Tuning BMI.NTf2 Ionic Liquid Concentration in Dental Adhesives Towards a Rational Design of Antibacterial Materials
Abstract
1. Introduction
2. Materials and Methods
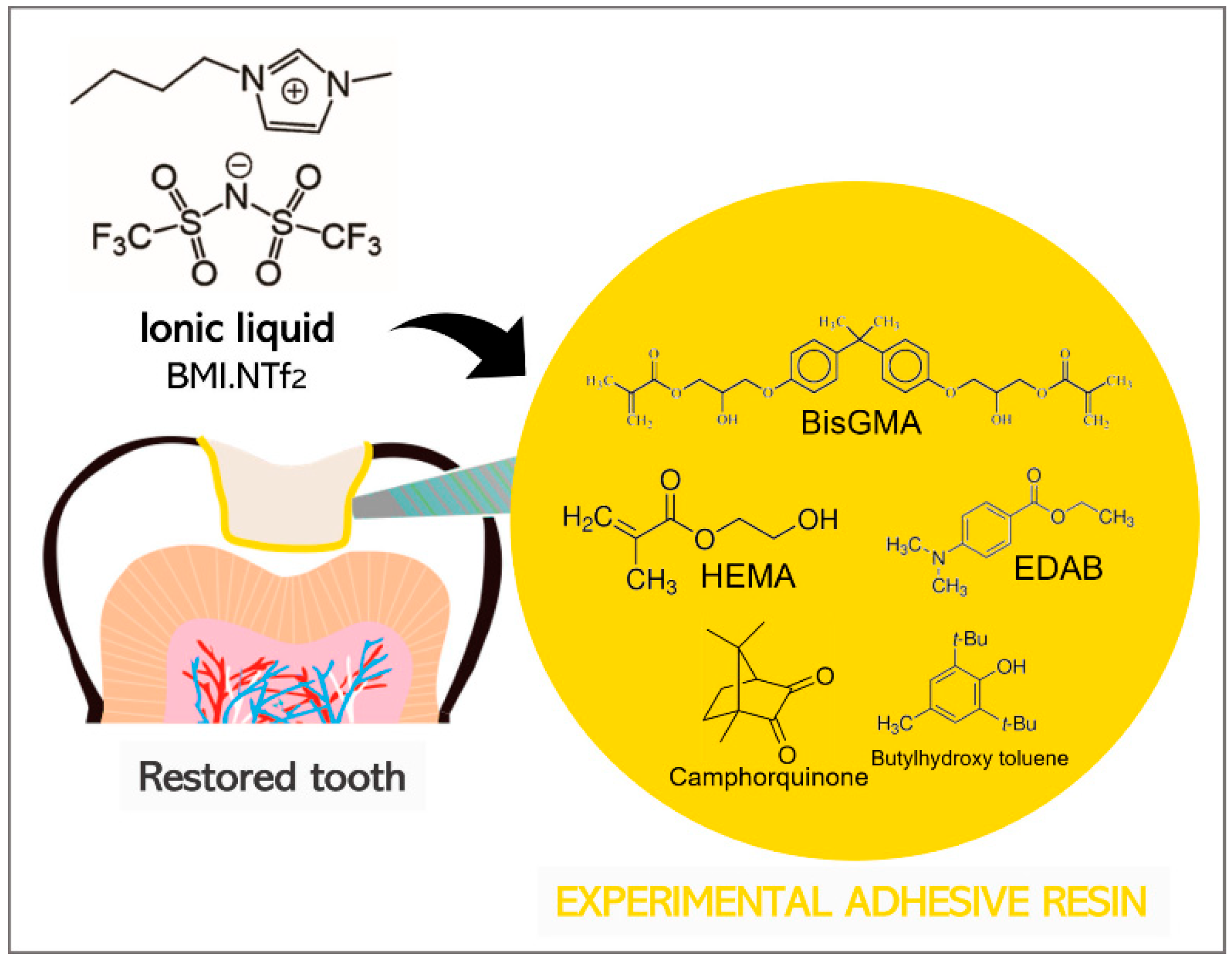
2.1. Formulation of Adhesive Resin Samples
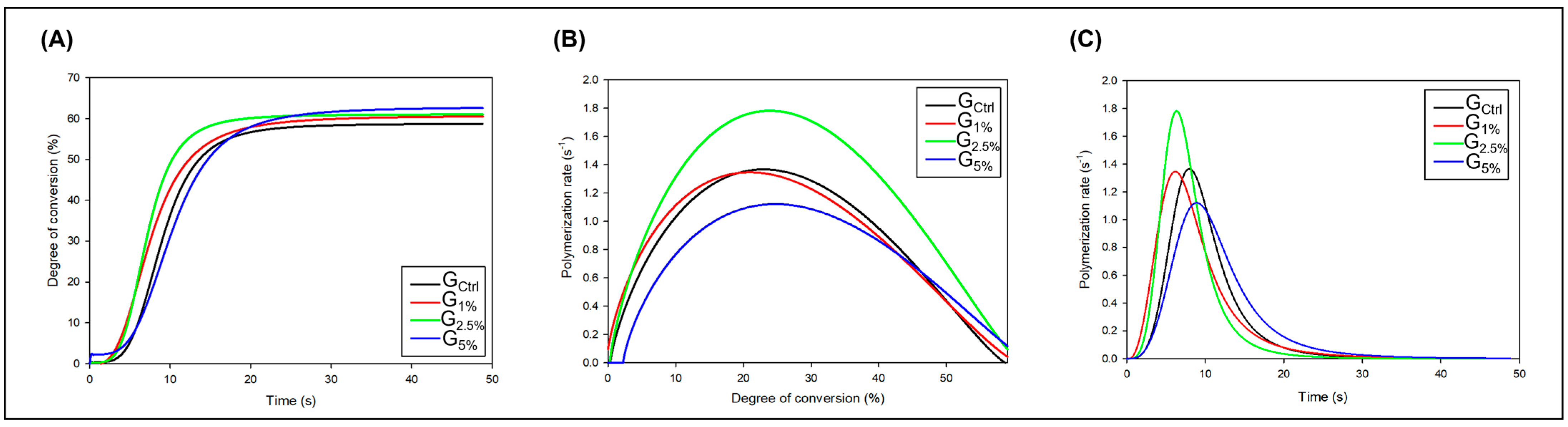
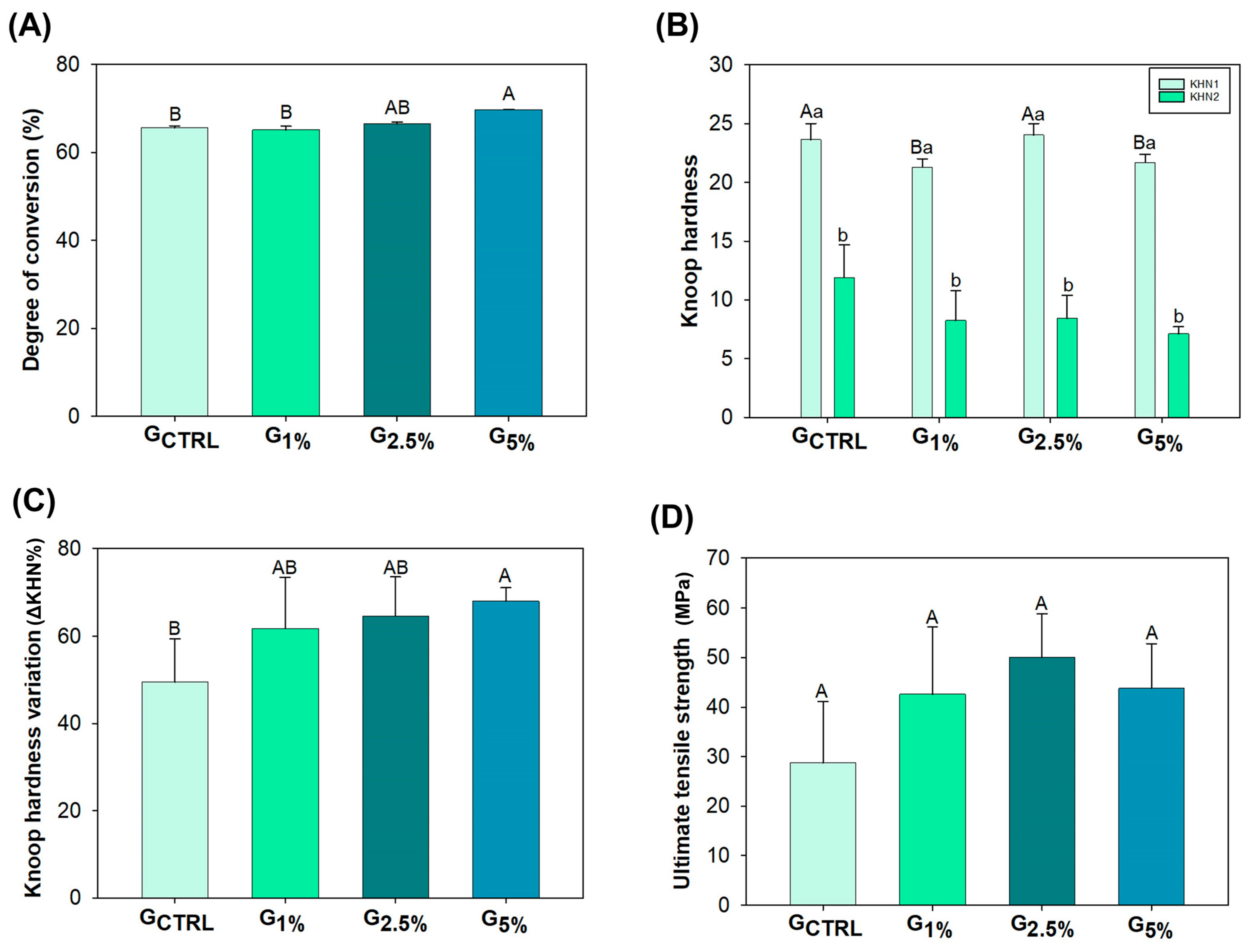
2.2. Polymerization Kinetics and Degree of Conversion (DC)
2.3. Softening in Solvent
2.4. Ultimate Tensile Strength
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing Bioactive Dental Resins for Restorative Dentistry. J. Dent. Res. 2023, 102, 220345231182357. [Google Scholar]
- Mallineni, S.K.; Sakhamuri, S.; Kotha, S.L.; AlAsmari, A.R.G.M.; AlJefri, G.H.; Almotawah, F.N.; Mallineni, S.; Sajja, R. Silver nanoparticles in dental applications: A descriptive review. Bioengineering 2023, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar]
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.P.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth-Adhesive Interface. Dent. Clin. N. Am. 2017, 61, 713–731. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano-and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar]
- Pendleton, J.N.; Gilmore, B.F. The antimicrobial potential of ionic liquids: A source of chemical diversity for infection and biofilm control. Int. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar]
- Weingärtner, H. Understanding ionic liquids at the molecular level: Facts, problems, and controversies. Angew. Chem. Int. Ed. Engl. 2008, 47, 654–670. [Google Scholar]
- Kitagawa, H.; Izutani, N.; Kitagawa, R.; Maezono, H.; Yamaguchi, M.; Imazato, S. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J. Dent. 2016, 47, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Souza, V.S.; Hellriegel, C.; Scholten, J.D.; Collares, F.M. Ionic liquid–stabilized titania quantum dots applied in adhesive resin. J. Dent. Res. 2019, 98, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Ferreira, C.J.; de Souza, V.S.; Leitune, V.C.B.; Samuel, S.M.W.; de Souza Balbinot, G.; de Souza da Motta, A.; Visioli, F.; Scholten, J.D.; Collares, F.M. Ionic liquid as antibacterial agent for an experimental orthodontic adhesive. Dent. Mater. 2019, 35, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Tu, Y.; Ren, H.; He, Y.; Ying, J.; Chen, Y. Interaction between microorganisms and dental material surfaces: General concepts and research progress. J. Oral Microbiol. 2023, 15, 2196897. [Google Scholar] [CrossRef]
- Bendary, I.M.; Garcia, I.M.; Collares, F.M.; Takimi, A.; Samuel, S.M.W.; Leitune, V.C.B. Wollastonite as filler of an experimental dental adhesive. J. Dent. 2020, 102, 103472. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef]
- Fugolin, A.P.P.; Pfeifer, C.S. New resins for dental composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Moraes, R.R.; Cavalcante, L.M.; Sinhoreti, M.A.C.; Correr-Sobrinho, L.; Consani, S. Cross-link density evaluation through softening tests: Effect of ethanol concentration. Dent. Mater. 2008, 24, 199–203. [Google Scholar] [CrossRef]
- Filho, J.D.N.; Poskus, L.T.; Guimarães, J.G.A.; Barcellos, A.A.L.; Silva, E.M. Degree of conversion and plasticization of dimethacrylate-based polymeric matrices: Influence of light-curing mode. J. Oral Sci. 2008, 50, 315–321. [Google Scholar] [CrossRef][Green Version]
- Selvaraj, M.; Mohaideen, K.; Sennimalai, K.; Gothankar, G.S.; Arora, G. Effect of oral environment on contemporary orthodontic materials and its clinical implications. J. Orthod. Sci. 2023, 12, 1. [Google Scholar] [PubMed]
- Hossain, M.I.; Shams, A.B.; Das Gupta, S.; Blanchard, G.J.; Mobasheri, A.; Hoque Apu, E. The Potential Role of Ionic Liquid as a Multifunctional Dental Biomaterial. Biomedicines 2023, 11, 3093. [Google Scholar] [CrossRef] [PubMed]
- Alluhaidan, T.; Qaw, M.; Garcia, I.M.; Montoya, C.; Orrego, S.; Melo, M.A. Seeking Endurance: Designing Smart Dental Composites for Tooth Restoration. Designs 2024, 8, 92. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Carvalho, E.O.; Correia, D.M.; Esperança, J.M.S.S.; Padrão, J.; Ivanova, K.; Hoyo, J.; Tzanov, T.; Lanceros-Mendez, S. Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli. ACS Appl. Bio Mater. 2022, 5, 5181–5189. [Google Scholar] [CrossRef]
- Hassanpour, M.; Torabi, S.M.; Afshar, D.; Kowsari, M.H.; Meratan, A.A.; Nikfarjam, N. Tracing the Antibacterial Performance of Bis-Imidazolium-based Ionic Liquid Derivatives. ACS Appl. Bio Mater. 2024, 7, 1558–1568. [Google Scholar] [CrossRef]
- Ben, C.; He, D.; Wu, Q.; Zhao, S.; Liu, D.; Li, S.; Guo, F.; Li, C.; Jin, L.; Wang, Q.; et al. Regulation of nanotopography of ionic liquid gel particles through the introduction of a second network and its impact on antibacterial properties. J. Colloid Interface Sci. 2025, 686, 864–877. [Google Scholar] [CrossRef]
- Bedair, H.M.; Hamed, M.; Mansour, F.R. New emerging materials with potential antibacterial activities. Appl. Microbiol. Biotechnol. 2024, 108, 515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, I.M.; Simionato, A.; Souza, V.S.; Scholten, J.D.; Melo, M.A.S.; Collares, F.M. Tuning BMI.NTf2 Ionic Liquid Concentration in Dental Adhesives Towards a Rational Design of Antibacterial Materials. Appl. Sci. 2025, 15, 3810. https://doi.org/10.3390/app15073810
Garcia IM, Simionato A, Souza VS, Scholten JD, Melo MAS, Collares FM. Tuning BMI.NTf2 Ionic Liquid Concentration in Dental Adhesives Towards a Rational Design of Antibacterial Materials. Applied Sciences. 2025; 15(7):3810. https://doi.org/10.3390/app15073810
Chicago/Turabian StyleGarcia, Isadora Martini, Andressa Simionato, Virgínia Serra Souza, Jackson Damiani Scholten, Mary Anne Sampaio Melo, and Fabrício Mezzomo Collares. 2025. "Tuning BMI.NTf2 Ionic Liquid Concentration in Dental Adhesives Towards a Rational Design of Antibacterial Materials" Applied Sciences 15, no. 7: 3810. https://doi.org/10.3390/app15073810
APA StyleGarcia, I. M., Simionato, A., Souza, V. S., Scholten, J. D., Melo, M. A. S., & Collares, F. M. (2025). Tuning BMI.NTf2 Ionic Liquid Concentration in Dental Adhesives Towards a Rational Design of Antibacterial Materials. Applied Sciences, 15(7), 3810. https://doi.org/10.3390/app15073810








