Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Allergen Preparations
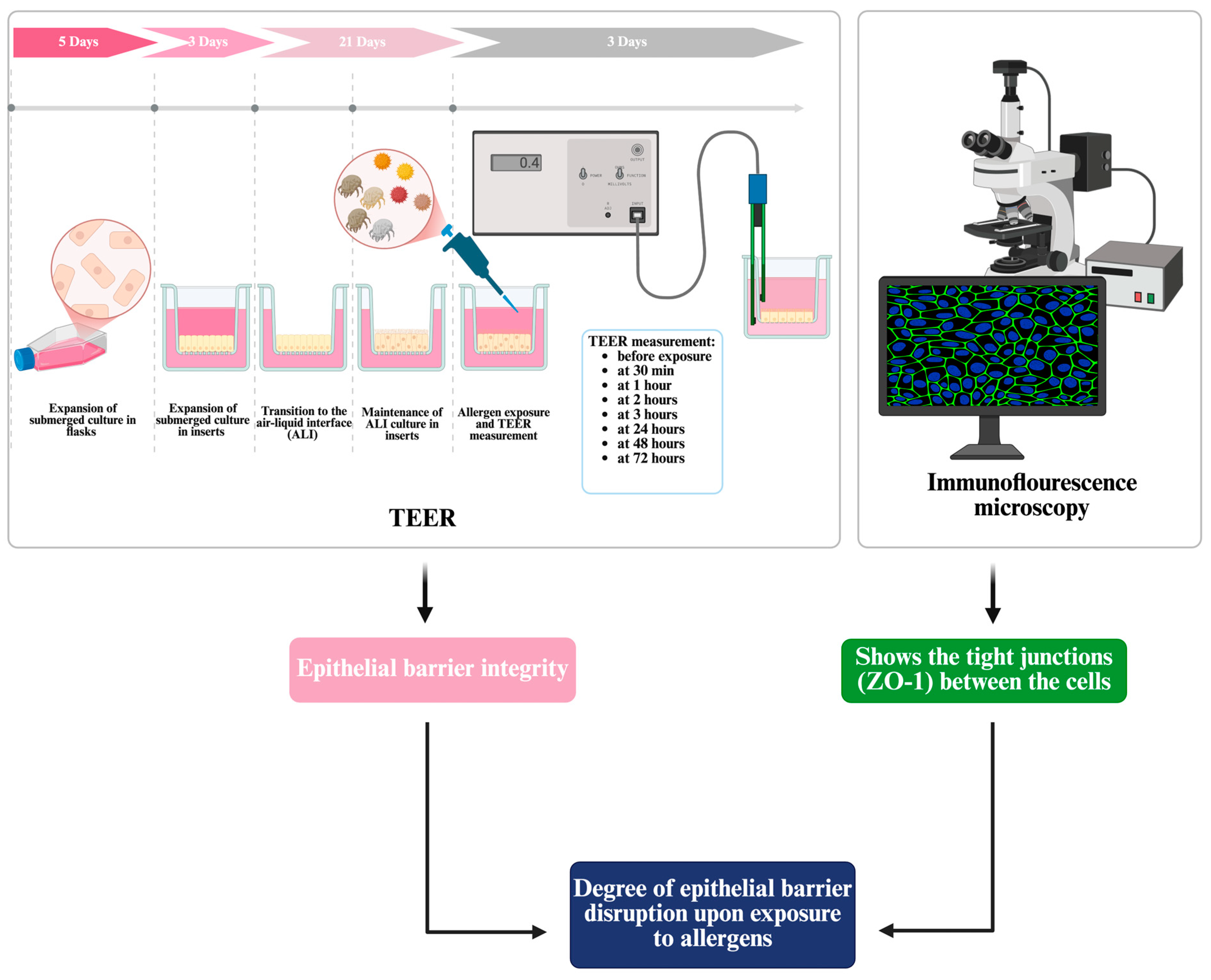
2.2. Culture of Differentiated Bronchial Epithelium at Air–Liquid Interface
2.3. Transepithelial Electrical Resistance (TEER) Measurement for the Assessment of Epithelial Barrier Integrity and Allergen-Induced Responses
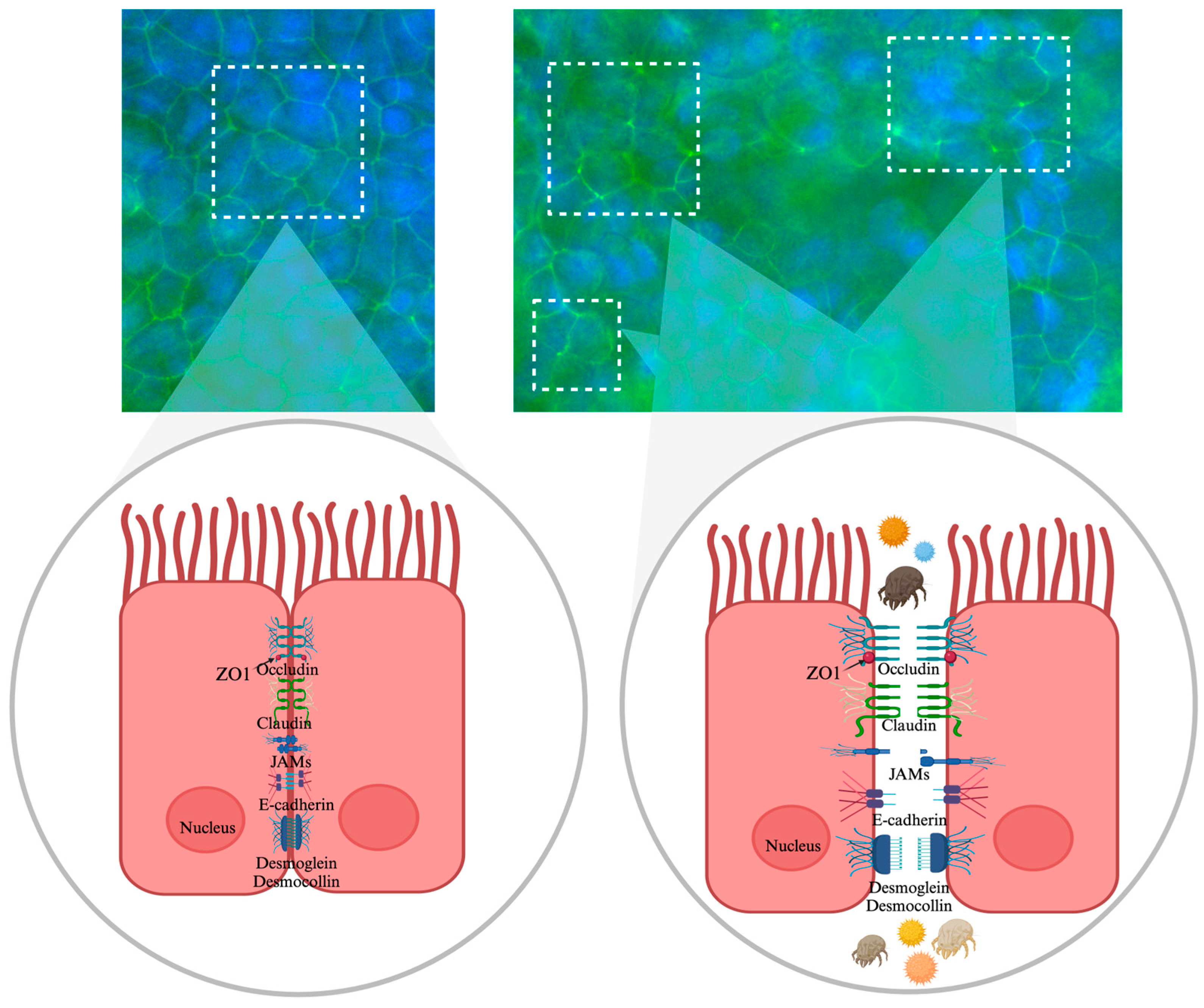
2.4. Assessment of Tight Junction Integrity Using Immunofluorescence with ZO-1
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Revealing the Impact of Allergen Exposure on Epithelial Barrier Integrity Through TEER Monitoring
3.2. Visualization of Tight Junction Integrity Through ZO-1 Immunofluorescence Imaging Following Allergen Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, S.; Nusrat, A. The Life and Death of Epithelia During Inflammation: Lessons Learned from the Gut. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 35–60. [Google Scholar] [CrossRef]
- Calvén, J.; Ax, E.; Rådinger, M. The Airway Epithelium—A Central Player in Asthma Pathogenesis. IJMS 2020, 21, 8907. [Google Scholar] [CrossRef]
- Haidar, L.; Bănărescu, C.F.; Uța, C.; Moldovan, S.I.; Zimbru, E.-L.; Zimbru, R.-I.; Ciurariu, E.; Georgescu, M.; Panaitescu, C. Pollen–Food Allergy Syndrome: Allergens, Clinical Insights, Diagnostic and Therapeutic Challenges. Appl. Sci. 2024, 15, 66. [Google Scholar] [CrossRef]
- Beerweiler, C.C.; Masanetz, R.K.; Schaub, B. Asthma and allergic diseases: Cross talk of immune system and environmental factors. Eur. J. Immunol. 2023, 53, 2249981. [Google Scholar] [CrossRef]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. A short guide to the tight junction. J. Cell Sci. 2024, 137, jcs261776. [Google Scholar] [CrossRef]
- Goktas, P.; Damadoglu, E. Future of allergy and immunology. Ann. Allergy Asthma Immunol. 2024, 134, 396–407. [Google Scholar] [CrossRef]
- Martens, K.; Hellings, P.W.; Steelant, B. Calu-3 epithelial cells exhibit different immune and epithelial barrier responses from freshly isolated primary nasal epithelial cells in vitro. Clin. Transl. Allergy 2018, 8, 40. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Heijink, I.H.; Kuchibhotla, V.N.S.; Roffel, M.P.; Maes, T.; Knight, D.A.; Sayers, I.; Nawijn, M.C. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020, 75, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska, D.; Bogawski, P.; Piotrowicz, K.; Bosiacka, B.; Grinn-Gofroń, A.; Berger, U.E.; Bonini, M.; Ceriotti, V.; Charalampopoulos, A.; Galán, C.; et al. Co-exposure to highly allergenic airborne pollen and fungal spores in Europe. Sci. Total Environ. 2023, 905, 167285. [Google Scholar] [CrossRef] [PubMed]
- Prodić, I.; Minić, R.; Stojadinović, M. The influence of environmental pollution on the allergenic potential of grass pollen. Aerobiologia 2024, 41, 3–16. [Google Scholar] [CrossRef]
- Chen, K.-W.; Marusciac, L.; Tamas, P.T.; Valenta, R.; Panaitescu, C. Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe. Int. Arch. Allergy Immunol. 2018, 176, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Zimbru, R.-I.; Zimbru, E.-L.; Ordodi, V.-L.; Bojin, F.-M.; Crîsnic, D.; Grijincu, M.; Mirica, S.-N.; Tănasie, G.; Georgescu, M.; Huțu, I.; et al. The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats. IJMS 2024, 25, 8868. [Google Scholar] [CrossRef]
- Buzan, M.; Zbîrcea, L.; Gattinger, P.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K. Complex IgE sensitization patterns in ragweed allergic patients: Implications for diagnosis and specific immunotherapy. Clin. Transl. All. 2022, 12, e12179. [Google Scholar] [CrossRef]
- Runswick, S.; Mitchell, T.; Davies, P.; Robinson, C.; Garrod, D.R. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007, 12, 834–842. [Google Scholar] [CrossRef]
- Zbîrcea, L.-E.; Buzan, M.-R.; Grijincu, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Relationship between IgE Levels Specific for Ragweed Pollen Extract, Amb a 1 and Cross-Reactive Allergen Molecules. IJMS 2023, 24, 4040. [Google Scholar] [CrossRef]
- Ogi, K.; Ramezanpour, M.; Liu, S.; Ferdoush Tuli, J.; Bennett, C.; Suzuki, M.; Fujieda, S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Der p 1 Disrupts the Epithelial Barrier and Induces IL-6 Production in Patients With House Dust Mite Allergic Rhinitis. Front. Allergy 2021, 2, 692049. [Google Scholar] [CrossRef]
- Grijincu, M.; Huțu, I.; Weber, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Physicochemical and immunological characterization of Amb a 12, a novel ragweed (Ambrosia artemisiifolia) pollen allergen. Mol. Immunol. 2023, 157, 18–29. [Google Scholar] [CrossRef]
- Lin, J.; Huang, N.; Li, J.; Liu, X.; Xiong, Q.; Hu, C.; Chen, D.; Guan, L.; Chang, K.; Li, D.; et al. Cross-reactive antibodies against dust mite-derived enolase induce neutrophilic airway inflammation. Eur. Respir. J. 2021, 57, 1902375. [Google Scholar] [CrossRef]
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Rezaee, F.; Georas, S.N. Breaking Barriers. New Insights into Airway Epithelial Barrier Function in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2014, 50, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Abu Khweek, A.; Kim, E.; Joldrichsen, M.R.; Amer, A.O.; Boyaka, P.N. Insights Into Mucosal Innate Immune Responses in House Dust Mite-Mediated Allergic Asthma. Front. Immunol. 2020, 11, 534501. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed]
- De Lagarde, V.M.; Chevalier, L.; Méausoone, C.; Cazier, F.; Dewaele, D.; Cazier-Dennin, F.; Janona, M.; Logie, C.; Achard, S.; André, V.; et al. Acute and repeated exposures of normal human bronchial epithelial (NHBE) cells culture to particles from a coloured pyrotechnic smoke. Environ. Toxicol. Pharmacol. 2024, 105, 104327. [Google Scholar] [CrossRef] [PubMed]
- Vinhas, R.; Cortes, L.; Cardoso, I.; Mendes, V.M.; Manadas, B.; Todo-Bom, A.; Pires, E.; Veríssimo, P. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides: Role of pollen proteases on allergic disorders. Allergy 2011, 66, 1088–1098. [Google Scholar] [CrossRef]
- Gaspar, R.; De Matos, M.R.; Cortes, L.; Nunes-Correia, I.; Todo-Bom, A.; Pires, E.; Veríssimo, P. Pollen Proteases Play Multiple Roles in Allergic Disorders. IJMS 2020, 21, 3578. [Google Scholar] [CrossRef]
- Zimbru, E.-L.; Zimbru, R.-I.; Ordodi, V.-L.; Bojin, F.-M.; Crîsnic, D.; Andor, M.; Mirica, S.-N.; Huțu, I.; Tănasie, G.; Haidar, L.; et al. Rosuvastatin Attenuates Vascular Dysfunction Induced by High-Fructose Diets and Allergic Asthma in Rats. Nutrients 2024, 16, 4104. [Google Scholar] [CrossRef]
- Movia, D.; Bruni-Favier, S.; Prina-Mello, A. In vitro Alternatives to Acute Inhalation Toxicity Studies in Animal Models—A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 549. [Google Scholar] [CrossRef]
- Blume, C.; Swindle, E.J.; Gilles, S.; Traidl-Hoffmann, C.; Davies, D.E. Low molecular weight components of pollen alter bronchial epithelial barrier functions. Tissue Barriers 2015, 3, e1062316. [Google Scholar] [CrossRef]
- Eisenhut, M. Reduction of Alveolar Epithelial Ion and Fluid Transport by Inflammatory Mediators. Am. J. Respir. Cell Mol. Biol. 2007, 36, 388–389. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Richter, K.; Fronius, M. Ion Transport by Pulmonary Epithelia. BioMed Res. Int. 2011, 2011, 174306. [Google Scholar] [CrossRef]
- Radbel, J.; Laskin, D.L.; Laskin, J.D.; Kipen, H.M. Disease-modifying treatment of chemical threat agent–induced acute lung injury. Ann. N. Y. Acad. Sci. 2020, 1480, 14–29. [Google Scholar] [CrossRef]
- Sharma, M.; Huber, E.; Arnesdotter, E.; Behrsing, H.P.; Bettmann, A.; Brandwein, D.; Constant, S.; Date, R.; Deshpande, A.; Fabian, E.; et al. Minimum information for reporting on the TEER (trans-epithelial/endothelial electrical resistance) assay (MIRTA). Arch. Toxicol. 2025, 99, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.J.; Zihni, C.; Krug, S.M.; Maraspini, R.; Otani, T.; Furuse, M.; Honigmann, A.; Balda, M.S.; Matter, K. ZO-1 Guides Tight Junction Assembly and Epithelial Morphogenesis via Cytoskeletal Tension-Dependent and -Independent Functions. Cells 2022, 11, 3775. [Google Scholar] [CrossRef] [PubMed]
- Steelant, B.; Seys, S.F.; Boeckxstaens, G.; Akdis, C.A.; Ceuppens, J.L.; Hellings, P.W. Restoring airway epithelial barrier dysfunction: A new therapeutic challenge in allergic airway disease. Rhin 2016, 54, 195–205. [Google Scholar] [CrossRef]
- Yazici, D.; Ogulur, I.; Pat, Y.; Babayev, H.; Barletta, E.; Ardicli, S.; Bel Imam, M.; Huang, M.; Koch, J.; Li, M.; et al. The epithelial barrier: The gateway to allergic, autoimmune, and metabolic diseases and chronic neuropsychiatric conditions. Semin. Immunol. 2023, 70, 101846. [Google Scholar] [CrossRef]
- Tamaș, T.-P.; Buzan, M.-R.; Zbîrcea, L.-E.; Cotarcă, M.-D.; Grijincu, M.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Ragweed Major Allergen Amb a 11 Recombinant Production and Clinical Implications. Biomolecules 2023, 13, 182. [Google Scholar] [CrossRef]
- Buzan, M.-R.; Grijincu, M.; Zbîrcea, L.-E.; Haidar, L.; Tamaș, T.-P.; Cotarcă, M.-D.; Tănasie, G.; Weber, M.; Babaev, E.; Stolz, F.; et al. Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen. IJMS 2024, 25, 5175. [Google Scholar] [CrossRef]
- Würtzen, P.A.; Hoof, I.; Christensen, L.H.; Váczy, Z.; Henmar, H.; Salamanca, G.; Lundegaard, C.; Lund, L.; Kráľova, T.; Brooks, E.G.; et al. Diverse and highly cross-reactive T-cell responses in ragweed allergic patients independent of geographical region. Allergy 2020, 75, 137–147. [Google Scholar] [CrossRef]
- Casset, A.; Mari, A.; Purohit, A.; Resch, Y.; Weghofer, M.; Ferrara, R.; Thomas, W.R.; Alessandri, C.; Chen, K.-W.; De Blay, F.; et al. Varying Allergen Composition and Content Affects the in vivo Allergenic Activity of Commercial Dermatophagoides pteronyssinus Extracts. Int. Arch. Allergy Immunol. 2012, 159, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Wang, J.; Xu, X.; Xiang, M.; Dou, J. PACAP38 improves airway epithelial barrier destruction induced by house dust mites allergen. Immunobiology 2019, 224, 758–764. [Google Scholar] [CrossRef]
- Dong, H.; Le, Y.; Wang, Y.; Zhao, H.; Huang, C.; Hu, Y.; Luo, L.; Wan, X.; Wei, Y.; Chu, Z.; et al. Extracellular heat shock protein 90α mediates HDM-induced bronchial epithelial barrier dysfunction by activating RhoA/MLC signaling. Respir. Res. 2017, 18, 111. [Google Scholar] [CrossRef]
- Post, S.; Nawijn, M.C.; Hackett, T.L.; Baranowska, M.; Gras, R.; Van Oosterhout, A.J.M.; Heijink, I.H. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax 2012, 67, 488–495. [Google Scholar] [CrossRef]
- Matsumura, Y. Role of Allergen Source-Derived Proteases in Sensitization via Airway Epithelial Cells. J. Allergy 2012, 2012, 903659. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. Interactions of airway epithelium with protease allergens in the allergic response: Interactions of airway epithelium with protease allergens. Clin. Exp. Allergy 2011, 41, 305–311. [Google Scholar] [CrossRef]
- Takai, T.; Ikeda, S. Barrier Dysfunction Caused by Environmental Proteases in the Pathogenesis of Allergic Diseases. Allergol. Int. 2011, 60, 25–35. [Google Scholar] [CrossRef]
- Lu, H.-F.; Zhou, Y.-C.; Yang, L.-T.; Zhou, Q.; Wang, X.-J.; Qiu, S.-Q.; Cheng, B.-H.; Zeng, X.-H. Involvement and repair of epithelial barrier dysfunction in allergic diseases. Front. Immunol. 2024, 15, 1348272. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Reidel, B.; Nelson, M.R.; Macdonald, J.K.; Kesimer, M.; Randell, S.H. Air-Liquid interface cultures to model drug delivery through the mucociliary epithelial barrier. Adv. Drug Deliv. Rev. 2023, 198, 114866. [Google Scholar] [CrossRef]
- Barron, S.L.; Wyatt, O.; O’Connor, A.; Mansfield, D.; Suzanne Cohen, E.; Witkos, T.M.; Strickson, S.; Owens, R.M. Modelling bronchial epithelial-fibroblast cross-talk in idiopathic pulmonary fibrosis (IPF) using a human-derived in vitro air liquid interface (ALI) culture. Sci. Rep. 2024, 14, 240. [Google Scholar] [CrossRef]
- Raby, K.L.; Michaeloudes, C.; Tonkin, J.; Chung, K.F.; Bhavsar, P.K. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front. Immunol. 2023, 14, 1201658. [Google Scholar] [CrossRef]
- Runft, S.; Färber, I.; Krüger, J.; Schöne, K.; Lehmbecker, A.; Baumgärtner, W. In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology. IJMS 2023, 24, 4987. [Google Scholar] [CrossRef]
- Leach, T.; Gandhi, U.; Reeves, K.D.; Stumpf, K.; Okuda, K.; Marini, F.C.; Walker, S.J.; Boucher, R.; Chan, J.; Cox, L.A.; et al. Development of a novel air–liquid interface airway tissue equivalent model for in vitro respiratory modeling studies. Sci. Rep. 2023, 13, 10137. [Google Scholar] [CrossRef]
- Ghio, A.J.; Dailey, L.A.; Soukup, J.M.; Stonehuerner, J.; Richards, J.H.; Devlin, R.B. Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part. Fibre Toxicol. 2013, 10, 25. [Google Scholar] [CrossRef]
- Waltl, E.E.; Selb, R.; Eckl-Dorna, J.; Mueller, C.A.; Cabauatan, C.R.; Eiwegger, T.; Resch-Marat, Y.; Niespodziana, K.; Vrtala, S.; Valenta, R.; et al. Betamethasone prevents human rhinovirus- and cigarette smoke- induced loss of respiratory epithelial barrier function. Sci. Rep. 2018, 8, 9688. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Gremmer, E.R.; Bannuscher, A.; Drasler, B.; Keshavan, S.; Rothen-Rutishauser, B.; Birk, B.; Verlohner, A.; Landsiedel, R.; Meldrum, K.; et al. Transferability and reproducibility of exposed air-liquid interface co-culture lung models. NanoImpact 2023, 31, 100466. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Invest. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Steelant, B.; Farré, R.; Wawrzyniak, P.; Belmans, J.; Dekimpe, E.; Vanheel, H.; Van Gerven, L.; Kortekaas Krohn, I.; Bullens, D.M.A.; Ceuppens, J.L.; et al. Impaired barrier function in patients with house dust mite–induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J. Allergy Clin. Immunol. 2016, 137, 1043–1053.e5. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ichikawa, T.; Numakura, T.; Yamada, M.; Koarai, A.; Fujino, N.; Murakami, K.; Yamanaka, S.; Sasaki, Y.; Kyogoku, Y.; et al. PGC-1α regulates airway epithelial barrier dysfunction induced by house dust mite. Respir. Res. 2021, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.G.; Lloyd, C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011, 32, 402–411. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Li, P.; Pang, K.; Liu, H.; Tian, L. Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases. Int. Arch. Allergy Immunol. 2023, 184, 481–501. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Gon, Y.; Hashimoto, S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol. Int. 2018, 67, 12–17. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Sun, L.; Ong, H.H.; Ye, J.; Xu, Y.; Wang, D. Updated epithelial barrier dysfunction in chronic rhinosinusitis: Targeting pathophysiology and treatment response of tight junctions. Allergy 2024, 79, 1146–1165. [Google Scholar] [CrossRef]
- Amison, R.T.; Page, C.P. Novel pharmacological therapies for the treatment of bronchial asthma. Minerva Med. 2022, 113, 31–50. [Google Scholar] [CrossRef]
- Chauhan, B.; Gupta, M.; Chauhan, K. Role of Antioxidants on the Clinical Outcome of Patients with Perennial Allergic Rhinitis. Allergy Rhinol. 2016, 7, ar.2016.7.0163. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Nadeem, A.; Al-Harbi, M.M.; Imam, F.; Al-Shabanah, O.A.; Ahmad, S.F.; Sayed-Ahmed, M.M.; Bahashwan, S.A. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int. Immunopharmacol. 2015, 26, 237–245. [Google Scholar] [CrossRef]
- Mansur, A.H.; Gonem, S.; Brown, T.; Burhan, H.; Chaudhuri, R.; Dodd, J.W.; Pantin, T.; Gore, R.; Jackson, D.; Menzies-Gow, A.; et al. Biologic therapy practices in severe asthma; outcomes from the UK Severe Asthma Registry and survey of specialist opinion. Clin. Exp. Allergy 2023, 53, 173–185. [Google Scholar] [CrossRef]
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751. [Google Scholar] [CrossRef]
- Rayner, R.E.; Wellmerling, J.; Makena, P.; Zhao, J.; Prasad, G.L.; Cormet-Boyaka, E. Transcriptomic Response of Primary Human Bronchial Cells to Repeated Exposures of Cigarette and ENDS Preparations. Cell Biochem. Biophys. 2022, 80, 217–228. [Google Scholar] [CrossRef] [PubMed]

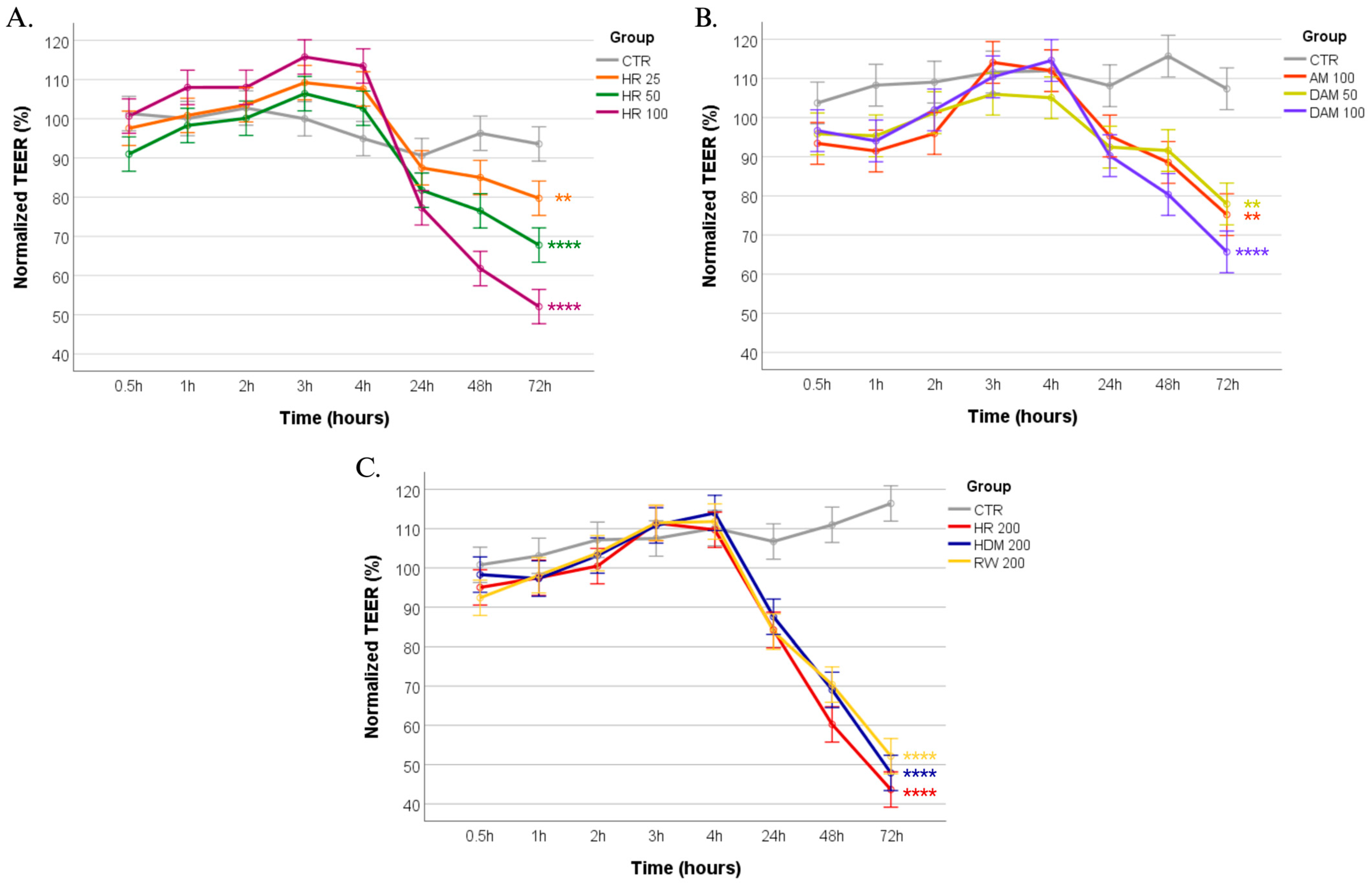

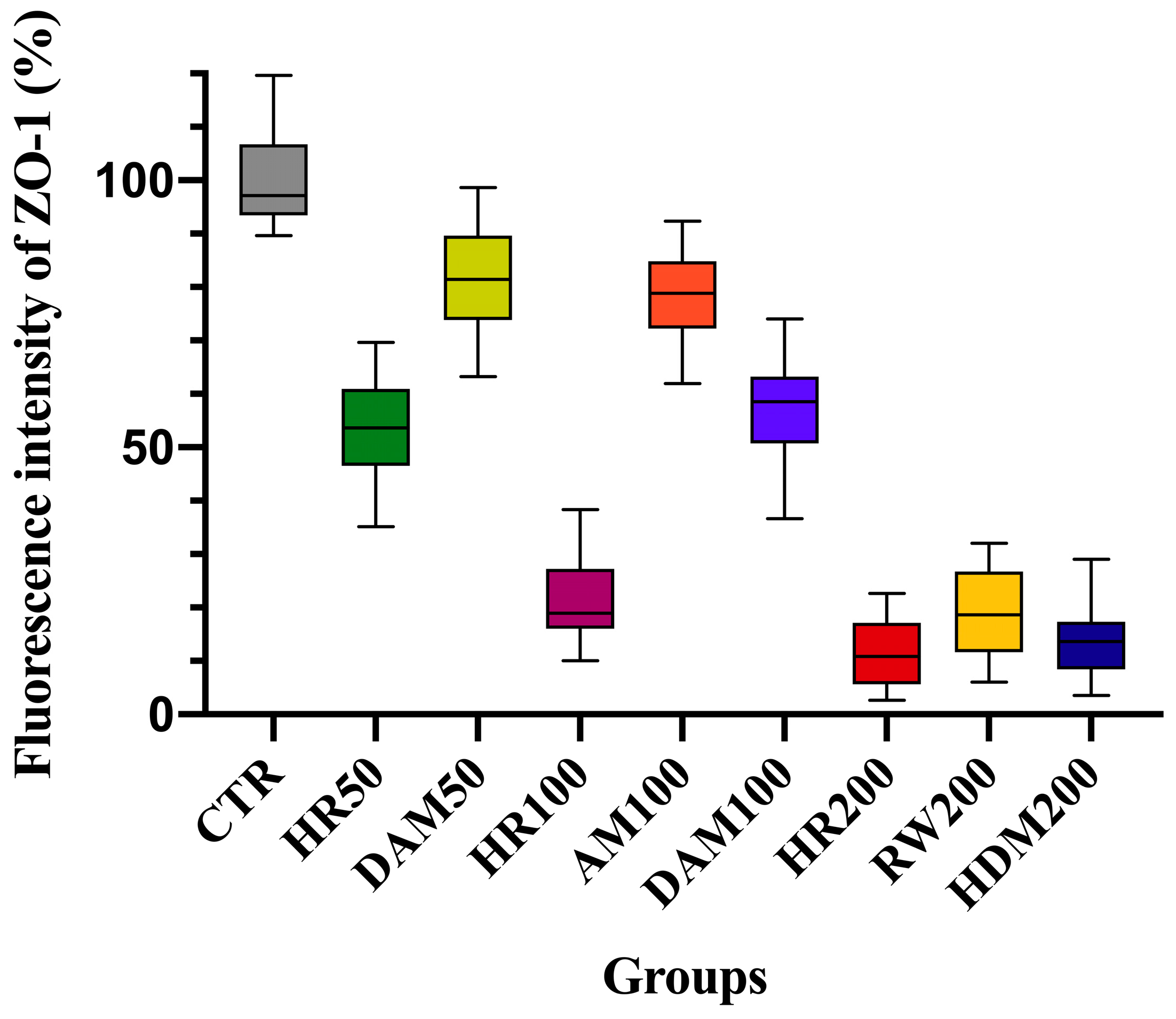
| Exposure Condition | Changes in Normalized TEER (%) at 72 h | Tight Junction Integrity (ZO-1 IF Staining *) | Implications for Airway Health |
|---|---|---|---|
| Control (no allergen) | 105.85% ± 6.19% | 0: Intact ZO-1 | Normal epithelial integrity |
| AM 100 (100 µg/mL) | 74.15% ± 6.14% | 1: Mild ZO-1 disruption | Mild barrier dysfunction, increased permeability |
| DAM 50 (50 µg/mL) | 76.54% ± 4.17% | 1: Mild ZO-1 disruption | Mild barrier dysfunction, increased permeability |
| DAM 100 (100 µg/mL) | 64.95% ± 5.05% | 2: Moderate ZO-1 disruption | Enhanced permeability, potential for allergen penetration |
| RW 200 (200 µg/mL) | 52.14% ± 4.88% | 3: Severe ZO-1 disruption | Marked permeability, greater potential for allergen penetration and for exacerbated allergic response |
| HDM 200 (200 µg/mL) | 47.87% ± 3.91% | 3: Severe ZO-1 disruption | Marked permeability, greater potential for allergen penetration and for exacerbated allergic response |
| HR 50 (RW + HDM 50 µg/mL) | 68.81% ± 3.27% | 2: Moderate ZO-1 disruption | Enhanced permeability, potential for allergen penetration |
| HR 100 (RW + HDM 100 µg/mL) | 51.86% ± 4.98% | 3: Severe ZO-1 disruption | Marked permeability, greater potential for allergen penetration and for exacerbated allergic response |
| HR 200 (RW + HDM 200 µg/mL) | 44.18% ± 6.19% | 3: Severe ZO-1 disruption | Marked permeability, greater potential for allergen penetration and for exacerbated allergic response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimbru, R.-I.; Grijincu, M.; Tănasie, G.; Zimbru, E.-L.; Bojin, F.-M.; Buzan, R.-M.; Tamaș, T.-P.; Cotarcă, M.-D.; Harich, O.O.; Pătrașcu, R.; et al. Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium. Appl. Sci. 2025, 15, 4113. https://doi.org/10.3390/app15084113
Zimbru R-I, Grijincu M, Tănasie G, Zimbru E-L, Bojin F-M, Buzan R-M, Tamaș T-P, Cotarcă M-D, Harich OO, Pătrașcu R, et al. Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium. Applied Sciences. 2025; 15(8):4113. https://doi.org/10.3390/app15084113
Chicago/Turabian StyleZimbru, Răzvan-Ionuț, Manuela Grijincu, Gabriela Tănasie, Elena-Larisa Zimbru, Florina-Maria Bojin, Roxana-Maria Buzan, Tudor-Paul Tamaș, Monica-Daniela Cotarcă, Octavia Oana Harich, Raul Pătrașcu, and et al. 2025. "Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium" Applied Sciences 15, no. 8: 4113. https://doi.org/10.3390/app15084113
APA StyleZimbru, R.-I., Grijincu, M., Tănasie, G., Zimbru, E.-L., Bojin, F.-M., Buzan, R.-M., Tamaș, T.-P., Cotarcă, M.-D., Harich, O. O., Pătrașcu, R., Haidar, L., Ciurariu, E., Marin, K. C., Păunescu, V., & Panaitescu, C. (2025). Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium. Applied Sciences, 15(8), 4113. https://doi.org/10.3390/app15084113








