Synergistic Antimicrobial Activity of Essential Oils and Vitamin C: Mechanisms, Molecular Targets and Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils and Ascorbic Acid
2.2. Gas Chromatography–Mass Spectrometry Analysis
2.3. Microbial Strains
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Determination of Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC)
2.6. Agar Dilution Method
2.7. Statistical Analysis
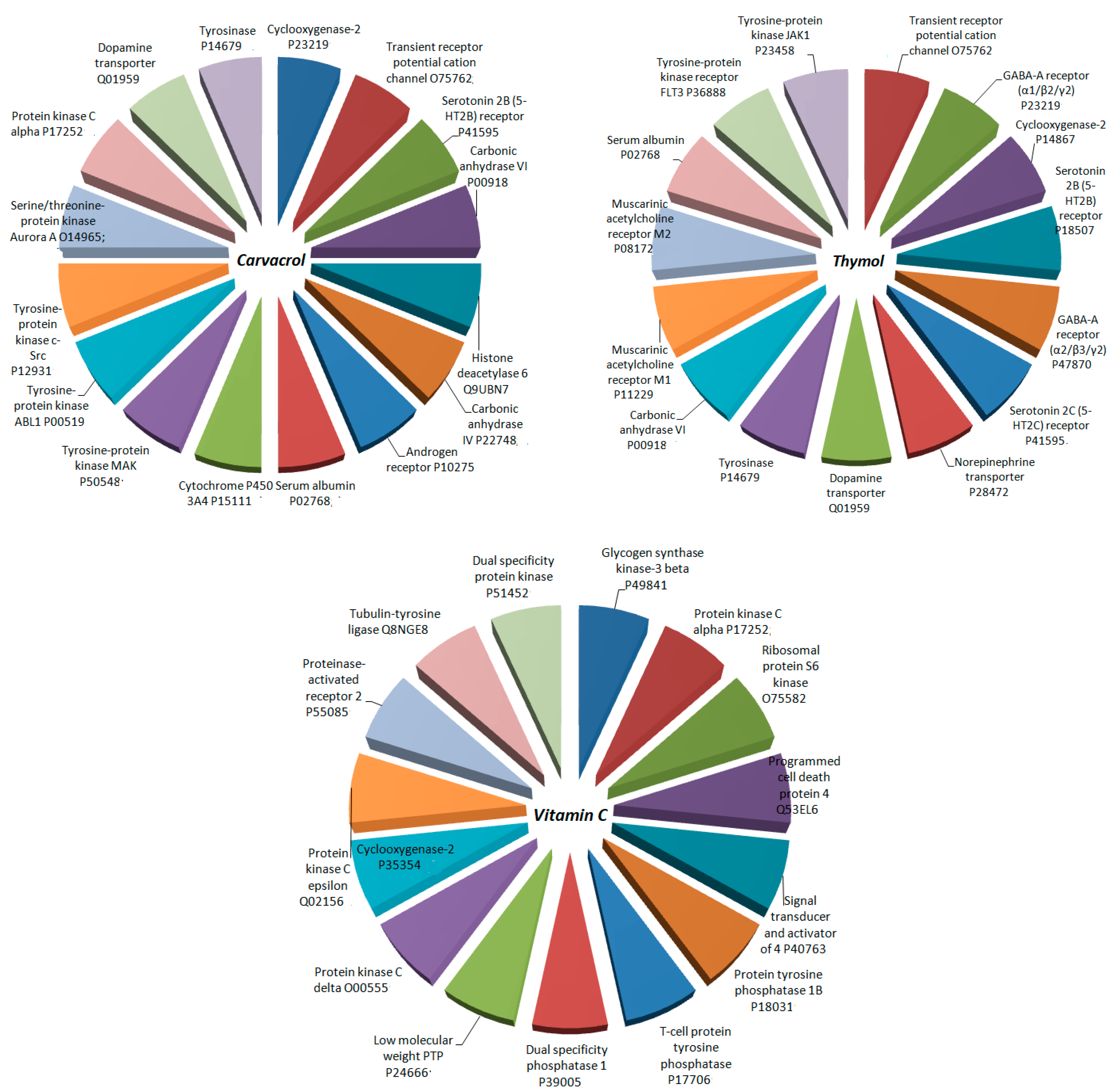
2.8. Host Modulation Effects Based on Target Prediction
3. Results
3.1. Gas Chromatography–Mass Spectrometry Analysis
3.2. Dilution Method in Liquid Medium
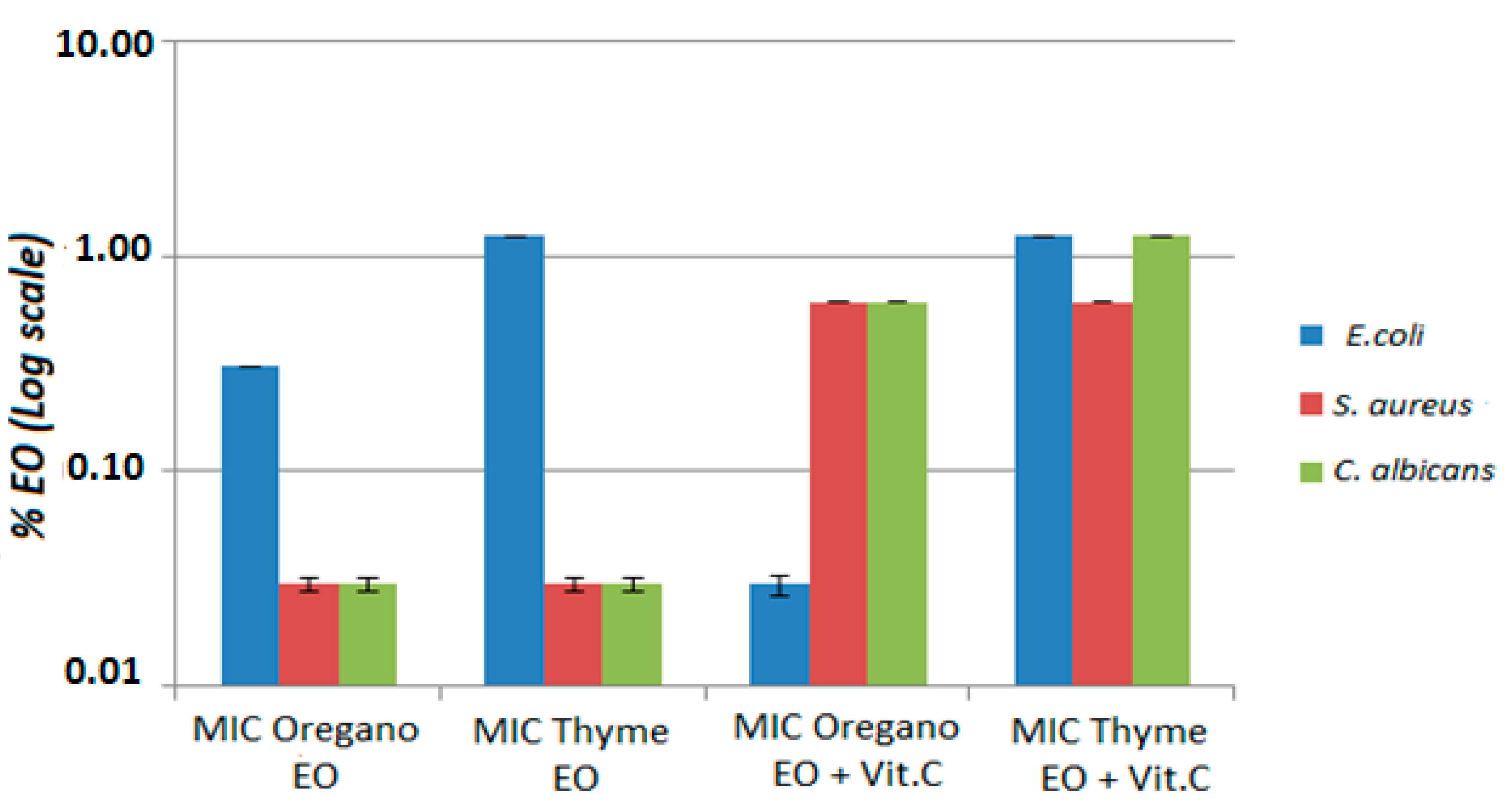
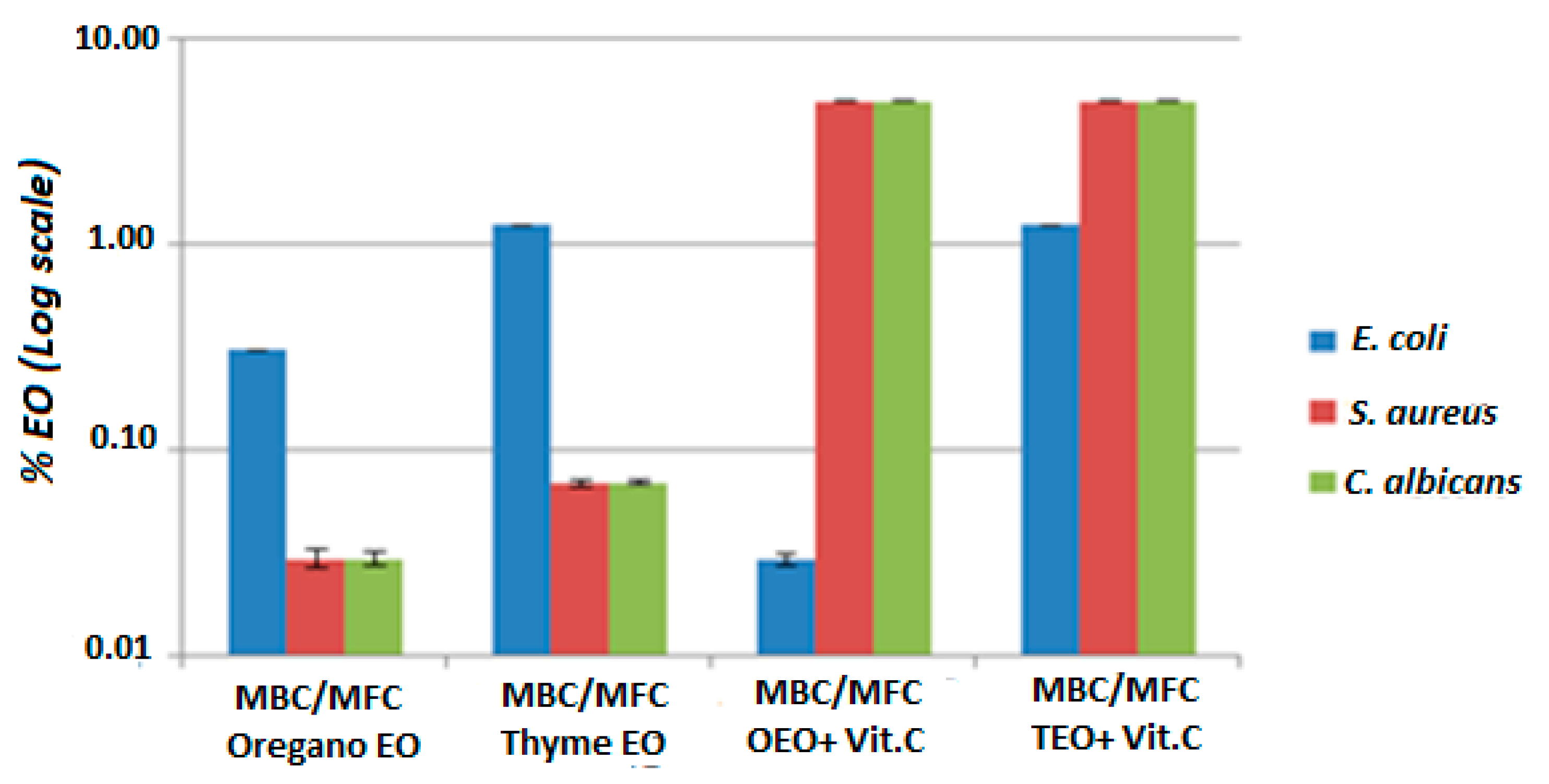
3.2.1. MIC and MBC Results for the Combination of Oregano Essential Oil and Vitamin C
3.2.2. MIC and MBC Results for the Combination of Thyme Essential Oil and Vitamin C
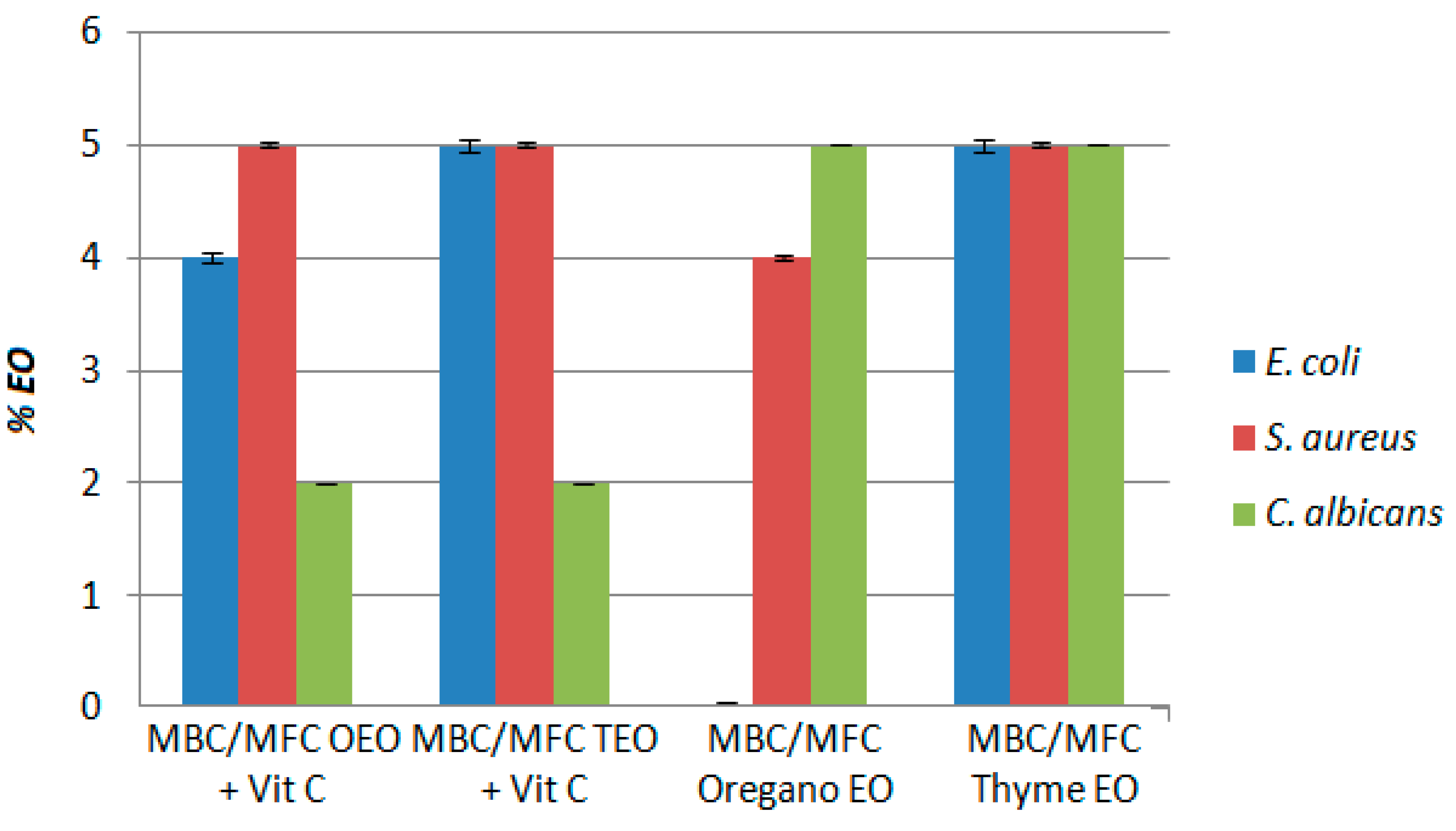
3.3. Determination of Antimicrobial Activityby the Agar Dilution Method
3.4. Statistical Analysis
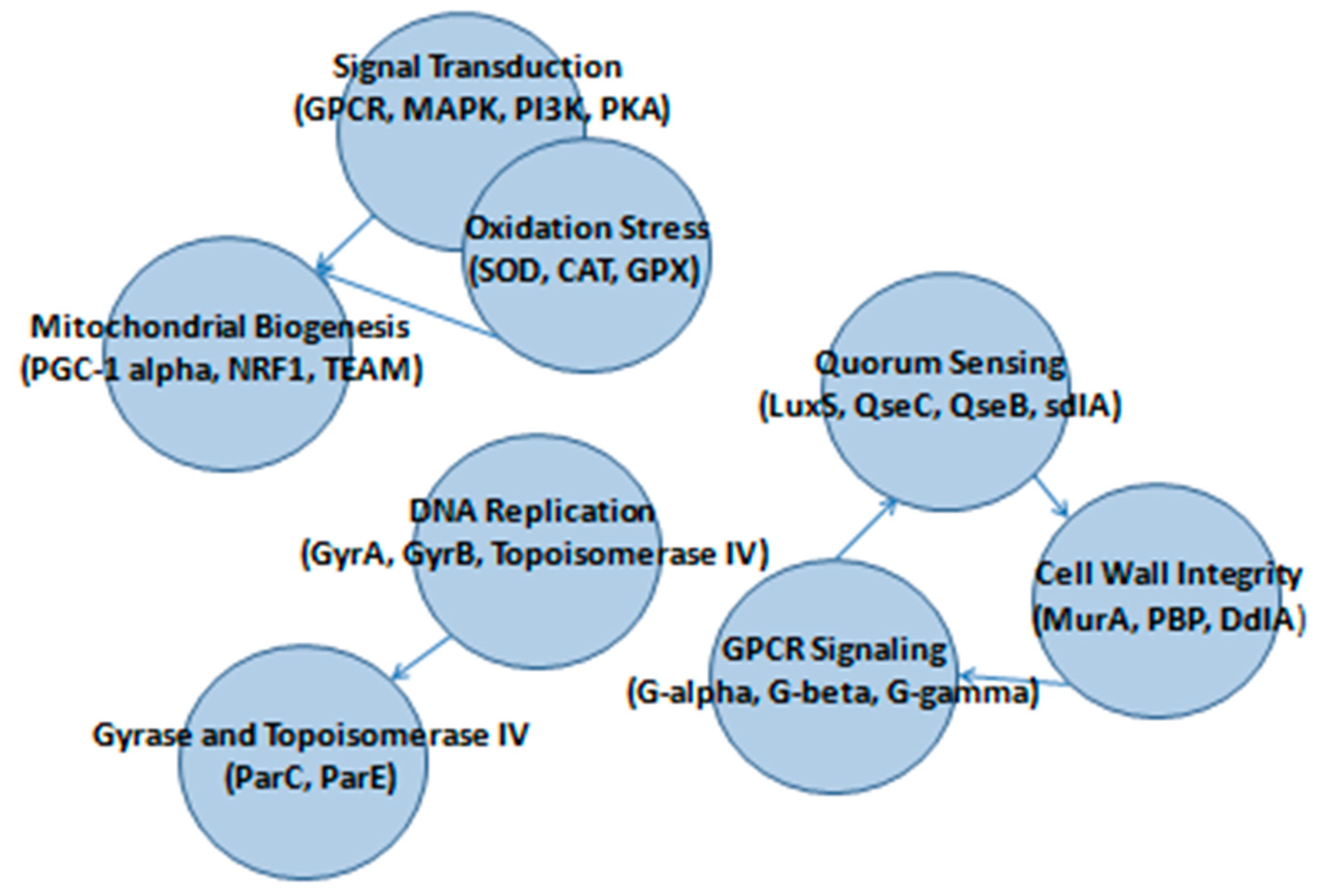
3.5. Host Modulation Effects Based on Target Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- The New EU One Health Action Plan Against Antimicrobial Resistance. 2017. Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_summary-action-plan_0.pdf (accessed on 10 April 2025).
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosami, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Read, A.F.; Woods, R.J. Antibiotic resistance management. Evol. Med. Public Health. 2014, 147. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Break throughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- O’Neil, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org (accessed on 10 April 2025).
- Rossiter, S.E.; Madison, H.F.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Boccolini, P.M.M.; Boccolini, C.S. Prevalence of complementary and alternative medicine (CAM) use in Brazil. BMC Complement. Med. Ther. 2020, 20, 51. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using the modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Stamova, S.; Mihaylova, S.; Ermenlieva, N.; Georgieva, E. In vitro study of antimicrobial activity of essential oils of Origanum vulgare against Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922. Scripta Sci. Pharm. 2021, 8, 34–37. [Google Scholar]
- Ermenlieva, N.; Georgieva, E.; Mihaylova, S.; Stamova, S.; Laleva, K.; Tsankova, G.; Tsvetkova, A. Synergistic interaction between Lamiaceae essential oils and antifungal drugs against Candida albicans ATCC10231. Farm. J. 2022, 70, 720–772. [Google Scholar]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, F.; Ji, B.; Pei, R.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Heckler, C.; Lemos, G.; Andrade, G.; Ribeiro, A.; Rocha, T.; Silva, C.; Rosa, C. Combined effect of carvacrol, thymol and nisin against pathogens. Ann. Braz. Acad. Sci. 2021, 93 (Suppl. 4), e20210550. [Google Scholar] [CrossRef]
- Mantzourani, I.; Plessa, S.; Alexopoulos, A.; Bekatorou, A. The antimicrobial effect of thymol and carvacrol in combination with organic acids against foodborne pathogens in chicken and beef meat fillets. Microorganisms 2025, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Kim, J.H.; Kang, J.S.; Lee, W.J.; Hwang, Y.I. Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro. Anat. Cell Biol. 2016, 49, 88–98. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Pereira, M.N.; Cetecioglu, Z.; Noriega, E.F.; Bover-Cid, S.; Hall, R.; Sauer, M. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J. Protective effects of natural antioxidants on inflammatory bowel disease: Thymol and its pharmacological properties. Antioxidants 2022, 11, 1947. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Lu, C.; Gong, Y.; Li, M.; Sun, S. Promising antifungal targets against Candida albicans based on ion homeostasis. Front. Cell Infect. Microbiol. 2018, 8, 286. [Google Scholar] [CrossRef]
- Yang, J.H.; Bening, S.C.; Collins, J.J. Antibiotic efficacy—Context matters. Curr. Opin. Microbiol. 2017, 39, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Brdová, D.; Ruml, T.; Viktorová, J. Mechanism of staphylococcal resistance to clinically relevant antibiotics. Drug Resist. Updat. 2024, 77, 101147. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans antifungal resistance and tolerance in bloodstream infections: The triad yeast–host–antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, G.; Kotzé, A. Eradication of antibiotic-resistant E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa with chlorine dioxide in vitro. Med. Res. Arch. 2023, 11, 4218. [Google Scholar] [CrossRef]
- Swiss Target Prediction. Available online: http://www.swisstargetprediction.ch (accessed on 10 April 2025).
- UniProt Database. Available online: https://www.uniprot.org (accessed on 10 April 2025).
- STRING Database. Available online: https://string-db.org (accessed on 10 April 2025).
- Reactome Pathway Database. Available online: https://reactome.org (accessed on 10 April 2025).
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Vitamin C inhibits Staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef]
- Evangelista-Martínez, Z.; Reyes-Vázquez, N.; Rodríguez-Buenfil, I. Antimicrobial evaluation of plant essential oils against pathogenic microorganisms: In vitro study of oregano oil combined with conventional food preservatives. Acta Univ. 2018, 28, 10–18. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C suppresses TNFα-induced NF-κB activation by inhibiting IκBα phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X. Vitamin C attenuates oxidative stress, inflammation, and apoptosis induced by acute hypoxia through the Nrf2/Keap1 signaling pathway in gibel carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C–induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef]
- Hatano, T.; Tsugawa, M.; Kusuda, M.; Taniguchi, S.; Yoshida, T.; Shiota, S.; Tsuchiya, T. Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid. Phytochemistry 2008, 69, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- McCarrell, E.M.; Gould, S.W.J.; Fielder, M.D.; Kelly, A.F.; ElSankary, W.; Naughton, D.P. Antimicrobial activities of pomegranate rind extracts: Enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med. 2008, 8, 64. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Wu, X.; Wang, L.; Zhao, M.; Yang, L. Ascorbic acid enhances the inhibitory effect of theasaponins against Candida albicans. Int. J. Mol. Sci. 2024, 25, 10661. [Google Scholar] [CrossRef]
- Altun, M.; Yapici, B.M. Determination of chemical compositions and antibacterial effects of selected essential oils against human pathogenic strains. An. Acad. Bras. Cienc. 2022, 94, e20210074. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Abderrahmane, D.; Boualem, S.; Boudarene, L.; Benseradj, F.; Aberrane, S.; Aitmoussa, S.; Chelghoum, C.; Lamari, L.; Sabaou, N.; Baaliouamer, A. In vitro synergistic/antagonistic antibacterial and anti-inflammatory effect of various extracts/essential oil from cones of Tetraclinis articulata (Vahl) Masters with antibiotic and anti-inflammatory agents. Ind. Crops Prod. 2014, 56, 60–66. [Google Scholar] [CrossRef]
- Buldain, D.; Buchamer, A.V.; Marchetti, M.L.; Aliverti, F.; Bandoni, A.; Mestorino, N. Combination of cloxacillin and essential oil of Melaleuca armillaris as an alternative against Staphylococcus aureus. Front. Vet. Sci. 2018, 5, 177. [Google Scholar] [CrossRef]
- Sumera, W.; Goc, A.; Niedzwiecki, A.; Rath, M. L-lysine and vitamin C work better in synergy against Escherichia coli and Acinetobacter baumannii. J. Cell. Med. Nat. 2023, 1, 1. [Google Scholar]
- Mortazavi, A.; Mohammad, P.K.H.; Beheshti, F.; Anaeigoudari, A.; Vaezi, G.; Hosseini, M. The effects of carvacrol on oxidative stress, inflammation, and liver function indicators in a systemic inflammation model induced by lipopolysaccharide in rats. Int. J. Vitam. Nutr. Res. 2023, 93, 111–121. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Bechara, N.; Flood, V.M.; Gunton, J.E. A systematic review on the role of vitamin C in tissue healing. Antioxidants 2022, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The nuclear factor-kappaB–interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Abdulwahid, A.H.; Mansouri, R.R.; Karimi, N.; Bostani, R.J.; Beiranvand, S.; Adelian, S.; Khorram, R.; Vafadar, R.; Hamblin, M.R. Targeting the NF-κB pathway as a potential regulator of immune checkpoints in cancer immunotherapy. Cell. Mol. Life Sci. 2024, 81, 106. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.-C.; Gong, J. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during LPS-induced inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Barak, T.H.; Eryilmaz, M.; Karaca, B.; Servi, H.; Ertekin, S.K.; Dinc, M.; Ustuner, H. Antimicrobial, anti-biofilm, anti-quorum sensing and cytotoxic activities of Thymbra spicata L. subsp. spicata essential oils. Antibiotics 2025, 14, 181. [Google Scholar] [CrossRef]
- Yap, P.; Yiap, B.; Ping, H.; Lim, S. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]

| No. Compound | Retention Time (Min) | % of Total Ion Current | Retention Time (Min) | % of Total Ion Current |
|---|---|---|---|---|
| Thyme EO | Oregano EO | |||
| α-Thujene | 9.11 | 1.38 | 9.05 | 0.12 |
| α-Pinene | 9.32 | 1.32 | 9.27 | 0.43 |
| Camphene | 9.84 | 1.19 | 9.79 | 0.16 |
| β-Pinene | 10.75 | 0.76 | 10.71 | 0.08 |
| β-Myrcene | 11.23 | 0.88 | 11.15 | 0.64 |
| α-Phellandrene | - | - | 11.16 | 0.11 |
| α-Terpinene | 12.06 | 1.18 | 12.02 | 0.90 |
| p-Cymene | 12.31 | 19.85 | 12.28 | 3.69 |
| Limonene | 12.47 | 0.80 | 12.40 | 0.13 |
| β-Phellandrene | - | - | 12.45 | 0.21 |
| ɣ-Terpinene | 13.41 | 12.64 | 13.37 | 3.52 |
| Sabinene hydrate | 13.79 | 0.57 | 13.73 | 0.16 |
| β-Linalool | 14.74 | 2.03 | 14.73 | 3.76 |
| Camphor | 16.12 | 1.17 | - | - |
| Terpinolen | - | - | 14.25 | 0.10 |
| Borneol | 16.90 | 1.81 | 16.87 | 0.71 |
| α-Terpineol | - | - | 17.60 | 0.24 |
| Terpinen-4-ol | 17.16 | 0.84 | - | - |
| Thymol methyl ether | 18.58 | 0.31 | - | - |
| Carvacrol, methyl ether | 18.84 | 1.39 | - | - |
| Thymol | 20.37 | 41.84 | 20.36 | 1.19 |
| Carvacrol | 20.75 | 2.14 | 20.75 | 81.20 |
| β-Caryophyllene | 23.77 | 2.88 | 23.70 | 0.97 |
| Aromadendrene | - | - | 24.18 | 0.15 |
| β-Bisabolene | - | - | 25.93 | 1.24 |
| δ-Cadinene | 26.19 | 0.64 | - | - |
| Caryophyllene oxide | 27.73 | 0.58 | 27.66 | 0.17 |
| MIC OEO+ Vit C (p-Value) | MIC TEO+Vit C (p-Value) | MIC Oregano EO (p-Value) | MIC Thyme EO (p-Value) | MBC/MFC OEO+ Vit C (p-Value) | MBC/MFC TEO+ Vit C (p-Value) | MBC/MFC Oregano EO (p-Value) | MBC/MFC Thyme EO (p-Value) | |
|---|---|---|---|---|---|---|---|---|
| E. coli | <0. 035% (p < 0.05) | <0.035% (p < 0.05) | 0.31% (p < 0.05) | 1.25% (p < 0.05) | <0.035% (p < 0.05) | <0.035% (p < 0.05) | 0.31% (p < 0.05) | 1.25% (p < 0.05) |
| S. aureus | <0.035% (p < 0.05) | <0.035% (p < 0.05) | 0.62% (p < 0.05) | 0.62% (p < 0.05) | <0.07% (p < 0.05) | <0.035% (p < 0.05) | >5% (p < 0.05) | 5% (p < 0.05) |
| C. albicans | <0.035% (p < 0.05) | <0.035% (p < 0.05) | 0. 62% (p < 0.05) | 1.25% (p < 0.05) | <0.07% (p < 0.05) | <0.035% (p < 0.05) | >5% (p < 0.05) | 5% (p < 0.05) |
| MBC/MFC OEO+ Vit C (p-Value) | MBC/MFC TEO+ Vit C (p-Value) |
MBC/MFC Oregano EO (p-Value) |
MBC/MFC Thyme EO (p-Value) | |
|---|---|---|---|---|
| E. coli | 4% (p ≈ 0.05) | 5% (p ≈ 0.05) | Lack of activity | 5% (p ≈ 0.05) |
| S. aureus | 5% (p ≈ 0.05) | 5% (p ≈ 0.05) | 4% (p ≈ 0.05) | 5% (p ≈ 0.05) |
| C. albicans | 2% (p ≈ 0.05) | 2% (p ≈ 0.05) | 5% (p ≈ 0.05) | 5% (p ≈ 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamova, S.; Ermenlieva, N.; Tsankova, G.; Nikolova, S.P.; Georgieva, E. Synergistic Antimicrobial Activity of Essential Oils and Vitamin C: Mechanisms, Molecular Targets and Therapeutic Potential. Appl. Sci. 2025, 15, 4294. https://doi.org/10.3390/app15084294
Stamova S, Ermenlieva N, Tsankova G, Nikolova SP, Georgieva E. Synergistic Antimicrobial Activity of Essential Oils and Vitamin C: Mechanisms, Molecular Targets and Therapeutic Potential. Applied Sciences. 2025; 15(8):4294. https://doi.org/10.3390/app15084294
Chicago/Turabian StyleStamova, Sylvia, Neli Ermenlieva, Gabriela Tsankova, Silviya P. Nikolova, and Emilia Georgieva. 2025. "Synergistic Antimicrobial Activity of Essential Oils and Vitamin C: Mechanisms, Molecular Targets and Therapeutic Potential" Applied Sciences 15, no. 8: 4294. https://doi.org/10.3390/app15084294
APA StyleStamova, S., Ermenlieva, N., Tsankova, G., Nikolova, S. P., & Georgieva, E. (2025). Synergistic Antimicrobial Activity of Essential Oils and Vitamin C: Mechanisms, Molecular Targets and Therapeutic Potential. Applied Sciences, 15(8), 4294. https://doi.org/10.3390/app15084294