Hematopoietic Stem Cells of Bone Marrow and Their Total RNA in Rat Liver Regeneration
Abstract
1. Introduction
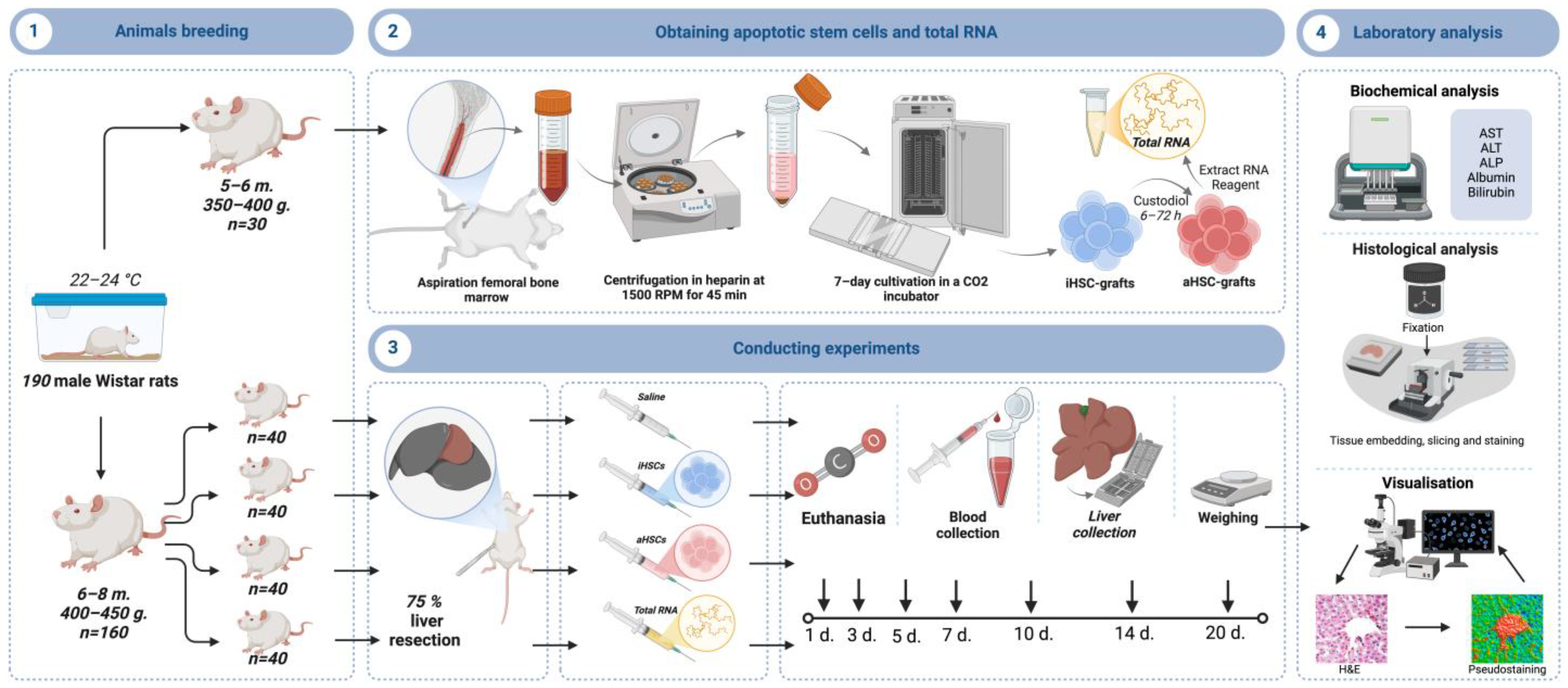
2. Materials and Methods
2.1. Animal Care and Extended Liver Resection
2.2. Stem Cell Obtaining
2.3. Obtaining Apoptotic Stem Cells and Total RNA
2.4. Experimental Design
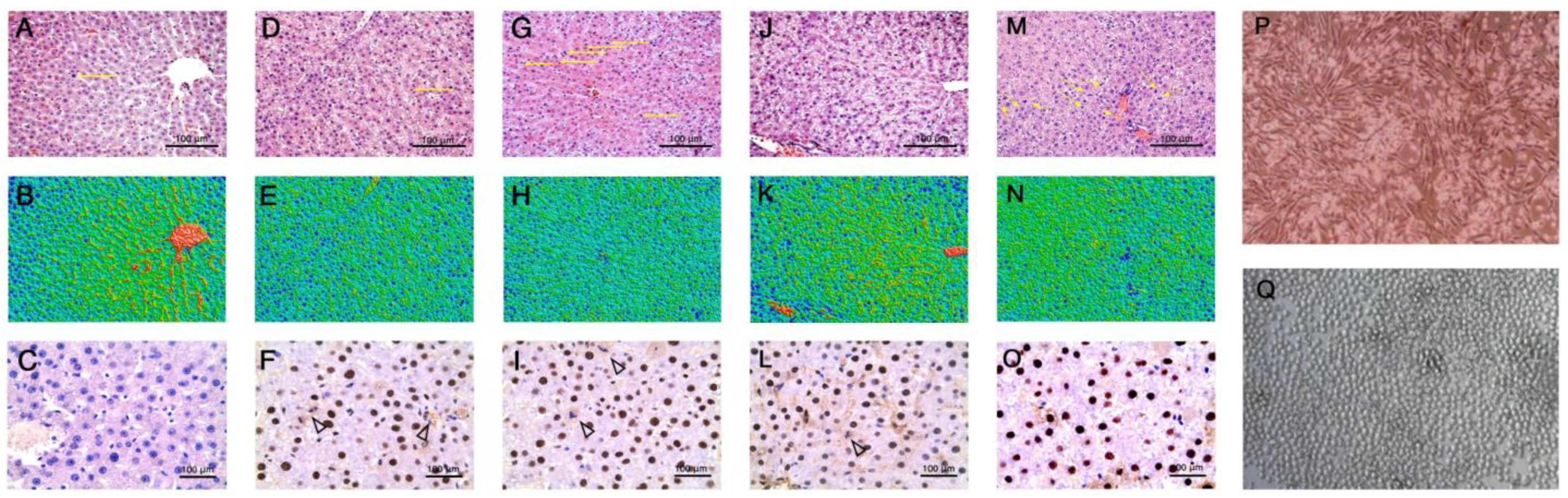
2.5. Histological Study
2.6. Statistics and Reproducibility
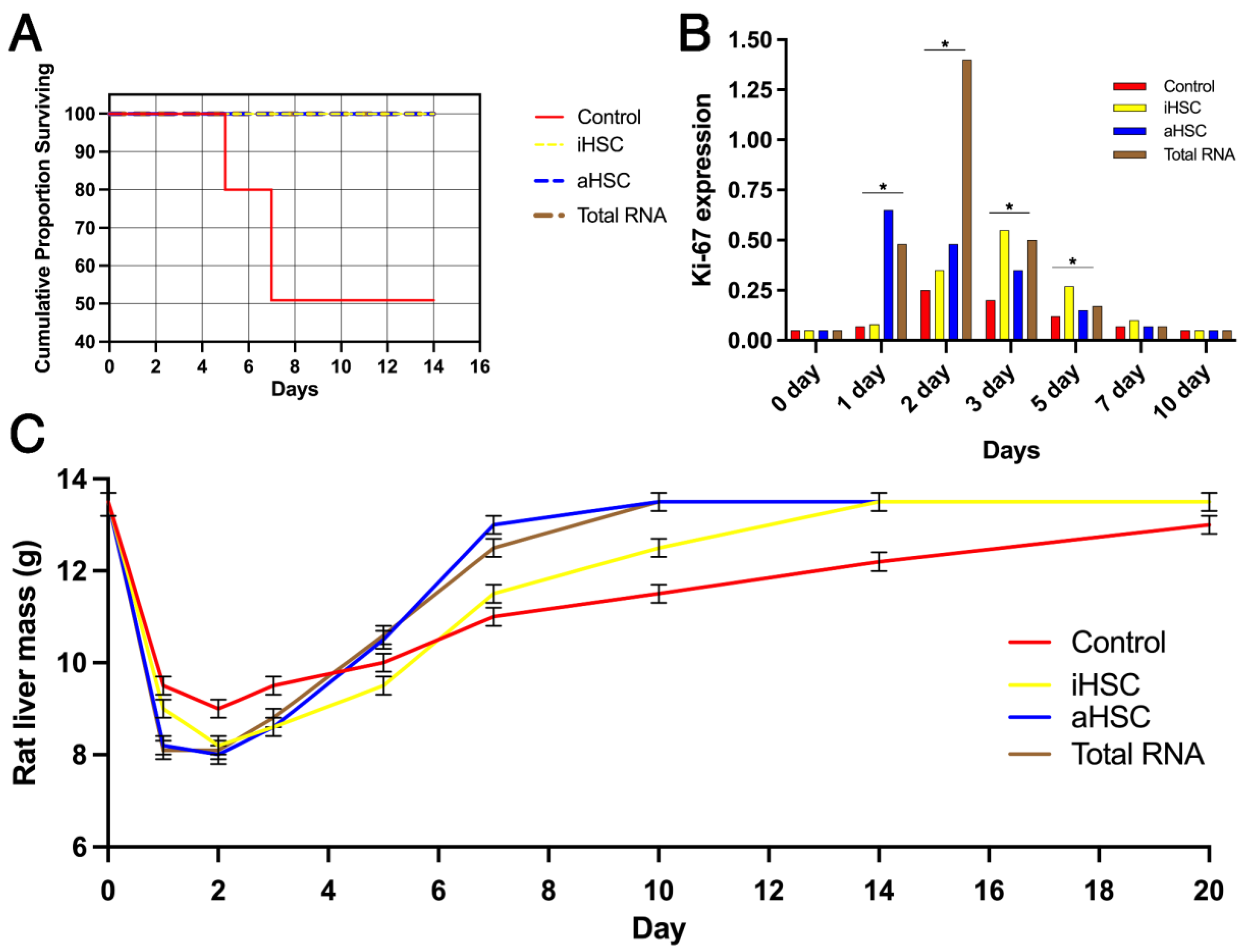
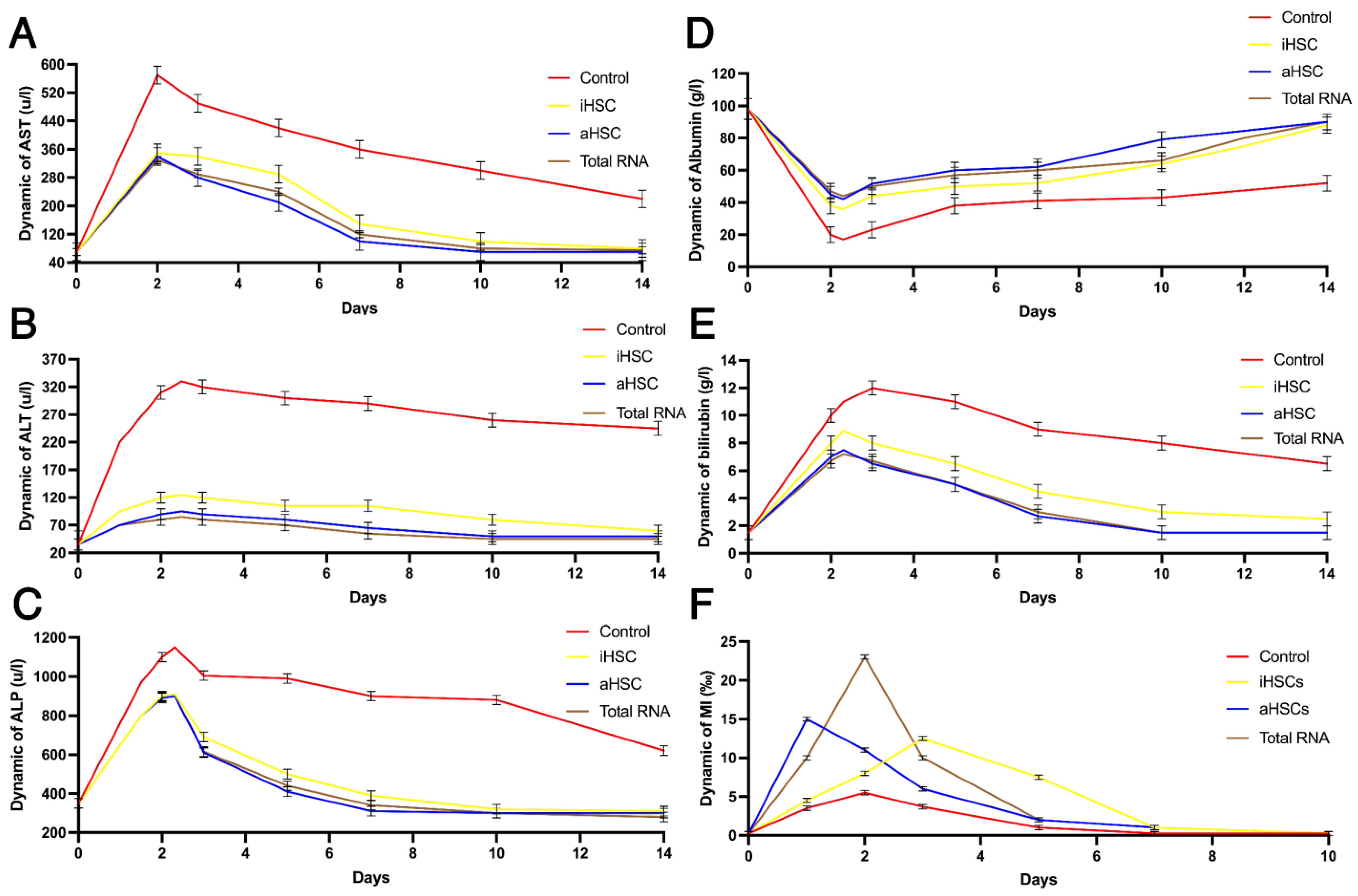
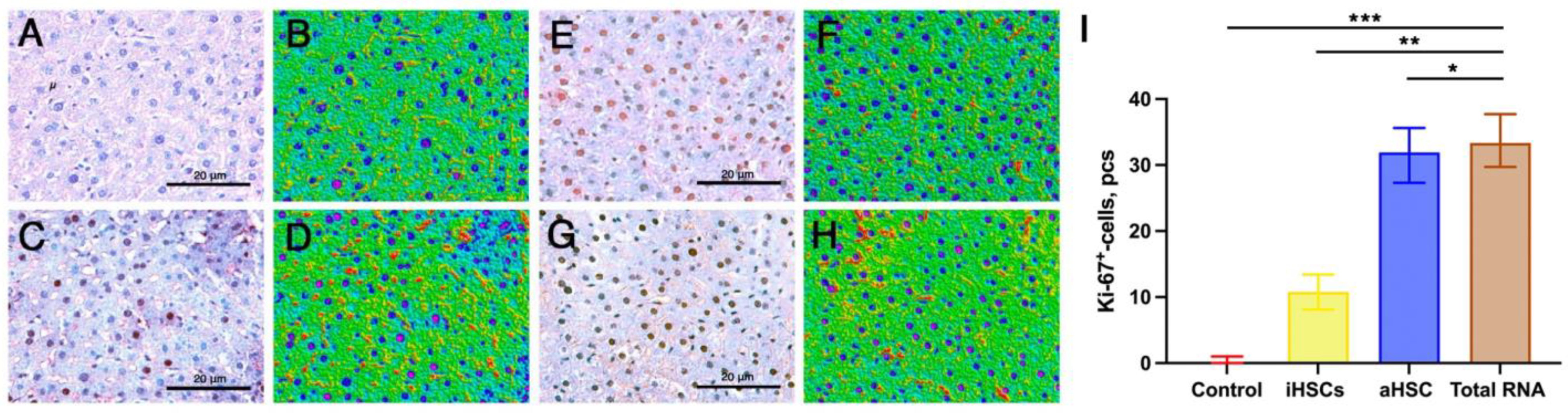
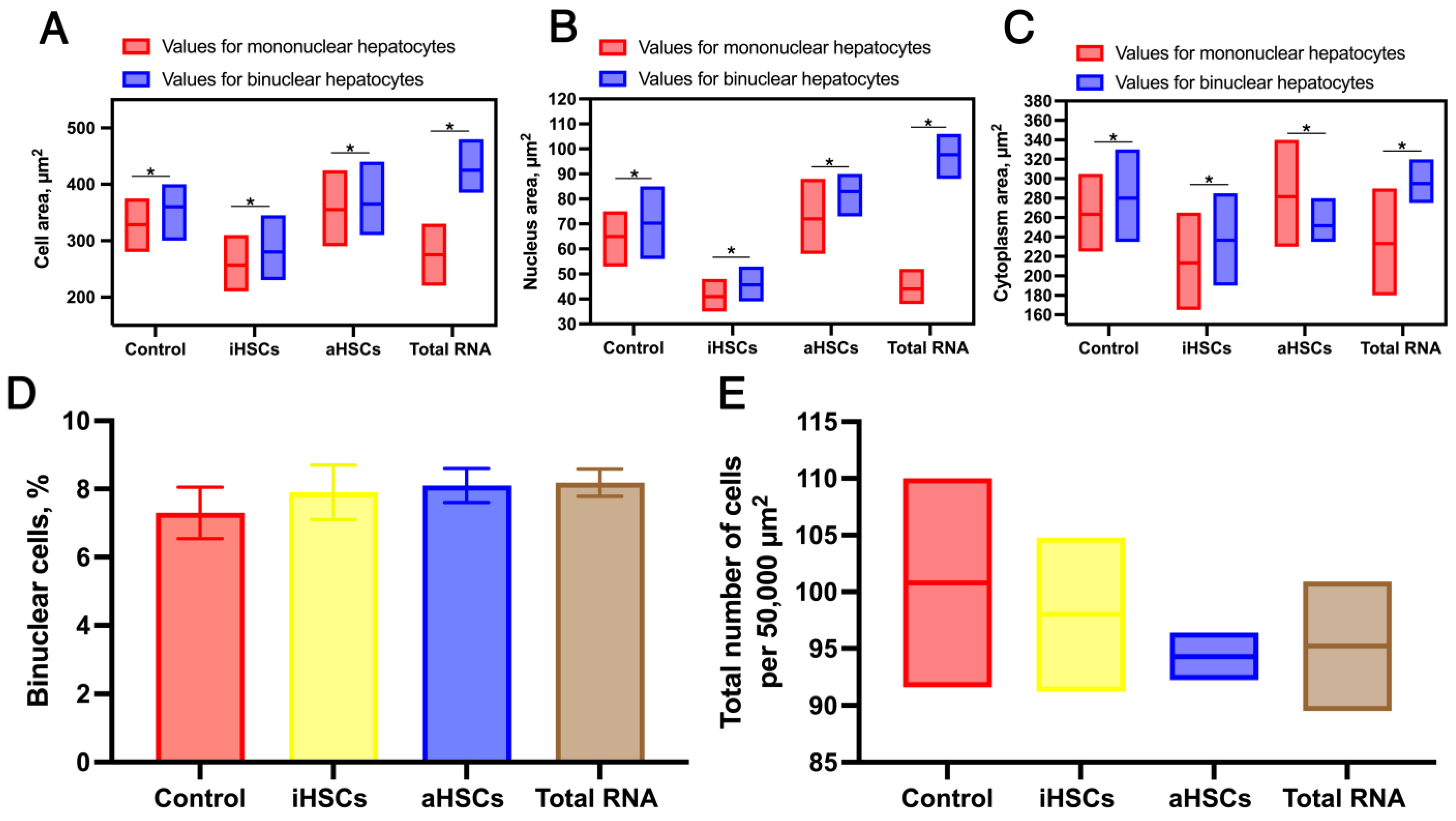
3. Results
4. Discussion
Limitations and Perspectives
- The study aimed to compare different HSC-originating modes to enhance regeneration. Thus, the methodology applied is not relevant for revealing all the mechanisms of HSC communication, and the ratio between paracrine, direct, and immune-mediated machinery remains unclear. An analysis of the RNA sequence in new proliferating cells in the liver after HSC-derived total RNA would help to elucidate the problematics.
- The study has not implied assessment of specific markers’ content by cellular and molecular methods, e.g., immunoblotting. These approaches are planned for future experiments in the field.
- The tumorigenic potential of aHSCs and total RNA is challenging. The experiment is to be widened for rat follow-up for several consecutive months after the liver recovery to estimate the rate of oncological risks before extrapolation to humans.
- Clinical implementation of the study results is limited due to legislative procedures. For instance, human stem cells are restricted for usage in certain countries’ jurisdictions, and the studies should absolutely respect the restrictions before planning any human extrapolation.
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.F.; Savage, T.M.; Ringham, O.R.; Arpaia, N. Treg-tissue cell interactions in repair and regeneration. J. Exp. Med. 2024, 221, e20231244. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, A.G.; Tishevskaya, N.V.; Gevorkyan, N.M. On the Morphogenetic Properties of RNA of Lymphoid and Stem Cells in Recovery Processes; RAMS, Scientific Research Institute of Human Morphology: Moscow, Russia, 2016. [Google Scholar]
- Tishevskaya, N.V.; Babaeva, A.G.; Gevorkyan, N.M. The role of lymphocytic RNA in intercellular information. Exchange and regulation of regenerative processes. Ross. Fiziol. Zhurnal Im. I.M. Sechenova 2016, 102, 1280–1301. (In Russian) [Google Scholar]
- Than, N.N.; Newsome, P.N. Stem cells for liver regeneration. QJM Mon. J. Assoc. Physicians 2014, 107, 417–421. [Google Scholar] [CrossRef]
- Liu, F.; Pan, X.; Chen, G.; Jiang, D.; Cong, X.; Fei, R.; Wei, L. Hematopoietic stem cells mobilized by granulocyte colony-stimulating factor partly contribute to liver graft regeneration after partial orthotopic liver transplantation. Liver Transplant. 2006, 12, 1129–1137. [Google Scholar] [CrossRef]
- Tsolaki, E.; Athanasiou, E.; Gounari, E.; Zogas, N.; Siotou, E.; Yiangou, M.; Anagnostopoulos, A.; Yannaki, E. Hematopoietic stem cells and liver regeneration: Differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells Mol. Dis. 2014, 53, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.; De, A.; Verma, N.; Kumari, S.; Leishangthem, B.; Sharma, R.R.; Kalra, N.; Grover, S.; Singh, V. Multiple cycles of granulocyte colony-stimulating factor in decompensated cirrhosis: A double-blind RCT. Hepatol. Int. 2022, 16, 1127–1136. [Google Scholar] [CrossRef]
- Siapati, E.K.; Roubelakis, M.G.; Vassilopoulos, G. Liver regeneration by hematopoietic stem cells: Have we reached the end of the road? Cells 2022, 11, 2312. [Google Scholar] [CrossRef]
- Bird, T.G.; Müller, M.; Boulter, L.; Vincent, D.F.; Ridgway, R.A.; Lopez-Guadamillas, E.; Lu, W.Y.; Jamieson, T.; Govaere, O.; Campbell, A.D.; et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 2018, 10, eaan1230. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ke, M.; Wang, T.; Wang, M.; Liu, W.; Du, Z.; Ren, Y.; Zhang, S.; Wu, Z.; et al. HSF2BP protects against acute liver injury by regulating HSF2/HSP70/MAPK signaling in mice. Cell Death Dis. 2022, 13, 830. [Google Scholar] [CrossRef]
- Fathi, E.; Valipour, B.; Jafari, S.; Kazemi, A.; Montazersaheb, S.; Farahzadi, R. The role of the hematopoietic stem/progenitor cells-derived extracellular vesicles in hematopoiesis. Heliyon 2024, 10, e35051. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Terashima, T.; Katagi, M.; Ohashi, N.; Nozaki, K.; Tsuji, A. Bone marrow-derived mononuclear cells ameliorate neurological function in chronic cerebral infarction model mice via improvement of cerebral blood flow. Cytotherapy 2023, 25, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Bauersachs, J.; Poole-Wilson, P.A.; Volk, H.D.; Anker, S.D. The dying stem cell hypothesis: Immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J. Am. Coll. Cardiol. 2005, 46, 1799–1802. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Beer, L.; Zimmermann, M.; Mitterbauer, A.; Ellinger, A.; Gruber, F.; Narzt, M.S.; Zellner, M.; Gyöngyösi, M.; Madlener, S.; Simader, E.; et al. Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration. Sci. Rep. 2015, 5, 16662. [Google Scholar] [CrossRef]
- Ankersmit, H.J.; Hoetzenecker, K.; Dietl, W.; Soleiman, A.; Horvat, R.; Wolfsberger, M.; Gerner, C.; Hacker, S.; Mildner, M.; Moser, B.; et al. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur. J. Clin. Investig. 2009, 39, 445–456. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef]
- Sirois, I.; Raymond, M.A.; Brassard, N.; Cailhier, J.F.; Fedjaev, M.; Hamelin, K.; Londono, I.; Bendayan, M.; Pshezhetsky, A.V.; Hébert, M.J. Caspase-3-dependent export of TCTP: A novel pathway for antiapoptotic intercellular communication. Cell Death Differ. 2011, 18, 549–562. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Huleihel, L.; Scarritt, M.E.; Badylak, S.F. The Influence of Extracellular RNA on Cell Behavior in Health, Disease and Regeneration. Curr. Pathobiol. Rep. 2017, 5, 13–22. [Google Scholar] [CrossRef]
- Gonikova, Z.Z.; Nikolskaya, A.O.; Kirsanova, L.A.; Shagidulin, M.Y.; Onischenko, N.A.; Sevastjanov, V.I. Investigation of regenerative and tissue-specific activity of tot al RNA of bone marrow cells. Russ. J. Transplantology Artif. Organs 2018, 20, 64–69. (In Russian) [Google Scholar] [CrossRef]
- Gonikova, Z.Z.; Nikolskaya, A.O.; Kirsanova, L.A.; Shagidulin, M.Y.; Onischenko, N.A.; Sevastjanov, V.I. The comparative analysis of the effectiveness of stimulation of liver regeneration by bone marrow cells and total RNA of these cells. Russ. J. Transplantology Artif. Organs 2019, 21, 113–121. (In Russian) [Google Scholar] [CrossRef]
- Geng, X.; Chang, C.; Zang, X.; Sun, J.; Li, P.; Guo, J.; Xu, G. Integrative proteomic and microRNA analysis of the priming phase during rat liver regeneration. Gene 2016, 575, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, J.; Chen, Y.; Chang, C.; Xu, C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genom. 2017, 18, 80. [Google Scholar] [CrossRef]
- Li, C.; Chang, L.; Chen, Z.; Liu, Z.; Wang, Y.; Ye, Q. The role of lncRNA MALAT1 in the regulation of hepatocyte proliferation during liver regeneration. Int J Mol Med. 2017, 39, 347–356. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Vorrink, S.U.; Moro, S.M.; Rezayee, F.; Nordling, Å.; Hendriks, D.F.G.; Bell, C.C.; Sison-Young, R.; Park, B.K.; Goldring, C.E.; et al. Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology 2016, 64, 1743–1756. [Google Scholar] [CrossRef]
- Qiao, J.; Yao, H.; Xia, Y.; Chu, P.; Li, M.; Wu, Y.; Li, W.; Ding, L.; Qi, K.; Li, D.; et al. Long non-coding RNAs expression profiles in hepatocytes of mice after hematopoietic stem cell transplantation. IUBMB Life 2016, 68, 232–241. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Rastegari, A.; Farokhi, M.; Dinarvand, R.; Hosseinkhani, H.; Ou, K.L.; Pack, D.W.; Mao, C.; Dinarvand, M.; Fatahi, Y.; et al. Prospects of siRNA applications in regenerative medicine. Int. J. Pharm. 2017, 524, 312–329. [Google Scholar] [CrossRef]
- Elchaninov, A.V.; Fatkhudinov, T.K. Mammalian Liver Regeneration: Intercellular Interactions; Nauka: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Saito, S.; Togo, S.; Morioka, D.; Matsuo, K.I.; Yoshimoto, N.; Nagano, Y.; Tanaka, K.; Kubota, T.; Nagashima, Y.; Shimada, H. A rat model of a repeat 70% major hepatectomy. J. Surg. Res. 2006, 134, 322–326. [Google Scholar] [CrossRef]
- Rumyantsev, A.G.; Maschan, A.A. Transplantation of Hematopoietic Stem Cells in Children; Medical information agency: Moscow, Russia, 2003. (In Russian) [Google Scholar]
- Baláz, P.; Matia, I.; Jackanin, S.; Rybárová, E.; Kron, I.; Pomfy, M.; Froněk, J.; Ryska, M. Preservation injury of jejunal grafts and its modulation by custodiol and University of Wisconsin perfusion solutions in Wistar rats. Eur. Surg. Res. 2004, 36, 192–197. [Google Scholar] [CrossRef]
- Baldo, G.; Giugliani, R.; Uribe, C.; Belardinelli, M.C.; Duarte, M.E.S.; Meurer, L.; da Silveira, T.R.; Matte, U. Bone marrow mononuclear cell transplantation improves survival and induces hepatocyte proliferation in rats after CCl4 acute liver damage. Dig. Dis. Sci. 2010, 55, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Macenko, M.; Niethammer, M.; Marron, J.S.; Borland, D.; Woosley, J.T.; Guan, X.; Schmitt, C.; Thomas, N.E. A method for normalizing histology slides for quantitative analysis. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; pp. 1107–1110. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Potapnev, M.P. Autophagy, apoptosis, cell necrosis and immune recognition of own and alien. Immunology 2014, 2, 95–102. (In Russian) [Google Scholar]
- Cheng, Y.; Wang, B.; Zhou, H.; Dang, S.; Jin, M.; Shi, Y.; Hao, L.; Yang, Z.; Zhang, Y. Autophagy is required for the maintenance of liver progenitor cell functionality. Cell Physiol. Biochem. 2015, 36, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, Y.S.; Lin, C.C.; Chen, Y.J.; Lo, G.H.; Lee, P.H.; Kuo, P.L.; Dai, C.Y.; Huang, J.F.; Chung, W.L.; et al. Amiodarone as an autophagy promoter reduces liver injury and enhances liver regeneration and survival in mice after partial hepatectomy. Sci. Rep. 2015, 5, 15807. [Google Scholar] [CrossRef]
- Lv, H.; Fan, X.; Wang, L.; Feng, H.; Ci, X. Daphnetin alleviates lipopolysaccharide/d-galactosamine-induced acute liver failure via the inhibition of NLRP3, MAPK and NF-κB, and the induction of autophagy. Int. J. Biol. Macromol. 2018, 119, 240–248. [Google Scholar] [CrossRef]
- Onishchenko, N.A.; Nikolskaya, A.O.; Shagidulin, M.Y. Cytogenetic recapitulation, induced by medical preparations, as the universal stage of formation of urgent protection against damage at organ transplantation. Pathol. Physiol. Exp. Ther. 2016, 60, 148–153. (In Russian) [Google Scholar] [CrossRef]
- Elchaninov, A.V.; Fatkhudinov, T.K.; Kananykhina, E.Y.; Arutyunyan, I.V.; Makarov, A.V.; Charyeva, I.G.; Bolshakova, G.B.; Sukhikh, G.T. Cell proliferation and death in hepatocytes after subtotal rat liver resection (In Russ). Clin Exp Morph. 2016, 3, 22–29. [Google Scholar]
- Gazizov, I.M.; Gumerova, A.A.; Kiyasov, A.P. Apoptosis in regenerative histogenesis of the liver after partial hepatectomy in rats. Genes Cells 2015, 10, 22–26. [Google Scholar] [CrossRef]
- Budd, R.C. Death receptors couple to both cell proliferation and apoptosis. J. Clin. Investig. 2002, 109, 437–441. [Google Scholar] [CrossRef]
- Jun-Tang, L.; Cong-Rui, W.; Pramanik, J.; Hui-Yong, Z.; Hui-Gen, F.; Bao-Sheng, Y.; Yu-Chang, L.I.; Cun-Shuan, X. Study on construction of cDNA libraries from rat normal liver and regeneration liver with smart technique. Indian J. Clin. Biochem. 2004, 19, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Hora, S.; Wuestefeld, T. Liver injury and regeneration: Current understanding, new approaches, and future perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Esser, H.; Huch, M.; Forbes, S. Liver regeneration and inflammation: From fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol. 2021, 22, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Zhu, H.; Zhang, M.; Pikiolek, M.; Ercan, C.; Li, J.; Huang, X.; Han, X.; Zhang, Z.; Lv, Z.; et al. Bipotent transitional liver progenitor cells contribute to liver regeneration. Nat. Genet. 2023, 55, 651–664. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Song, P.; Zhi-Qiang, F.; Zhen-Sheng, Y.; Jing-Jing, L.; Guo-Rui, D.; Hua, H.; Lin, W. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Gatzidou, E.; Mantzourani, M.; Giaginis, C.; Giagini, A.; Patsouris, E.; Kouraklis, G.; Theocharis, S. Augmenter of liver regeneration gene expression in human colon cancer cell lines and clinical tissue samples. J. B.U.ON. 2015, 20, 84–91. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishchenko, N.; Shagidulin, M.; Gonikova, Z.; Nikolskaya, A.; Kirsanova, L.; Venediktov, A.; Pokidova, K.; Kuzmin, E.; Kuznetsova, N.; Kozlov, I.; et al. Hematopoietic Stem Cells of Bone Marrow and Their Total RNA in Rat Liver Regeneration. Appl. Sci. 2025, 15, 3782. https://doi.org/10.3390/app15073782
Onishchenko N, Shagidulin M, Gonikova Z, Nikolskaya A, Kirsanova L, Venediktov A, Pokidova K, Kuzmin E, Kuznetsova N, Kozlov I, et al. Hematopoietic Stem Cells of Bone Marrow and Their Total RNA in Rat Liver Regeneration. Applied Sciences. 2025; 15(7):3782. https://doi.org/10.3390/app15073782
Chicago/Turabian StyleOnishchenko, Nina, Murat Shagidulin, Zalina Gonikova, Alla Nikolskaya, Ludmila Kirsanova, Artem Venediktov, Ksenia Pokidova, Egor Kuzmin, Natalia Kuznetsova, Igor Kozlov, and et al. 2025. "Hematopoietic Stem Cells of Bone Marrow and Their Total RNA in Rat Liver Regeneration" Applied Sciences 15, no. 7: 3782. https://doi.org/10.3390/app15073782
APA StyleOnishchenko, N., Shagidulin, M., Gonikova, Z., Nikolskaya, A., Kirsanova, L., Venediktov, A., Pokidova, K., Kuzmin, E., Kuznetsova, N., Kozlov, I., Telyshev, D., Kartashkina, N., Varentsov, V., Timofeeva, M., Sevastjanov, V., Elchaninov, A., Piavchenko, G., & Gautier, S. (2025). Hematopoietic Stem Cells of Bone Marrow and Their Total RNA in Rat Liver Regeneration. Applied Sciences, 15(7), 3782. https://doi.org/10.3390/app15073782










