Mechanistic Studies of H2 Adsorption and Diffusion in Low-Rank Coals: A Discussion on Geologic Hydrogen Storage
Abstract
1. Introduction
2. Materials, Experimental and Simulation Setting
2.1. Coal Sample
2.2. Experimental Methods
2.3. Simulation Details
2.4. Calculation Methods
3. Results and Discussions
3.1. XPS Analysis
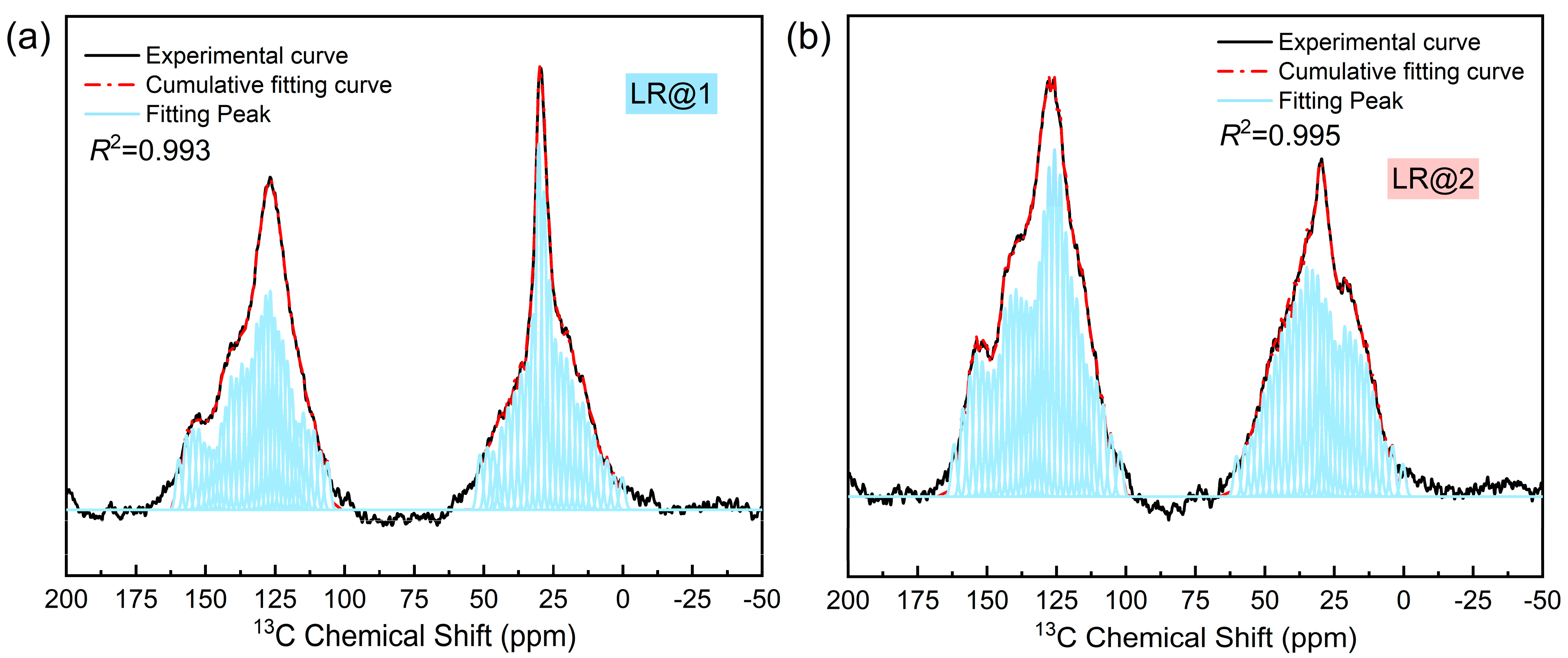
3.2. 13C NMR Spectra Analysis
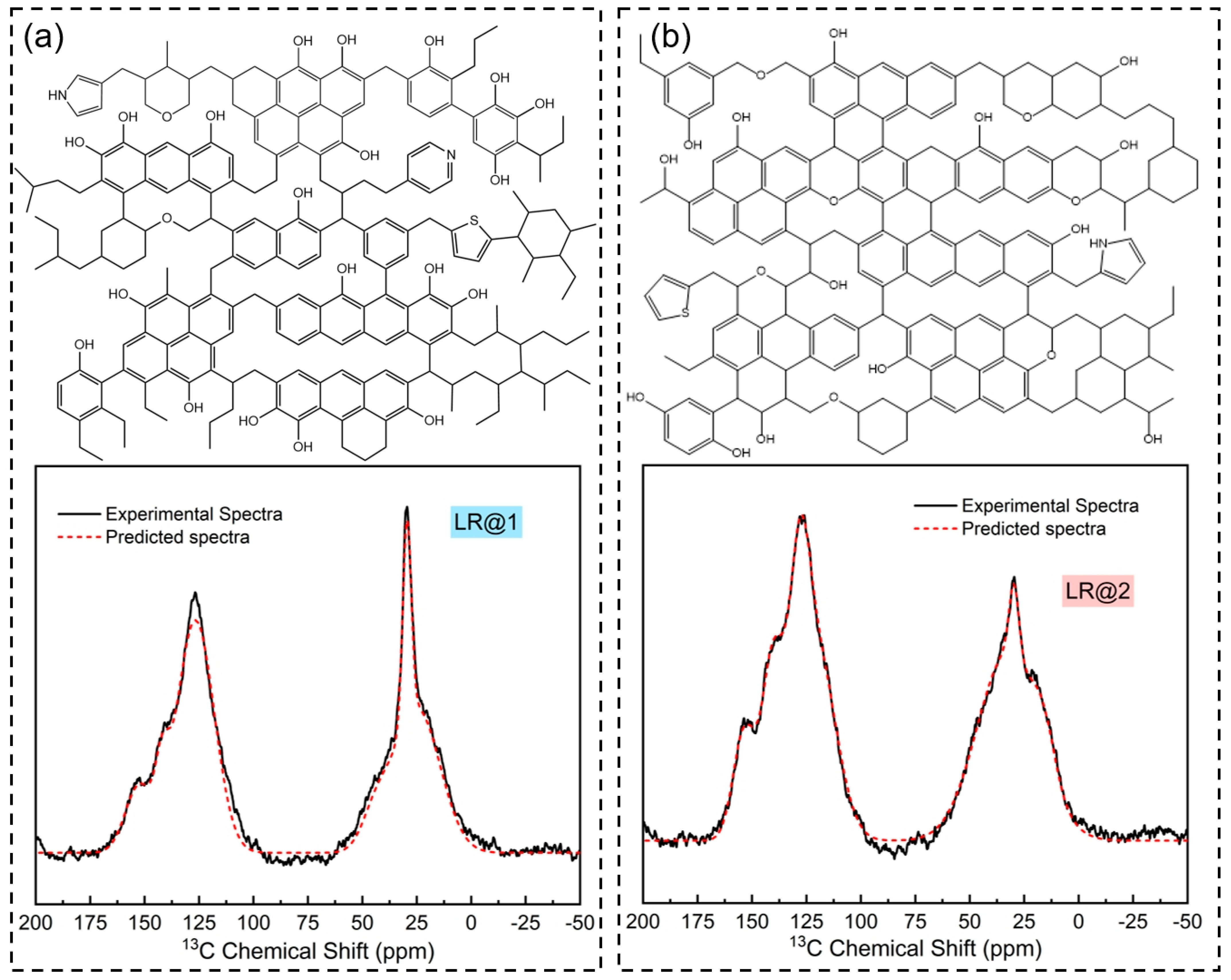
3.3. Molecular Structure Construction of Low-Rank Coal
3.4. DFT Calculations
3.5. Adsorption and Diffusion of H2 in a Microporous Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bade, S.O.; Gyimah, E.; Tomomewo, O.; Josephs, R.; Omojiba, T.; Aluah, R. Assessing the potential of large-scale geological hydrogen storage in North Dakota’s Bakken Formation: A case study integrating wind-powered hydrogen production. Renew. Energy 2024, 237, 121906. [Google Scholar]
- Gasanzade, F.; Witte, F.; Tuschy, I.; Bauer, S. Integration of geological compressed air energy storage into future energy supply systems dominated by renewable power sources. Energy Convers. Manag. 2023, 277, 116643. [Google Scholar]
- Tarkowski, R.; Czapowski, G. Salt domes in Poland—Potential sites for hydrogen storage in caverns. Int. J. Hydrogen Energy 2018, 43, 21414–21427. [Google Scholar]
- Tarkowski, R.; Lankof, L.; Luboń, K.; Michalski, J. Hydrogen storage capacity of salt caverns and deep aquifers versus demand for hydrogen storage: A case study of Poland. Appl. Energy 2024, 355, 122268. [Google Scholar]
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2019, 21, 241–258. [Google Scholar]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A review of hydrogen/rock/brine interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, 101066. [Google Scholar]
- Oni, B.A.; Bade, S.O.; Sanni, S.E.; Orodu, O.D. Underground hydrogen storage in salt caverns: Recent advances, modeling approaches, barriers, and future outlook. J. Energy Storage 2025, 107, 114951. [Google Scholar]
- Zhao, Z.; Yang, W.; Zhao, Z.; Xu, W.; Gong, D.; Jin, H.; Song, W.; Liu, G.; Zhang, C.; Huang, S. Research progresses in geological theory and key exploration areas of coal-formed gas in China. Pet. Explor. Dev. 2024, 51, 1435–1450. [Google Scholar]
- Huang, L.; Hou, Z.; Fang, Y.; Luo, J.; Wu, L.; Wang, Q.; Guo, Y.; Zhang, X.; Shi, T.; Liu, J. The development, frontier and prospect of Large-Scale Underground Energy Storage: A bibliometric review. J. Energy Storage 2024, 103, 114293. [Google Scholar]
- Liu, A.; Liu, S. Hydrogen sorption and diffusion in coals: Implications for hydrogen geo-storage. Appl. Energy 2023, 334, 120746. [Google Scholar]
- Tellez-Juárez, M.; Fierro, V.; Zhao, W.; Fernández-Huerta, N.; Izquierdo, M.; Reguera, E.; Celzard, A. Hydrogen storage in activated carbons produced from coals of different ranks: Effect of oxygen content. Int. J. Hydrogen Energy 2014, 39, 4996–5002. [Google Scholar]
- Iglauer, S.; Abid, H.; Al-Yaseri, A.; Keshavarz, A. Hydrogen Adsorption on Sub-Bituminous Coal: Implications for Hydrogen Geo-Storage. Geophys. Res. Lett. 2021, 48, e2021GL092976. [Google Scholar]
- Kumar, J.; Mendhe, V.A.; Kamble, A.D.; Bannerjee, M.; Mishra, S.; Singh, B.D.; Mishra, V.K.; Singh, P.K.; Singh, H. Coalbed methane reservoir characteristics of coal seams of south Karanpura coalfield, Jharkhand, India. Int. J. Coal Geol. 2018, 196, 185–200. [Google Scholar]
- Sun, Y.; Wang, L.; Wang, R.; Zheng, S.; Liao, X.; Zhu, Z.; Zhao, Y. Insight on microscopic mechanisms of CH4 and CO2 adsorption of coal with different ranks. Fuel 2022, 330, 125715. [Google Scholar]
- Muhammad, A.B. Thermal evolution of aliphatic and aromatic moieties of asphaltenes from coals of different rank: Possible implication to the molecular architecture of asphaltenes. Chin. J. Geochem. 2015, 34, 422–430. [Google Scholar]
- Zhang, F.; Jiang, J.; Wang, C.; Cheng, Y.; Dong, X.; Wu, J. Influence of components on methane micropore filling capacity of low-rank coal. Powder Technol. 2025, 449, 120363. [Google Scholar]
- Meng, Y.; Li, Z. Experimental comparisons of gas adsorption, sorption induced strain, diffusivity and permeability for low and high rank coals. Fuel 2018, 234, 914–923. [Google Scholar]
- Wang, G.; Chen, W. Molecular simulations of hydrogen adsorption on coal with different ranks: Implications for hydrogen geo-storage. Int. J. Hydrogen Energy 2024, 51, 10–20. [Google Scholar]
- Mirzaei, S.; Ahmadpour, A.; Shahsavand, A.; Pourb, A.; Leila Lotfikatoolic Asild, A.; Pouladie, B.; Arash, A. Experimental and simulation study of the effect of surface functional groups decoration on CH4 and H2 storage capacity of microporous carbons. Appl. Surf. Sci. 2020, 533, 147487. [Google Scholar]
- Han, S.; Huang, J.; Wang, J.; Jin, Z. Hydrogen sorption capacity and mechanism in coals: Implications for natural hydrogen exploration. Int. J. Hydrogen Energy 2025, 112, 511–530. [Google Scholar]
- Li, Y.; Dai, C.; Meng, S.; Wu, J. Molecular structure characterization of coal with different coalification degrees: A combined study from FT-IR, Raman, 13C NMR spectroscopy and modelling. J. Mol. Struct. 2024, 1310, 138354. [Google Scholar] [CrossRef]
- Jia, M.C.; Su, S.; He, L.M.; Chen, Y.; Xu, K.; Jiang, L.; Xu, J.; Wang, Y.; Hu, S.; Xiang, J. Experimental and density functional theory study on role of calcium in NO reduction by NH3 on char surface during ammonia co-firing with pulverized coal. Energy 2023, 285, 129484. [Google Scholar] [CrossRef]
- Arif, M.; Abid, H.R.; Keshavarz, A.; Jones, F.; Iglauer, S. Hydrogen storage potential of coals as a function of pressure, temperature, and rank. J. Colloid Interface Sci. 2022, 620, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Y.; Yao, W.; Jiang, X.; Jiang, X. Molecular Characterization of Henan Anthracite Coal. Energy Fuels 2019, 33, 6215–6225. [Google Scholar] [CrossRef]
- Jia, J.; Song, H.; Jia, P.; Wang, D.; Zhao, D.; Xing, Y. Molecular simulation of the adsorption and diffusion of CO2, CH4, and N2 in alkali metal-doped low/medium-rank coal. Fuel 2025, 383, 133900. [Google Scholar] [CrossRef]
- Bai, Y.; Lin, H.-F.; Li, S.-G.; Yan, M.; Long, H. Molecular simulation of N2 and CO2 injection into a coal model containing adsorbed methane at different temperatures. Energy 2021, 219, 119686. [Google Scholar] [CrossRef]
- Jia, J.; Song, H.; Jia, P.; Wang, D. Construction of molecular structure of low/middle coal rank and its adsorption mechanism of CO2 after N/S/P doping. J. Environ. Chem. Eng. 2024, 12, 112741. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 2014, 293, 293–298. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar]
- Ping, A.; Xia, W.; Peng, Y.; Xie, G. Construction of bituminous coal vitrinite and inertinite molecular assisted by 13C NMR, FTIR and XPS. J. Mol. Struct. 2020, 1222, 128959. [Google Scholar]
- Lu, J.; Wang, X.; Li, H.; Shi, S.; Yang, W.; Lu, Y.; Shao, S.; Ye, Q. Molecular insights into the methane adsorption capacity of coal under microwave irradiation based on solid-state 13C-NMR and XPS. Fuel 2023, 339, 127484. [Google Scholar]
- Ghosh, A.K.; Bandopadhyay, A.K. Formation of thermogenic gases with coalification: FTIR and DFT examination of vitrinite rich coals. Int. J. Coal Geol. 2020, 219, 103379. [Google Scholar]
- Cao, W.; Huang, Y.; Li, D.; Chen, W.; Qie, Z.; Pi, X.; Du, Q.; Lai, X.; Li, Y. N/S co-doped microporous zeolite-templated carbon for efficient CO2 adsorption and separation. J. Energy Inst. 2023, 106, 101159. [Google Scholar]
- Jumabaev, A.; Holikulov, U.; Hushvaktov, H.; Issaoui, N.; Absanov, A. Intermolecular interactions in ethanol solution of OABA: Raman, FTIR, DFT, M062X, MEP, NBO, FMO, AIM, NCI, RDG analysis. J. Mol. Liq. 2023, 377, 121552. [Google Scholar]
- Li, X.; Sun, X.; Walters, C.C.; Zhang, T. H2, CH4 and CO2 adsorption on Cameo coal: Insights into the role of cushion gas in hydrogen geological storage. Int. J. Hydrogen Energy 2024, 50, 879–892. [Google Scholar]
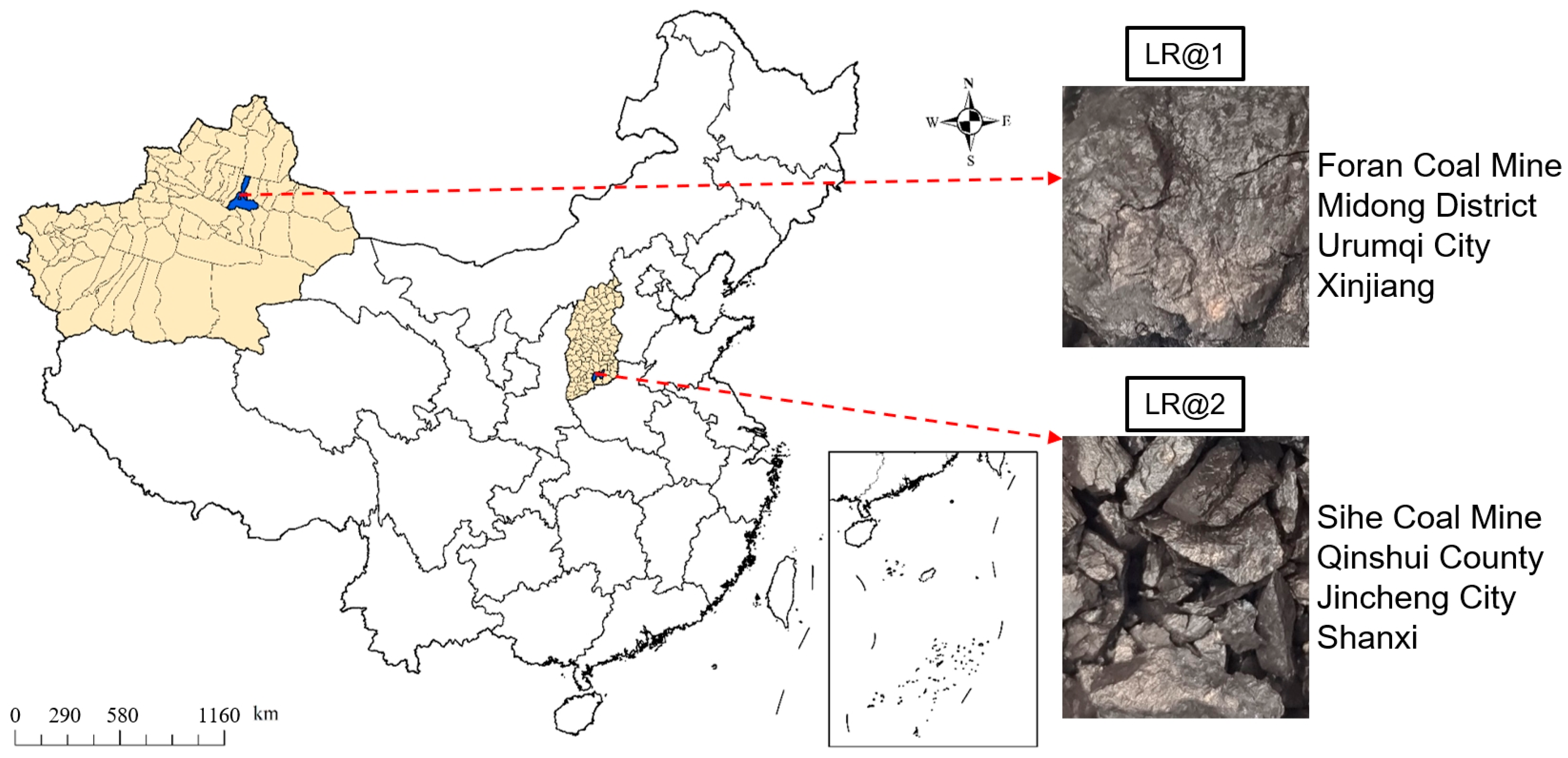



| Coal Sample | Elemental Content (%) | Density (g/cm3) | Maximum Vitrinite Reflectance (R0,max, %) | ||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | |||
| LR@1 | 79.96 | 7.35 | 10.84 | 0.87 | 0.98 | 1.16 | 0.43 |
| LR@2 | 80.06 | 6.25 | 12.03 | 0.51 | 1.15 | 1.18 | 0.56 |
| Coal Sample | Elements | |||||
|---|---|---|---|---|---|---|
| N | S | |||||
| Peak Value | Elemental Structure | Relative Content (%) | Peak Value | Elemental Structure | Relative Content (%) | |
| LR@1 | 399.38 | Pyridine | 64.37 | 164.62 | Thiophene | 47.63 |
| 402.58 | Pyrrole | 35.63 | 168.37 | Sulfone | 23.42 | |
| 169.57 | Sulfoxide | 28.95 | ||||
| LR@2 | 398.87 | Pyridine | 32.99 | 164.08 | Thiophene | 51.01 |
| 400.88 | Pyrrole | 42.12 | 168.42 | Sulfone | 48.99 | |
| 403.20 | Oxidized nitrogen | 24.89 | ||||
| Sample | faB | faH | faP | faS | faN | fal* | falH | falO | fal | fa | fa′ | XBP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR@1 | 13.16 | 28.60 | 9.26 | 6.68 | 29.09 | 3.78 | 27.95 | 0.88 | 42.31 | 57.69 | 57.69 | 0.295 |
| LR@2 | 12.75 | 27.91 | 7.69 | 12.34 | 32.77 | 3.72 | 20.04 | 3.85 | 39.32 | 60.68 | 60.68 | 0.266 |
| Typology | Amount | Typology | Amount | ||
|---|---|---|---|---|---|
| LR@1 | LR@2 | LR@1 | LR@2 | ||
 | 4 | 5 | 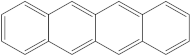 | 1 | 1 |
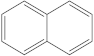 | 1 | 1 | 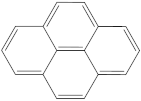 | 2 | 2 |
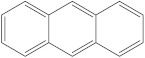 | 2 | 1 | 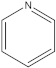 | 1 | 0 |
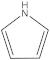 | 1 | 1 | 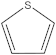 | 1 | 1 |
| Coal Sample | Actual Elemental Content (%) | Calculated Elemental Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | C | H | O | N | S | |
| LR@1 | 79.96 | 7.35 | 10.84 | 0.87 | 0.98 | 78.39 | 8.26 | 11.51 | 0.86 | 0.98 |
| LR@2 | 80.06 | 6.25 | 12.03 | 0.51 | 1.15 | 79.81 | 7.02 | 11.73 | 0.43 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Fu, S.; Jia, J.; Song, H.; Tian, H. Mechanistic Studies of H2 Adsorption and Diffusion in Low-Rank Coals: A Discussion on Geologic Hydrogen Storage. Appl. Sci. 2025, 15, 3932. https://doi.org/10.3390/app15073932
Gao X, Fu S, Jia J, Song H, Tian H. Mechanistic Studies of H2 Adsorption and Diffusion in Low-Rank Coals: A Discussion on Geologic Hydrogen Storage. Applied Sciences. 2025; 15(7):3932. https://doi.org/10.3390/app15073932
Chicago/Turabian StyleGao, Xiaoxu, Sixin Fu, Jinzhang Jia, Hailong Song, and Hao Tian. 2025. "Mechanistic Studies of H2 Adsorption and Diffusion in Low-Rank Coals: A Discussion on Geologic Hydrogen Storage" Applied Sciences 15, no. 7: 3932. https://doi.org/10.3390/app15073932
APA StyleGao, X., Fu, S., Jia, J., Song, H., & Tian, H. (2025). Mechanistic Studies of H2 Adsorption and Diffusion in Low-Rank Coals: A Discussion on Geologic Hydrogen Storage. Applied Sciences, 15(7), 3932. https://doi.org/10.3390/app15073932







