Author Contributions
Supervision, metadata design, database, web portal and data retriever, T.M. (Takahiro Matsumoto); data curation, T.M. (Takahiro Matsumoto) and M.M. (Masashi Matsumoto); data infrastructure and sample preparation protocol, S.Y.; XAFS protocol, T.K.; HAXPES protocol, M.M. (Mayeesha Marium), D.F. and T.M. (Tetsuya Miyazawa); XRD protocol, J.K. and H.T.; PDF protocol, Y.M. and Y.W.; SAXS protocol, H.I. (Hiroyuki Iwamoto) and A.M.; guidance on data analysis, T.U.; project administration and funding acquisition, H.I. (Hideto Imai), Y.S. and Y.U.; leading writing and editing of the draft, T.M. (Takahiro Matsumoto); writing draft for sample preparation protocol, S.Y.; writing draft for XAFS protocol, T.K.; writing draft for HAXPES protocol, M.M. (Mayeesha Marium); writing draft for XRD protocol, J.K.; writing draft for PDF protocol, Y.W.; writing draft for SAXS protocol, H.I. (Hiroyuki Iwamoto); writing draft for data analysis example, K.U.; review, T.M. (Takahiro Matsumoto), S.Y., T.K., M.M. (Mayeesha Marium), J.K., Y.W., H.I. (Hiroyuki Iwamoto), T.U., K.U. and Y.S. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Overview of the FC-Platform, an analysis and evaluation platform for PEFCs. This figure illustrates the platform’s architecture, highlighting its role in integrating cross-disciplinary expertise and facilitating the development of advanced materials and methods for PEFCs.
Figure 1.
Overview of the FC-Platform, an analysis and evaluation platform for PEFCs. This figure illustrates the platform’s architecture, highlighting its role in integrating cross-disciplinary expertise and facilitating the development of advanced materials and methods for PEFCs.
Figure 2.
Experimental setup for CV measurements. The setup includes an RHE, a platinum counter electrode, and a gold plate working electrode coated with catalyst ink. The figure illustrates the arrangement of electrodes, electrolyte solution, and measurement equipment used to perform CV scans under controlled conditions.
Figure 2.
Experimental setup for CV measurements. The setup includes an RHE, a platinum counter electrode, and a gold plate working electrode coated with catalyst ink. The figure illustrates the arrangement of electrodes, electrolyte solution, and measurement equipment used to perform CV scans under controlled conditions.
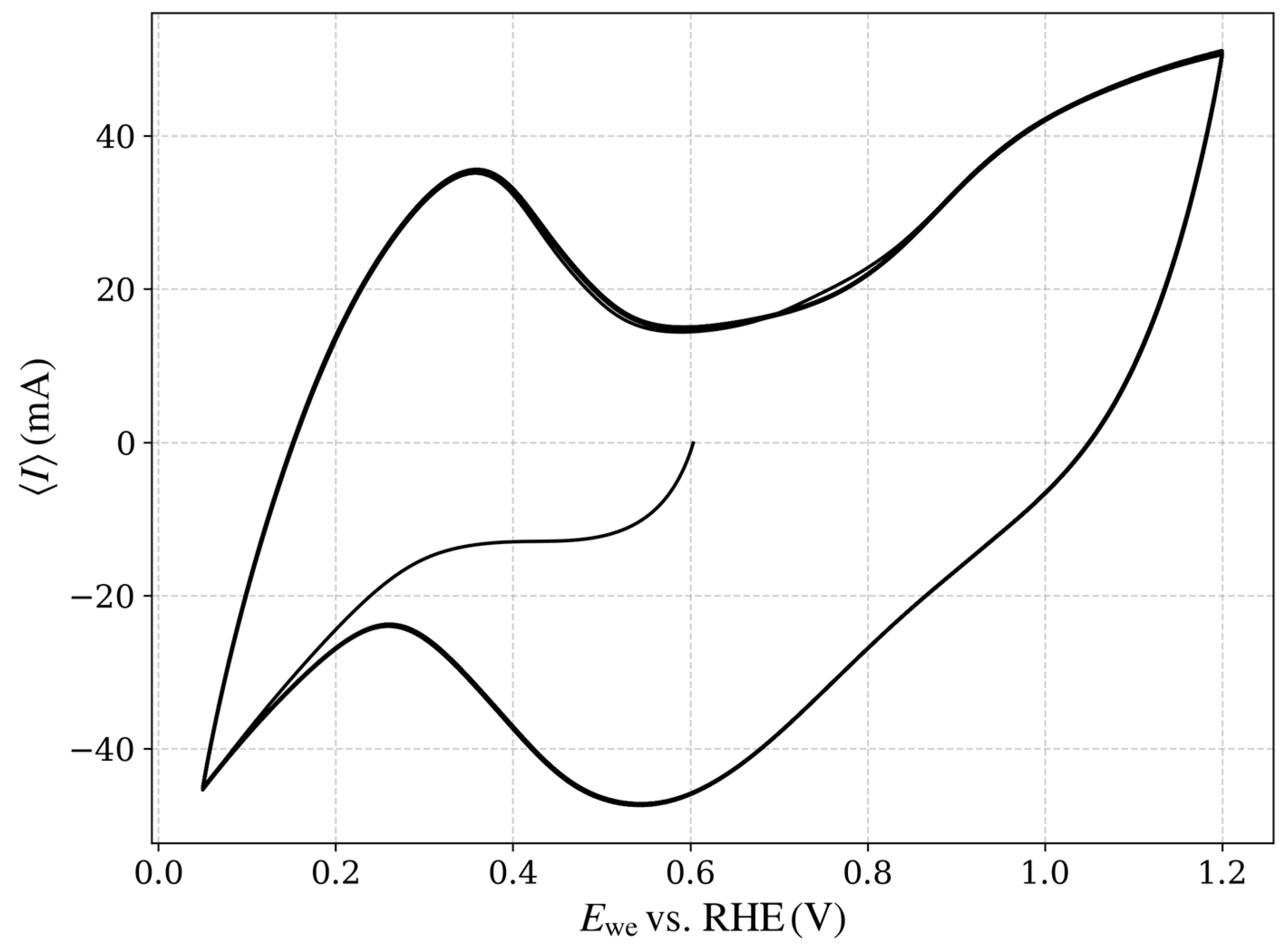
Figure 3.
Representative CV results for TEC10V30E (supplied by TANAKA Precious Metals [TANAKA PRECIOUS METAL TECHNOLOGIES Co., Ltd., Tokyo, Japan]) under an operational potential range of 0.05–1.2 V vs. the RHE at a scan rate of 50 mV/s. The plot shows the relationship between the working electrode potential (Ewe) relative to RHE and the resulting current (I), highlighting key electrochemical responses within the operational range.
Figure 3.
Representative CV results for TEC10V30E (supplied by TANAKA Precious Metals [TANAKA PRECIOUS METAL TECHNOLOGIES Co., Ltd., Tokyo, Japan]) under an operational potential range of 0.05–1.2 V vs. the RHE at a scan rate of 50 mV/s. The plot shows the relationship between the working electrode potential (Ewe) relative to RHE and the resulting current (I), highlighting key electrochemical responses within the operational range.
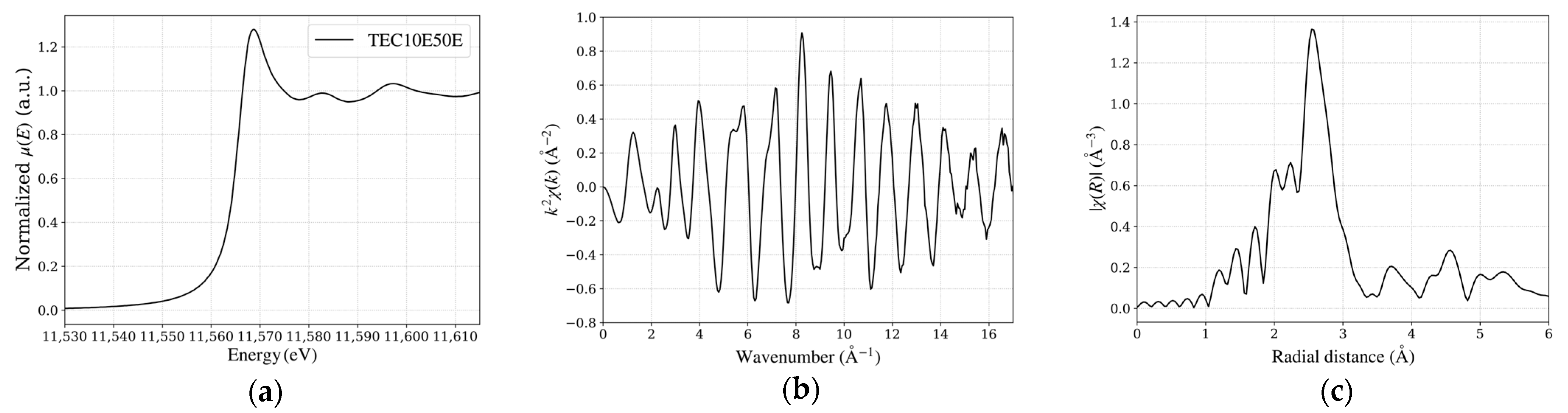
Figure 4.
Pt L3-edge XAFS spectra of TEC10E50E, a hydrogen-reduced sample supplied by TANAKA Precious Metals. (a) X-ray absorption near edge structure spectrum at the Pt L3-edge, indicating the electronic state of Pt. (b) EXAFS spectrum in k-space, showing oscillations related to local structures. (c) Fourier-transformed EXAFS spectrum in R-space, illustrating the radial distribution of neighboring atoms around Pt.
Figure 4.
Pt L3-edge XAFS spectra of TEC10E50E, a hydrogen-reduced sample supplied by TANAKA Precious Metals. (a) X-ray absorption near edge structure spectrum at the Pt L3-edge, indicating the electronic state of Pt. (b) EXAFS spectrum in k-space, showing oscillations related to local structures. (c) Fourier-transformed EXAFS spectrum in R-space, illustrating the radial distribution of neighboring atoms around Pt.
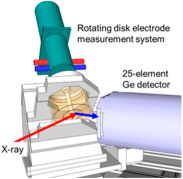
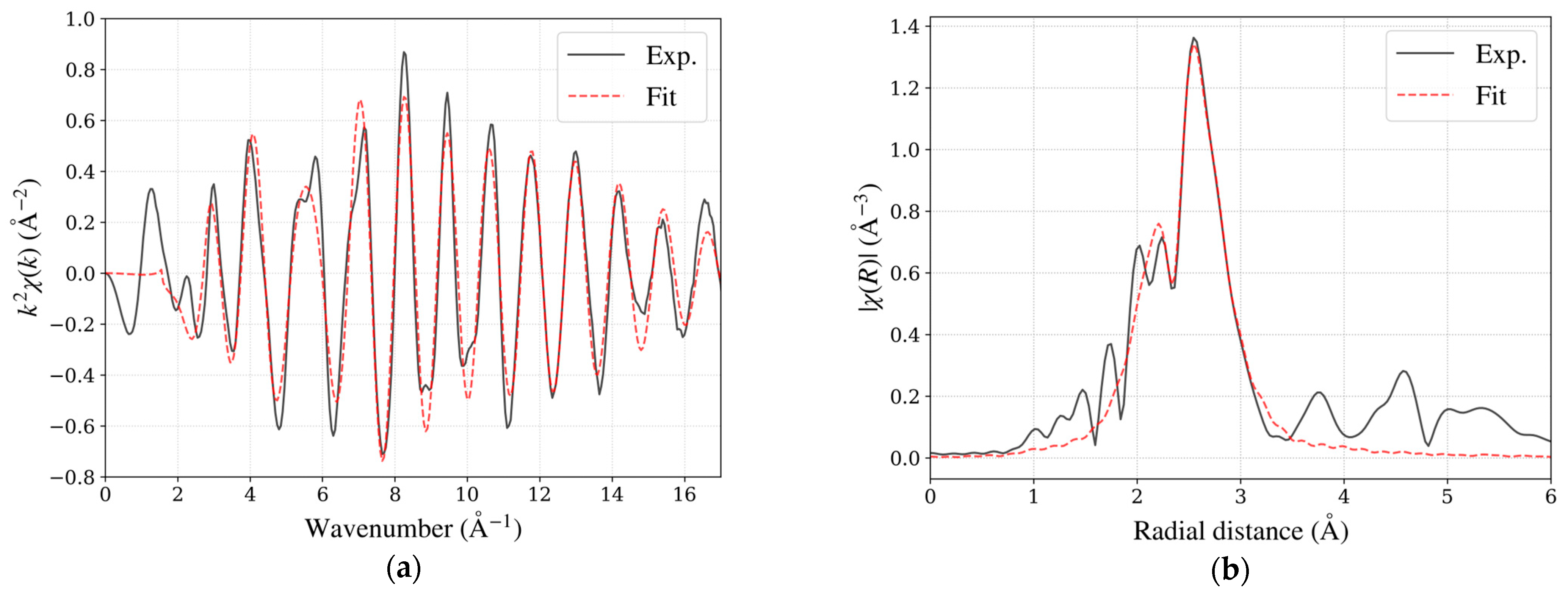
Figure 5.
(a) EXAFS spectrum in k-space for the Pt L3-edge of TEC10E50E, a hydrogen-reduced sample supplied by TANAKA Precious Metals. (b) Magnitudes of Fourier-transformed spectrum in R-space. Experimental data (black solid line, Exp.) and fitting result (red dashed line, Fit) are shown.
Figure 5.
(a) EXAFS spectrum in k-space for the Pt L3-edge of TEC10E50E, a hydrogen-reduced sample supplied by TANAKA Precious Metals. (b) Magnitudes of Fourier-transformed spectrum in R-space. Experimental data (black solid line, Exp.) and fitting result (red dashed line, Fit) are shown.
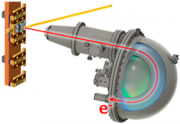
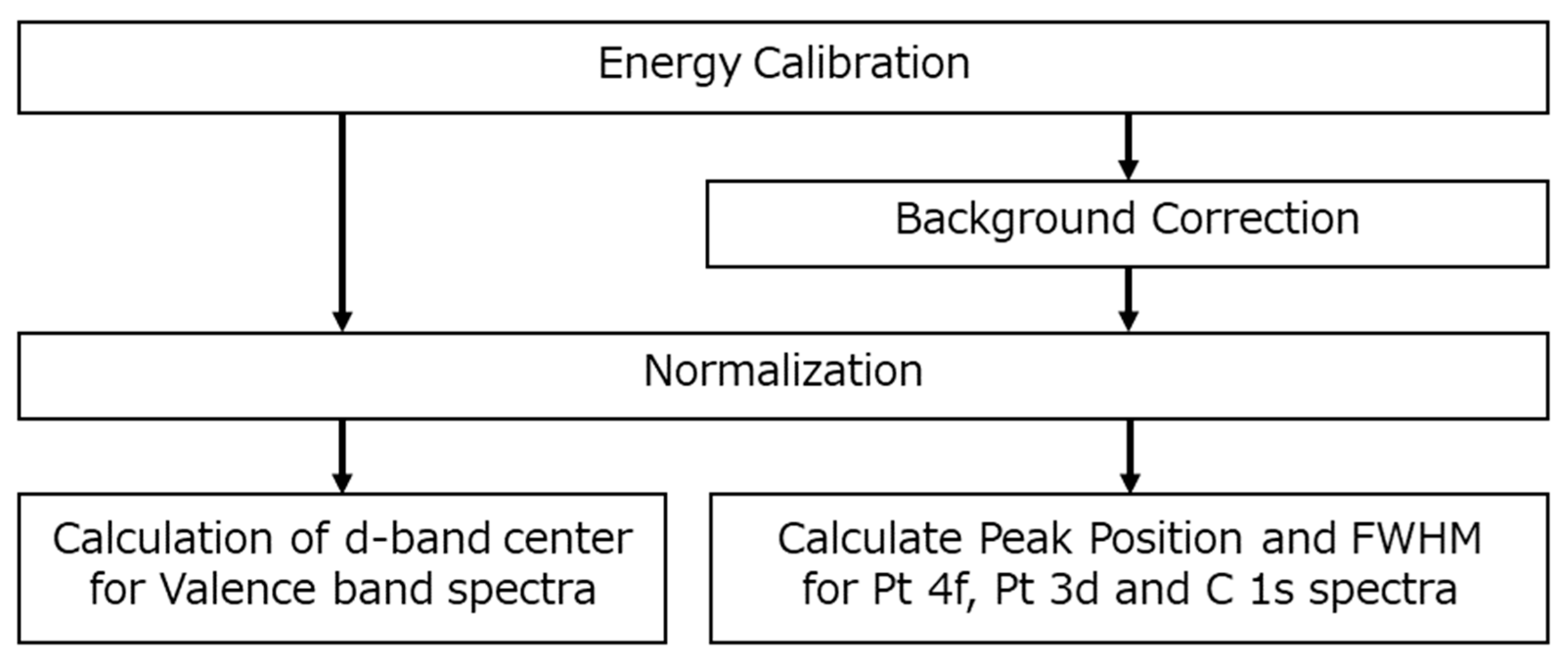
Figure 6.
Workflow for HAXPES spectra analysis, illustrating the sequential steps from energy calibration to background correction and normalization. The calculation of d-band center for valence band spectra uses energy calibration and normalization, while peak positions with FWHM for core-level spectra also include background correction.
Figure 6.
Workflow for HAXPES spectra analysis, illustrating the sequential steps from energy calibration to background correction and normalization. The calculation of d-band center for valence band spectra uses energy calibration and normalization, while peak positions with FWHM for core-level spectra also include background correction.
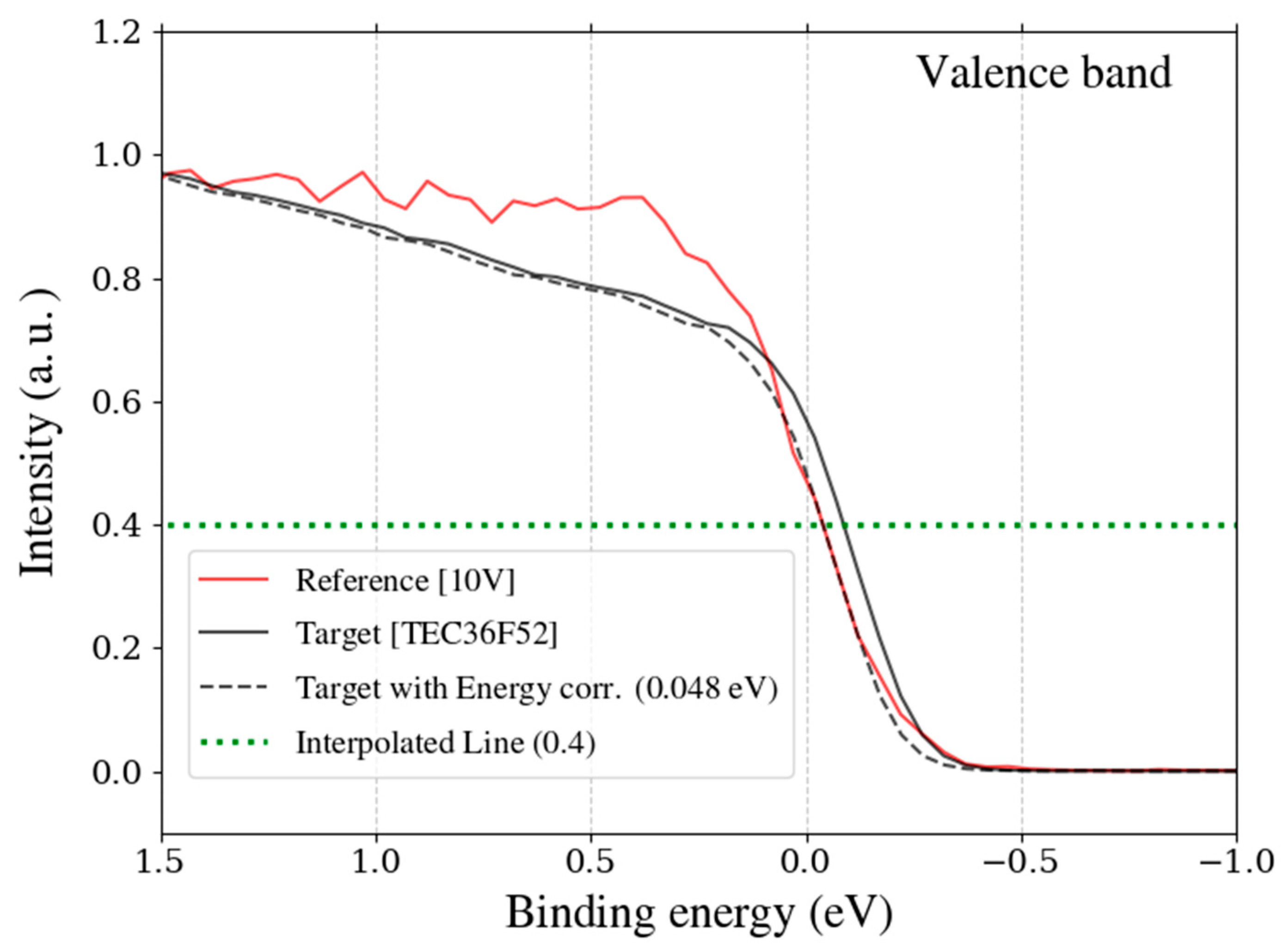
Figure 7.
Binding energy profiles of the valence band for the reference sample (10 V, red solid line) and the target sample (TEC36F52, black solid line), both provided by TANAKA Precious Metals. The intensity distributions are normalized. The interpolated binding energy is indicated by a green dashed line at an intensity of 0.4. The black dashed line represents the target sample data after energy correction, where the binding energy has been calibrated using the energy offset at the interpolated point, aligning it with the reference sample.
Figure 7.
Binding energy profiles of the valence band for the reference sample (10 V, red solid line) and the target sample (TEC36F52, black solid line), both provided by TANAKA Precious Metals. The intensity distributions are normalized. The interpolated binding energy is indicated by a green dashed line at an intensity of 0.4. The black dashed line represents the target sample data after energy correction, where the binding energy has been calibrated using the energy offset at the interpolated point, aligning it with the reference sample.
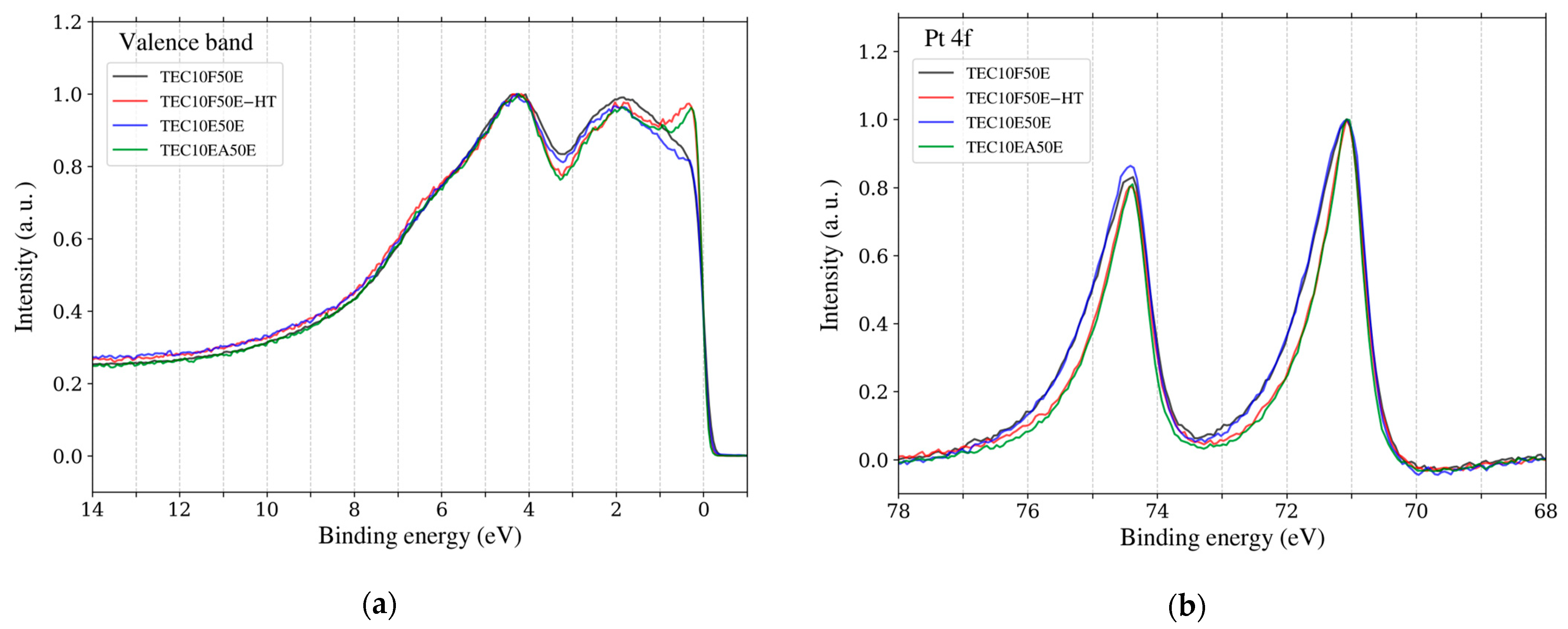
Figure 8.
(a) Valence band spectra after energy calibration and normalization. (b) Pt 4f spectra after energy calibration, background correction and normalization. Each plot represents data for different samples: TEC10EF50E (black), TEC10F50E-HT (red), TEC10E50E (blue), and TEC10EA50E (green). All samples are hydrogen-reduced and supplied by TANAKA Precious Metals.
Figure 8.
(a) Valence band spectra after energy calibration and normalization. (b) Pt 4f spectra after energy calibration, background correction and normalization. Each plot represents data for different samples: TEC10EF50E (black), TEC10F50E-HT (red), TEC10E50E (blue), and TEC10EA50E (green). All samples are hydrogen-reduced and supplied by TANAKA Precious Metals.
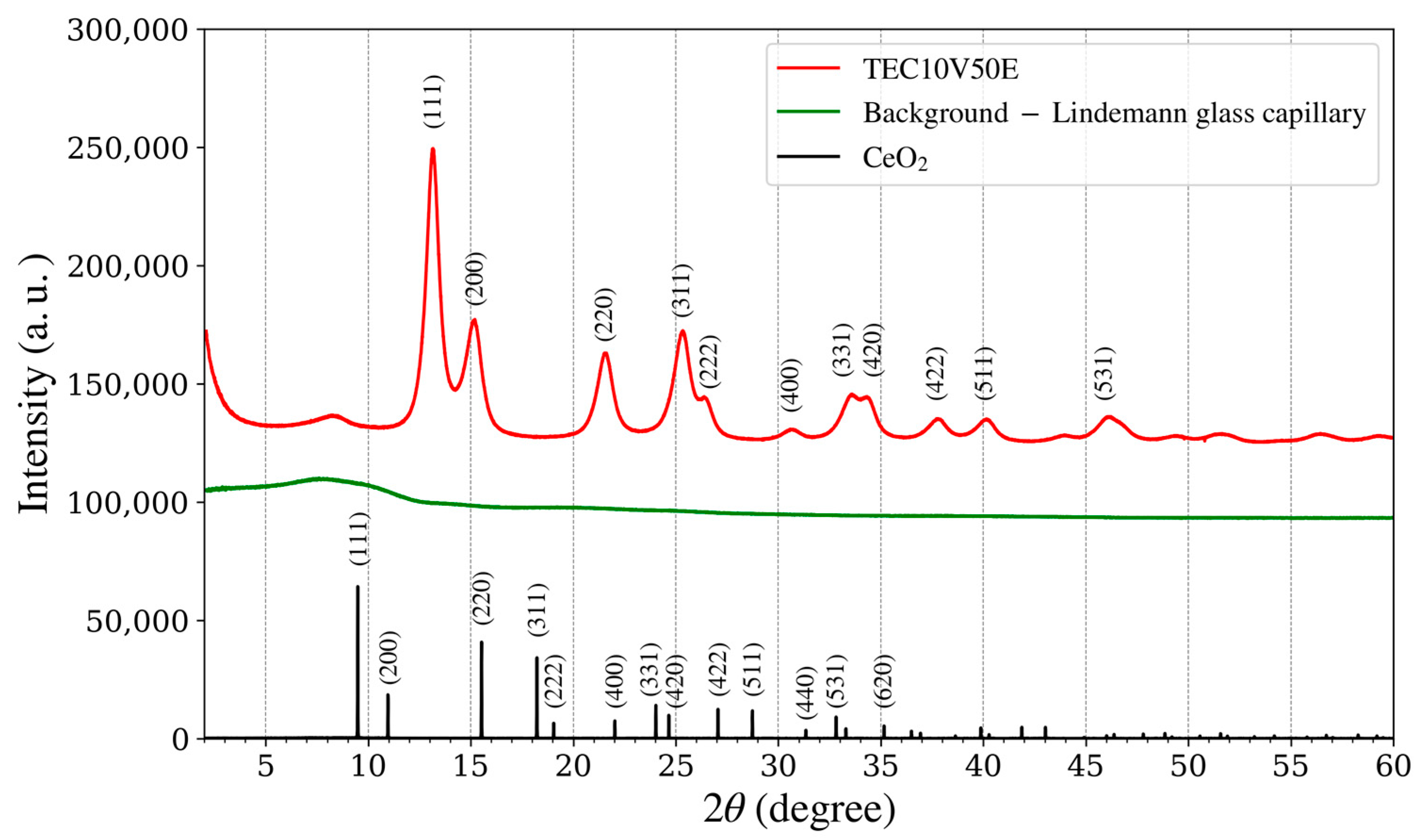
Figure 9.
XRD spectra of the platinum catalyst as a standard sample (TEC10V50E, red line), supplied by TANAKA Precious Metals; background of an empty Lindemann glass capillary (green line) and cerium oxide (black line).
Figure 9.
XRD spectra of the platinum catalyst as a standard sample (TEC10V50E, red line), supplied by TANAKA Precious Metals; background of an empty Lindemann glass capillary (green line) and cerium oxide (black line).
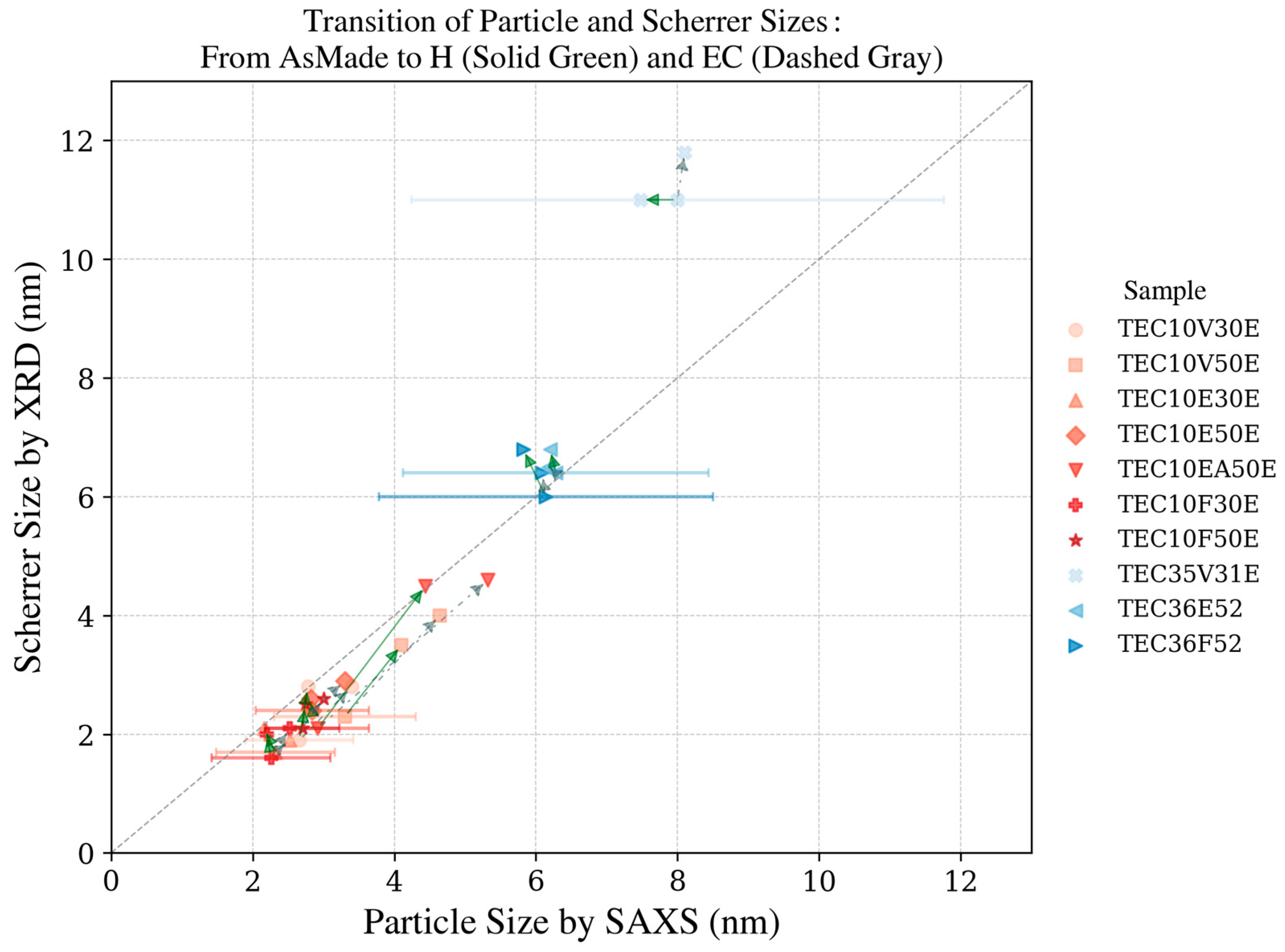
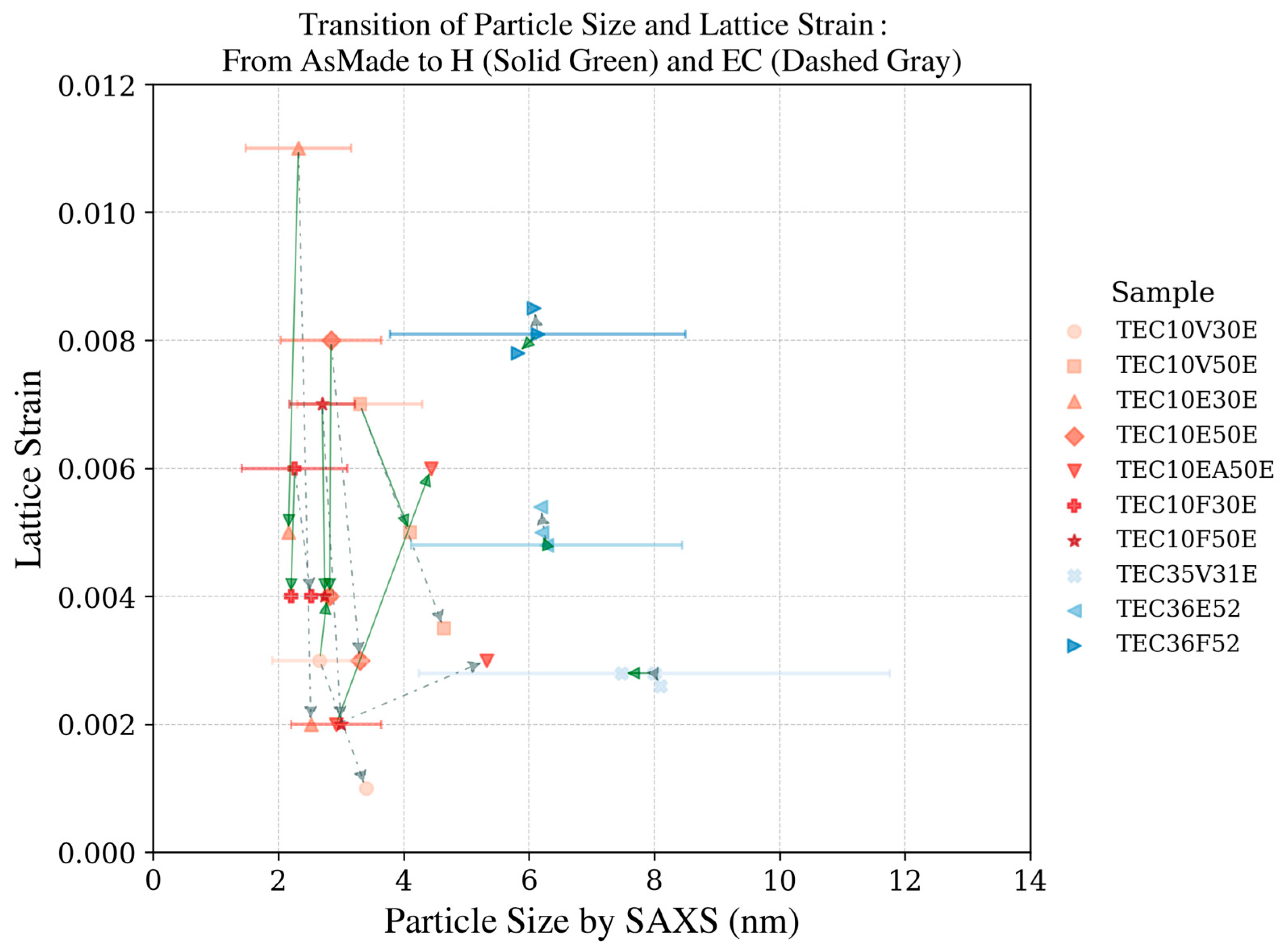
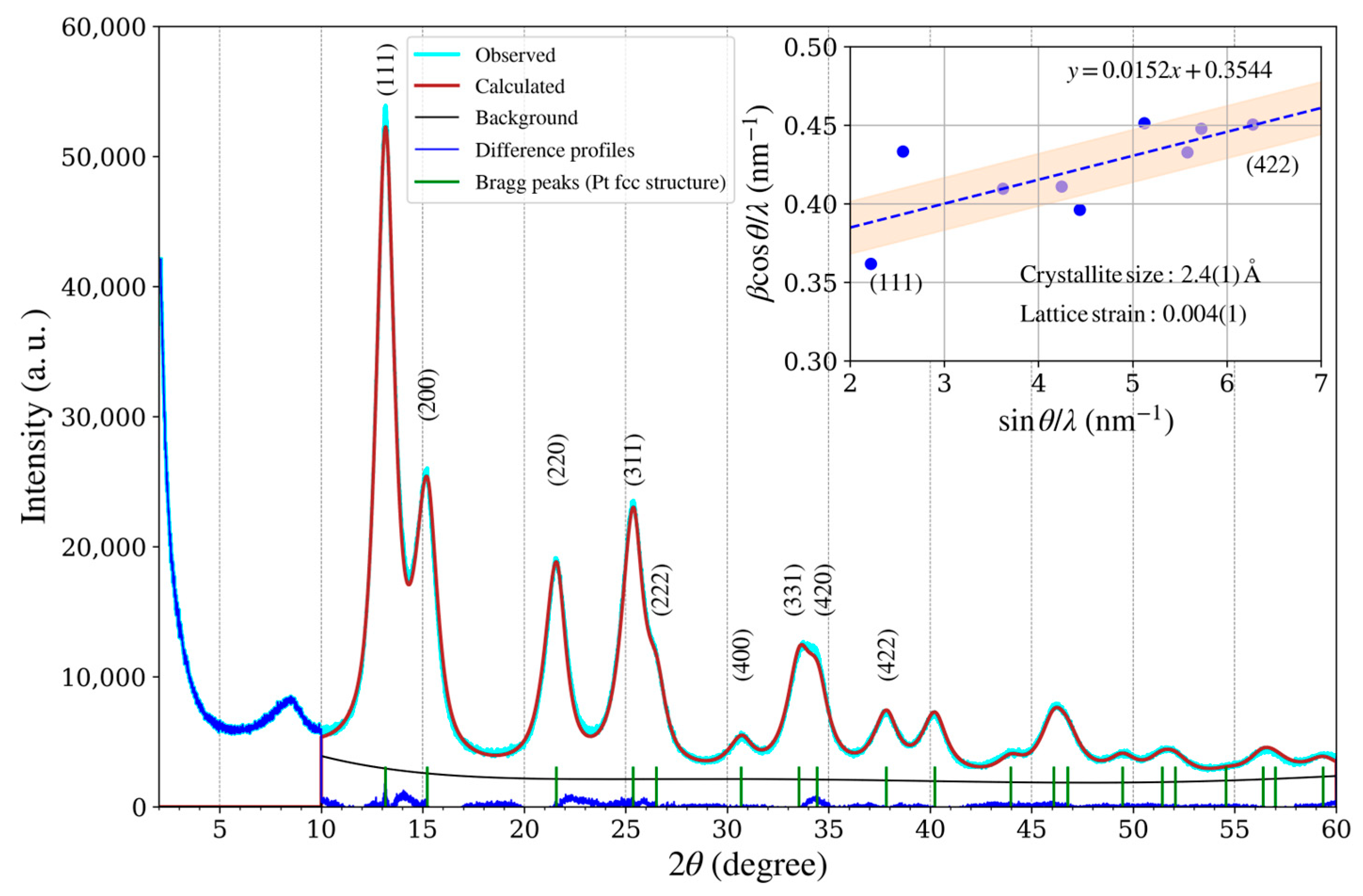
Figure 10.
XRD pattern of the platinum catalyst (TEC10V50E, supplied by TANAKA Precious Metals) and plot of Williamson–Hall analysis (inset).
Figure 10.
XRD pattern of the platinum catalyst (TEC10V50E, supplied by TANAKA Precious Metals) and plot of Williamson–Hall analysis (inset).
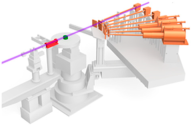
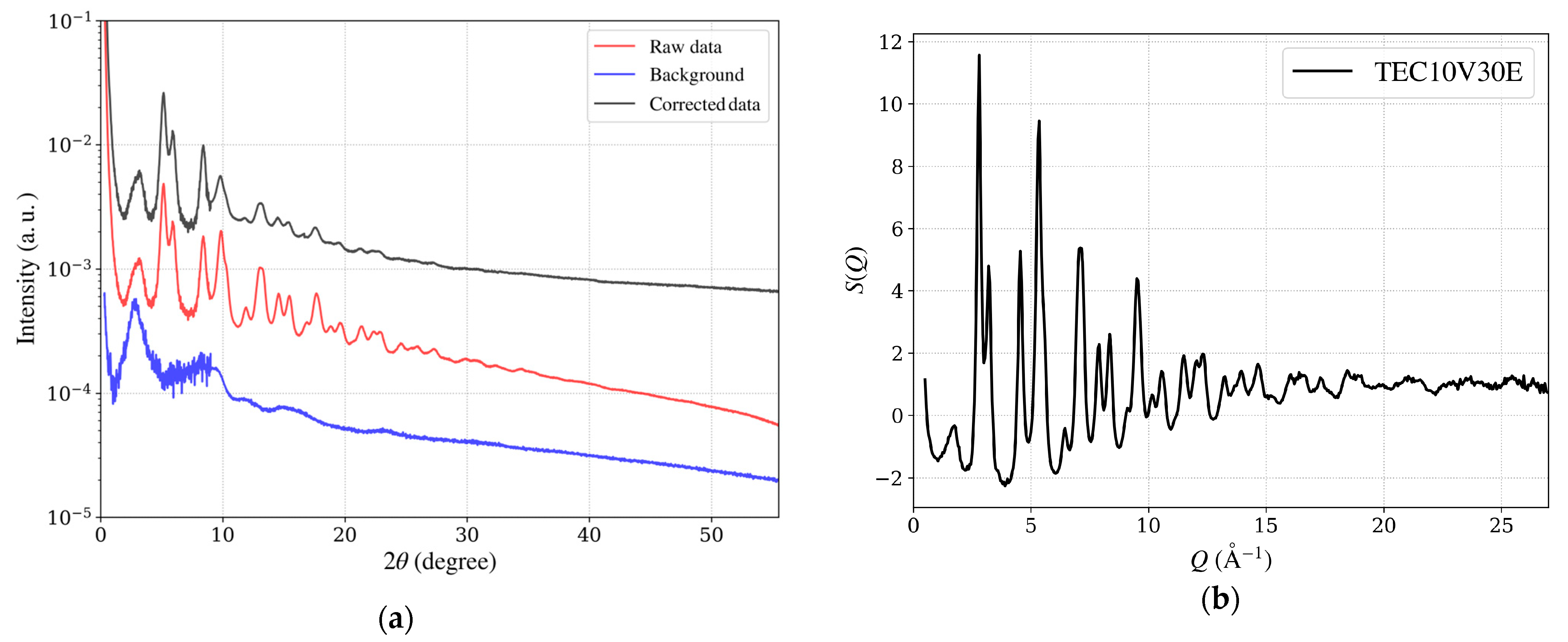
Figure 11.
(a) Intensity as a function of 2θ for the hydrogen-reduced TEC10V30E sample, supplied by TANAKA Precious Metals. The red line (Iobs) represents raw data, and the blue line (IBG) shows background data from a vacant quartz capillary, obtained by combining measurements from seven detectors positioned at different angles. The black line (Icorrected) indicates the corrected data, obtained after applying background subtraction and corrections for absorption and polarization. (b) The structure factor S(Q), emphasizing high Q oscillations.
Figure 11.
(a) Intensity as a function of 2θ for the hydrogen-reduced TEC10V30E sample, supplied by TANAKA Precious Metals. The red line (Iobs) represents raw data, and the blue line (IBG) shows background data from a vacant quartz capillary, obtained by combining measurements from seven detectors positioned at different angles. The black line (Icorrected) indicates the corrected data, obtained after applying background subtraction and corrections for absorption and polarization. (b) The structure factor S(Q), emphasizing high Q oscillations.
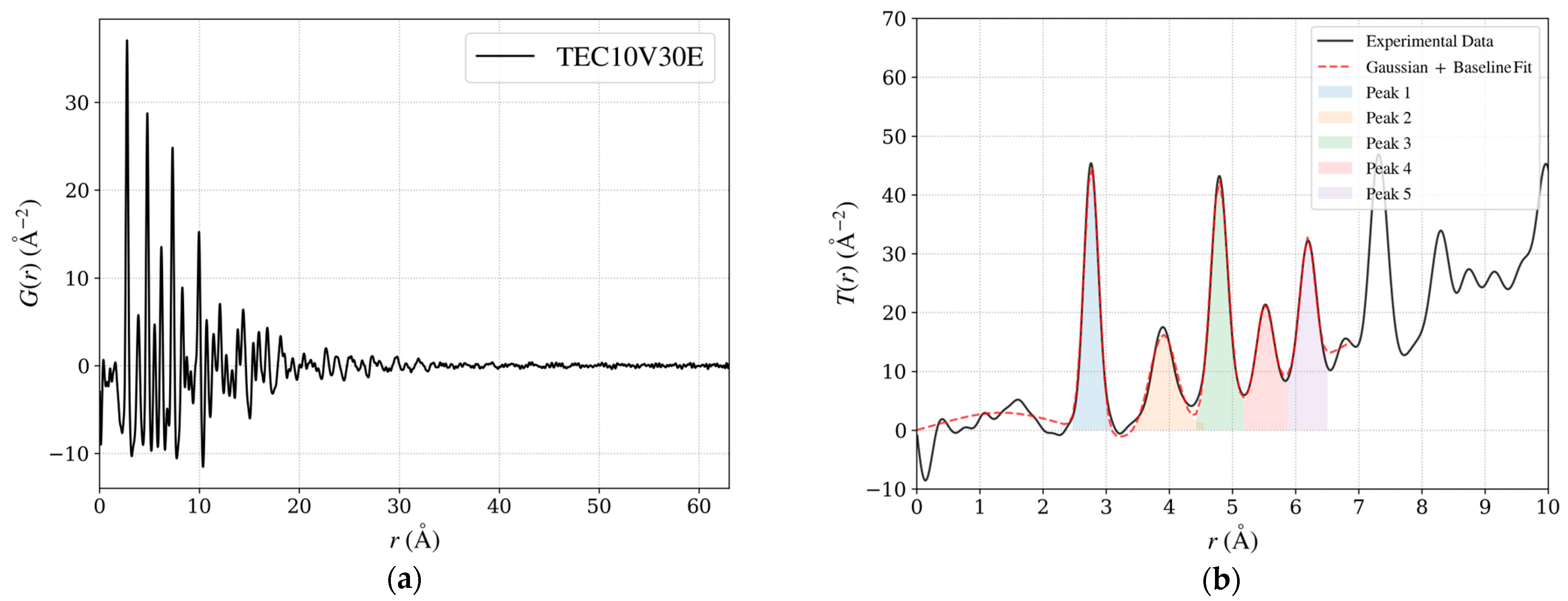
Figure 12.
(a) PDF G(r) for the standard sample TEC10V30E, a hydrogen-reduced material supplied by TANAKA Precious Metals. (b) T(r), where each peak is fitted using a Gaussian function combined with a baseline. The integration range for each peak, corresponding to ±3σ from the peak center where T(r) > 0, is highlighted with distinct background colors. The integrated values represent the relative coordination numbers, offering insights into local structural trends.
Figure 12.
(a) PDF G(r) for the standard sample TEC10V30E, a hydrogen-reduced material supplied by TANAKA Precious Metals. (b) T(r), where each peak is fitted using a Gaussian function combined with a baseline. The integration range for each peak, corresponding to ±3σ from the peak center where T(r) > 0, is highlighted with distinct background colors. The integrated values represent the relative coordination numbers, offering insights into local structural trends.
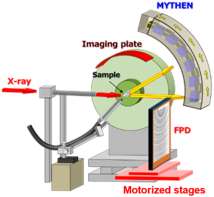
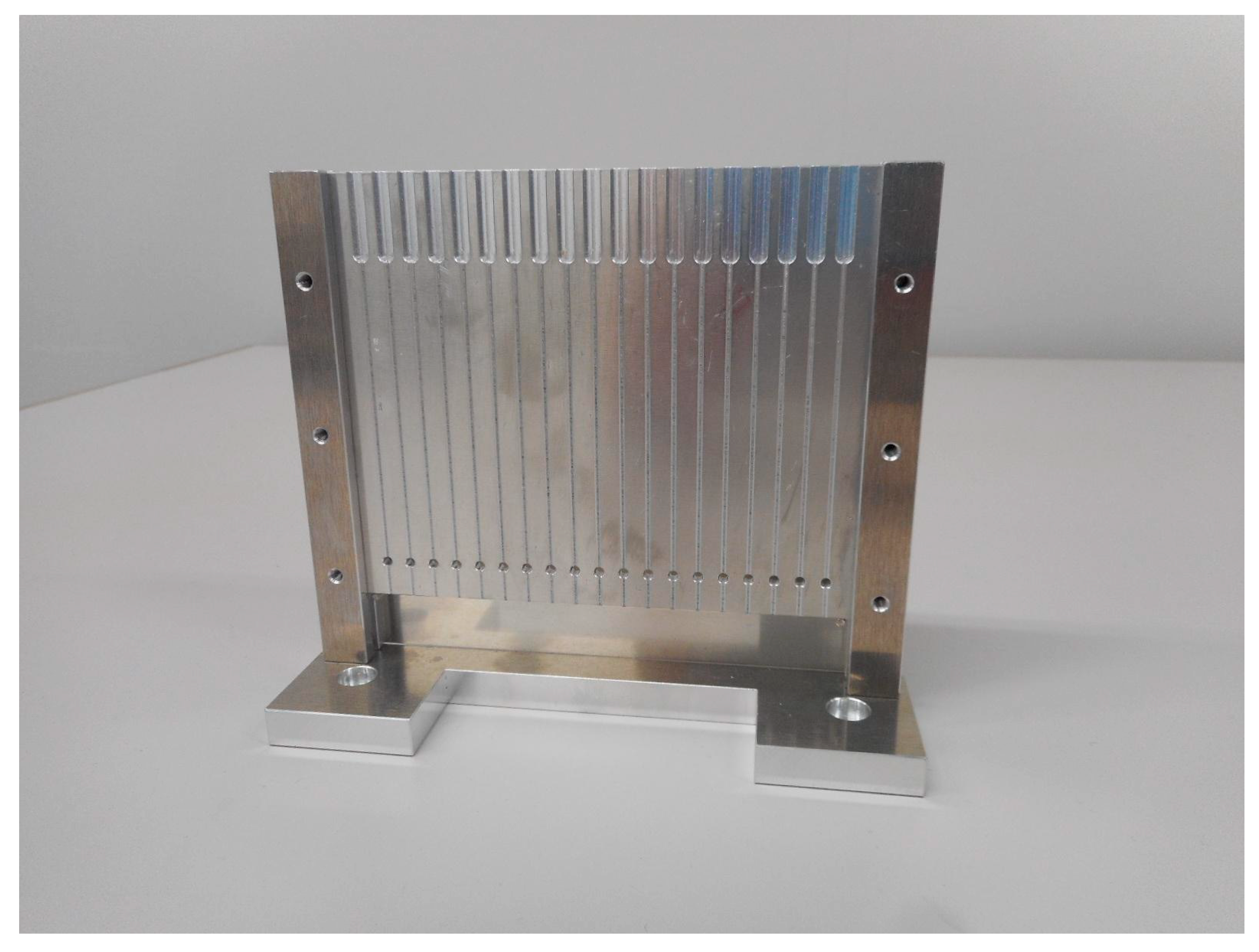
Figure 13.
Custom-designed cassette for holding capillaries during SAXS measurements. Each capillary is placed in a vertical groove, and X-rays pass through a small aperture aligned with the sample position, ensuring stable and reproducible sample orientation for scattering measurements.
Figure 13.
Custom-designed cassette for holding capillaries during SAXS measurements. Each capillary is placed in a vertical groove, and X-rays pass through a small aperture aligned with the sample position, ensuring stable and reproducible sample orientation for scattering measurements.
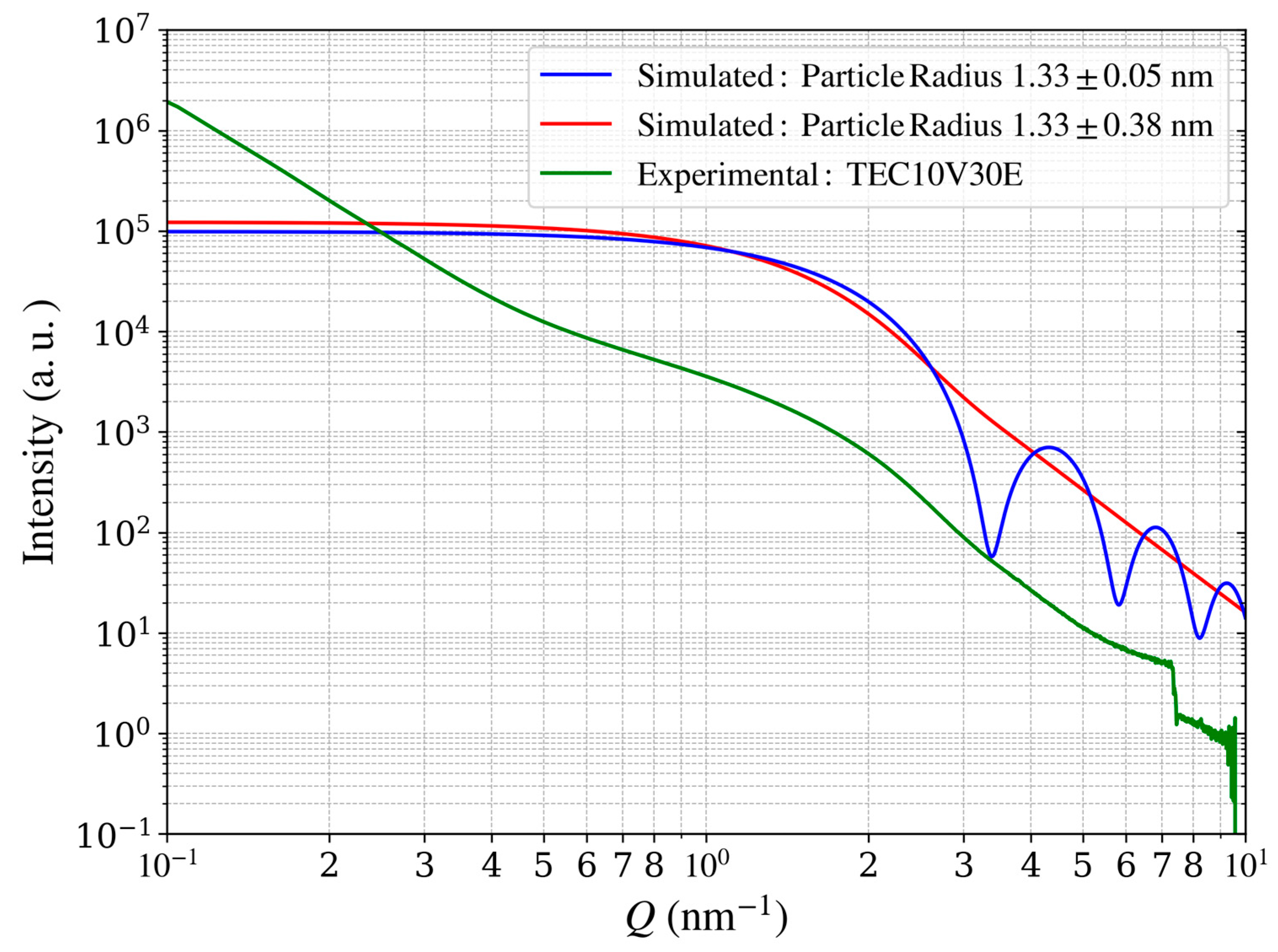
Figure 14.
Measured and simulated scattering intensity profiles of Pt catalysts. The green curve shows experimental data for carbon-supported Pt catalysts (TEC10V30E, supplied by TANAKA Precious Metals), while the blue and red curves represent simulated profiles of Pt catalysts without carbon support, sharing an identical mean particle radius (1.33 nm). Varying the radius distribution (standard deviations of 0.05 and 0.38 nm) illustrates the effect of particle size dispersion on the scattering profile.
Figure 14.
Measured and simulated scattering intensity profiles of Pt catalysts. The green curve shows experimental data for carbon-supported Pt catalysts (TEC10V30E, supplied by TANAKA Precious Metals), while the blue and red curves represent simulated profiles of Pt catalysts without carbon support, sharing an identical mean particle radius (1.33 nm). Varying the radius distribution (standard deviations of 0.05 and 0.38 nm) illustrates the effect of particle size dispersion on the scattering profile.
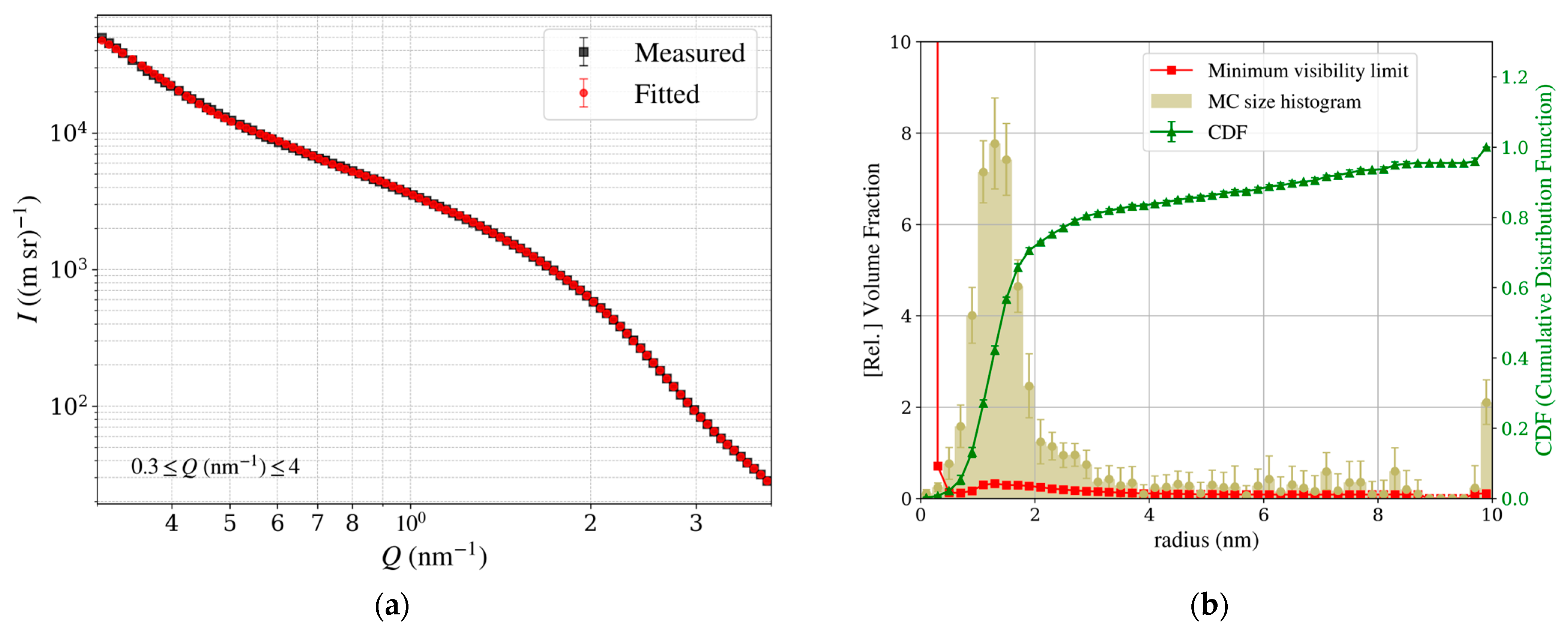
Figure 15.
Results of the McSAS analysis of the Pt particle size distribution in TEC10V30E, supplied by TANAKA Precious Metals. (a) Measured scattering profile in the Guinier region (black) with the fitted curve (red), showing excellent agreement, with the fitted curve predominantly obscuring the measured data. (b) Particle size distribution as a histogram, with a Gaussian fit yielding a mean particle radius of 1.33 ± 0.38 nm, where the value is expressed as mean ± S.D., reflecting the structural uniformity of the catalyst nanoparticles.
Figure 15.
Results of the McSAS analysis of the Pt particle size distribution in TEC10V30E, supplied by TANAKA Precious Metals. (a) Measured scattering profile in the Guinier region (black) with the fitted curve (red), showing excellent agreement, with the fitted curve predominantly obscuring the measured data. (b) Particle size distribution as a histogram, with a Gaussian fit yielding a mean particle radius of 1.33 ± 0.38 nm, where the value is expressed as mean ± S.D., reflecting the structural uniformity of the catalyst nanoparticles.
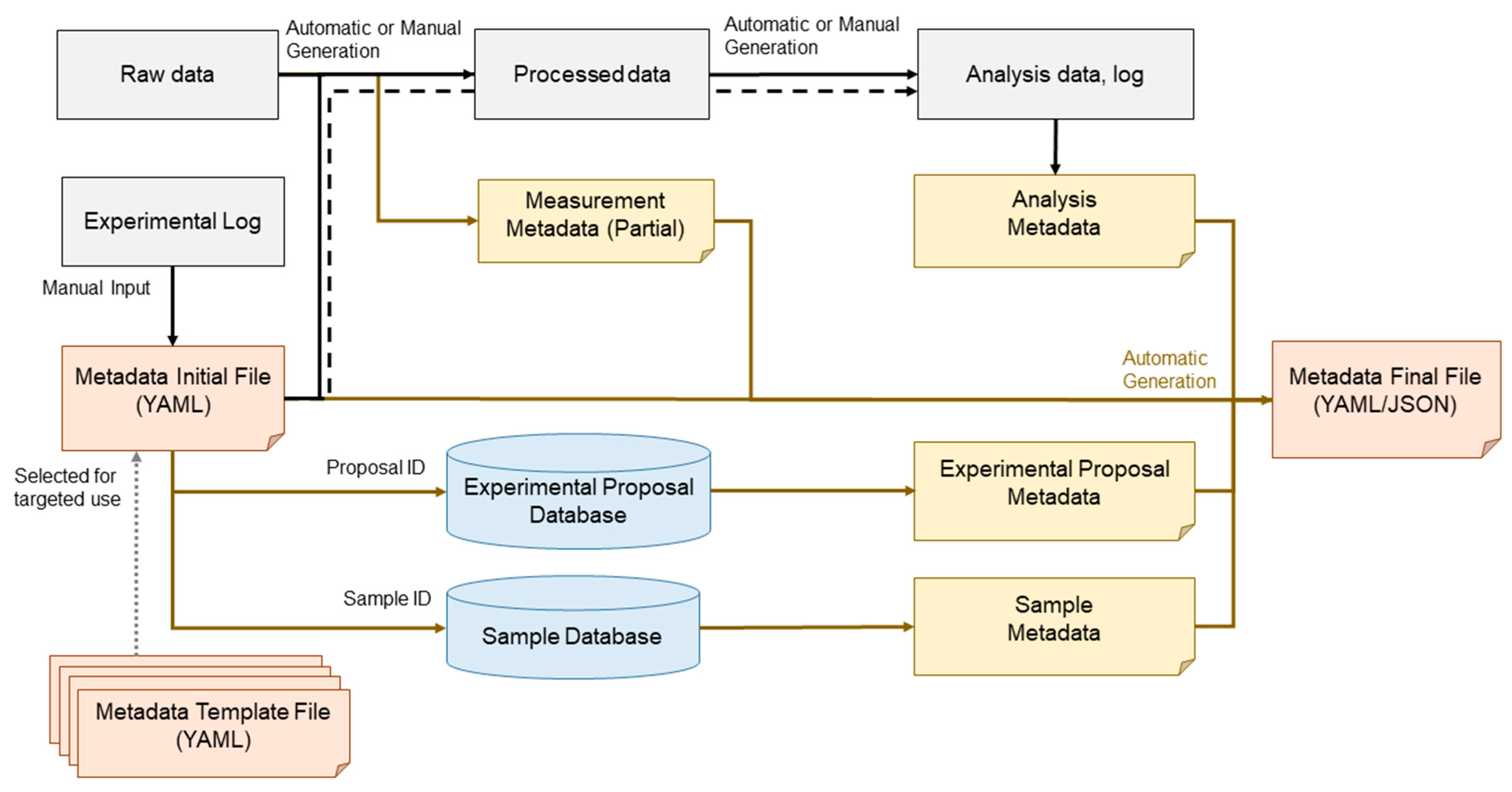
Figure 16.
Overview of metadata, processed data, and analysis data generation workflow. This figure illustrates the sequential steps from raw data acquisition to metadata annotation, data processing, and subsequent analysis, ensuring data traceability, reproducibility, and integration within the research framework.
Figure 16.
Overview of metadata, processed data, and analysis data generation workflow. This figure illustrates the sequential steps from raw data acquisition to metadata annotation, data processing, and subsequent analysis, ensuring data traceability, reproducibility, and integration within the research framework.
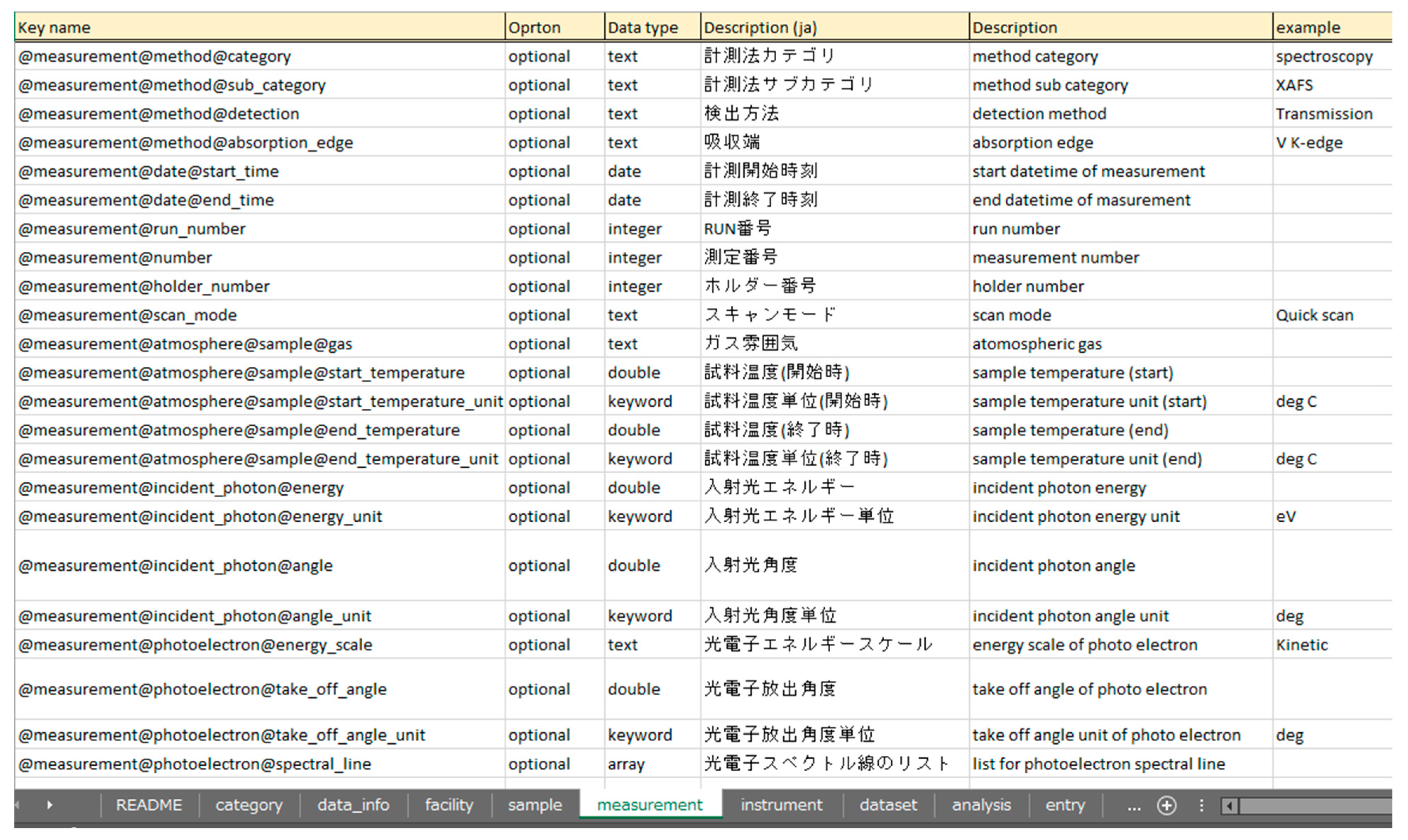
Figure 17.
Metadata schema defined in an Excel file. Each category (data_info, facility, sample, measurement, instrument, dataset, analysis, entry) is represented as a separate sheet, detailing mandatory/optional status, data types, descriptions, and usage examples. A corresponding JSON schema is automatically generated from the Excel metadata to enable streamlined validation.
Figure 17.
Metadata schema defined in an Excel file. Each category (data_info, facility, sample, measurement, instrument, dataset, analysis, entry) is represented as a separate sheet, detailing mandatory/optional status, data types, descriptions, and usage examples. A corresponding JSON schema is automatically generated from the Excel metadata to enable streamlined validation.
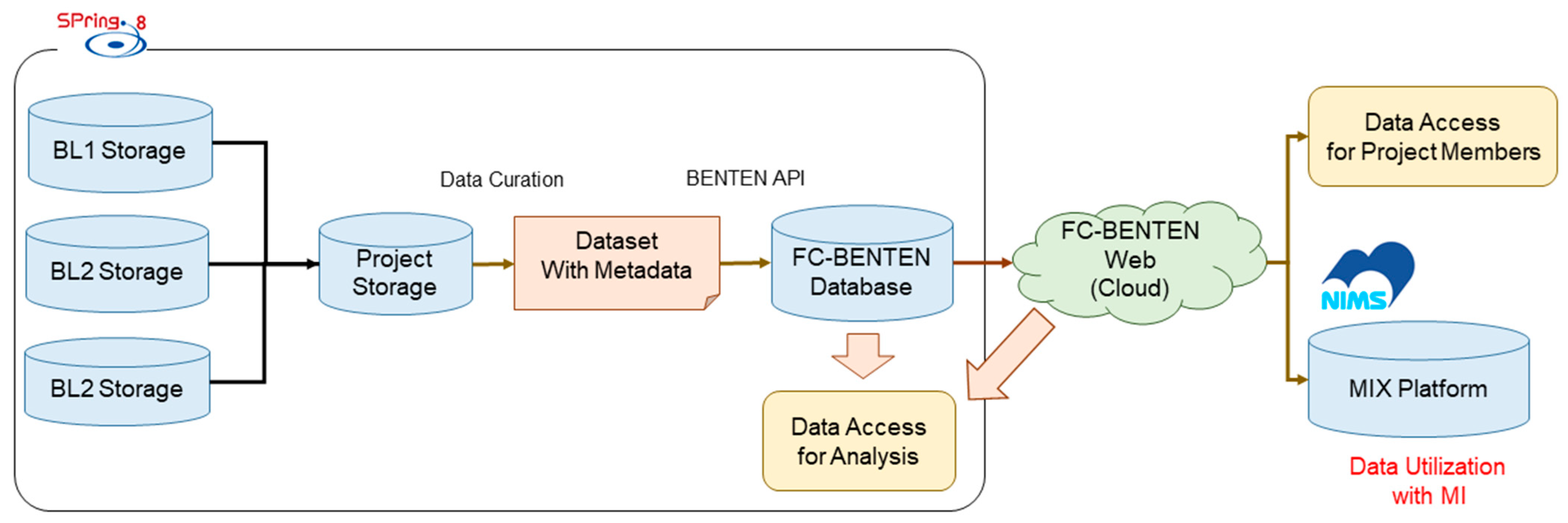
Figure 18.
Overview of data management using the BENTEN platform. Beamline-collected datasets are curated and annotated with metadata before database registration. The curated data are made available for analysis via a web interface and can be transferred to the NIMS MIX platform for advanced MI-based evaluations.
Figure 18.
Overview of data management using the BENTEN platform. Beamline-collected datasets are curated and annotated with metadata before database registration. The curated data are made available for analysis via a web interface and can be transferred to the NIMS MIX platform for advanced MI-based evaluations.
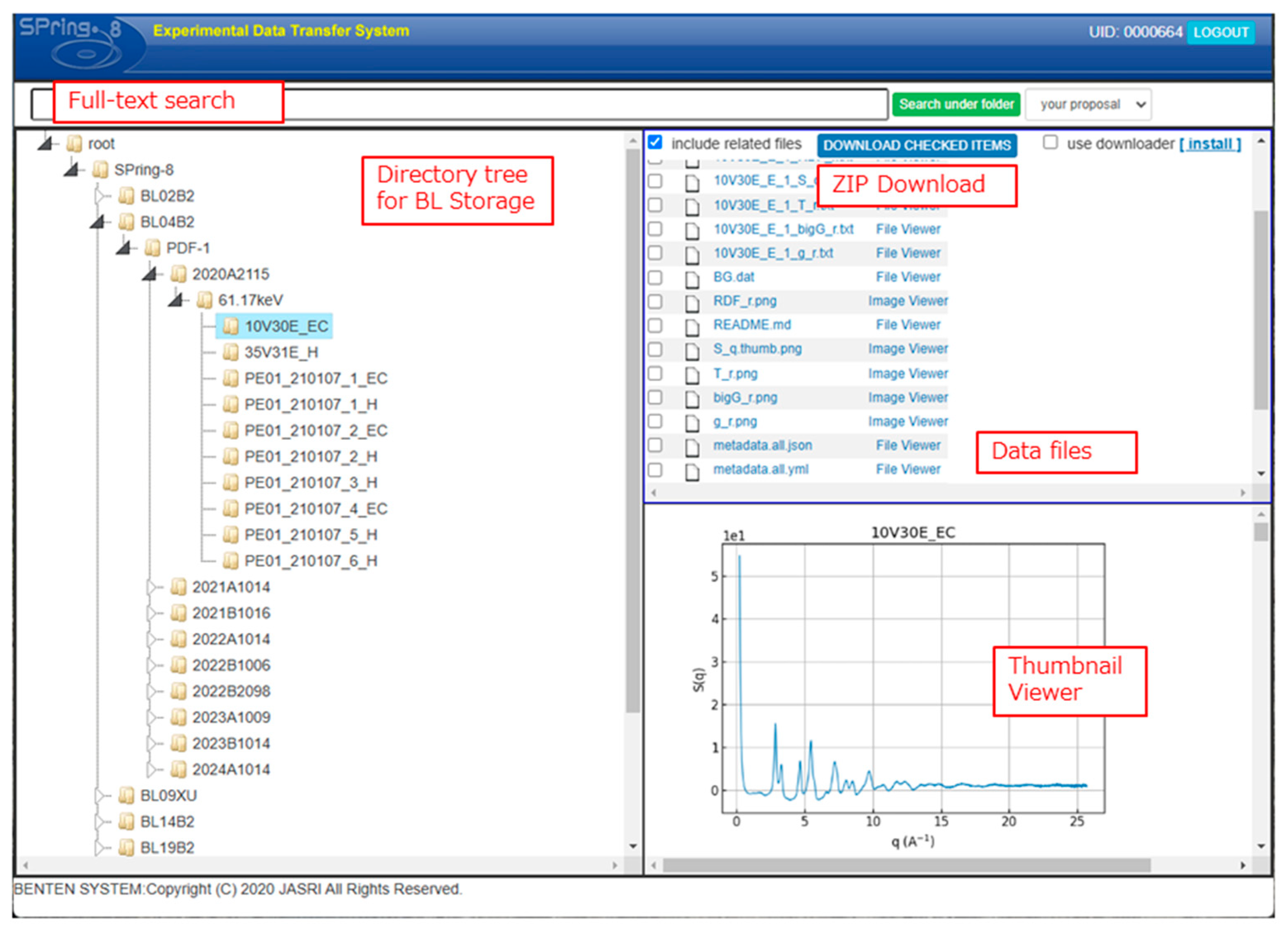
Figure 19.
FC-BENTEN web interface for data access (
https://fcbenten.jasri.jp/, accessed on 26 March 2025). The directory structure on the left panel and full-text search functionalities streamline dataset navigation. The upper-right pane lists files, while the lower-right pane displays thumbnails or spectra previews, facilitating rapid and informed dataset selection.
Figure 19.
FC-BENTEN web interface for data access (
https://fcbenten.jasri.jp/, accessed on 26 March 2025). The directory structure on the left panel and full-text search functionalities streamline dataset navigation. The upper-right pane lists files, while the lower-right pane displays thumbnails or spectra previews, facilitating rapid and informed dataset selection.
Table 2.
Metadata elements categorized by sample type, focusing on those submitted for analysis. This table identifies required metadata fields common to all samples as well as those specific to catalysts and electrolyte membranes. Comprehensive metadata ensures accurate documentation of sample characteristics and experimental context.
Table 2.
Metadata elements categorized by sample type, focusing on those submitted for analysis. This table identifies required metadata fields common to all samples as well as those specific to catalysts and electrolyte membranes. Comprehensive metadata ensures accurate documentation of sample characteristics and experimental context.
| Category | Element | Remarks |
|---|
| Common | Sample ID | Unique identifier for the sample |
| Sample Name | Designated name of the sample |
| Sample Preparation Date | Date the sample was prepared |
| Responsible Person | Name and affiliation of the person responsible for the sample |
| Remarks | Additional notes or observations |
| For Catalysts | Catalyst Structure | Structural details of the catalyst |
| Catalyst Loading | Weight percentage (wt%) |
| Elemental Composition | Composition of elements in the catalyst |
| Crystal Structure and Homogeneity | Information on crystallinity and uniformity |
| Catalyst Particle Size | Average particle size (nm) |
| Support Material and Surface Treatment | Type of support material and any applied surface treatments |
| Loading Method | Method used to load the catalyst onto the support |
| Synthesis Conditions | Raw materials and synthesis details |
| Additives and Surface Treatments | Details of additives, surface modifications, and their quantities |
| Post-Treatment Processes | Information on processes such as heat or acid treatments |
| Aging Conditions | Presence and details of any aging treatment |
| For Electrolyte Membranes | Chemical Structure | Molecular structure of the material |
| Membrane Structure | Morphology of the membrane |
| Membrane Thickness | Measured in micrometers (μm) |
| Ion Exchange Capacity | Equivalent weight (EW) |
| Membrane Resistance | Electrical resistance (Ω·cm2) |
| Gas Permeability | Permeability in cm3/(cm2·s·kPa) |
| Glass Transition/Softening Temperature | Temperature (°C) |
| Water Content and Volume Expansion | Hydration levels and volumetric changes (%) |
| Durability | Durability metrics under specified conditions |
Table 3.
Selected analytical metadata and utilized data for key measurement techniques. This table summarizes key analytical metadata elements (e.g., coordination number, binding energy, lattice constant, particle size distribution) and the corresponding utilized data (e.g., normalized μt, intensity profiles, G(r)), highlighting the information derived from each measurement technique and its role in data analysis and interpretation.
Table 3.
Selected analytical metadata and utilized data for key measurement techniques. This table summarizes key analytical metadata elements (e.g., coordination number, binding energy, lattice constant, particle size distribution) and the corresponding utilized data (e.g., normalized μt, intensity profiles, G(r)), highlighting the information derived from each measurement technique and its role in data analysis and interpretation.
| Measurement Technique | Analytical Metadata Elements | Utilized Data |
|---|
| XAFS | Coordination Number, Bond Distance, Debye–Waller Factor, R factor | Normalized μt, χ(k), χ(R) |
| HAXPES | Binding Energy Peak and Width (FWHM), d-band center | Binding Energy Profile |
| XRD | Crystal Structure Phase, Lattice Constant, Atomic Displacement, Occupancy Rate, Crystallite Diameter, Rwp, S | Intensity Profile |
| PDF | Interatomic Distance, Relative Coordination Number, Peak Area (1st–5th peaks) from T(r) | S(Q), G(r), T(r), g(r), RDF(r) |
| SAXS | Particle Radius, Size Distribution, and Width | Intensity Profile and Particle Size Distribution |
Table 4.
Overview of datasets in the FC-BENTEN database, showcasing its comprehensive data coverage and research utility. This table summarizes the number of analysis-requested samples, corresponding datasets, and total datasets collected using various measurement techniques, including XAFS, HAXPES, XRD, PDF, SAXS, and CV. The “Total” for “Number of Analysis-Requested Samples” (567) includes overlapping samples that were analyzed using multiple measurement techniques. When excluding such overlaps, the number of unique samples amounts to 202. The data exemplify the database’s systematic approach to capturing diverse analytical parameters, supporting advanced research into the structural and electronic properties of PEFC materials.
Table 4.
Overview of datasets in the FC-BENTEN database, showcasing its comprehensive data coverage and research utility. This table summarizes the number of analysis-requested samples, corresponding datasets, and total datasets collected using various measurement techniques, including XAFS, HAXPES, XRD, PDF, SAXS, and CV. The “Total” for “Number of Analysis-Requested Samples” (567) includes overlapping samples that were analyzed using multiple measurement techniques. When excluding such overlaps, the number of unique samples amounts to 202. The data exemplify the database’s systematic approach to capturing diverse analytical parameters, supporting advanced research into the structural and electronic properties of PEFC materials.
| Measurement Technique | Number of Analysis-Requested Samples | Number of Datasets for Analysis-Requested Samples | Total Number of Datasets |
|---|
| XAFS | 79 | 196 | 272 |
| HAXPES | 85 | 873 | 1298 |
| XRD | 144 | 234 | 358 |
| PDF | 110 | 178 | 235 |
| SAXS | 128 | 205 | 366 |
| CV (Electrochemical) | 21 | 21 | 43 |
| Total | 567 | 1707 | 2572 |
Table 5.
Sample list showing Pt (wt%), Co (wt%), C (wt%), and carbon support types for the analyzed materials. All samples were obtained from TANAKA Precious Metals. Samples TEC35V31E, TEC36E52, and TEC36F52 were Pt–Co-based, whereas the other samples were Pt-based. The Carbon supports were classified as solid spheres (Vulcan XC72, Acetylene black) or hollow spheres (Ketjenblack, Graphitized Ketjenblack).
Table 5.
Sample list showing Pt (wt%), Co (wt%), C (wt%), and carbon support types for the analyzed materials. All samples were obtained from TANAKA Precious Metals. Samples TEC35V31E, TEC36E52, and TEC36F52 were Pt–Co-based, whereas the other samples were Pt-based. The Carbon supports were classified as solid spheres (Vulcan XC72, Acetylene black) or hollow spheres (Ketjenblack, Graphitized Ketjenblack).
| Sample Name | Pt (wt%) | Co (wt%) | C (wt%) | Carbon Support |
|---|
| TEC10V30E | 28.9 | 0.0 | 71.1 | Vulcan XC72 |
| TEC10V50E | 46.5 | 0.0 | 53.5 | Vulcan XC72 |
| TEC10E30E | 30.0 | 0.0 | 70.0 | Vulcan XC72 |
| TEC10E50E | 46.3 | 0.0 | 53.7 | Ketjenblack |
| TEC10EA50E | 46.8 | 0.0 | 53.2 | Graphitized Ketjenblack |
| TEC10F30E | 27.2 | 0.0 | 72.8 | Acetylene black |
| TEC10F50E | 46.3 | 0.0 | 53.7 | Acetylene black |
| TEC35V31E | 29.6 | 2.2 | 68.2 | Vulcan XC72 |
| TEC36E52 | 46.2 | 5.2 | 48.6 | Ketjenblack |
| TEC36F52 | 47.4 | 4.9 | 47.7 | Acetylene black |