Analysis of the Impact of the Addition of Alphitobius diaperinus Larval Powder on the Physicochemical, Textural, and Sensorial Properties of Shortbread Cookies
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Flours and Mixtures Prepared for Baking Crumbly Cookies
2.2. Baking and Evaluation of the Obtained Products
2.3. Proximal Analysis of Flours and Cookies
2.4. Color Measurement
2.5. Texture Properties of Cookies
2.6. Sensorial Evaluation
2.7. Statistical Analysis
3. Results and Discussion
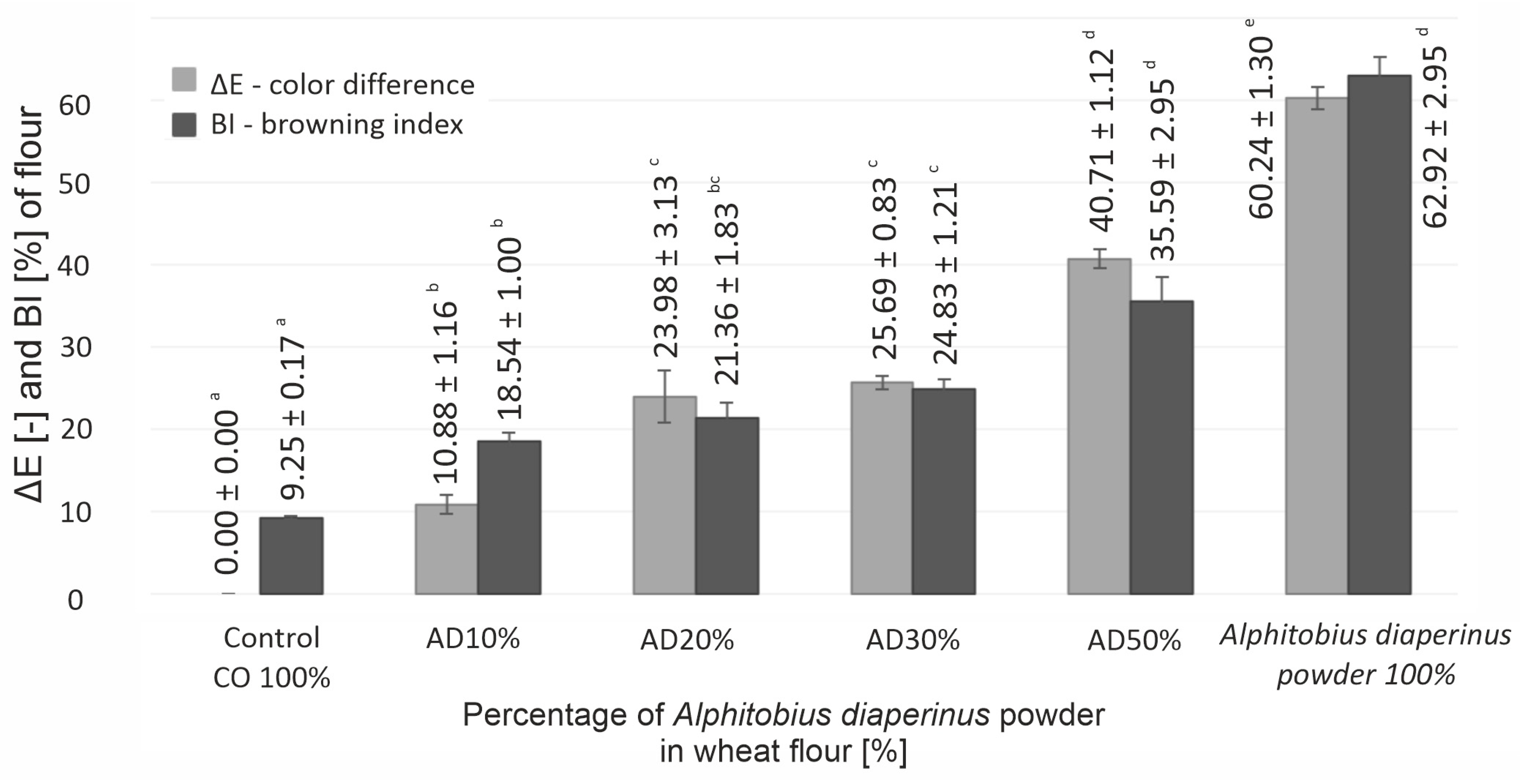
3.1. Physicochemical Properties of Flour and Baking Mix
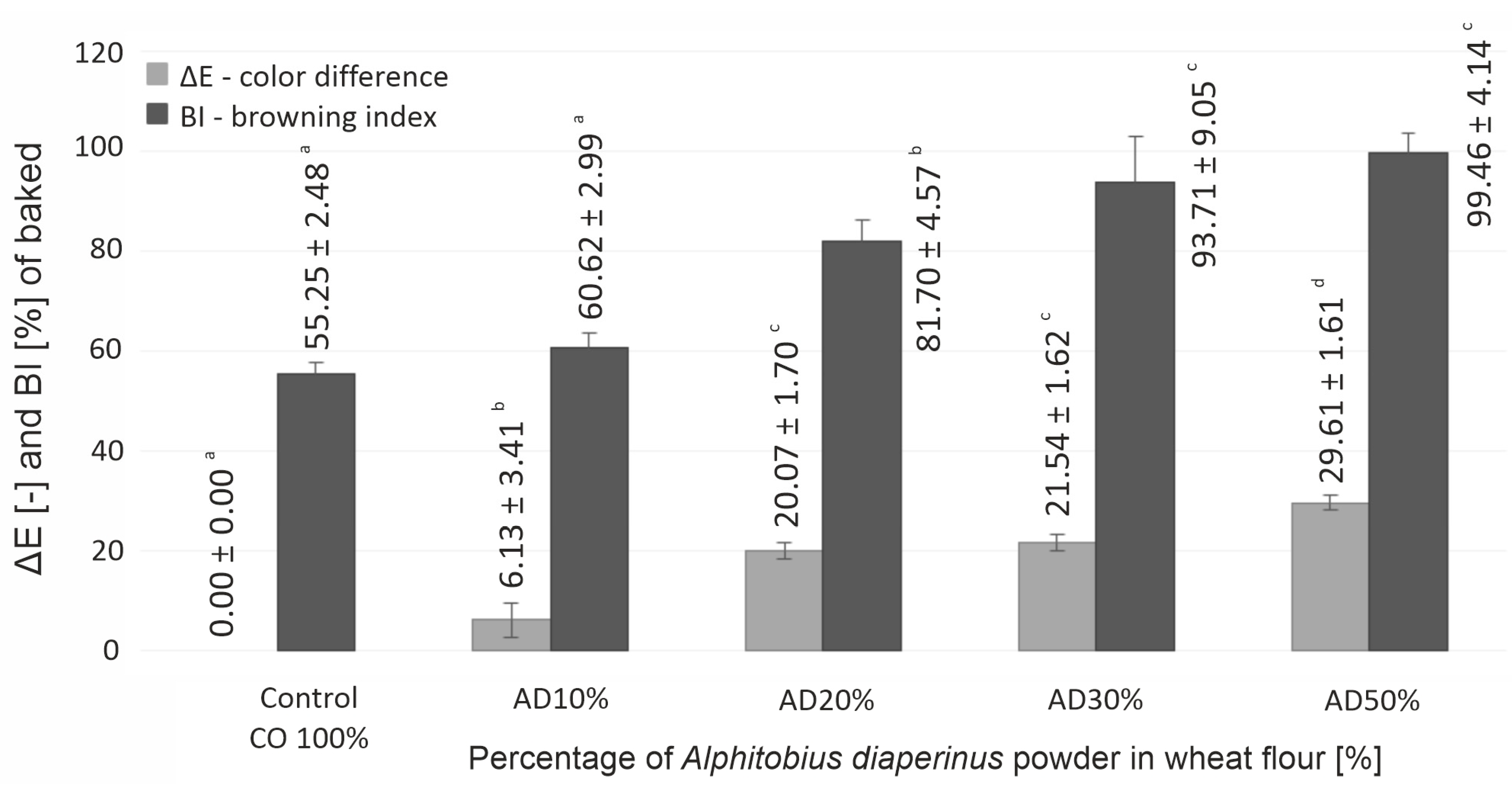
3.2. Physicochemical Properties of Cookies
Sensorial Property Evaluation of Shortcrust Biscuits with the Addition of Flour from Alphitobius diaperinus
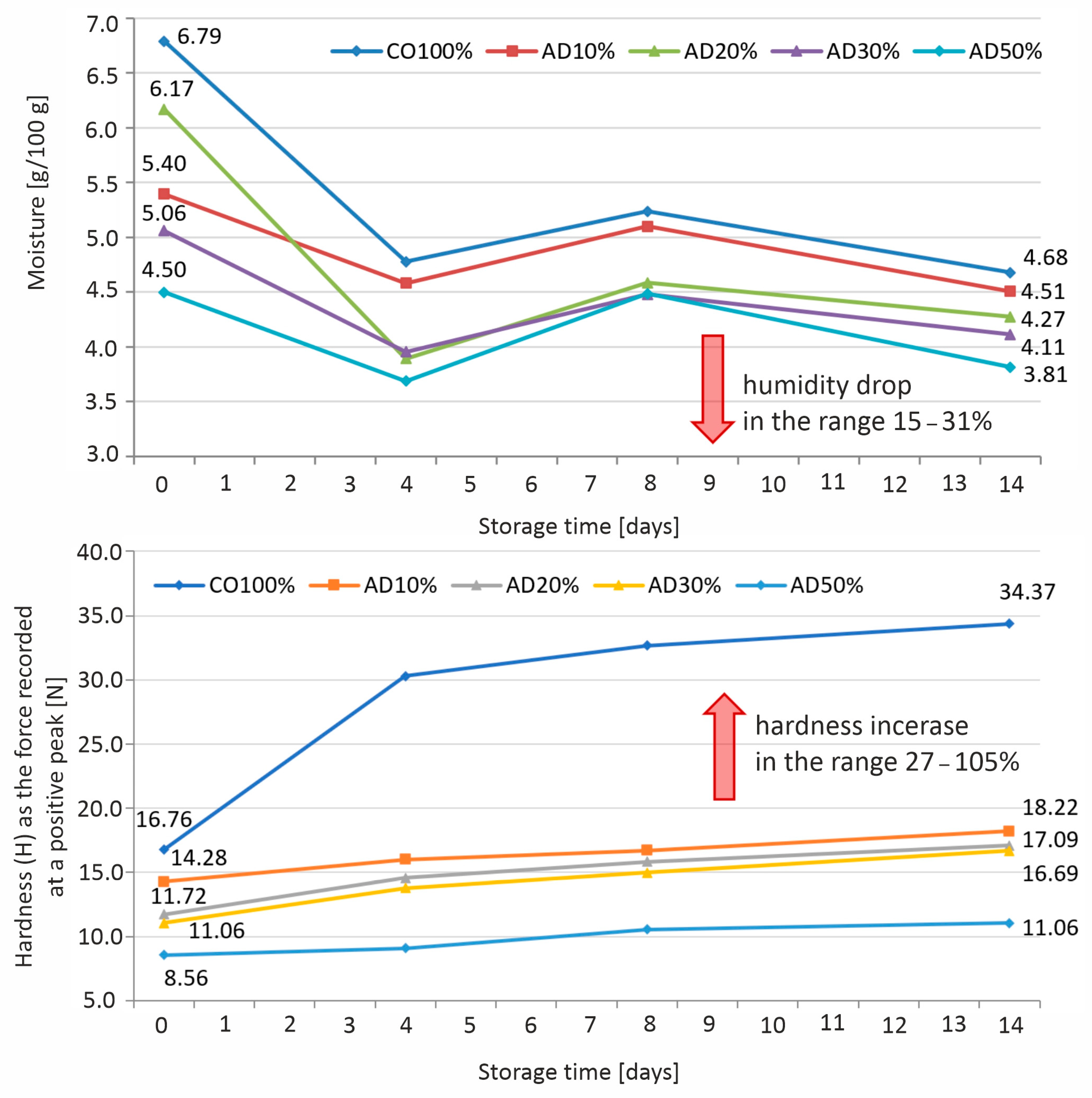
3.3. Changes in the Moisture Content and Hardness of Biscuits During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of frozen and freeze-dried formulations of the lesser mealworm (Alphitobius, diaperinus larva) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, 7325. [Google Scholar] [CrossRef]
- EU Commission. Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder Forms of Alphitobius Diaperinus Larvae (Lesser mealworm) as a Novel Food and Amending Implementing Regulation (EU); EU Commission: Brussels, Belgium, 2023. [Google Scholar]
- Orkusz, A.; Wolańska, W.; Harasym, J.; Piwowar, A.; Kapelko, M. Consumers’ Attitudes Facing Entomophagy: Polish Case Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 2427. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U. Nutritional, physiochemical, and antioxidative characteristics of shortcake biscuits enriched with Tenebrio molitor flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef]
- Homann, A.M.; Ayieko, M.A.; Konyole, S.O.; Roos, N. Acceptability of biscuits containing 10% cricket (Acheta domesticus) compared to milk biscuits among 5–10-year-old Kenyan schoolchildren. J. Insects Food Feed 2017, 3, 1–10. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture; FAO: Rome, Italy, 2013; ISSN 0081-4539. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Egas-Montenegro, E. Edible insects: A food alternative for the sustainable development of the planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A.; Liceaga, A.M.; Santiago-López, L.; Vallejo-Cordoba, B. An insight to fermented edible insects: A global perspective and prospective. Food Res. Int. 2020, 137, 109750. [Google Scholar] [CrossRef]
- Biró, B.; Sipos, M.A.; Kovács, A.; Badak-Kerti, K.; Pásztor-Huszár, K.; Gere, A. Cricket-Enriched Oat Biscuit: Technological Analysis and Sensory Evaluation. Foods 2020, 9, 1561. [Google Scholar] [CrossRef]
- Vantomme, P. Way Forward to Bring Insects in the Human Food Chain. J. Insects Food Feed 2015, 1, 121–129. [Google Scholar] [CrossRef]
- Van Huis, A. Nutrition and health of edible insectsvan. Curr. Opin. Clin. Nutr. 2020, 23, 228–231. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Dos Santos Aguilar, J.G. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Tech. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Çabuk, B. Influence of grasshopper (Locusta migratoria) and mealworm (Tenebrio molitor) powders on the quality characteristics of protein rich muffins: Nutritional, physicochemical, textural and sensory aspects. J. Food Meas. Charact. 2021, 15, 3862–3872. [Google Scholar] [CrossRef]
- Carcea, M. Quality and Nutritional/Textural Properties of Durum Wheat Pasta Enriched with Cricket Powder. Foods 2020, 9, 1298. [Google Scholar] [CrossRef]
- Gonzalez, C.M.; Garzon, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Foote, N. UE: Food Agency Approves Mealworms for Consumption; EURACTIV: Brussels, Belgium, 2021; Available online: https://www.euractiv.pl/section/rolnictwowpr/news/owady-unia-europejska-zywnosc-larwa-macznika-bialko-efsa-bezpieczenstwo-zywnosci/ (accessed on 10 February 2025).
- Carriço-Sá, B.; Teixeira, C.S.S.; Villa, C.; Mendes, E.; Ferreira, I.M.P.L.V.O.; Mafra, I.; Costa, J. Protein extraction from edible insects: Implications for IgE-binding capacity. Food Chem. 2024, 468, 142453. [Google Scholar] [CrossRef] [PubMed]
- Lampova, B.; Kopecka, A.; Smíd, P.; Kulma, M.; Kurecka, M.; Ogrinc, N.; Heath, D.; Kourimska, L.; Doskocil, I. Evaluating protein quality in edible insects: A comparative analysis of house cricket, yellow mealworm, and migratory locust using DIAAS methodologies. LWT 2024, 213, 117062. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Bailey, D.; Pinckaers, P.J.; van Loon, L.J. Ketone bodies and exercise performance: The next magic bullet or merely hype? Sports Med. 2017, 47, 383–391. [Google Scholar]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H.K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia-Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Koseckova, P.; Zverina, O.; Pechova, M.; Krulíkova, M.; Duborska, E.; Borkovcov, M. Mineral profile of cricket powders, some edible insect species and their implication for gastronomy. J. Food Compos. Anal. 2022, 107, 104340. [Google Scholar] [CrossRef]
- Lu, M.; Zhu Ch Smetana, S.; Zhao, M.; Zhang, H.; Zhang, F.; Du, Y. Minerals in edible insects: A review of content and potential for sustainable sourcing. Food Sci. Hum. Wellness 2024, 13, 65–74. [Google Scholar] [CrossRef]
- Mancini, A.; Dreassi, E.; Botta, M.; Tarchi, F.; Francardi, V. Bioaccumulation risk assessment of aflatoxin b1, ochratoxin and fumonisin b1 in tenebrio molitor larvae. REDIA 2020, 103, 101–108. [Google Scholar] [CrossRef]
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Protect. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- Amorello, D.; Barreca, S.; Orecchio, S. Critical evaluation of hazardous pollutants in edible insects: Asimple review. J. Food Compos. Anal. 2024, 135, 106675. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Świątek, Z. Subcellular partitioning of cadmium and zinc in mealworm beetle (Tenebrio molitor) larvae exposed to metal-contaminated flour. Ecotox. Environ. Safe 2016, 133, 82–89. [Google Scholar] [CrossRef]
- Looy, H.; Dunkel, F.V.; Wood, J.R. How then shall we eat? Insect-eating attitudes and sustainable foodways. Agric. Hum. Values 2014, 31, 131–141. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel food or potential improvers for wheat flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Biró, B.; Gere, A.; Fodor, R.; Pásztor-Huszár, K.; Szedljak, I. Buckwheat-pasta enriched with silkworm powder: Technological analysis and sensory evaluation. LWT 2019, 116, 108542. [Google Scholar] [CrossRef]
- Florowska, A.; Florowski, T. Insects—The food of the future. Food Ind. 2020, 74, 32–36. [Google Scholar] [CrossRef]
- van Huis, A. New Sources of Animal Proteins: Edible Insects. New Asp. Meat Qual. 2017, 443–461. [Google Scholar] [CrossRef]
- Borges, S.; Sousa, P.; Pintado, M. Insects as Food Sources; Universidade Católica Portuguesa, CBQF—Centro de Biotecnologia e Química Fina—Laboratório Associado, Escola Superior de Biotecnologia: Porto, Portugal, 2023. [Google Scholar]
- Piwowarek, P. Polskie ciasta—Niezakłócony rozwój. Raport Banku Pekao SA. 2023. Available online: https://www.portalspozywczy.pl/slodycze-przekaski/wiadomosci/polskie-ciastka-niezaklocony-rozwoj-raport-banku-pekao-sa,223773.html (accessed on 10 February 2025).
- Krygier, K.; Żbikowska, A. The impact of fat on selected properties of sponge cake. ŻYWNOŚĆ Nauka Technol. Jakość 2002, 32, 47–57. [Google Scholar]
- Slover, H.T.; Thompson, R.H.; Davis, C.S.; Merola, G.V. Lipids in margarines and margarine-like foods. J. Am. Oil Chem. Soc. 1985, 62, 775–786. [Google Scholar] [CrossRef]
- Toshtay, K.; Auyezov, A.; Azat, S.; Busquets, R. Trans fatty acids and saturated fatty acids in margarines and spreads in Kazakhstan: Study period 2015–2021. Food Chem. 2025, X, 102246. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The effect of trans fatty acids on human health: Regulation and consumption patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Abramovič, H.; Zlatić, E.; Demšar, L.; Piskernik, S.; Schreiner, M.; Vidrih, R. Content of trans-fatty acid isomers in bakery products on the Slovenian market. LWT 2021, 143, 111095. [Google Scholar] [CrossRef]
- Żbikowska, A.; Rutkowska, J. Skład kwasów tłuszczowych a jakość i przydatność technologiczna tłuszczów do pieczenia. ŻYWNOŚĆ Nauka Technol. Jakość 2008, 4, 90–95. [Google Scholar]
- Żbikowska, A.; Krygier, K. Wpływ zawartości izomerów trans w tłuszczach na jakość ciast wysokotłuszczowych. ŻYWNOŚĆ Nauka Technol. Jakość 2003, 2, 207–216. [Google Scholar]
- AOAC 992.15-1992; Crude Protein in Meat and Meat Products. AOAC International: Rockville, MD, USA, 1996.
- AOAC 925.09-1925; Solids (Total) and Loss on Drying (Moisture). AOAC International: Rockville, MD, USA, 2015.
- AOAC Official Method 939.05; Fat Acidity—Grains Titrimetric Method. AOAC International: Rockville, MD, USA, 2013.
- AOAC 923.03-1923; Ash of Flour. Direct Method. AOAC International: Rockville, MD, USA, 2005.
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Piepiórka-Stepuk, J.; Wojtasik-Kalinowska, I.; Sterczyńska, M.; Mierzejewska, S.; Stachnik, M.; Jakubowski, M. The effect of heat treatment on bioactive compounds and color of selected pumpkin cultivars. LWT 2023, 175, 114469. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Nutritional, sensory, and texture quality of bread and cookie enriched with house cricket (Acheta domesticus) Powder. J. Food Process. Pres. 2020, 44, e14601. [Google Scholar] [CrossRef]
- Tappi, S.; Glicerina, V.; Ragni, L.; Dettori, A.; Romani, S.; Rocculi, P. Physical and structural properties of honey crystallized by static and dynamic processes. J. Food Eng. 2021, 292, 110316. [Google Scholar] [CrossRef]
- Conforti, P.A.; Lupano, C.E.; Malacalza, N.H.; Arias, V.; Castells, C.B. Crystallization of honey at −20 °C. Int. J. Food Prop. 2006, 9, 99–107. [Google Scholar] [CrossRef]
- Dolik, K.; Kubiak, M.S. Instrumentalny test analizy profilu tekstury w badaniu jakości wybranych produktów spożywczych. Eng. Sci. Technol. 2013, 3, 35–44. [Google Scholar]
- ISO 4121:2003; Sensory analysis—Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization: Geneva, Switzerland, 2003.
- PN-ISO 3972:2011; Sensory Analysis—Methodology—Method of Investigating Sensitivity of Taste. International Organization for Standardization: Geneva, Switzerland, 2011.
- PN-ISO 5496:2006; Sensory Analysis—Methodology—Initiation and Training of Assessors in the Detection and Recognition of Odours. International Organization for Standardization: Geneva, Switzerland, 2006.
- Talens, C.; Lago, M.; Sim′o-Boyle, L.; Odriozola-Serrano, I.; Ibargüen, M. Desirability-based optimization of bakery products containing pea, hemp and insect flours using mixture design methodology. LWT 2022, 168, 113878. [Google Scholar] [CrossRef]
- Amoah, I.; Cobbinah, J.C.; Yeboah, J.A.; Essiam, F.A.; Lim, J.J.; Tandoh, M.A.; Rush, E. Edible insect powder for enrichment of bakery products—A review of nutritional, physical characteristics and acceptability of bakery products to consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Sriprablom, J.; Kitthawee, S.; Suphantharika, M. Functional and physicochemical properties of cookies enriched with edible insect (Tenebrio molitor and Zophobas atratus) powders. J. Food Meas. Charact. 2022, 16, 2181–2190. [Google Scholar] [CrossRef]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Alvarez-Rivera, G.; Viejo, J.L.; Martin-Cabrejas, M.A. Investigating edible insects as a sustainable food source: Nutritional value and techno-functional and physiological properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef] [PubMed]
- Althwab, S.A.; Alhomaid, R.M.; Ali, R.F.M.; Mohammed El-Anany, A.; Mousa, H.M. Effect of migratory locust (Locusta migratoria) powder incorporation on nutritional and sensorial properties of wheat flour bread. Brit. Food J. 2021, 123, 3576–3591. [Google Scholar] [CrossRef]
- Rumbos, I.; Karapanagiotidis, I.; Mente, E.; Athanassiou, G. The lesser mealworm 1887 Alphitobius diaperinus: A noxious pest or a promising nutrient source? Rev. Aquacult. 2019, 11, 1418–1437. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Fernandez-Lopez, J.; Perez-Alvarez, J.A.; Viuda-Martos, M. Effect of drying processes in the chemical, physico-chemical, techno-functional and antioxidant properties of flours obtained from house cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451–1458. [Google Scholar] [CrossRef]
- Djouadi, A.; Sales, J.R.; Carvalho, M.O.; Raymundo, A. Development of healthy protein-rich crackers using tenebrio molitor flour. Foods 2022, 11, 702. [Google Scholar] [CrossRef]
- Awobusuyi, T.D.; Pillay, K.; Siwela, M. Consumer Acceptance of Biscuits Supplemented with a Sorghum–Insect Meal. Nutrients 2020, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schonlechner, R.; Rawdkuen, S. Whole wheat bread enriched with cricket powder as an alternative protein. Foods 2022, 11, 2142. [Google Scholar] [CrossRef]
- Haber, M.; Mishyna, M.; Martinez, J.J.I.; Benjamin, O. The influence of grasshopper (Schistocerca gregaria) powder enrichment on bread nutritional and sensorial properties. LWT 2019, 115, 108395. [Google Scholar] [CrossRef]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Ribas-Agusti, A.; Martin-Belloso, O.; Soliva-Fortuny, R.; Elez-Martinez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Marcia, J.; Pournaki, S.K.; Borrás-Linares, I.; Lozano-Sanchez, J.; Fernandez, I.M. Formulation of Protein-Rich Chocolate Chip Cookies Using Cricket (Acheta domesticus) Powder. Foods 2022, 11, 3275. [Google Scholar] [CrossRef]
- Autio, K.; Laurikainen, T. Relationships between flour/dough microstructure and dough handling and baking properties. Trends Foods Sci. Technol. 1997, 6, 181–185. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Ortolá, M.D.; Martínez-Catalá, M.; Del Carmen, A.Y.; Castelló, M.L. Physicochemical and sensory properties of biscuits formulated with Tenebrio molitor and Alphitobius diaperinus flours. J. Texture Stud. 2022, 53, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gantner, M.; Król, K.; Piotrowska, A.; Sionek, B.; Sadowska, A.; Kulik, K.; Wiącek, M. Adding mealworm (Tenebrio molitor L.) powder to wheat bread: Effects on physicochemical, sensory and microbiological qualities of the end-product. Molecules 2022, 27, 6155. [Google Scholar] [CrossRef] [PubMed]
- Khuenpet, K.; Pakasap, C.; Vatthanakul, S.; Kitthawee, S. Effect of larval-stage mealworm (Tenebrio molitor) powder on qualities of bread. Int. J. Agric. Technol. 2020, 16, 283–296. Available online: https://www.thaiscience.info/view_content.asp?id=10992804 (accessed on 20 May 2024).
- Kourimská, L.; Adamková, A. Jakość odżywcza i sensoryczna owadów jadalnych. Dz. NFS 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R. Protein fortification with mealworm (Tenebrio molitor L.) powder: Effect on textural, microbiological, nutritional and sensory features of bread. PLoS ONE 2019, 14, e0211747. [Google Scholar] [CrossRef]



| Fat [g/100 g] | Protein [g/100 g] | Carbs [g/100 g] | Includes Sugars [g/100 g] | Calories [kcal] | |
|---|---|---|---|---|---|
| AD | 26.2 | 62.0 | 2.7 | 0.5 | 505.0 |
| WF | 1.0 | 11.0 | 71.0 | 2.4 | 342.0 |
| Baking Mixture Variant | Proportion of Wheat Flour in the Baking Mixture WF [%] | Proportion of Alphitobius diaperinus Powder in the Baking Mixture AD [%] | Proportion of Alphitobius diaperinus Powder in the Raw Dough AD [%] |
|---|---|---|---|
| CO—Control sample | 100 | 0 | 0 |
| AD10% | 90 | 10 | 5 |
| AD20% | 80 | 20 | 10 |
| AD30% | 70 | 30 | 15 |
| AD50% | 50 | 50 | 25 |
| CO: Control | Insect Powder (%, w/w Flour Basis) AD: Alphitobius diaperinus | |||||
|---|---|---|---|---|---|---|
| Wheat Flour 100% | AD10% | AD20% | AD30% | AD50% | AD100% | |
| Moisture | 12.09 ± 0.01 f | 11.33 ± 0.01 e | 10.89 ± 0.01 d | 10.13 ± 0.02 c | 7.60 ± 0.02 b | 4.14 ± 0.01 a |
| Ash [%] | 0.49 ± 0.01 a | 0.64 ± 0.10 a | 0.98 ± 0.10 a | 1.34 ± 0.04 b | 2.03 ± 0.03 c | 3.67 ± 0.01 e |
| CIE L* | 98.89 ± 1.32 d | 89.81 ± 0.96 c | 75.47 ± 2.95 b | 74.14 ± 0.58 b | 59.04 ± 0.65 a | 58.59 ± 1.29 a |
| CIE a* | 0.87 ± 0.05 a | 2.36 ± 0.12 b | 2.81 ± 0.49 bc | 3.07 ± 0.10 c | 3.72 ± 0.49 d | 6.12 ± 0.37 e |
| CIE b* | 8.45 ± 0.24 a | 14.19 ± 0.79 bc | 13.19 ± 0.68 b | 14.95 ± 0.62 cd | 16.15 ± 1.27 d | 25.57 ± 0.92 e |
| Chroma ∆C* | 8.50 ± 0.24 a | 14.38 ± 0.80 bc | 13.49 ± 0.69 b | 15.26 ± 0.62 cd | 16.58 ± 1.32 d | 26.30 ± 0.85 e |
| Hue ∆H* [°] | 84.13 ± 0.47 c | 80.56 ± 0.09 b | 78.39 ± 1.97 a | 77.98 ± 0.34 a | 77.04 ± 1.08 a | 76.52 ± 1.14 a |
| Control | Insect Powder (%, w/w Flour Basis) AD: Alphitobius diaperinus | ||||
|---|---|---|---|---|---|
| Sample | Wheat Flour 100% | AD10% | AD20% | AD30% | AD50% |
| Protein [g/100 g] | 7.73 ± 0.11 a | 9.81 ± 0.14 b | 11.63± 0.03 c | 14.27 ± 0.02 d | 18.81 ± 0.09 e |
| Moisture [g/100 g] | 6.79 ± 0.16 e | 5.40 ± 0.08 c | 6.17 ± 0.02 d | 5.06 ± 0.09 b | 4.50 ± 0.09 a |
| Acidity [%] | 0.08 ± 0.00 a | 0.80 ± 0.02 b | 1.44 ± 0.02 c | 2.27 ± 0.20 d | 3.24 ± 0.03 e |
| Hardness [N] | 16.76 ± 0.65 d | 14.28 ± 1.05 c | 11.72 ± 0.87 b | 11.06 ± 0.93 ab | 8.56 ± 1.84 a |
| Color | |||||
| CIE L* | 69.61 ± 0.49 d | 63.97 ± 2.89 c | 50.43 ± 1.10 b | 49.12 ± 1.59 b | 41.45 ± 1.23 a |
| CIE a* | 5.34 ± 0.40 a | 7.04 ± 0.90 b | 10.34 ± 0.93 c | 11.67 ± 0.61 cd | 12.90 ± 0.60 d |
| CIE b* | 27.99 ± 1.06 c | 26.83 ± 0.90 bc | 25.27 ± 0.70 b | 27.05 ± 1.28 bc | 22.98 ± 1.12 a |
| Chroma ∆C* | 28.50 ± 1.05 bc | 27.75 ± 0.76 abc | 27.31 ± 0.79 ab | 29.46 ± 1.38 c | 26.37 ± 0.87 a |
| Hue ∆H* [°] | 79.18 ± 0.86 d | 75.28 ± 2.13 c | 67.76 ± 1.82 b | 66.66 ± 0.61 b | 60.64 ± 2.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzejewska, S.; Domiszewski, Z.; Piepiórka-Stepuk, J.; Bielicka, A.; Szpicer, A.; Wojtasik-Kalinowska, I. Analysis of the Impact of the Addition of Alphitobius diaperinus Larval Powder on the Physicochemical, Textural, and Sensorial Properties of Shortbread Cookies. Appl. Sci. 2025, 15, 4269. https://doi.org/10.3390/app15084269
Mierzejewska S, Domiszewski Z, Piepiórka-Stepuk J, Bielicka A, Szpicer A, Wojtasik-Kalinowska I. Analysis of the Impact of the Addition of Alphitobius diaperinus Larval Powder on the Physicochemical, Textural, and Sensorial Properties of Shortbread Cookies. Applied Sciences. 2025; 15(8):4269. https://doi.org/10.3390/app15084269
Chicago/Turabian StyleMierzejewska, Sylwia, Zdzisław Domiszewski, Joanna Piepiórka-Stepuk, Anna Bielicka, Arkadiusz Szpicer, and Iwona Wojtasik-Kalinowska. 2025. "Analysis of the Impact of the Addition of Alphitobius diaperinus Larval Powder on the Physicochemical, Textural, and Sensorial Properties of Shortbread Cookies" Applied Sciences 15, no. 8: 4269. https://doi.org/10.3390/app15084269
APA StyleMierzejewska, S., Domiszewski, Z., Piepiórka-Stepuk, J., Bielicka, A., Szpicer, A., & Wojtasik-Kalinowska, I. (2025). Analysis of the Impact of the Addition of Alphitobius diaperinus Larval Powder on the Physicochemical, Textural, and Sensorial Properties of Shortbread Cookies. Applied Sciences, 15(8), 4269. https://doi.org/10.3390/app15084269









