The Effect of Low-Temperature Heat Treatment on the Physicochemical Properties of Bovine Semitendinosus Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Preparation
2.2. Heat Treatments
2.3. pH Value Evaluation
2.4. Spectrometric Quantification of Basic Meat Composition
2.5. Color Measurement
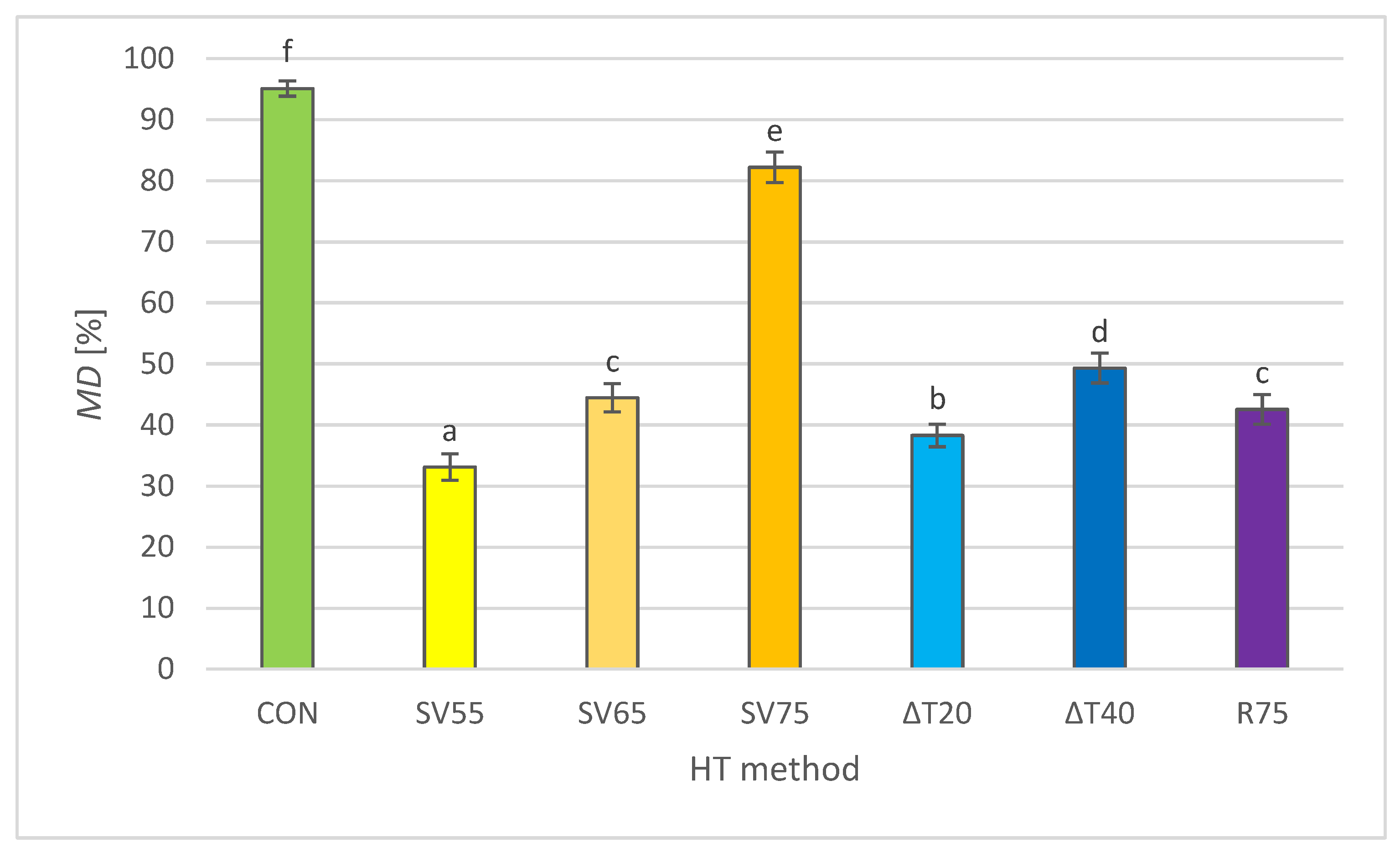
2.6. Myoglobin Denaturation
2.7. Cooking Loss
2.8. Warner–Bratzler Shear Force Determination
2.9. Consumer Acceptance
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Raw Material—Beef ST
3.2. Cooking Loss and WBSF
3.3. Color Changes
3.4. Myoglobin Denaturation Changes
3.5. Consumer Acceptance Rating of ST Muscle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Kondjoyan, A.; Oillic, S.; Portanguen, S.; Gros, J.-B. Combined heat transfer and kinetic models to predict cooking loss during heat treatment of beef meat. Meat Sci. 2013, 95, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Latoch, A.; Moczkowska-Wyrwisz, M.; Sałek, P.; Czarniecka-Skubina, E. Effect of Marinating in Dairy-Fermented Products and Sous-Vide Cooking on the Protein Profile and Sensory Quality of Pork Longissimus Muscle. Foods 2023, 12, 3257. [Google Scholar] [CrossRef]
- Wereńska, M. Effect of different sous-vide cooking temperature-time combinations on the functional and sensory properties of goose meat. Poultry Sci. 2024, 103, 103701. [Google Scholar] [CrossRef]
- Półtorak, A.; Wyrwisz, J.; Moczkowska, M.; Marcinkowska-Lesiak, M.; Stelmasiak, A.; Rafalska, U.; Wierzbicka, A.; Sun, D.W. Microwave vs. convection heating of bovine Gluteus Medius muscle: Impact on selected physical properties of final product and cooking yield. Int. J. Food Sci. Technol. 2015, 50, 958–965. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Interventions of two-stage thermal sous-vide cooking on the toughness of beef semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef]
- Rodas-González, A.; Larsen, I.L.; Uttaro, B.; Juárez, M.; Parslow, J.; Aalhus, J.L. Determination of optimum oven cooking procedures for lean beef products. Food Sci. Nutr. 2015, 24, 475–485. [Google Scholar] [CrossRef]
- Ko, S.; Yoo, S.-H.; Lee, S.; Cho, S.; KIM, K.-H.; Hwang, R. Effect of Long Low Temperature-Short High Temperature Cooking Cycle on Physicochemical Properties of Beef. Food Sci. Technol. Res. 2011, 17, 11–16. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, Ageing and Temperature Influence the Changes in Texture, Cooking Loss and Shrinkage of Cooked Beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef]
- Del Pulgar, J.S.; Gazquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Zikirov, E. The effects of sous-vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT-Food Sci. Technol. 2015, 64, 120–125. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Effect of cooking method on mechanical properties, color and structure of beef muscle (M. pectoralis). J. Food Eng. 2007, 80, 813–821. [Google Scholar] [CrossRef]
- Moczkowska, M.; Półtorak, A.; Montowska, M.; Pospiech, E.; Wierzbicka, A. The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci. 2017, 130, 7–15. [Google Scholar] [CrossRef]
- Stanisławczyk, R.; Żurek, J.; Rudy, M.; Gil, M.; Krajewska, A.; Dziki, D. Effect of Time and Temperature in Sous-Vide Heat Treatment on Selected Physicochemical Properties of Horsemeat. Processes 2023, 11, 3126. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Moczkowska, M.; Kurek, M.A.; Karp, S.; Atanasov, A.G.; Wierzbicka, A. Evaluation of WBSF, Color, Cooking Loss of Longissimus Lumborum Muscle with Fiber Optic Near-Infrared Spectroscopy (FT-NIR), Depending on Aging Time. Molecules 2019, 24, 757. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors influencing internal color of cooked meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Gašperlin, L.; Žlender, B.; Abram, V. Colour of beef heated to different temperatures as related to meat ageing. Meat Sci. 2001, 59, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yıkmış, S.; Aksu, H.; Çöl, B.G.; Demirçakmak, İ.L. Evaluation of Sous-Vide Technology in Gastronomy. Int. J. Agric. Life Sci. 2018, 4, 226–231. [Google Scholar] [CrossRef]
- Bowers, L.J.; Dikeman, M.E.; Murray, L.; Stroda, S.L. Cooked yields, color, tenderness, and sensory traits of beef roasts cooked in an oven with steam generation versus a commercial convection oven to different endpoint temperatures. Meat Sci. 2012, 92, 97–106. [Google Scholar] [CrossRef]
- Dominguez-Hernandez, E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Półtorak, A.; Poławska, E.; Pierzchała, M.; Jóźwik, A.; Zalewska, M.; Zaremba, R.; Wierzbicka, A. The impact of heat treatment methods on the physical properties and cooking yield of selected muscles from Limousine breed cattle. Anim. Sci. Pap. Rep. 2012, 30, 339–351. [Google Scholar]
- Cho, S.; Kang, S.M.; Seong, P.; Kang, G.; Kim, Y.; Kim, J.; Lee, S.; Kim, S. Effect of Aging Time on Physicochemical Meat Quality and Sensory Property of Hanwoo Bull Beef. Korean J. Food Sci. Anim. Resour. 2016, 36, 68–76. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Półtorak, A.; Zalewska, M.; Zaremba, R.; Wierzbicka, A. Analysis of relationship between basic composition, pH, and physical properties of selected bovine muscles. Bull. Vet. Inst. Pulawy 2012, 56, 403–409. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2008, 48, 137–159. [Google Scholar] [CrossRef]
- Li, S.; Ma, R.; Pan, J.; Lin, X.; Dong, X.; Yu, C. Combined effects of aging and low temperature, long time heating on pork toughness. Meat Sci. 2019, 150, 33–39. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Moczkowska, M.; Kurek, M.; Stelmasiak, A.; Półtorak, A.; Wierzbicka, A. Influence of 21 days of vacuum-aging on color, bloom development, and WBSF of beef semimembranosus. Meat Sci. 2016, 122, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.R.; Naveena, B.M.; Muthukumar, M.; Vaithiyanathan, S. Colour, myoglobin denaturation and storage stability of raw and cooked mutton chops at different end point cooking temperature. J. Food Sci. Technol. 2014, 51, 970–975. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, Y.; Yang, X.; Mao, Y.; Luo, X.; Hopkins, D.L.; Niu, L.; Liang, R. Sous vide cooking improved the physicochemical parameters of hot-boned bovine semimembranosus muscles. Meat Sci. 2023, 206, 109326. [Google Scholar] [CrossRef]
- Patinho, I.; Cavalcante, C.L.; Saldaña, E.; Gagaoua, M.; Behrens, J.H.; Contreras-Castillo, C.J. Assessment of beef sensory attributes and physicochemical characteristics: A comparative study of intermediate versus normal ultimate pH striploin cuts. Food Res. Int. 2024, 175, 113778. [Google Scholar] [CrossRef]
- PN-EN ISO 11136:2017-08; Analiza Sensoryczna. Metodyka. Ogólne Wytyczne Przeprowadzania Testów Hedonicznych z Konsumentami na Obszarze Kontrolowanym. Polish Committee for Standardization: Warszawa, Poland, 2017. (In Polish)
- PN-EN ISO 5492:2009; Analiza Sensoryczna. Terminologia. Polish Committee for Standardization: Warszawa, Poland, 2009. (In Polish)
- Chmiel, M.; Slowinski, M.; Dasiewicz, K.; Florowski, T. Application of a computer vision system to classify beef as normal or dark, firm, and dry. J. Anim. Sci. 2012, 90, 4126–4130. [Google Scholar] [CrossRef]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between meat color of DFD beef and other quality attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef]
- Tkacz, K.; Modzelewska-Kapituła, M.; Petracci, M.; Zduńczyk, W. Improving the quality of sous-vide beef from Holstein-Friesian bulls by different marinades. Meat Sci. 2021, 182, 108639. [Google Scholar] [CrossRef]
- Li, Z.; He, Q.; Lai, J.; Lin, J.; Wu, S.; Guo, Z.; Zheng, H. Effect of stepwise cooking on the water-retention capacity and protein denaturation degree of chicken breast. Int. J. Gastron. Food Sci. 2024, 38, 101012. [Google Scholar] [CrossRef]
- Chinzorig, O.; Hwang, I. Mechanical texture profile of Hanwoo muscles as a function of heating temperatures. J. Anim. Sci. Technol. 2018, 60, 22. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Calkins, C.R. Ranking beef muscles for Warner–Bratzler shear force and trained sensory panel ratings from Published literature. J. Food Qual. 2011, 34, 195–203. [Google Scholar] [CrossRef]
- Panea, B.; Astiz, C.S.; Olleta, J.; Civit, D. Effect of ageing method, ageing period, cooking method and sample thickness on beef textural characteristics. Span. J. Agric. Res. 2008, 6, 25–32. [Google Scholar] [CrossRef]
- Ishiwatari, N.; Fukuoka, M.; Sakai, N. Effect of protein denaturation degree on texture and water state of cooked meat. J. Food Eng. 2013, 117, 361–369. [Google Scholar] [CrossRef]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.U.; De Palo, P.; Korma, S.A. Dynamic alterations in protein, sensory, chemical, and oxidative properties occurring in meat during thermal and non-thermal processing techniques: A comprehensive review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef]
- Moczkowska, M.; Półtorak, A.; Wierzbicka, A. The effect of ageing on changes in myofibrillar protein in selected muscles in relation to the tenderness of meat obtained from cross-breed heifers. Int. J. Food Sci. Technol. 2017, 52, 1375–1382. [Google Scholar] [CrossRef]
- Barbera, S.; Tassone, S. Meat cooking shrinkage: Measurement of a new meat quality parameter. Meat Sci. 2006, 73, 467–474. [Google Scholar] [CrossRef]
- Modzelewska-Kapituła, M.; Tkacz, K.; Zduńczyk, W.; Ozturk-Kerimoglu, B.; Nogalski, Z. Is Prolonged Ageing a Necessity for Improving the Quality of Sous-Vide Cooked Beef? Appl. Sci. 2024, 14, 5180. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, X.; Li, X.; Wang, W.; Wang, S.; Pan, J.; Dong, X.; Li, S. Effect of cooking temperatures on meat quality, protein carbonylation and protein crosslinking of beef packed in high oxygen atmosphere. LWT-Food Sci. Technol. 2022, 154, 112633. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, Y.; Luo, X.; Zhang, Y.M.; Zhu, L.X.; Xu, B.C.; Hopkins, D.L.; Liang, R.R. Influence of oxygen concentration on the fresh and internal cooked color of modified atmosphere packaged dark-cutting beef stored under chilled and superchilled conditions. Meat Sci. 2022, 188, 108773. [Google Scholar] [CrossRef]
- Beyer, E.S.; Farmer, K.J.; Kidwell, E.G.; Davis, S.G.; Harr, K.M.; Chao, M.D.; Zumbaugh, M.D.; Vipham, J.L.; Hunt, M.C.; O’Quinn, T.G. Change in Myoglobin Denaturation and Physiochemical Properties Among Three Degrees of Doneness and Three Beef Whole Muscles. Meat Muscle Biol. 2024, 8, 16919. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2002, 71, 100–121. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Stanisławczyk, R.; Duma-Kocan, P. Effect of Traditional Cooking and Sous Vide Heat Treatment, Cold Storage Time and Muscle on Physicochemical and Sensory Properties of Beef Meat. Molecules 2022, 27, 7307. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, Z.B.; Thomson, P.C.; Ha, M.; Campbell, M.A.; McGill, D.M.; Friend, M.A.; Warner, R.D. Effect of sous vide cooking and ageing on tenderness and water-holding capacity of low-value beef muscles from young and older animals. Meat Sci. 2021, 175, 108435. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Mean ± SD |
|---|---|
| pH | 5.69 ± 0.25 |
| Water, % | 75.51 ± 1.08 |
| Fat, % | 1.36 ± 0.38 |
| Protein, % | 22.89 ± 0.86 |
| Total connective tissue, % | 1.34 ± 0.39 |
| L* | 49.85 ± 3.18 |
| a* | 23.93 ± 2.63 |
| b* | 12.58 ± 2.12 |
| HT Method | CT (Min) | CL (%) | WBSF (N) |
|---|---|---|---|
| CON | 47.24 ± 3.54 | 37.12 ± 2.56 e | 44.29 ± 3.45 d |
| SV55 | 360 * | 22.55 ± 2.84 b | 26.47 ± 3.18 a |
| SV65 | 240 * | 26.28 ± 3.08 c | 28.26 ± 3.85 ab |
| SV75 | 180 * | 31.19 ± 2.87 d | 32.84 ± 3.26 c |
| ΔT20 | 135.25 ± 8.28 | 18.78 ± 3.12 a | 29.65 ± 2.98 b |
| ΔT40 | 82.21 ± 5.28 | 19.54 ± 3.28 a | 33.68 ± 3.77 c |
| R75 | 116.22 ± 11.26 | 21.32 ± 2.49 ab | 29.84 ± 2.46 b |
| HT Method | PC-S | CC-S | ΔE | |||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |||
| CON | 56.54 a, Y | 5.34 a, X | 8.26 a, X | 53.33 a, X | 8.24 a, Y | 9.16 a, X | 4.48 f | |
| SD | ±0.66 | ±0.84 | ±0.58 | ±1.43 | ±0.41 | ±0.32 | ±0.64 | |
| SV55 | 57.12 ab, X | 20.01 f, X | 11.58 d, X | 56.56 b, X | 20.42 e, X | 12.15 c, X | 0.86 a | |
| SD | ±1.84 | ±0.46 | ±0.82 | ±2.19 | ±0.83 | ±0.65 | ±0.28 | |
| SV65 | 58.84 ab, X | 15.83 d, X | 10.11 c, X | 57.94 b, X | 16.27 c, X | 10.98 b, X | 1.35 b | |
| SD | ±1.01 | ±0.59 | ±1.02 | ±1.67 | ±1.21 | ±0.38 | ±0.49 | |
| SV75 | 62.68 c, X | 8.12 b, X | 9.22 b, X | 61.15 d, X | 9.03 b, Y | 9.88 a, X | 1.97 c | |
| SD | ±1.18 | ±0.73 | ±0.26 | ±1.04 | ±0.41 | ±0.38 | ±0.35 | |
| ΔT20 | 59.75 b, X | 19.12 e, X | 10.28 c, X | 59.29 c, X | 19.57 e, X | 11.12 b, X | 1.49 b | |
| SD | ±0.49 | ±0.95 | ±0.46 | ±0.85 | ±0.59 | ±0.38 | ±0.38 | |
| ΔT40 | 62.22 c, X | 13.14 c, X | 11.24 d, X | 61.98 d, X | 15.52 c, Y | 12.54 c, Y | 2.68 e | |
| SD | ±1.08 | ±0.49 | ±0.75 | ±1.47 | ±1.06 | ±0.43 | ±0.47 | |
| R75 | 60.83 b, X | 16.21 d, X | 12.02 e, X | 60.75 cd, X | 18.32 d, Y | 12.81 c, X | 2.29 d | |
| SD | ±1.15 | ±0.94 | ±0.44 | ±1.22 | ±0.29 | ±0.47 | ±0.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyrwisz, J.; Moczkowska-Wyrwisz, M.; Kurek, M. The Effect of Low-Temperature Heat Treatment on the Physicochemical Properties of Bovine Semitendinosus Muscle. Appl. Sci. 2025, 15, 4146. https://doi.org/10.3390/app15084146
Wyrwisz J, Moczkowska-Wyrwisz M, Kurek M. The Effect of Low-Temperature Heat Treatment on the Physicochemical Properties of Bovine Semitendinosus Muscle. Applied Sciences. 2025; 15(8):4146. https://doi.org/10.3390/app15084146
Chicago/Turabian StyleWyrwisz, Jarosław, Małgorzata Moczkowska-Wyrwisz, and Marcin Kurek. 2025. "The Effect of Low-Temperature Heat Treatment on the Physicochemical Properties of Bovine Semitendinosus Muscle" Applied Sciences 15, no. 8: 4146. https://doi.org/10.3390/app15084146
APA StyleWyrwisz, J., Moczkowska-Wyrwisz, M., & Kurek, M. (2025). The Effect of Low-Temperature Heat Treatment on the Physicochemical Properties of Bovine Semitendinosus Muscle. Applied Sciences, 15(8), 4146. https://doi.org/10.3390/app15084146







