Abstract
In this study, a straightforward and cost-effective HPLC-UV method was developed for the rapid determination of vitamin D2 and ergosterol in mushrooms. These bioactive components are known to play a significant role in the nutritional value of mushrooms, particularly in the production of mushroom-based food supplements. The method, designed for routine analysis, involves a simple sample preparation process combining saponification and solid–liquid extraction, followed by HPLC-UV detection. High recovery rates (97–99%) were achieved by the method, with limits of detection (LOD) and quantitation (LOQ) of 0.1 mg/kg dry weight and 0.5 mg/kg dry weight, respectively. The enrichment of vitamin D₂ content in mushroom powders through UV irradiation was also investigated. In Agaricus bisporus, vitamin D₂ levels increased from an initial 1.92 mg/kg to 4.66 mg/kg following heat treatment at 100 °C, and reached a maximum of 28.13 mg/kg when heat treatment was combined with UV irradiation. In contrast, Lentinula edodes exhibited an initial vitamin D₂ content of 7–8.5 mg/kg, with the highest levels achieved through UV treatment alone, which also preserved ergosterol content. These findings highlight species-specific differences in vitamin D₂ conversion and present an effective approach for enhancing the nutritional profile of mushroom-based products, while providing a reliable analytical tool for quality control.
1. Introduction
White button (Agaricus bisporus) and shiitake (Lentinula edodes) mushrooms are two of the most popular and most widely grown mushrooms in the world. According to Royse et al. [1], the genus Lentinula contributes 22% of the world’s cultivated mushroom industry, while Agaricus accounts for 15% of production. Numerous scientific studies have been conducted on the nutritional value and medicinal benefits of shiitake mushrooms, increasing interest in their consumption and, consequently, their production [2]. At the turn of the millennium, China dominated global shiitake production and has since accounted for 95% of the world’s Lentinula edodes output. As a result, shiitake became the most popular cultivated mushroom, overtaking the white button mushroom in popularity. The production of Agaricus bisporus is also most prominent in China; however, its growth rate is less intense compared to shiitake or certain exotic mushroom species. Nonetheless, China remains the world’s largest Agaricus bisporus producer [2]. However, this species is widely produced globally and remains a preferred choice in Western countries.
While mushrooms are rich in several essential macronutrients, they also contain biologically active compounds, making them excellent food sources. In addition to their high protein content, mushrooms can serve as valuable sources of vitamin D2 and ergosterol [3,4]. Since plants do not contain vitamin D, mushrooms are an important component of vegan nutrition. Although there are five types of vitamin D (numbered 2 to 6), the most important dietary forms are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) [5]. Both are produced from provitamin D sterols through UV exposure (Figure 1). One of these provitamins, ergosterol, found in fungi and yeast, promotes the formation of vitamin D2 [6], while 7-dehydrocholesterol, found in the skin, photolyzes into previtamin D3 [7].
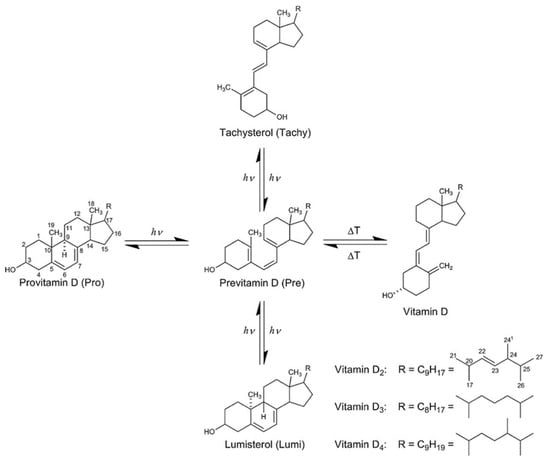
Figure 1.
Formation of vitamin D. Numbers indicate the conventional numbering of carbon atoms in the organic molecules.
UV-treated mushroom powders from A. bisporus have recently been approved as a source of vitamin D in various food products, including breakfast cereals, vegetable juices, calcium-fortified fruit juices, grain products, and plant- or soy-based products marketed as milk, cheese, or yogurt alternatives [8]. The photolysis of ergosterol by UV light synthesizes not only previtamin D2 but also tachysterol and lumisterol. According to Havinga et al. [9], 85% of ergosterol is converted to pre-ergocalciferol, while the remainder forms over-irradiation products. Wittig et al. [5] identified these over-irradiation products as irreversibly formed suprasterols derived from vitamin D2 and toxisterols derived from previtamin D2. Havinga et al. [9] further stated that prolonged irradiation of previtamin D results in a photochemical conversion to tachysterol and lumisterol, which remain in equilibrium with previtamin D. Vitamin D production can also be thermally initiated from previtamin D [6]. Studies have explored these equilibrium reactions and have sought optimal conditions for vitamin D2 production. Factors influencing vitamin D2 conversion in mushrooms include the wavelength of UV light, irradiation time, distance from the light source, temperature, and the processing conditions and methods applied to the mushrooms [10,11,12].
UV light is categorized into three subregions: UV-C (190–290 nm), UV-B (290-320 nm), and UV-A (320–400 nm). Several studies have utilized all three types of UV light in mushroom irradiation, yet no consensus has been reached on which subregion is most effective for previtamin D2 conversion. Teichmann et al. [13] found that the yield of vitamin D2 was highest with UV-B irradiation, but similar results were achieved with UV-C light. They identified UV-A radiation as the least effective. Contradictory findings have also been reported [14,15]. Wittig et al. [5] discovered that 295 nm (in the UV-B region) was the optimal wavelength for previtamin D2 conversion, aligning with the conclusions of Wu and Cardwell [16,17]. Regarding the yields of tachysterol and lumisterol, Hu et al. [14] reported that shorter wavelengths promoted tachysterol production, while longer wavelengths favored lumisterol formation. Despite these ambiguous results, UV-B light appears to produce the greatest amount of previtamin D2 with the least formation of byproducts when applied during mushroom irradiation. Since vitamin D2 synthesis from previtamin D2 is thermally initiated (Figure 1), higher conversion rates can be achieved by increasing the temperature [13,14,15,16].
Although prolonged irradiation up to 2 h can increase vitamin D₂ production, studies suggest that extended exposure may lead to photodegradation of vitamin D [15]. Optimizing the preparation of mushroom samples prior to UV irradiation is therefore critical. Jasinghe and Perera [15] investigated the effect of moisture content on vitamin D₂ conversion in shiitake mushrooms and found that the most efficient conversion occurs at a moisture content of 78–80%. Their study also highlighted that the surface exposure of the sample plays a significant role in conversion efficiency.
There is a demand from both academic and industrial sectors for an accurate and simple method for the analysis of vitamin D2 and its provitamin. The typical approach for the quantitation of vitamin D involves high-performance liquid chromatography with mass spectrometric detection (HPLC-MS), following alkaline saponification and cleanup steps prior to chromatography [18,19]. Given the complexity and cost of LC-MS instrumentation, HPLC-UV methods have also been developed recently for the rapid analysis of vitamin D2 [5,18,20].
While LC-MS is the most sensitive and selective technique, its high operational cost limits its accessibility for routine food industry applications. In contrast, HPLC-UV methods offer a more practical and cost-effective alternative, providing sufficient sensitivity and accuracy for vitamin D₂ analysis in mushroom-based food products. Previous studies have demonstrated the feasibility of HPLC-DAD for vitamin D₂ quantitation, but further optimization is needed to improve the method’s robustness and applicability in routine quality control.
In this paper, an accurate HPLC-DAD method was developed for the simultaneous analysis of vitamin D₂ and ergosterol. The method was validated in terms of linearity, precision, recovery, and detection limits to ensure its reliability for practical applications. Additionally, a straightforward UV irradiation method was established, involving the direct treatment of mushroom powders under controlled conditions. This approach aims to provide an optimized, scalable method for enhancing vitamin D₂ content in mushroom-based food products while ensuring a reliable analytical tool for its quantitation.
2. Materials and Methods
2.1. Chemicals and Standards
Cultivated mushrooms for the experiments were provided by Új Champignons Ltd., Budapest, Hungary. Vitamin D2 standard (>98.0% purity), vitamin D3 internal standard (>98.0% purity), and ergosterol standard were obtained from Sigma–Aldrich, Hamburg, Germany. HPLC-grade methanol, HPLC-grade water and other solvents were sourced from VWR International Ltd., Debrecen, Hungary.
2.2. Preparation of Dry Mushroom Powder
Fresh whole mushrooms were thoroughly washed under running water, then dried at an ambient temperature for seven days in perforated baskets, and placed in a dark, dry environment. Once dried, the mushrooms were cut into smaller pieces using a stainless-steel chopper (ZBP-7610, Zurrichberg, Shanghai, China) and ground into a fine powder using a grain mill (Widu 2 Electric Grain Mill, WIDU, Brockel, Germany).
2.3. UV Irradiation of Dry Mushroom Powder
For UV irradiation, 10 g of mushroom powder was treated using a UVC-15W-G13-254 nm germicidal lamp (Ledvance GmbH, Garching bei München, Germany). The lamp was installed in a custom-designed wooden box built specifically for irradiation experiments. Mushroom powders were evenly distributed in 95 mm Petri dishes and placed inside the irradiation box, maintaining a 20 cm distance between the samples and the light source. The irradiation was carried out for 10 min.
2.4. Heat Treatment of Dry Mushroom Powder
Mushroom powders were subjected to heat treatment in a laboratory drying cabinet (Heraeus, Hanau, Germany) at 100 °C for 10 h. Samples were collected at 0, 1, 2, 5, and 10 h to monitor changes in vitamin D₂ and ergosterol content.
2.5. Sample Preparation for HPLC Analysis
2.5.1. Saponification of the Sample
Initially, 500 mg of mushroom powder was placed into a screw-cap tube (VWR, Debrecen, Hungary). Subsequently, 4 mL of a 10% (w/w) potassium hydroxide (KOH) solution was added, and the mixture was vortexed until the sample became homogeneous. The tube was then heated at 80 °C for 1 h in a thermostatic bath (Huber, Offenburg, Germany), with vortex mixing every 15 min to ensure consistent saponification. Shaking was avoided to prevent foaming. This process facilitated the release of vitamin D2 and ergosterol from the cytosolic lipid particles.
2.5.2. Extraction of Vitamin D2 and Ergosterol
Following saponification, the sample was centrifuged at 4000 rpm for 5 min (Eppendorf Centrifuge 5702R, Eppendorf, Hamburg, Germany) to achieve phase separation. The upper layer was carefully transferred to a new screw-cap plastic tube.
For liquid–liquid extraction, 1 mL of distilled water, 2 mL of n-hexane, 1 mL of methanol, and an additional 1 mL of methanol containing a known concentration (10 µg/mL) of vitamin D3 as an internal standard were added. Vitamin D3 was deliberately introduced as an internal standard due to its structural similarity to vitamin D2 and its absence in mushrooms, allowing it to serve as a surrogate analyte for evaluating the efficiency of the extraction process [18]. The mixture was manually shaken vigorously for 1 min to ensure thorough mixing.
To aid phase separation, the mixture was centrifuged again at 4000 rpm for 1 min. The upper n-hexane layer, containing the extracted compounds, was carefully removed using a pipette and transferred to a 10 mL sample vial (VWR, Debrecen, Hungary). The n-hexane was then evaporated under a gentle stream of nitrogen gas until dryness, ensuring no wet or oily residue remained. This extraction process was repeated four times, each time adding 1 mL of n-hexane to the residual aqueous phase to maximize the recovery of vitamin D2 and ergosterol. After the final evaporation, the remaining residue was dissolved in 1 mL of methanol, mixed thoroughly using a vortex mixer, and transferred to an HPLC vial for subsequent analysis.
2.6. HPLC Analysis of Vitamin D2 and Ergosterol
Quantitative analysis of vitamin D2 and ergosterol was conducted using a Thermo Scientific Ultimate 3000 high-performance liquid chromatography (HPLC) system equipped with a diode array detector (Thermo Fisher Scientific, Waltham, MA, USA). Isocratic elution was performed on a Hypersil Gold 250 × 4.6 mm 5 μ (Thermo Scientific, Waltham, MA, USA) chromatographic column, utilizing a mobile phase composed of water and methanol in a 2:98 ratio. The flow rate was maintained at 1.5 mL/min, with the column temperature set at 40 °C. A 20 μL sample volume was injected in full-loop mode. Detection of vitamin D₂ and ergosterol was achieved at a wavelength of 265 nm. Data acquisition and processing were managed using Chromeleon v.7 software.
2.7. Limit of Detection and Limit of Quantitation
To determine the instrument’s limit of quantitation (LOQ) for vitamin D2, serial dilutions of a 1.0 mg/mL certified standard solution were analyzed. The LOQ was defined as the lowest concentration producing a peak with a signal-to-noise ratio of at least 10. The limit of detection (LOD) was calculated as the concentration corresponding to a signal-to-noise ratio of 3, using the following standard equations:
where σ is the standard deviation of the response, and S is the slope of the calibration curve.
LOQ = 10 × σ/S
LOD = 3.3 × σ/S
This HPLC method provided reliable quantitation of vitamin D₂ and ergosterol in mushroom samples, facilitating the assessment of their concentrations following various treatments. The determined LOD and LOQ values were 0.1 mg/kg and 0.5 mg/kg dry weight, respectively, ensuring sufficient sensitivity for the analysis of mushroom-derived samples.
2.8. Data Analysis
Statistical analysis was performed with Microsoft Excel version 2412. Comparison of the samples was carried out using a two-sample t-test with unequal variances. p-values lower than 0.05 were considered statistically significant.
3. Results and Discussion
The primary aim of this study was to develop a time-efficient, cost-effective laboratory-scale method utilizing simple equipment to enhance the vitamin D content of mushrooms. Given their global popularity and extensive cultivation, we selected white button and shiitake mushrooms for our experiments.
3.1. HPLC-DAD Method Development for the Analysis of Vitamin D2 and Ergosterol
A novel, rapid HPLC-DAD method was developed for the simultaneous analysis of vitamin D2 and ergosterol, tailored for quality control in mushroom cultivation and the production of mushroom-based food supplements and functional foods. This cost-effective method, described in detail in Section 2, was thoroughly investigated and integrated into routine analyses within mushroom cultivation processes.
To assess the method’s effectiveness, commercially available cultivated mushrooms were analyzed. The developed HPLC method was subsequently applied to UV-irradiated mushroom samples. The analytical procedure involved straightforward sample preparation, including saponification and solid–liquid extraction, followed by HPLC-UV determination.
Under the conditions applied, the retention times were 5.68 min for vitamin D₂ and 6.66 min for ergosterol (Figure 2 and Figure 3). Quantitation was based on calibration curves generated from triplicate injections of standard solutions, with manual integration of all peaks.
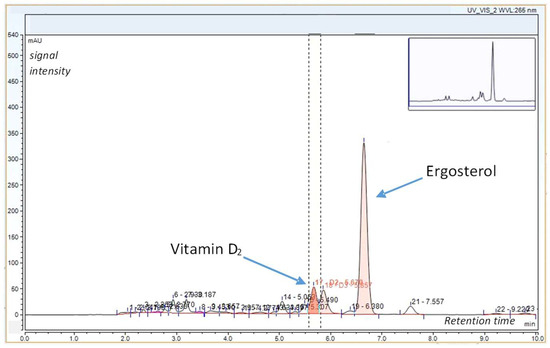
Figure 2.
Chromatogram of vitamin D2 and ergosterol in a white button mushroom sample.
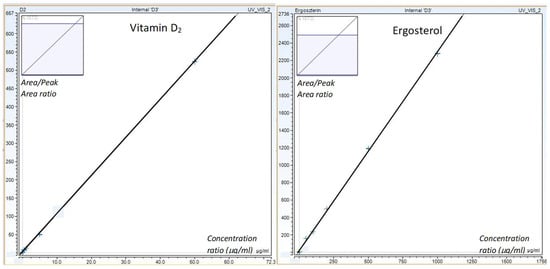
Figure 3.
Calibration curves of vitamin D2 and ergosterol (vitamin D2: 0.05, 0.1, 0.5, 1, 5, 10, and 50 mg/L; ergosterol: 0.05, 0.1, 0.5, 1, 5, 10, 50, 100, 200, 500, 1000, and 2000 mg/L).
The method demonstrated high efficiency in analyzing different cultivated mushrooms, yielding results comparable to LC-MS measurements, with differences remaining below 5% (Table 1). The extraction efficiency of vitamin D₂ was inferred by monitoring the recovery of the spiked vitamin D₃ internal standard, based on the assumption that both compounds exhibit similar physicochemical behavior under the applied conditions. The method achieved high recovery rates of 97–99%, which aligns with previous studies reporting similarly efficient extraction and quantitation methods for sterols in food matrices [21,22]. The method also exhibited a limit of detection (LOD) of 0.1 mg/kg dry weight and a limit of quantitation (LOQ) of 0.5 mg/kg dry weight, both well within the required analytical range.

Table 1.
Average values of vitamin D₂ and ergosterol content in cultivated mushroom samples analyzed by HPLC-DAD.
Our findings indicated that the average vitamin D₂ content in untreated white button mushrooms ranged from 1.5 to 4.5 mg/kg dry weight, while shiitake mushrooms contained between 7 and 8.5 mg/kg dry weight. The higher vitamin D2 content in shiitake mushrooms can be attributed to differences in cultivation conditions: white button mushrooms are grown in the dark, whereas shiitake cultivation involves several hours of artificial illumination.
3.2. UV Irradiation of Mushroom Powders
The primary objective was to optimize UV irradiation conditions to enhance the vitamin D₂ and ergosterol content of mushroom powders, with the aim of developing an efficient technology for producing mushroom-based dietary supplements. It is well-established that UV irradiation facilitates the conversion of ergosterol to previtamin D2, which is subsequently transformed into ergocalciferol (vitamin D₂) [10,12]. Additionally, it has been shown that heat treatment increases the conversion of previtamin D₂ to vitamin D2 [6].
In the experiments, UV irradiation was combined with heat treatment to achieve higher vitamin D₂ concentrations in mushroom powders. Mushroom powders, dried at room temperature, were subjected to heat treatment at 100 °C, followed by UV irradiation. The results for white button and shiitake mushrooms are summarized in Table 2 and Table 3, respectively.

Table 2.
Effect of heat treatment and UV irradiation on vitamin D2 and ergosterol content in white button mushroom powder.

Table 3.
Effect of heat treatment and UV irradiation on vitamin D2 and ergosterol content in shiitake mushroom powder.
For A. bisporus, heat treatment alone resulted in a gradual increase in vitamin D₂ content, reaching 4.66 mg/kg from an initial 1.92 mg/kg after 10 h at 100 °C. Both 5 and 10 h long heat treatments resulted in significant enhancement in vitamin D2 content. When heat treatment was followed by UV irradiation, a substantial further increase in vitamin D2 levels was observed, with the highest concentration recorded (28.13 mg/kg) after 10 h of heating followed by UV exposure. In the case of white button mushrooms, the combination of 10 h heat treatment and UV irradiation resulted in the highest vitamin D2 concentration, but a significant decrease in ergosterol concentration was also observed.
In contrast, L. edodes exhibited different responses. Heat treatment led to a similar gradual increase in vitamin D₂ levels but after 2 h, further heat treatment did not lead to significant enhancement. Subsequent UV irradiation resulted in a comparatively smaller increase in contrast to A. bisporus. Prolonged heating followed by UV exposure led to diminishing returns in vitamin D2 enhancement. This observation underscores the biochemical complexity of vitamin D2 formation from provitamin D2, influenced by equilibrium dynamics.
In both mushroom species, a significant decrease in ergosterol content was observed following treatment. This reduction suggests that in shiitake mushroom powder, the formation of other sterol compounds may be favored, leading to a less pronounced increase in vitamin D2 levels post-UV irradiation. In the case of shiitake mushrooms, the highest vitamin D2 levels can be achieved by UV irradiation without heat treatment, during which ergosterol levels also remain high.
These findings highlight the importance of optimizing both UV irradiation and heat treatment parameters to maximize vitamin D2 content in mushroom powders, considering species-specific responses and the intricate biochemical pathways involved.
4. Conclusions
The effects of heat treatment and UV irradiation on the concentrations of vitamin D₂ and ergosterol in mushroom powders were investigated in this study. Considering the practical feasibility of industrial applications, a 10 min UV treatment and a heating temperature of 100 °C were identified as optimal conditions to balance efficiency, cost-effectiveness, and product integrity. These parameters ensure a viable enrichment process without excessive energy consumption or unfavorable degradation effects.
The results demonstrated that combining these treatments significantly enhances vitamin D₂ levels, particularly in Agaricus bisporus. Heat treatment alone increased vitamin D₂ content from 1.92 mg/kg to 4.66 mg/kg after 10 h at 100 °C, while the combination of heat and UV treatment resulted in a 14-fold increase, reaching 28.13 mg/kg. In contrast, Lentinula edodes exhibited a different response, where UV treatment alone led to the highest vitamin D₂ levels (28.61 mg/kg) while better preserving ergosterol content. This highlights species-specific differences in vitamin D₂ conversion and suggests that optimal processing conditions should be tailored accordingly.
Additionally, a straightforward HPLC-DAD method was developed for the simultaneous analysis of vitamin D₂ and ergosterol. The method demonstrated high recovery rates (97–99%), a limit of detection (LOD) of 0.1 mg/kg dry weight, and a limit of quantitation (LOQ) of 0.5 mg/kg dry weight, confirming its robustness and precision.
The observed ergosterol degradation in A. bisporus after prolonged heat and UV exposure suggests that excessive processing may reduce sterol availability, potentially affecting the overall nutritional quality of the product. In contrast, L. edodes retained higher ergosterol levels when subjected to UV treatment alone, making it a preferable approach for preserving both vitamin D2 and sterol content in this mushroom.
These findings provide valuable insights for optimizing vitamin D2 enrichment strategies in mushroom-based food products. The combined application of heat and UV treatment offers a scalable and efficient approach for industrial vitamin D2 fortification, particularly for A. bisporus, while L. edodes may benefit more from UV-only treatment. However, further research should explore long-term stability, bioavailability, and consumer acceptability to ensure the effectiveness of these treatments in commercial applications.
Author Contributions
Conceptualization, J.B., C.C., M.V. and C.V.; methodology, J.B., A.M. and M.V.; validation, J.R. and C.C.; formal analysis, J.B. and C.C.; investigation, J.B. and A.M.; resources, J.B., J.R., C.C., M.V. and C.V.; data curation, J.B., A.M. and C.C.; writing—original draft preparation, J.B., M.V., C.V. and C.C.; writing—review and editing, J.B., C.C., M.V. and C.V.; visualization, C.C.; supervision, C.C. and M.V.; project administration, C.C., M.V. and C.V.; funding acquisition, C.C., M.V., J.R. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants 2020-1.1.2-PIACI-KFI-2020-00100 and 2023-1.1.1-PIACI_FÓKUSZ-2024-00044 from the National Research, Development and Innovation Office, Hungary. Additional backing came from the Doctoral Student Scholarship Program of the Co-operative Doctoral Program of the Ministry of Innovation and Technology, funded by the National Research, Development and Innovation Fund (grant No. KDP-2023-C2298833 to J. Bajzát).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are presented within the manuscript.
Conflicts of Interest
Authors Judit Bajzát, András Misz and Csaba Csutorás was employed by the company Új Champignons Ltd. Author József Rácz was employed by the company Magyar Gomba Kertész Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPLC | High-performance liquid chromatography |
| UV | Ultraviolet |
| LOD | Limits of detection |
| LOQ | Limits of quantitation |
| HPLC-MS | High-performance liquid chromatography–mass spectrometry |
| HPLC-DAD | High-performance liquid chromatography–diode array detection |
| SD | Standard deviation |
References
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2017. [Google Scholar]
- Li, C.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Yu, H.T.; Kao, J.P.; Yang, C.C.; Chiang, S.S.; Mishchuk, D.O.; Mau, J.J.; Slupsky, C.M. Consumption of vitamin D2 enhanced mushrooms is associated with improved bone health. J. Nutr. Biochem. 2015, 26, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Starck, C.; Cassettari, T.; Wright, J.; Petocz, P.; Beckett, E.; Fayet-Moore, F. Mushrooms: A food-based solution to vitamin D deficiency to include in dietary guidelines. Front. Nutr. 2024, 11, 1384273. [Google Scholar] [CrossRef] [PubMed]
- Wittig, M.; Krings, U.; Berger, R.G. Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J. Food Compos. Anal. 2013, 31, 266–274. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.J.; Palmieri, T.; Young, A.R. Physical determinants of vitamin D photosynthesis: A review. JBMR Plus 2021, 5, e10460. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. In Food Additives Permitted for Direct Addition to Food for Human Consumption (Vitamin D2 Mushroom Powder); 21 CFR Part 172; FDA: Silver Spring, MD, USA, 2020; Volume 85, pp. 41916–41920.
- Havinga, E.; de Kock, R.J.; Rappoldt, M.P. The photochemical interconversions of provitamin D, lumisterol, previtamin D and tachysterol. Tetrahedron 1960, 11, 276–284. [Google Scholar] [CrossRef]
- Salemi, S.; Saedisomeolia, A.; Azimi, F.; Zolfigol, S.; Mohajerani, E.; Mohammadi, M.; Yaseri, M. Optimizing the production of vitamin D in white button mushrooms (Agaricus bisporus) using ultraviolet radiation and measurement of its stability. LWT-Food Sci. Technol. 2021, 137, 110401. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dalbehera, M.M.; Maiti, S.; Bisht, R.S.; Balam, N.B.; Panigrahi, S.K. Investigation of agro-forestry and construction demolition wastes in alkali-activated fly ash bricks as sustainable building materials. Waste Manag. 2023, 159, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Daly, A.; Strobel, N.; Jakobsen, J.; Black, L.J. Effect of air-drying on the generation of vitamin D2 and 25-hydroxyvitamin D2 by pulsed UV irradiation in button mushroom (Agaricus bisporus). J. Food Compos. Anal. 2023, 115, 105034. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT-Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Hu, C.; Feng, Z.; Chen, W.; Shi, H. Comparison of ergosterol and vitamin D2 in mushrooms Agaricus bisporus and Cordyceps militaris using ultraviolet irradiation directly on dry powder or in ethanol suspension. ACS Omega 2021, 6, 29506–29515. [Google Scholar] [CrossRef] [PubMed]
- Jasinghe, V.J.; Perera, C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Wu, W.J.; Ahn, B.Y. Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) Using response surface methodology. PLoS ONE 2014, 9, e95359. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.; James, A.; Black, L. A review of mushrooms as a potential source of dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Piironen, V.I.; Uusi-Rauva, E.J.; Koivistoinen, P.E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. [Google Scholar] [CrossRef]
- Huang, M.; Cadwallader, A.; Heltsley, R. Mechanism of error caused by isotope-labeled internal standard: Accurate method for simultaneous measurement of vitamin D and pre-vitamin D by liquid chromatography/tandem mass spectroscopy. Rapid Commun. Mass Spectrom. 2014, 28, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- McGinty, R.C.; Phillips, K.M. Quantitation of total vitamin D2 and D4 in UV-exposed mushrooms using HPLC with UV detection after novel two-step solid phase extraction. Food Chem. 2024, 439, 138091. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Clausen, I.; Leth, T.; Ovesen, L. A new method for the determination of vitamin D3 and 25-hydroxyvitamin D3 in meat. J. Food Compos. Anal. 2004, 17, 777–787. [Google Scholar] [CrossRef]
- Amithabh, G.S.; Kumar, T.; Gireesh Kumar, M.P.; Kaviya, S.; Baskar, B. Development and validation of a novel HPLC-UV method for quantifying vitamin D forms and precursors in vegetable oils after exposure to sunlight and UV radiation. Acta Chromatogr. 2024. published online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).