NPs, and their alloy variants, have gained increasing industrial interest [2]. Researchers have been striving to develop environmentally sustainable and cost-effective metal NP synthesis methods, particularly green synthesis using plant extracts. This method generates minimally hazardous byproducts and requires no stringent reaction conditions [3]. Plant extract components, particularly phenols and flavonoids, serve as reducing agents that convert metal ions into metal NPs while forming an organic layer that serves as a capping and stabilizing agent. This process enhances the water stability, biocompatibility, biological safety, pharmacological efficacy, and decontamination ability of metal NPs [4,5]. Various plant parts, such as leaves, roots, seeds, fruits, bark, and flowers, have been used in the synthesis of metal NPs [6]. Melaleuca quinquenervia leaf extract (MQLE) is rich in phenolic acids such as gallic acid, ellagic acid, 3-O-methylellagic acid, caffeic acid, vanillic acid, ferulic acid, and rosmarinic acid, as well as flavonoids such as luteolin, kaempferol, quercetin, catechin, kaempferol-3-O-glucoside, quercetin-3-O-glucuronopyranoside, apigenin, rutin, hesperidin, and naringin [7]. These compounds facilitate the reduction in metal ions into stable metal NPs.
The synthesis of metal NPs from plant extracts typically occurs in three stages: (1) the activation stage, characterized by the reduction in metal ions and the nucleation of reduced metal ions, during which the plant extract serves as a reducing agent; (2) the growth stage, characterized by the spontaneous aggregation of smaller particles into larger ones (Ostwald ripening); and (3) the stabilization stage, during which the plant extract serves as a stabilizing agent [8]. These stages aim to minimize pollution, enable rapid synthesis, and produce NPs with low cytotoxicity and broad-spectrum efficacy. To achieve these goals, advanced methods such as microwave-assisted synthesis and ultrasound-assisted synthesis have been developed. The aforementioned techniques reduce the duration of NP synthesis, ensure the production of relatively small particle sizes, and enhance the physiological activity of NPs [9,10].
Au and Ag NPs have distinct advantages and disadvantages. Au NPs exhibit minimal to no bactericidal activity and have high raw material costs but exhibit biocompatibility, low toxicity, and strong antioxidant properties, which contribute to their pronounced anti-inflammatory effects and decontamination abilities [11,12]. By contrast, Ag NPs exhibit strong antibacterial activity and moderate antioxidant activity but pose toxicity risks and exhibit low catalytic activity, which reduce their pollutant removal efficiency [13,14]. To capitalize on the benefits of both metals, Au-Ag alloy NPs can be synthesized using microwave-assisted techniques and MQLE. In this study, we initially performed microwave-assisted extraction to obtain MQLE, which was subsequently used as both a reducing agent and a capping agent in the microwave-assisted green synthesis of Au-Ag alloy NPs. Our approach facilitated the rapid production of Au-Ag alloy NPs while enhancing their diverse physiological activities.
2. Materials and Methods
2.1. Materials
Freshly fallen M. quinquenervia leaves were collected from Taipei City, Taiwan, and identified by Professor Bau-Yuan Hu [7]. The Gram-negative bacteria Escherichia coli ATCC 8739 and Pseudomonas aeruginosa ATCC 9027, the Gram-positive bacterium Staphylococcus aureus ATCC 6538, and the fungal strains Candida albicans ATCC 10231 and Aspergillus brasiliensis ATCC 16404 were obtained from the Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan. Tryptic Soy Broth (TSB) (Neogen, Lansing, MI, USA) was used to cultivate E. coli, P. aeruginosa, and S. aureus, whereas Sabouraud Dextrose Broth (SDB) (Neogen, MI, USA) was used to cultivate C. albicans and A. brasiliensis. The HaCaT cell line was provided by Professor Guey-Horng Wang, faculty member at Xiamen Medical College. The CCD966SK (BCRC 60153) and Raw264.7 (BCRC 60001) cell lines were obtained from the BCRC. HaCaT and Raw264.7 cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% streptomycin–penicillin solution (DMEM-FSP), whereas CCD966SK cells were cultivated in Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (MEM-F). The chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified. Mammalian protein extraction reagent was obtained from Thermo Scientific (Waltham, MA, USA). Primary antibodies against interleukin (IL)-6, tumor necrosis factor (TNF)-α, cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and β-actin were sourced from Santa Cruz Biotechnology (Paso Robles, CA, USA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2. Preparation of MQLE
To prepare MQLE, M. quinquenervia leaves were washed with distilled water and dried at 50 °C for 2 h. The dried leaves were crushed through a 0.5 mm sieve, and 10 g of the resultant powder was mixed with 200 mL of distilled water (liquid/solid ratio: 20 mL/g). To obtain a crude extract, the mixture was irradiated at 80 °C for 180 s in a microwave digestion apparatus (SINEO, Shanghai Sineo Microwave Chemistry Technology Co., Ltd., Shanghai, China) with a power output of 700 W and a frequency of 2.45 GHz [7]. The crude extract was centrifuged at 5000 rpm for 15 min and filtered through Whatman No. 1 filter paper. The filtrate—MQLE—was used as a bioreductant in the synthesis of metal NPs. The extract was lyophilized using a shelf freeze dryer (Uniss Corp., Taipei City, Taiwan) and stored for use as a positive control in activity assessments.
2.3. Synthesis of Au, Ag, and Au-Ag Alloy NPs
To synthesize Au, Ag, and Au-Ag alloy NPs, 10 mL of MQLE (prepared in water) was mixed with 50 mL of 10−3 M HAuCl4 (prepared in water, with a final pH of 2.6), 10−3 M AgNO3 (prepared in water, with a final pH of 4.7), or an equimolar mixture of HAuCl4 and AgNO3, resulting in a final concentration of 10−3 M (prepared in water, with a final pH of 5.6). Afterward, each mixture was subjected to microwave irradiation at 700 W and 2.45 GHz for varying durations (0–70 s). To recover NPs, the mixture was centrifuged at 12,000 rpm for 20 min. The supernatant was discarded, and the pellet was collected and dispersed in distilled water. This purification process was repeated thrice. Finally, the pellet was dried in a vacuum oven at 80 °C for 6 h to obtain NP powder.
A colorimetric change in the mixture indicated the formation of NPs. The surface plasmon resonance (SPR) absorption spectrum of each NP solution was recorded using a UV–Vis spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan). To determine the reduction efficiency of MQLE, the concentrations of Au(III) and Ag(I) in the solution were periodically measured using atomic absorption spectrometry (AA-6300, Shimadzu, Japan). During the reaction process, the reduction potential and pH of the solution were monitored using a digital potential/pH meter (TES-1381K, TES Electrical Electronic Corp., Taipei, Taiwan). For the evaluation of NP stability during storage, a 25 mg/L Au-Ag alloy NPs solution was prepared in 100 mL of distilled water and stored at 5 °C. A 1 mL aliquot was extracted every month to analyze the wavelength position and bandwidth of the SPR signal.
2.4. Analysis of NP Characteristics
To characterize the resultant NP powder, Au, Ag, and Au-Ag alloy NPs synthesized at irradiation durations of 50, 60, and 60 s were subjected to X-ray diffraction (XRD) analysis, thermogravimetric (TG) analysis, field emission scanning electron microscopy (FE-SEM), and energy-dispersive X-ray spectroscopy (EDS). XRD analysis was performed through Cu Kα radiation at a scanning rate of 2° min−1 and a 2θ value of 10–80° by using a Bruker X-ray diffractometer (Billerica, MA, USA). TG analysis was performed in a nitrogen atmosphere at a heating rate of 10 °C/min by using a PerkinElmer TG analyzer (Waltham, WA, USA). Morphological characteristics, including NP size and shape, were analyzed using FE-SEM (JEOL, Tokyo, Japan). Elemental composition was analyzed using an EDS detector (Oxford Ultim Max 100, Oxford Instruments, Abingdon, UK).
2.5. Assessment of Cytotoxicity and Wound Healing Capacity
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess the viability of NP-exposed HaCaT, CCD966SK, and Raw264.7 cells [15]. In brief, the cells were seeded (density: 3 × 105 cells/mL) in 24-well plates and incubated at 37 °C under 5% CO2 for 24 h. HaCaT and Raw264.7 cells were maintained in DMEM-FSP, whereas CCD966SK cells were maintained in MEM-F. Subsequently, various Au-Ag alloy NP concentrations (20–140 mg/L) were added to the plates and incubated for 24 h. Then, the culture medium was discarded, the cells were rinsed, and fresh culture medium was added. Next, 10 μL of 0.02% MTT solution was added to each well and incubated for 4 h. After the MTT solution was discarded and 0.1 mL of DMSO was added to solubilize the resulting crystals. Absorbance was measured at 570 nm by using an Epoch microplate reader (Winooski, VT, USA). Cell viability was calculated by comparing absorbance between the experimental and control groups.
Wound healing capacity was assessed following the methods of Nguyen et al. (2021) and Danna et al. (2022) [16,17], with slight modifications. In brief, HaCaT and CCD966SK cells were seeded (density: 5 × 105 cells/well) in 12-well plates and incubated for 24 h. Then, the cells were scratched with a stile tip and incubated with 0–25 mg/L concentrations of Au-Ag alloy NPs for 24 h to facilitate cell repair. Next, the cells were fixed with FineFIX solution and stained with toluidine blue O. Wound width was measured immediately after injury and after 24 h of repair by using a Lionheart FX automated microscope (BioTek Instruments Inc., Winooski, VT, USA) with Gen5 software (version 3). The degree of wound closure was quantified by measuring the scratch width of 30 wounded HaCaT cells and cell density within the wound area for 30 wounded CCD966SK cells at both 0 and 24 h. A 25 mg/L MQLE concentration was used as a control treatment in this experiment.
2.6. Assessment of Antioxidant Activity
Antioxidant activity was assessed using several indicators, such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) [18]. The DPPH free radical scavenging activity of various NPs—chemically synthesized Au NPs (Che-AuNPs; product reference: 741965), chemically synthesized Ag NPs (Che-AgNPs; CAS number 7440-22-4), bio-capped Ag NPs, bio-capped Au NPs, and bio-capped Au-Ag alloy NPs—was evaluated as follows: 2 mL of NP solution or MQLE at different concentrations was mixed with 0.5 mL of 0.25 mM DPPH solution in ethanol and incubated in the dark for 30 min. Absorbance was measured at 517 nm by using an UV–Vis spectrophotometer. The percentage of scavenging activity was plotted against concentration to derive half-maximal inhibitory concentration (IC50) values [19].
The ABTS free radical scavenging assay was performed following a previously described method [20]. ABTS solution (7 mM) was mixed with an equal volume of potassium persulfate (2.45 mM) and allowed to react in the dark for 16 h. The resultant ABTS+• solution was diluted until its absorbance reached 0.7 at 734 nm. Then, 20 μL of NP solution or MQLE at various concentrations was mixed with 180 μL of the ABTS+• solution and incubated for 2 h. Absorbance was measured at 734 nm by using a microplate reader. The percentage of ABTS+• scavenging along with IC50 values was calculated. All measurements were performed in triplicate.
2.7. Assessment of Antimicrobial Activity
In this study, E. coli, P. aeruginosa, S. aureus, C. albicans, and A. brasiliensis were used to assess the antimicrobial activity of various NPs. Biosynthesized and chemically synthesized NPs were compared in terms of antimicrobial activity. In brief, 1 mL of NP solution at different concentrations was mixed with 1 mL of bacterial suspension (containing 5.0 × 106 CFU/mL; derived from 18 h bacterial culture) and 8 mL of TSB in a test tube. This mixture was incubated at 37 °C at 180 rpm (shaking). The minimum inhibitory concentration (MIC) was calculated as the lowest concentration at which the solution in the test tube remained clear after 24 h of incubation [21].
To assess antifungal activity, 1 mL of NP solution at different concentrations was mixed with 1 mL of a fungal suspension (2.0 × 106 spores/mL or CFU/mL; derived from 5- to 7-day fungal culture) and 8 mL of freshly prepared SDB in a test tube. This mixture was incubated at 22.5 °C for 5 to 7 days at 150 rpm (shaking). Then, the antifungal activity of the NPs was evaluated using conventional serial dilution and plating techniques. The concentration at which the NPs exhibited at least 99.9% fungicidal efficiency was regarded as the minimum fungicidal concentration (MFC) [22].
2.8. Assessment of Anti-Inflammatory Activity
The production of NO, IL-6, TNF-α, COX-2, and LOX in lipopolysaccharide (LPS)-stimulated Raw264.7 cells was assessed following a method outlined by Rahmawati et al. (2024) [23]. In brief, Raw264.7 cells were cultured in DMEM-FSP at 37 °C under 5% CO2. The culture medium was replenished every 24 h. The cells were seeded (density: 5 × 104 cells/well) in 24-well plates containing DMEM-FSP plus 2.5 mg/L LPS. Different concentrations of Au-Ag alloy NPs were added to the plates. Two untreated control groups were used. In one group, Raw264.7 cells were cultivated in only DMEM-FSP (negative control). In the other group, the cells were cultivated in DMEM-FSP plus LPS (positive control). After 24 h of incubation, the cells were centrifuged. The supernatant was collected. To evaluate NO production, the supernatant was mixed with an equal volume of Griess reagent and incubated for 10 min. Absorbance was measured at 540 nm by using a microplate reader. NO levels were quantified from a standard curve generated using NaNO2 solution, as described by Divate and Chung (2017) [24].
Western blotting was performed to measure the levels of IL-6, TNF-α, COX-2, and LOX in LPS-stimulated Raw264.7 cells [25]. In brief, the cells were lysed using mammalian protein extraction reagent. The cell lysate was incubated on ice for 15 min and then centrifuged at 12,500 rpm for 10 min at 4 °C. The supernatant was collected. Protein concentration in the supernatant was determined through the Bradford assay. The extracted protein (30 μg) was separated through sodium dodecyl sulfate polyacrylamide gel (10%) electrophoresis. The resultant protein bands were transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline (TBS)–Tween 20 (0.1%) for 2 h. After washing with TBS–Tween 20, the membrane was incubated with primary antibodies against IL-6, TNF-α, COX-2, LOX, or β-actin for 1 h at room temperature. After three 5 min washes with TBS–Tween 20, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Then, a chemiluminescent reagent was added to generate a detectable signal. Protein bands were visualized using the LAS3000 Luminescent Image Analyzer (Fujifilm Life Science, Tokyo, Japan). Notably, β-actin was used as a control to ensure equal protein loading.
2.9. Assessment of Catalytic Activity
Each NP solution (50 mL; 25 mg/L) was incubated with various dyes (100 mg/L)—Victoria blue R (VBR), malachite green (MG), methylene blue (MB), reactive yellow 4G (RY4G), Congo red (CR), methyl orange (MO), and rhodamine B (RhB)—for 2 h in the dark at 100 rpm to establish adsorption–desorption equilibrium. Then, the solution was exposed to visible light for 12 min. From the solution, 300-μL samples were collected at 30 s intervals to determine the catalytic activity of the NPs by using a UV-Vis spectrophotometer. The specific monitoring wavelengths for various dyes were as follows: VBR, 615 nm; MG, 660 nm; MB, 665 nm; RY4G, 485 nm; CR, 498 nm; MO, 464 nm; and RhB, 554 nm. Background noise was subtracted using deionized water as a reference standard.
3. Results and Discussion
3.1. Reduction Reaction of Metal Ions
After the addition of MQLE, the color of the HAuCl4 solution changed from light brown to purple and eventually to deep ruby red with increasing irradiation time, indicating successful synthesis of Au NPs [26]. Similarly, the color of the AgNO3 solution changed from light brown to intense brown, confirming the synthesis of Ag NPs [27]. Furthermore, the mixture containing both HAuCl4 and AgNO3 changed color from light brown to dark brown [28].
Figure 1A presents the reduction efficiency and reduction potential of the Au ion, Ag ion, and Au-Ag solutions over prolonged irradiation times. The Au ion solution was completely reduced to NPs within 50 s, whereas the Ag ion solution and the mixed solution were completely reduced within 60 s. This rapid reduction process (50–60 s) under microwave irradiation was significantly more efficient than the prolonged time (several hours) required for biogenic production using only plant extracts, microorganisms, and biopolymers [29]. Given that NP formation follows first-order reaction kinetics, the rate constants (k) for the Au ion solution, Ag ion solution, and mixed solution were 0.1093, 0.0947, and 0.0746 s−1, respectively, indicating that the rate of reduction was the highest for the Au ion solution. The reduction potential of the Au-Ag solution increased from 15.2 to 54.8 mV, suggesting that biomolecules in MQLE, such as phytochemicals, facilitated the reduction in metal ions to NPs. The pH of the Au-Ag solution decreased from 5.6 to 2.6, likely due to the release of H+ ions during the oxidation process facilitated by certain reductants in MQLE [28]. Notably, before irradiation, the reduction efficiency of the metal ions was approximately 35%. Therefore, even in the absence of microwave irradiation, MQLE could reduce metal ions.
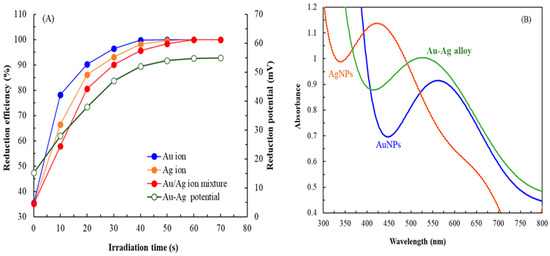
Figure 1.
(A) Reduction efficiency of different metal ions and corresponding reduction potentials in the Au-Ag solution at different irradiation times. (B) SPR peaks for Au NPs, Ag NPs, and Au-Ag alloy NPs were observed at 562, 426, and 528 nm, respectively; the peaks were recorded at irradiation times of 50, 60, and 60 s, respectively.
Figure 1B presents the SPR spectra of different NPs. The SPR spectra of Au NPs, Ag NPs, and Au-Ag alloy NPs were recorded at irradiation times of 50, 60, and 60 s, respectively. The absorption peaks for Au NPs and Ag NPs were observed at 562 and 426 nm, respectively. By contrast, the SPR spectrum of Au-Ag alloy NPs exhibited a single peak at 528 nm, positioned between the absorption peaks of Au NPs and Ag NPs, suggesting the formation of alloy NPs rather than a mere mixture of distinct metal particles [30]. This observation differs slightly from the SPR peaks obtained for chemically synthesized NPs, likely attributable to the effects of organic NP conjugates, which can induce either a red shift or a blue shift in the SPR peak [5]. Similar results have been reported by studies where NPs were synthesized using mannosylerythritol lipid and citrus peel extract [31,32].
Regarding stability, the absorbance and spectral bandwidth of the SPR signal of Au-Ag alloy NPs, obtained at an irradiation time of 60 s, exhibited no significant changes over an 8-month storage period. However, a reduction in absorbance was observed in month 9, suggesting the formation of aggregates [33]. Nonetheless, the spectral bandwidth remained unchanged. After 12 months, alterations in both absorbance and bandwidth, along with a red shift, were observed, indicating particle enlargement and NP instability [34]. These findings suggest that Au-Ag alloy NPs can maintain stability for at least 8 months.
3.2. Characteristics of NP Powder
Figure 2A presents the powder XRD patterns of Au, Ag, and Au-Ag alloy NPs. The XRD patterns of all NPs exhibited four distinct diffraction peaks at 2θ values of 38.2°, 43.5°, 65.1°, and 77.9°, corresponding to the characteristic diffractions of the (111), (200), (220), and (311) planes, respectively, in accordance with the face-centered cubic structure [35]. The Au-Ag alloy NPs had similarities with both Au and Ag NPs, attributable to their closely related lattice constants (Au: JCPDS 4-0783; Ag: JCPDS 4-0784).
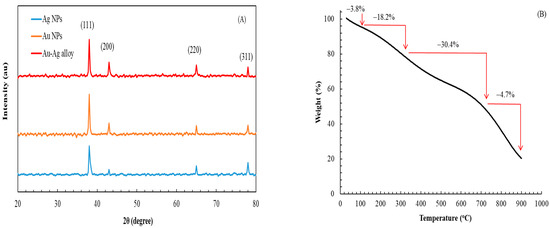
Figure 2.
(A) XRD patterns of Ag, Au, and Au-Ag alloy NPs. (B) TG curve of Au-Ag alloy NPs heated from 30 to 900 °C in a N2 atmosphere.
Figure 2B presents the TG curve for Au-Ag alloy NPs. The weight loss observed in these NPs could be categorized into four stages, with weight losses of 3.8%, 18.2%, 30.4%, and 24.7% occurring at temperatures of <100, 100–320, 320–750, and 750–900 °C, culminating in a total weight loss of 77.1%. At the first stage, weight loss likely resulted from the desorption of water molecules adsorbed on the NP surfaces. At the second stage, weight loss likely resulted from the thermal degradation of small molecules from plant residues loosely bound to the NPs. At the third stage, weight loss likely resulted from the desorption of bioactive molecules derived from MQLE, which played a crucial role in the reduction and capping processes during NP synthesis [28]. At the final stage, weight loss likely resulted from the decomposition of residual compounds. The significant weight loss observed in stages 2–4 indicates the presence of a substantial amount of MQLE on the NP surfaces. Given that Au-Ag alloy NPs were in situ capped with bioactive compounds in MQLE—for example, polyphenols, flavonoids, and terpenoids [7], they exhibited a stable capping function that enhanced their applicability across industrial sectors.
FE-SEM revealed no significant morphological differences among Au, Ag, and Au-Ag alloy NPs; the NPs predominantly exhibited elliptical and spherical shapes. The average diameters of Au, Ag, and Au-Ag alloy NPs were 42 ± 2.6, 26 ± 1.3, and 37 ± 2.2 nm, respectively. The literature suggests that most NPs synthesized using green methods with Jasminum sambac, Barleria prionitis, Plumbago zeylanica, and citrus peel extracts predominantly exhibit spherical or elliptical geometries [28,30,32,36]. However, only a limited number of NPs derived from Gloriosa superba and Hubertia ambavilla extracts exhibit alternative shapes [3,11]. In the present study, the average particle size of Au-Ag alloy NPs was slightly larger than that of NPs synthesized using chemical methods or those derived from J. sambac, G. superba, Trapa peels, and Tamarix aphylla extracts [3,28,37,38,39]; this difference may be attributable to the accelerated reduction process (60 s).
EDS confirmed that Au constituted 86.61% of Au NPs, whereas Ag constituted 85.23% of Ag NPs (Table 1). Thus, Au and Ag were the predominant components in corresponding NPs. In Au-Ag alloy NPs, Au and Ag were present at proportions of 43.56% and 40.21%, respectively. Trace amounts of C and O were detected, likely because of the capping of the NPs by MQLE-derived phytochemicals. Furthermore, the distribution of Au and Ag was more uniform in Au-Ag alloy NPs synthesized using MQLE than in those synthesized using J. sambac extract (50.74% Au vs. 29.99% Ag) or G. superba extract (2.49% Au vs. 49.79% Ag) [3,28]. This uniformity suggests that the Au-Ag alloy NPs synthesized in this study exhibit enhanced pharmacological activity.

Table 1.
EDS analysis of various NPs (element % by mass).
3.3. Cytotoxic Effects of Au-Ag Alloy NPs
Metal NPs, particularly Ag NPs, frequently exhibit cytotoxic properties, which can be advantageous in specific purposes, such as the suppression of cancer cell proliferation [12,26,36]. However, their cytotoxicity raises safety concerns regarding their use in health care, cosmeceuticals, and environmental contexts. The cytotoxic effects of metal NPs are influenced by their physicochemical properties, such as size, shape, surface charge, bio-capping, concentration, and exposure duration [31]. The cytotoxic effects of Au-Ag alloy NPs on various cell types after 24 h exposure are presented in Figure 3. The concentration of Au-Ag alloy NPs was negatively correlated with cell viability. Raw264.7 cells exhibited the least sensitivity to these NPs, whereas CCD966SK cells exhibited the highest sensitivity. Considering a cell viability threshold of 80% as the safety limit, we determined the safe concentration for HaCaT and CCD966SK cells to be 80 mg/L. On the basis of a curve depicting the correlation between cell viability and NP concentration, the IC50 values for the cytotoxic effects of Au-Ag alloy NPs on HaCaT and CCD966SK cells were determined to be 120.87 and 112.61 mg/L, respectively.
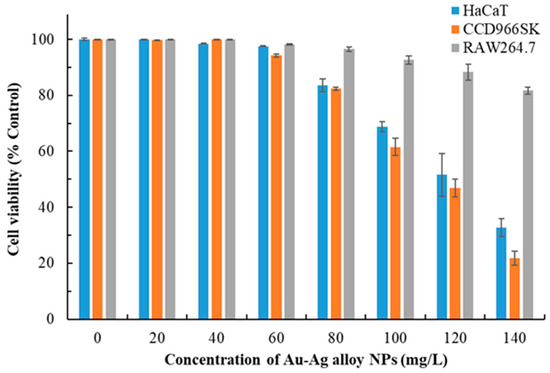
Figure 3.
Cytotoxic effects of Au-Ag alloy NPs on HaCaT, CCD966SK, and Raw264.7 cells 24 h after exposure. Data are presented in terms of mean ± standard deviation values. Each experiment was performed in triplicate.
Abbai et al. (2016) found that Ag NPs synthesized using Siberian ginseng stem extract exhibited considerable toxicity toward HaCaT cells, reducing cell viability to 45% at a concentration of 100 mg/L [26]. By contrast, Au NPs derived from the same plant extract exhibited relatively low toxicity toward HaCaT cells. Navya et al. (2019) reported that Au-Ag alloy NPs synthesized using isonicotinylhydrazide exerted strong cytotoxic effects on mouse skin fibroblast cells [40]. Furthermore, Das et al. (2023) noted that Au-Ag alloy NPs synthesized using sericin protein reduced the viability of HaCaT cells to <80% when at a concentration of >25 mg/L [41]. The cytotoxicity of these NPs has been attributed to the elevated proportion of Ag within the composite NPs [42]. Together, the findings indicate that careful selection of plant extracts, such as MQLE, as reducing and capping agents can mitigate cytotoxicity and expand the potential applications of Au-Ag alloy NPs.
3.4. Antioxidant Activity of Various NPs
Table 2 presents the IC50 values for the DPPH and ABTS free radical scavenging activities of various NPs and MQLE. The IC50 value represents the concentration at which 50% of all free radicals are scavenged [43], with lower values indicating superior scavenging activity. The findings revealed that Che-Au NPs and Che-Ag NPs exhibited inferior free radical scavenging activity than did bio-capped NPs. Furthermore, Au NPs exhibited greater free radical scavenging activity than Ag NPs. Bio-capped Au-Ag alloy NPs exhibited commendable DPPH and ABTS free radical scavenging activities, with IC50 values of 14.6 ± 1.5 and 10.6 ± 0.9 mg/L, respectively. Das et al. (2023) reported that the IC50 values for the DPPH and ABTS free radical scavenging activities of Au-Ag alloy NPs synthesized using sericin ranged from 45 to 16.4 mg/L [41]. Ghosh et al. (2022) stated that the IC50 value for the DPPH free radical scavenging activity of Au-Ag alloy NPs synthesized using Polyalthia longifolia leaf extract was 55.8 mg/L [13]. Mujahid et al. (2024) found that the IC50 value for the DPPH free radical scavenging activity of Au-Ag alloy NPs synthesized using T. aphylla bark extract was 222.3 mg/L [39]. MQLE exhibited strong scavenging activity against DPPH and ABTS free radicals, with IC50 values of 134.7 ± 12.5 and 78.2 ± 8.3 mg/L, respectively, outperforming Che-Ag NPs. The IC50 values for the DPPH and ABTS free radical scavenging activities of various positive controls (rutin, butylated hydroxytoluene, and ascorbic acid) were 13.6–24.6 and 8.2–16.4 mg/L, respectively, suggesting that Au-Ag alloy NPs synthesized using MQLE hold promise for industrial applications requiring antioxidant activity. The pronounced antioxidant capacity of bimetallic NPs is likely attributable to synergistic interactions between the metals and the active phytochemicals that cap the NPs [44].

Table 2.
IC50 values (mg/L) for the DPPH and ABTS free radical scavenging effects of various NPs and MQLE.
Studies using MTT assays have indicated that the safe concentration for Au-Ag alloy NPs is 80 mg/L, with an IC50 value < 15 mg/L for free radical scavenging. Thus, Au-Ag alloy NPs are safe and effective agents for applications in health care and cosmeceuticals, particularly for their ability to scavenge free radicals in cells. Furthermore, these NPs can serve as carriers in drug delivery systems and molecular imaging technologies [26].
3.5. Antimicrobial Activity of Various NPs
Metal NPs, particularly Ag NPs, are well known for their antimicrobial properties [28,45]. Au NPs have traditionally been considered less effective than Ag NPs in this regard [3,26]. However, recent evidence suggests that Au NPs can exhibit antibacterial effects similar to those of Ag NPs when functionalized with suitable capping agents [41]. If low-toxicity Au-Ag alloy NPs exhibit substantial antimicrobial activity, they may be used in burn treatment and wound healing.
Table 3 presents the MIC and MFC values for the antimicrobial activities of various NPs and MQLE. Che-Au NPs exhibited limited antimicrobial activity. MIC and MFC values for biosynthesized Au NPs were 50–75 and 75–100 mg/L, respectively. Although Che-Ag NPs effectively inhibited microbial growth, biosynthesized Ag NPs were more potent than Che-Ag NPs. Biosynthesized Au-Ag alloy NPs exhibited remarkable antimicrobial activity, with MIC and MFC values of 2.5–10 and 15–20 mg/L, respectively.

Table 3.
MIC and MFC values (mg/L) for the antimicrobial activities of various NPs and MQLE.
Amina et al. (2020) reported an MIC value of 480 mg/L for the antibacterial activity (against P. aeruginosa and S. aureus) of Au-Ag alloy NPs synthesized using Asparagus racemosus root extract [46]. Rezk et al. (2022) indicated an MIC value of 31.25 mg/L for the antibacterial activity (against P. aeruginosa) of Au-Ag alloy NPs synthesized using propolis extract [47]. Das et al. (2023) reported an MIC value of 25–50 mg/L for the activity of sericin-mediated Au-Ag alloy NPs against various foodborne pathogens [41]. Ojo et al. (2016) reported that the antifungal activity of Au-Ag alloy NPs synthesized using cell-free extract of Bacillus safensis against A. niger was 83.33% at 200 mg/L [48]. Compared with the literature, the Au-Ag alloy NPs synthesized in the present study exhibited promising antimicrobial activity.
MQLE exhibited moderate antimicrobial potential, suggesting that the observed activity partially stemmed from the components of the capping agent. Notably, Au-Ag alloy NPs exhibit enhanced antimicrobial activity when combined with antibiotics against E. coli, S. aureus, P. aeruginosa, and C. albicans [28], inhibiting biofilm formation [3]. In addition, these NPs exhibit strong antifungal activity against plant pathogenic fungi [13]. The increased efficacy of bio-capped Au-Ag alloy NPs may be attributable to the synergistic effects of Au NPs and Ag NPs or bioactive compounds in the bio-capping agent [28].
Das et al. (2023) elucidated the mechanism underlying the antibacterial effect of Au-Ag alloy NPs [41]. These NPs first induce membrane damage, leading to the leakage of intracellular DNA and RNA, ultimately resulting in bacterial death. Singh et al. (2022) suggested that this mechanism encompasses the degradation of the cell membrane, the disruption of homeostasis, and the induction of oxidative stress [29].
3.6. Anti-Inflammatory Activity of Au-Ag Alloy NPs
Figure 4 presents the results of Western blotting, indicating the levels of various inflammation-related proteins in LPS-stimulated Raw264.7 cells treated with various concentrations of Au-Ag alloy NPs. The concentration of Au-Ag alloy NPs was negatively correlated with the production of these proteins (Figure 4A). Thus, anti-inflammatory activity increased with increasing NP concentration (Figure 4B). The strongest anti-inflammatory activities were observed against NO, IL-6, TNF-α, COX-2, and LOX, corresponding to percent values of 90.2% ± 3.2%, 68.1% ± 1.5%, 96.3% ± 3.8%, 95.8% ± 0.4%, and 81.3% ± 1.2%, respectively. The IC50 values for the anti-inflammatory effects of Au-Ag alloy NPs on NO, IL-6, TNF-α, and LOX were 29.13, 35.41, 9.45, and 15.96 mg/L, respectively; corresponding values for the positive control, indomethacin (a potent nonsteroidal anti-inflammatory drug), were 22.47, 40.17, 10.21, and 8.28 mg/L, respectively. These findings underscore the anti-inflammatory potential of Au-Ag alloy NPs, with the most pronounced activity observed against COX-2, where a concentration of 20 mg/L achieved an anti-inflammatory activity of >95%.
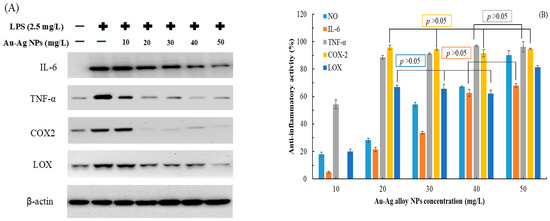
Figure 4.
(A) Levels of IL-6, TNF-α, COX-2, and LOX proteins in LPS-stimulated Raw264.7 cells incubated with various concentrations of Au-Ag alloy NPs at 37 °C for 24 h. β-Actin was used as the internal control for Western blotting. (B) Effects of Au-Ag alloy NPs on the levels of NO, IL-6, TNF-α, COX-2, and LOX in LPS-treated Raw264.7 cells. Data are presented in terms of mean ± standard deviation values (n = 3). A p value of >0.05 indicates a nonsignificant between-group difference. Data are expressed as means and standard deviations of three independent experiments.
Au-Ag alloy NPs synthesized using procyanidins exhibited significant anti-inflammatory effects and facilitated tissue repair in animal models of periodontal inflammation [49]. Conversely, Au-Ag alloy NPs synthesized using A. racemosus root extract inhibited TNF-α production but did not significantly affect IL-6 production in LPS-stimulated THP1 cells [46]. This discrepancy may be attributable to differences in bioactive plant extracts serving as reducing and capping agents during NP synthesis [50].
3.7. Wound Healing Capacity of Au-Ag Alloy NPs
Au-Ag alloy NPs exhibited prominent antioxidant and antimicrobial activities (Table 2 and Table 3). Therefore, they can promote wound healing and cell/tissue repair [51]. Figure 5 depicts the wound healing capacity of Au-Ag alloy NPs. Both HaCaT and CCD966SK cells exhibited considerable wound healing with increasing concentrations of Au-Ag alloy NPs. Specifically, the rate of wound healing in HaCaT cells was 36.5% ± 2.4% for control treatment. By contrast, for treatment with 20 mg/L Au-Ag alloy NPs, this rate was 72.5% ± 2.3% within 24 h (highest rate). The proliferation density of CCD966SK cells was 57.4 ± 1.2 cells per wound area after control treatment. After treatment with 20 mg/L Au-Ag alloy NPs, the proliferation density increased to 124.1 ± 3.2 cells per wound area, representing a 2.16-fold increase compared with the density in the control group. However, no significant difference was observed between the 15 and 20 mg/L concentrations of Au-Ag alloy NPs (p > 0.05). Gubitosa et al. (2020) demonstrated that bio-capped NPs effectively promoted wound healing without causing cytotoxicity [52]. In HaCaT and CCD966SK cells, the wound healing capacity of 20 mg/L MQLE (positive control group) was 52.1% ± 1.4% and 90.3 ± 2.6 cells per wound area, respectively. The wound closure rate of 25 mg/L sericin-mediated Au-Ag alloy NPs was 63.38% for HaCaT cells [41]. Therefore, MQLE-mediated Au-Ag alloy NPs are more effective than sericin-mediated Au-Ag alloy NPs. Notably, considerable wound recovery (in vivo) was observed in an animal model treated with an Ag–Au bimetallic nonwoven mat [53].
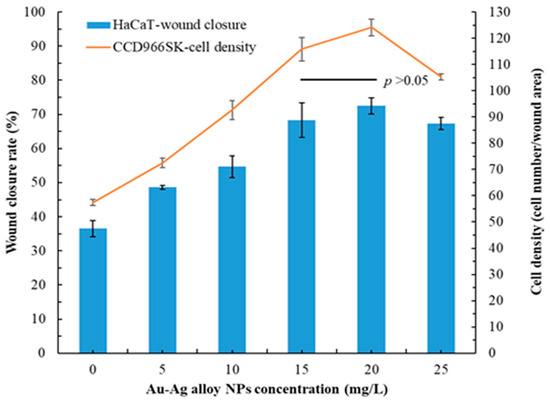
Figure 5.
Wound healing capacity of Au-Ag alloy NPs. Data are presented in terms of mean ± standard deviation values (n = 30). A p value of >0.05 indicates a nonsignificant between-group difference.
3.8. Catalytic Activity of Various NPs for Dyes
Under visible light irradiation, the degradation efficiency of various dye solutions increased with reaction time, ultimately reaching a plateau. When Au-Ag alloy NPs were used, the degradation efficiency of VBR stabilized at the 5th minute, achieving complete degradation (100%). The degradation efficiency of MO and MG plateaued at the 8th minute, each exceeding 99.97%, whereas that of RY4G reached a plateau at the 9th minute, with an efficiency exceeding 99.96%. Furthermore, the degradation efficiency of MB, CR, and RhB stabilized at the 11th minute, each exceeding 99.93%.
The NP-mediated decolorization reaction of dyes followed pseudo-first-order kinetics [54]. To calculate apparent rate constants for catalytic reactions involving various NPs, the logarithm of dye concentration was plotted against reaction time. Table 4 presents the apparent rate constants for the degradation of different dyes by Au, Ag, and Au-Ag alloy NPs. Notably, Au NPs exhibited the highest catalytic activity, followed by Au-Ag alloy NPs and Ag NPs. Numerous studies have demonstrated that the stability and catalytic performance of Ag NPs are inferior to those of other metal NPs [55]. Nevertheless, the Ag NPs synthesized in our study retained a certain level of catalytic activity, likely because of the presence of the MQLE coating on their surface, which facilitated efficient electron transfer between MQLE and the dyes [48].

Table 4.
Apparent rate constants (min−1) for the degradation of various dyes by Au, Ag, and Au-Ag alloy NPs.
VBR was the most effectively degraded dye, followed by MO, MG, RY4G, MB, CR, and RhB. The challenges associated with degradation primarily stem from the complexity of the chemical structures and the solubility of the dyes [54,56]. In the degradation of MG, the catalytic activities of Au-Ag alloy NPs synthesized using cell-free extracts of B. safensis and those synthesized using Deinococcus radiodurans protein extract were 92.6% and 83.68%, respectively [48,57]. The apparent rate constant for chlorpyriphos degradation by Au-Ag alloy NPs synthesized using P. longifolia leaf extract was 0.405 d−1 [13]. The apparent rate constants for MO and MB degradation by chemically synthesized Au-Ag alloy NPs, utilizing NaBH4 as a reducing agent, were 0.888 and 0.69 min−1, respectively [58]. Typically, three methods are used for the degradation of dyes with NPs: NaBH4 addition, ultraviolet light irradiation, and visible light irradiation [59]. Among these methods, visible light irradiation is the most convenient and sustainable option. The green-synthesized NPs investigated in this study effectively degraded various dyes under visible light irradiation, achieving degradation efficiency higher than or similar to that reported in the literature, which highlights the advantages of this synthesis technology.
4. Conclusions
We developed a simple, rapid, and green method for synthesizing Au-Ag alloy NPs. For this, we integrated microwave-assisted technology with MQLE. The observed reduction in the pH and increase in the redox potential of the Ag–Au solution during irradiation highlighted MQLE as a reducing agent. Alterations in the TG curve further supported the role of MQLE as a capping agent in the synthesis of Au-Ag alloy NPs. Successful synthesis of these NPs was also corroborated by their SPR peak at 528 nm, color change, XRD patterns, and EDS results. The uniform distribution of Au and Ag within Au-Ag alloy NPs, along with effective capping by MQLE, may reduce the cytotoxicity and enhance the biological activity of these bimetallic NPs. Au-Ag alloy NPs exhibit significant antimicrobial activity and lower raw material costs compared to Au NPs. Additionally, they demonstrate superior biosafety, enhanced antioxidant activity, increased anti-inflammatory properties, and improved catalytic activity when contrasted with Ag NPs. Furthermore, the rapid synthesis (60 s) and extended storage stability (8 months) enhance the viability of Au-Ag alloy NPs for industrial applications. The Au-Ag alloy NPs synthesized in this study have various potential applications, such as in health care, cosmeceuticals, biomedicine, agriculture, and environmental protection.
Author Contributions
Conceptualization, Y.-C.C. and H.-W.L.; methodology, J.-Y.L.; validation, T.-K.L., J.-T.K. and Y.-L.L.; formal analysis, T.-K.L.; investigation, T.-K.L., J.-Y.L., J.-T.K., Y.-L.L. and H.-W.L.; resources, T.-K.L. and J.-Y.L.; data curation, J.-T.K. and Y.-L.L.; writing—original draft preparation, T.-K.L., Y.-C.C. and H.-W.L., writing—review and editing, J.-T.K., Y.-C.C. and H.-W.L.; supervision, Y.-C.C. and H.-W.L.; funding acquisition, Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council, grant number NSTC 113-2622-E-157-001 and NSTC 112-2313-B-157-001-MY3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Chi-Hsiang Tang and Yueh-Te Lin for providing the authors with partially analytical measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi. J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Adamska, E.; Grobelna, B. Medical applications of silver and gold nanoparticles and core-shell nanostructures based on silver or gold core: Recent progress and innovations. ChemMedChem 2024, 19, e202300672. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.R.; Birla, S.S.; Yadav, A.P.; Abd-Elsalam, K.A. Potential role of biological systems in formation of nanoparticles: Mechanism of synthesis and biomedical applications. Curr. Nanosci. 2013, 9, 576–587. [Google Scholar] [CrossRef]
- Ditta, S.A.; Yaqub, A.; Tanvir, F.; Rashid, M.; Ullah, R.; Zubair, M.; Ali, S.; Anjum, K.M. Gold nanoparticles capped with L-glycine, L-cystine, and L-tyrosine: Toxicity profiling and antioxidant potential. J. Mater. Sci. 2023, 58, 2814–2837. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Leu, J.Y.; Lai, Y.L.; Chang, Y.C.; Chung, Y.C.; Liu, H.W. Application of microwave-assisted water extraction (MAWE) to fully realize various physiological activities of Melaleuca quinquenervia leaf extract. Plants 2024, 13, 3362. [Google Scholar] [CrossRef]
- Madkour, L.H. Ecofriendly green biosynthesized of metallic nanoparticles: Bio-reduction mechanism, characterization and pharmaceutical applications in biotechnology industry. Glob. Drugs Therap. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Kustov, L.; Vikanova, K. Synthesis of metal nanoparticles under microwave irradiation: Get much with less energy. Metals 2023, 13, 1714. [Google Scholar] [CrossRef]
- Badrillah, N.; Susanti, D.; Kamil, T.K.T.M.; Swandiny, G.F.; Widyastuti, Y.; Zaini, E.; Taher, M. Silver nanoparticles biogenically synthesised using Maclurodendron porteri extract and their bioactivities. Heliyon 2024, 10, e25454. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Ningaraju, S.; Munawer, U.; Raghavendra, V.B.; Balaji, K.S.; Melappa, G.; Brindhadevi, K.; Pugazhendhi, A. Chaetomium globosum extract mediated gold nanoparticle synthesis and potent anti-inflammatory activity. Anal. Biochem. 2021, 612, 113970. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rana, D.; Sarkar, P.; Roy, S.; Kumar, A.; Naskar, J.; Kole, R.K. Ecological safety with multifunctional applications of biogenic mono and bimetallic (Au-Ag) alloy nanoparticles. Chemosphere 2022, 288 Pt 2, 132585. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Unlocking the potential of silver nanoparticles: From synthesis to versatile bio-applications. Pharmaceutics 2024, 16, 1232. [Google Scholar] [CrossRef]
- Wang, G.H.; Lin, Y.M.; Kuo, J.T.; Lin, C.P.; Chang, C.F.; Hsieh, M.C.; Cheng, C.Y.; Chung, Y.C. Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.H.; Ahn, S.H.; Choi, M.J.; Yang, I.J.; Shin, H.M. Puerarin improves dexamethasone-impaired wound healing in vitro and in vivo by enhancing keratinocyte proliferation and migration. Appl. Sci. 2021, 11, 9343. [Google Scholar] [CrossRef]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-inflammatory and wound healing properties of leaf and rhizome extracts from the medicinal plant Peucedanum ostruthium (L.) W.D.J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel platinum-palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar]
- Wu, L.C.; Chen, C.Y.; Cheng, C.Y.; Dai, H.; Ai, Y.; Lin, C.H.; Chung, C.Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity: Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Miyaji, M.; Kurane, R.; Takahara, Y. Antifungal activity of components of essential oils. Agric. Biol. Chem. 1981, 45, 945–952. [Google Scholar]
- Rahmawati, S.I.; Indriani, D.W.; Ningsih, F.N.; Hardhiyuna, M.; Firdayani, F.; Ahmadi, P.; Rosyidah, A.; Septiana, E.; Dharmayanti, N.L.P.I.; Bayu, A.; et al. Dual anti-inflammatory activities of COX-2/5-LOX driven by kratom alkaloid extracts in lipopolysaccharide-induced RAW 264.7 cells. Sci. Rep. 2024, 14, 28993. [Google Scholar] [CrossRef] [PubMed]
- Divate, R.D.; Chung, Y.C. In vitro and in vivo assessment of anti-inflammatory and immunomodulatory activities of Xylaria nigripes mycelium. J. Funct. Foods 2017, 35, 81–89. [Google Scholar] [CrossRef]
- Choi, Y.H.; Choi, Y.S.; Kim, Y.K.; Rahman, M.S.; Pradeep, G.C.; Yoo, J.C.; Suh, J.W. A multifunctional alanine-rich anti-inflammatory peptide BCP61 showed potent inhibitory effects by inhibiting both NF-κB and MAPK expression. Inflammation 2017, 40, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Abbai, R.; Mathiyalagan, R.; Markus, J.; Kim, Y.J.; Wang, C.; Singh, P.; Ahn, S.; Farh, M.; Yang, D.C. Green synthesis of multifunctional silver and gold nanoparticles from the oriental herbal adaptogen: Siberian ginseng. Int. J. Nanomedicine 2016, 11, 3131–3143. [Google Scholar]
- Ghosh, S.; Chacko, M.J.; Harke, A.N.; Gurav, S.P.; Joshi, K.A. Barleria prionitis Leaf mediated synthesis of silver and gold nanocatalysts. J. Nanomed. Nanotechnol. 2016, 7, 394. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au-Ag alloy nanoparticles using J. sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Singh, C.; Mehata, A.K.; Priya, V.; Malik, A.K.; Setia, A.; Suseela, M.N.L.; Vikas; Gokul, P.; Samridhi; Singh, S.K.; et al. Bimetallic Au–Ag nanoparticles: Advanced nanotechnology for tackling antimicrobial resistance. Molecules 2022, 27, 7059. [Google Scholar] [CrossRef]
- Okazaki, K.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S.; Torimoto, T. Single-step synthesis of gold-silver alloy nanoparticles in ionic liquids by a sputter deposition technique. Chem. Commun. 2008, 6, 691–693. [Google Scholar] [CrossRef]
- Bakur, A.; Niu, Y.; Kuang, H. Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. AMB Express 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mei, S.; Ma, H.; Chen, X. Ultrasound-assisted green synthesis of gold nanoparticles using citrus peel extract and their enhanced anti-inflammatory activity. Ultrason. Sonochem. 2022, 83, 105940. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Corrêa, A.; Contreras, L.A.; Keijok, W.J.; Barcelos, D.H.F.; Pereira, A.C.H.; Kitagawa, R.R.; Scherer, R.; de Oliveira Gomes, D.C.; da Silva, A.R.; Endringer, D.C.; et al. Virola oleifera-capped gold nanoparticles showing radical-scavenging activity and low cytotoxicity. Mater. Sci. Eng. C 2018, 91, 853–858. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Khrennikov, D.E.; Gerasimov, V.S.; Ershov, A.E.; Polyutov, S.P.; Karpov, S.V.; Ågren, H. Nature of the anomalous size dependence of resonance red shifts in ultrafine plasmonic nanoparticles. J. Phys. Chem. C 2022, 126, 16804–16814. [Google Scholar] [CrossRef]
- Shin, Y.; Bae, I.T.; Arey, B.W.; Exarhos, G.J. Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. J. Phys. Chem. C 2008, 112, 4844–4848. [Google Scholar] [CrossRef]
- Priya Velammal, S.; Devi, T.A.; Amaladhas, T.P. Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostruct. Chem. 2016, 6, 247–260. [Google Scholar] [CrossRef]
- Sun, L.; Luan, W.; Shan, Y.J. A composition and size controllable approach for Au-Ag alloy nanoparticles. Nanoscale Res. Lett. 2012, 7, 225. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, A.K.; Sharma, S.; Khan, I.; Sharma, D.K.; Shamsi, A.; Santhosh Kumar, T.R.; Seervi, M. Biosynthesized composites of Au-Ag nanoparticles using Trapa peel extract induced ROS-mediated p53 independent apoptosis in cancer cells. Drug Chem. Toxicol. 2019, 42, 43–53. [Google Scholar] [CrossRef]
- Mujahid, S.; Ambreen, N.; Yaseen, M.; Ihtesham, M.; Mohammed Khan, K.; Nasimullah Qureshi, M. Metallic nanoentities: Bio-engineered silver, gold, and silver/gold bimetallic nanoparticles for biomedical applications. Heliyon 2024, 10, e37481. [Google Scholar] [CrossRef]
- Navya, P.N.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294. [Google Scholar] [CrossRef]
- Das, G.; Seo, S.; Yang, I.J.; Nguyen, L.T.H.; Shin, H.S.; Patra, J.K. Sericin mediated gold/silver bimetallic nanoparticles and exploration of its multi-therapeutic efficiency and photocatalytic degradation potential. Environ. Res. 2023, 229, 115935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Luo, Y.; Liu, P.; Li, Y.; Yue, J.; Jiang, L. Atomic-engineering Au-Ag nanoalloys for screening antimicrobial agents with low toxicity towards mammalian cells. Colloids Surf. B Biointerfaces 2021, 204, 111831. [Google Scholar] [CrossRef] [PubMed]
- Reviana, R.; Usman, A.N.; Raya, I.; Dirpan, A.; Arsyad, A.; Fendi, F. Analysis of antioxidant activity on cocktail honey products as female pre-conception supplements. Gac. Sanit. 2021, 35 (Suppl. S2), S202–S205. [Google Scholar] [CrossRef]
- Sharma, C.; Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Srivastava, M.M. Single-step green route synthesis of Au/Ag bimetallic nanoparticles using clove buds extract: Enhancement in antioxidant bio-efficacy and catalytic activity. Mater. Sci. Eng. C 2020, 116, 111153. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Khan, S.A.; Qayum, M.; Khan, M.A.; Anwar, Y.; Akhtar, K.; Asiri, A.M.; Khan, S.B. Green synthesis of antibacterial bimetallic Ag–Cu nanoparticles for catalytic reduction of persistent organic pollutants. J. Mater. Sci. Mater. Electron. 2018, 29, 20840–20855. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and immunomodulatory potentials of biosynthesized Ag, Au, Ag-Au bimetallic alloy nanoparticles using the Asparagus racemosus root extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Rezk, N.; Abdelsattar, A.S.; Makky, S.; Hussein, A.H.; Kamel, A.G.; El-Shibiny, A. New formula of the green synthesised Au@Ag core@shell nanoparticles using propolis extract presented high antibacterial and anticancer activity. AMB Express 2022, 12, 108. [Google Scholar] [CrossRef]
- Ojo, S.A.; Lateef, A.; Azeez, M.A.; Oladejo, S.M.; Akinwale, A.S.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; et al. Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of Bacillus safensis LAU 13: Antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Trans. Nanobiosci. 2016, 15, 433–442. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Huangfu, H.; Chen, S.; Qin, Q.; Ren, S.; Zhang, Y.; Fu, L.; Zhou, Y. Highly efficient photothermal branched Au-Ag nanoparticles containing procyanidins for synergistic antibacterial and anti-inflammatory immunotherapy. Biomater. Sci. 2023, 11, 1335–1349. [Google Scholar] [CrossRef]
- Tran, T.H.M.; Wang, R.; Kim, H.; Kim, Y.J. The anti-inflammation and skin moisturizing effects of Boehmeria tricuspis-mediated biosynthesized gold nanoparticles in human keratinocytes. Front. Pharmacol. 2023, 14, 1258057. [Google Scholar] [CrossRef]
- Soliman, W.E.; Elsewedy, H.S.; Younis, N.S.; Shinu, P.; Elsawy, L.E.; Ramadan, H.A. Evaluating antimicrobial activity and wound healing effect of rod-shaped nanoparticles. Polymers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Laurenzana, A.; Fibbi, G.; Veiga-Villauriz, C.; Fanelli, F.; Fracassi, F.; Onzo, A.; Bianco, G.; et al. Biomolecules from snail mucus (Helix aspersa) conjugate gold nanoparticles, exhibiting potential wound healing and anti-inflammatory activity. Soft Matter 2020, 16, 10876–10888. [Google Scholar] [CrossRef]
- Bai, M.Y.; Ku, F.Y.; Shyu, J.F.; Hayashi, T.; Wu, C.C. Evaluation of polyacrylonitrile nonwoven mats and silver-gold bimetallic nanoparticle-decorated nonwoven mats for potential promotion of wound healing in vitro and in vivo and bone growth in vitro. Polymers 2021, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Mbarek, W.B.; Escoda, L.; Saurina, J.; Pineda, E.; Alminderej, F.M.; Khitouni, M.; Suñol, J.J. Nanomaterials as a sustainable choice for treating wastewater: A review. Materials 2022, 15, 8576. [Google Scholar] [CrossRef] [PubMed]
- Alula, M.T.; Aragaw, B.A.; Modukanele, S.T.; Yang, J. Enhanced catalytic activity of silver nanoparticles loaded into Fe3O4 nanoparticles towards reduction of 4-nitrophenol, degradation of organic dyes and oxidation of o-phenylenediamine. Inorg. Chem. Commun. 2021, 127, 108504. [Google Scholar] [CrossRef]
- Mutukwa, D.; Taziwa, R.T.; Khotseng, L. A review of plant-mediated ZnO nanoparticles for photodegradation and antibacterial applications. Nanomaterials 2024, 14, 1182. [Google Scholar] [CrossRef]
- Weng, Y.; Li, J.; Ding, X.; Wang, B.; Dai, S.; Zhou, Y.; Pang, R.; Zhao, Y.; Xu, H.; Tian, B.; et al. Functionalized gold and silver bimetallic nanoparticles using Deinococcus radiodurans protein extract mediate degradation of toxic dye malachite green. Int. J. Nanomed. 2020, 15, 1823–1835. [Google Scholar] [CrossRef]
- Sabahat, S.; Nazish, Y.; Akhtar, A.; Shahid, A. Nanoengineering of mono (Au, Ag) and bimetallic (Ag-Au) alloy nanoparticles for dye degradation and toxicity assessment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 321, 124705. [Google Scholar] [CrossRef]
- Sharma, A.; Sunny, S.; Arulraj, J.; Hegde, G. Exploring the efficiency of green synthesized silver nanoparticles as photocatalysts for organic dye degradation: Unveiling key insights. Nano Express 2024, 5, 022002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).