Monitorization of Cleaning Processes from Digital Video Spatiotemporal Analysis: Cleaning Kinetics from Characteristic Times
Abstract
1. Introduction
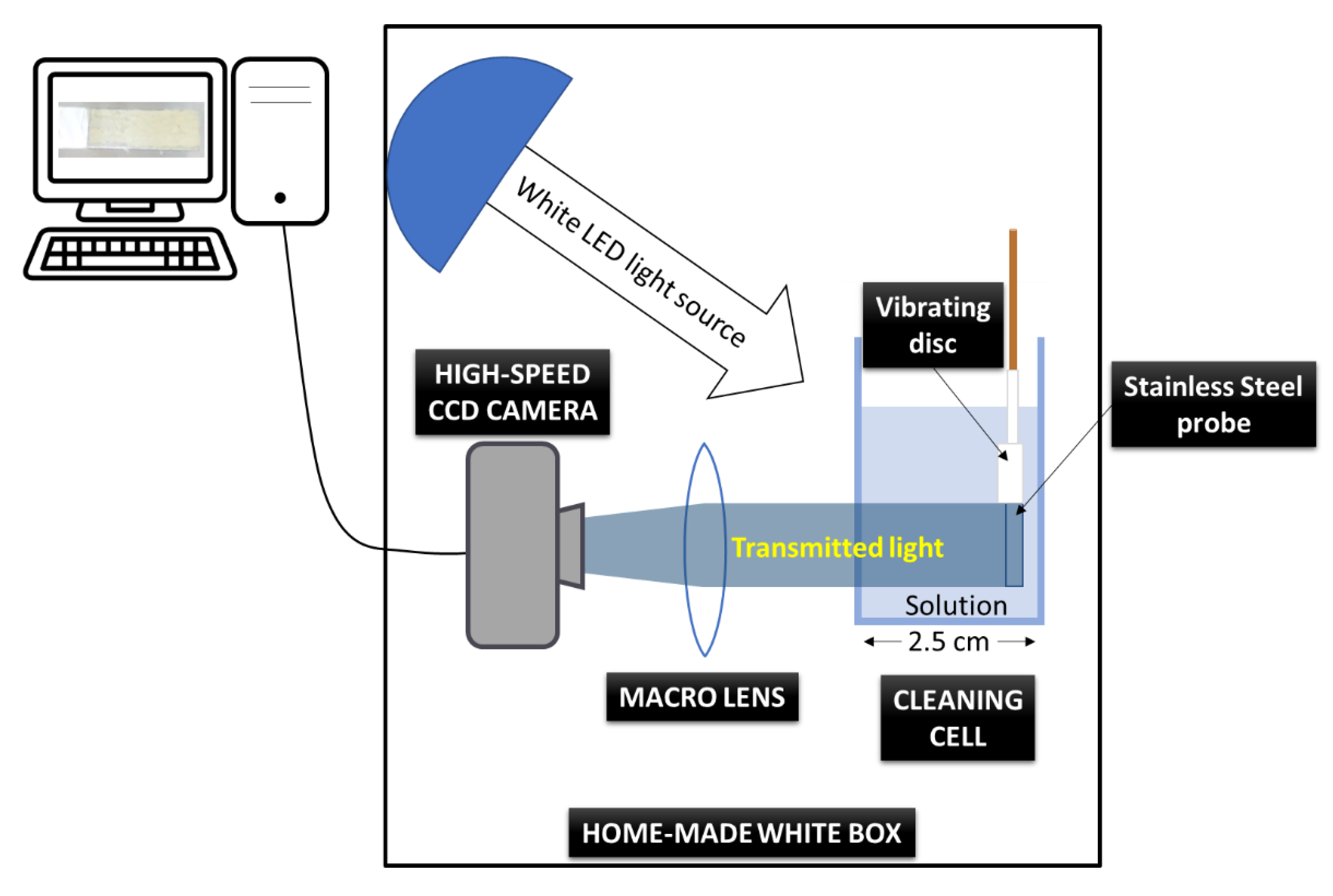
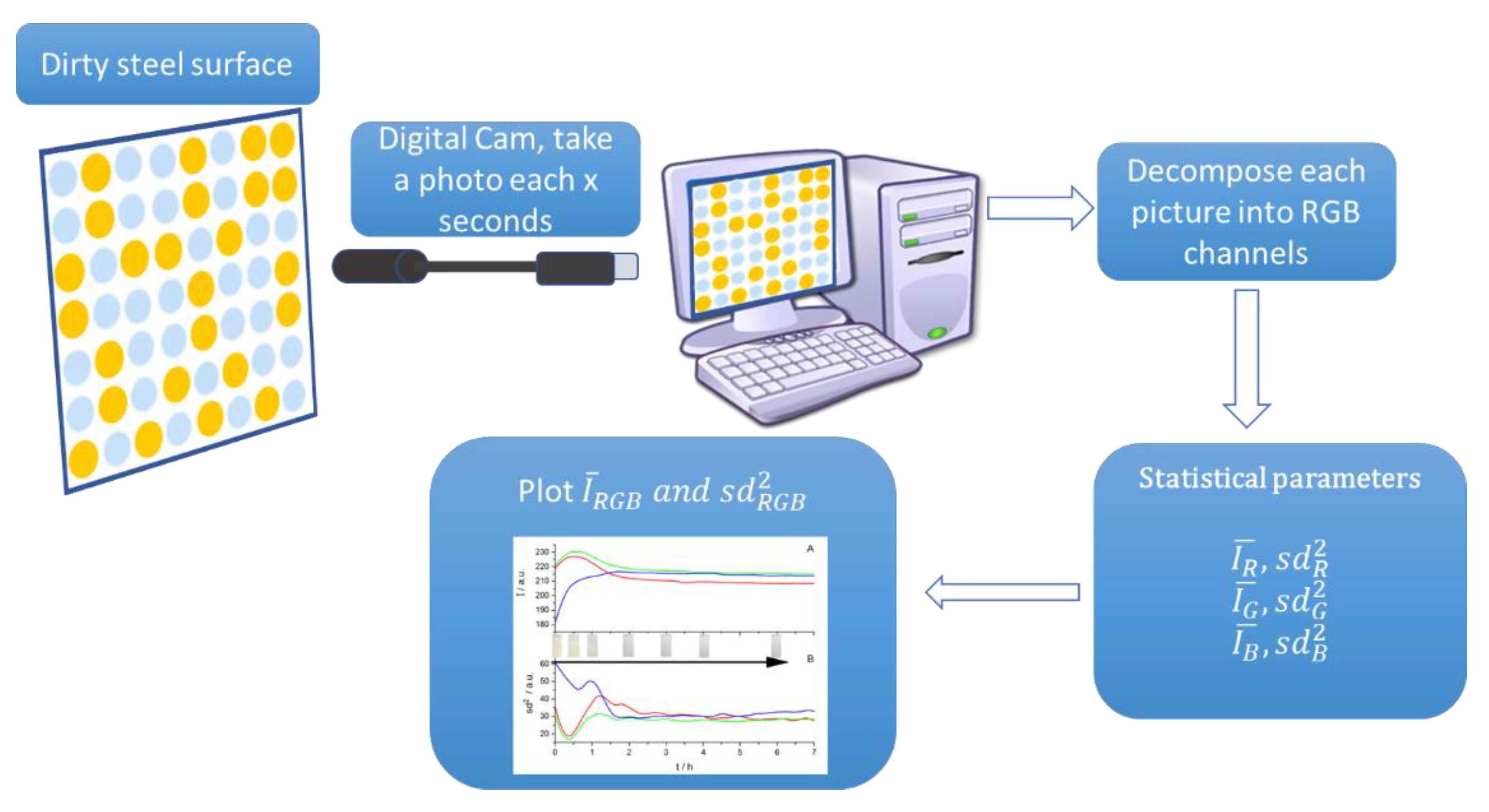
2. Materials and Methods
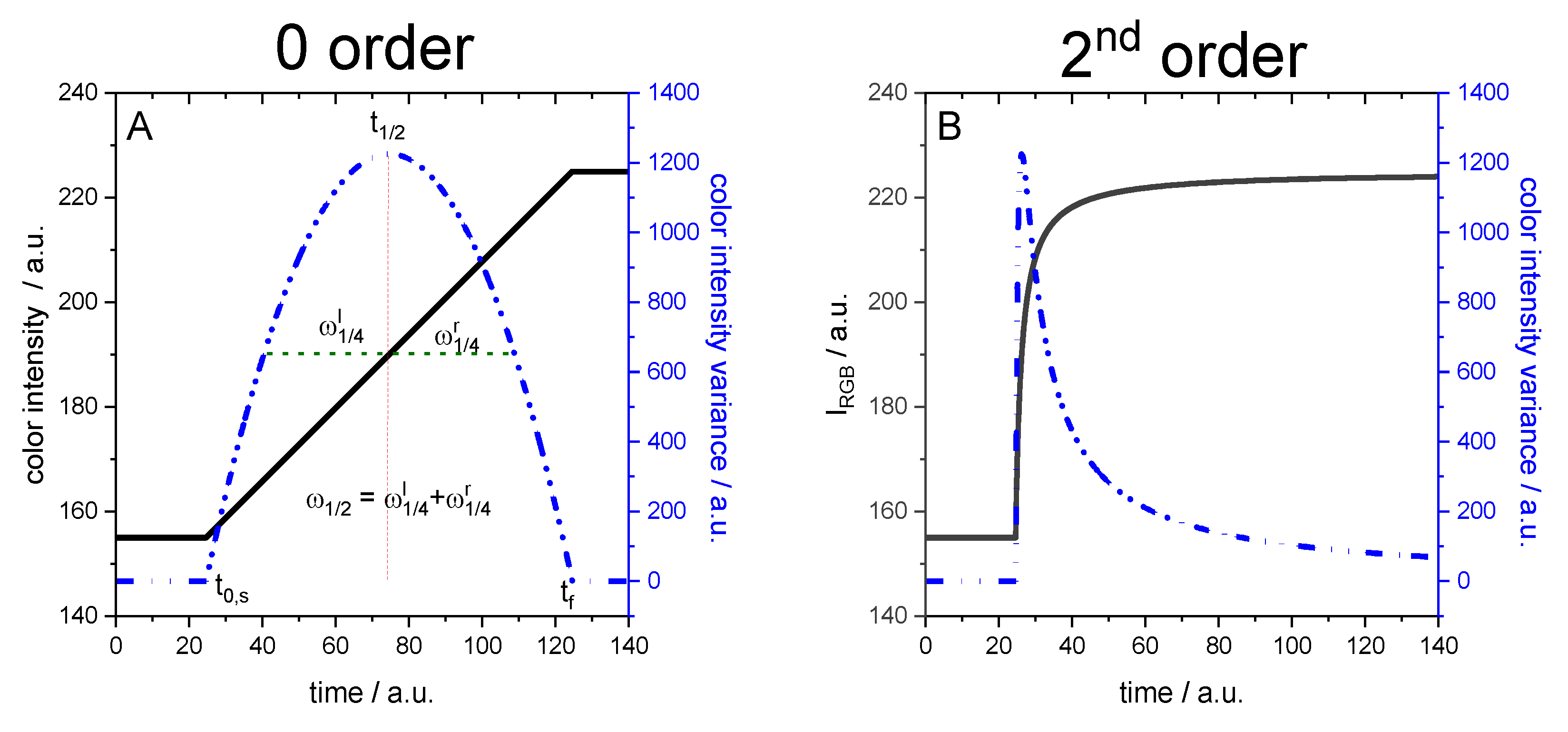
Modelization
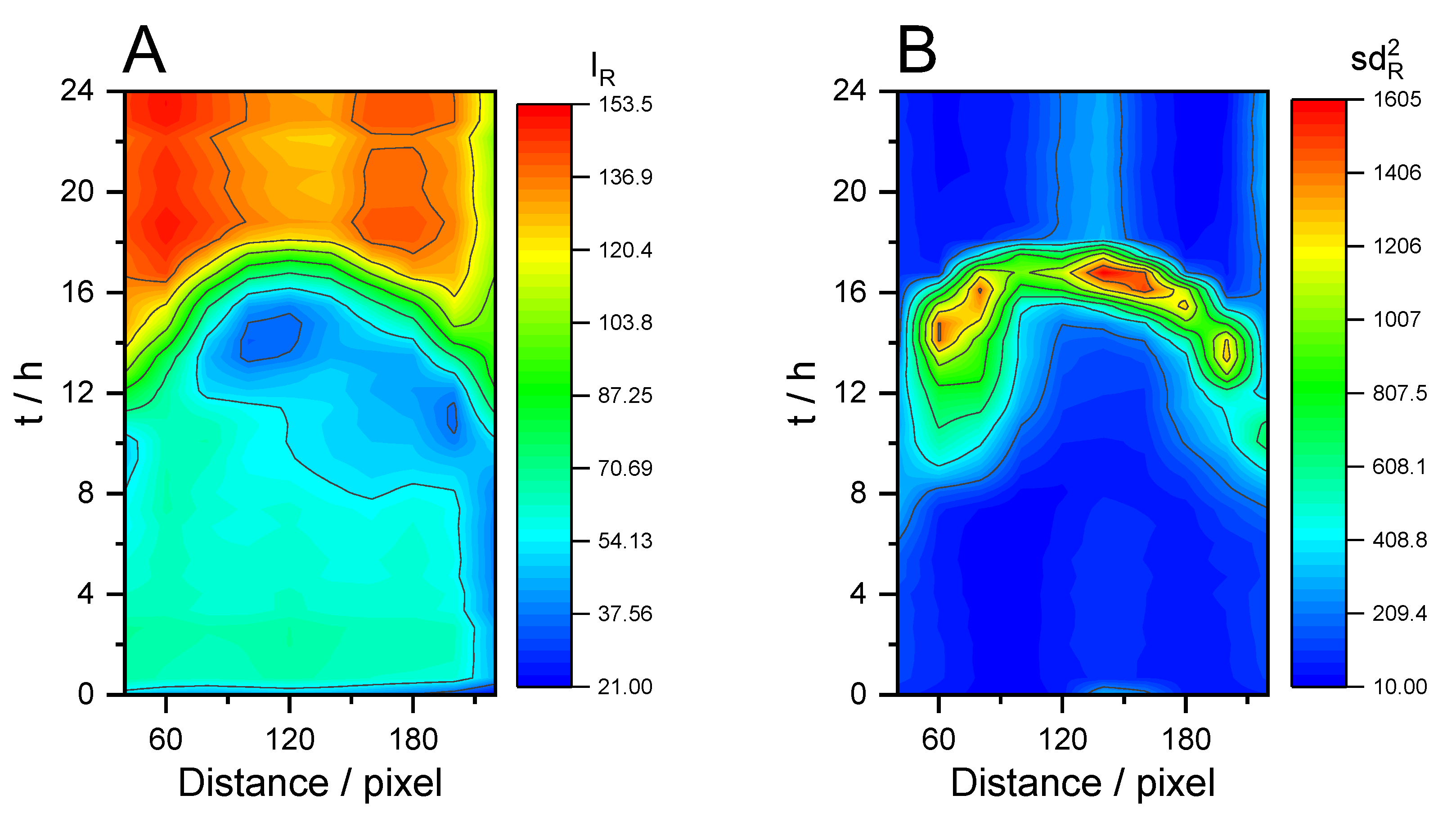
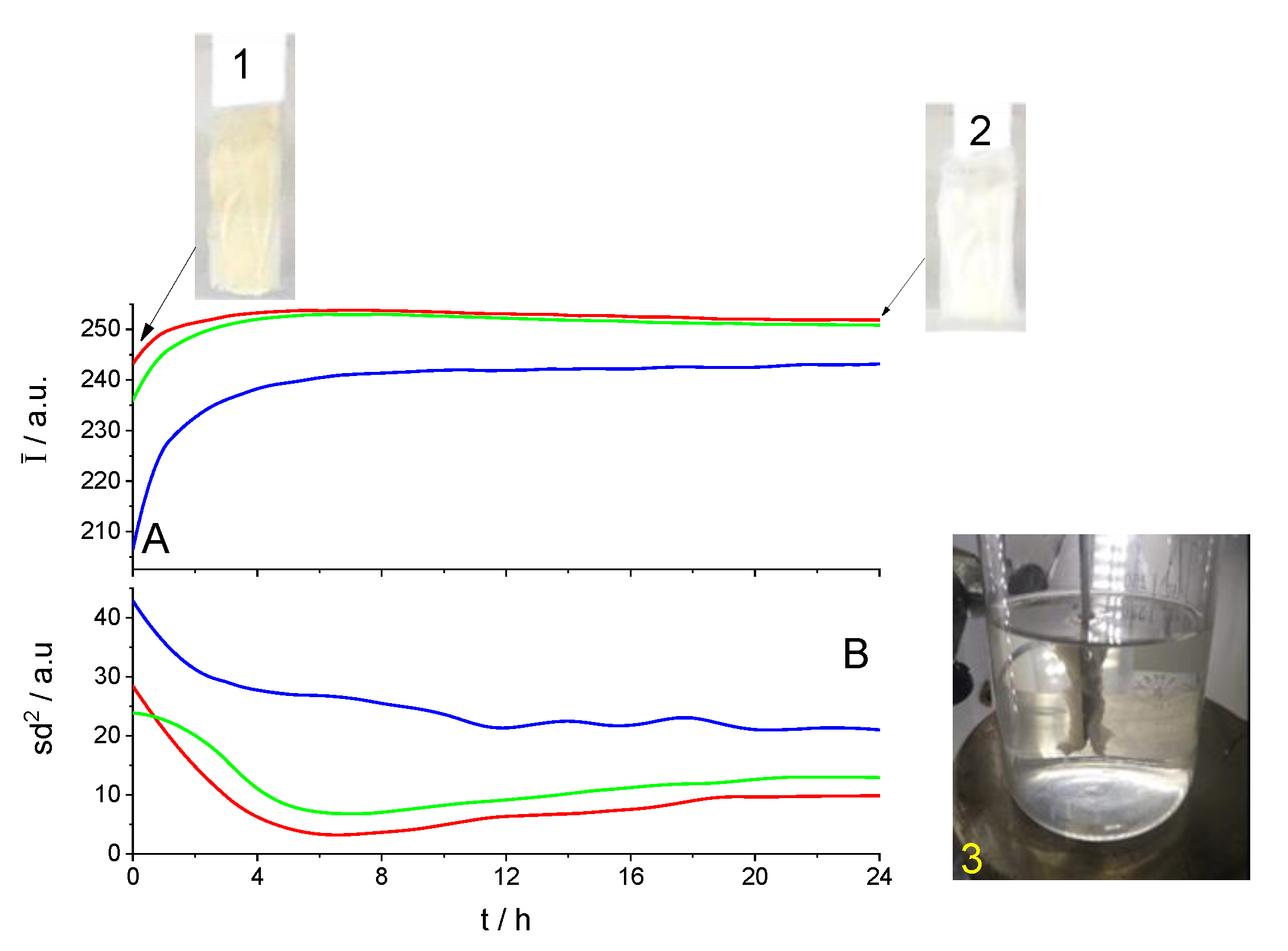
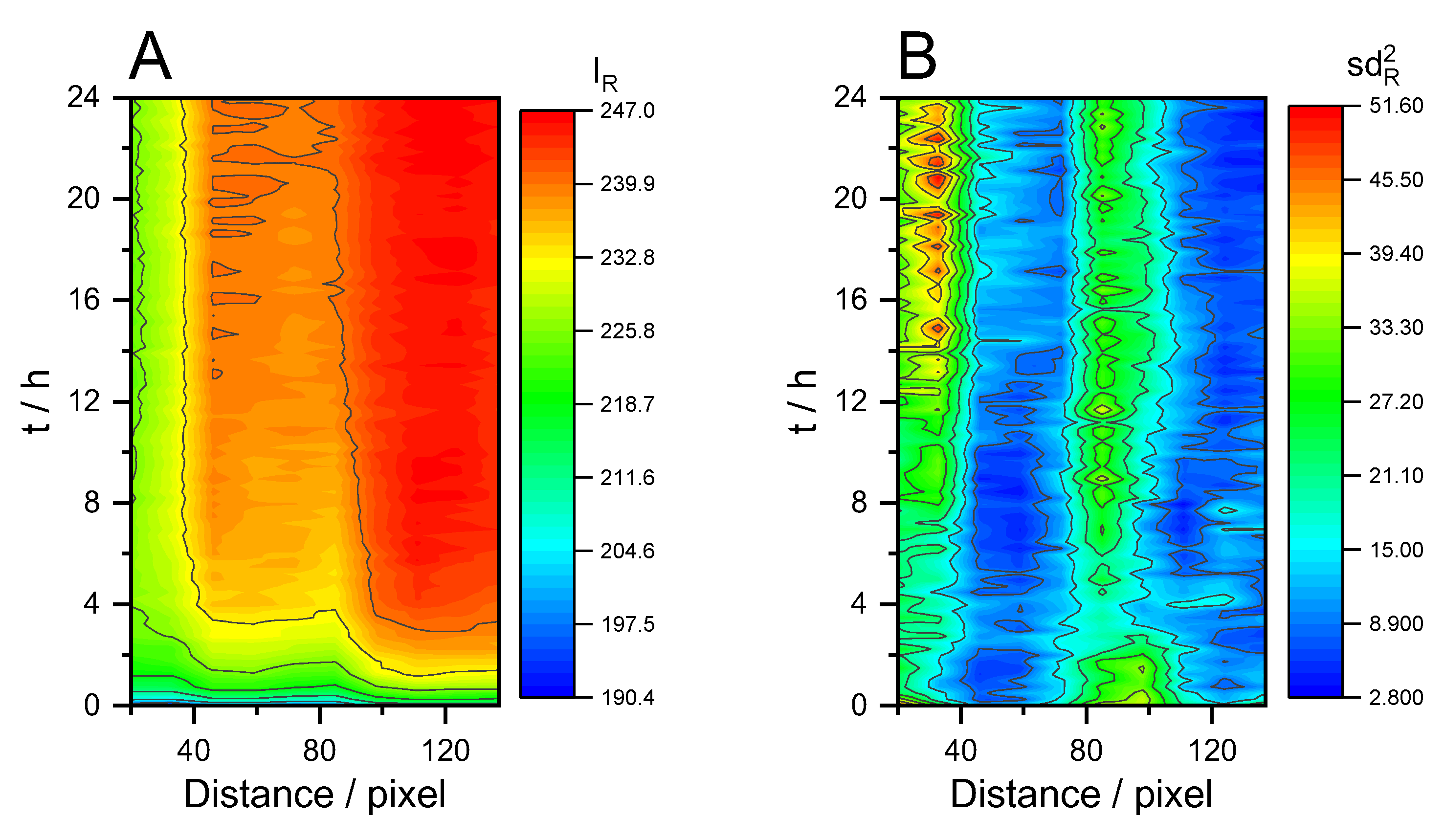
3. Results and Discussion
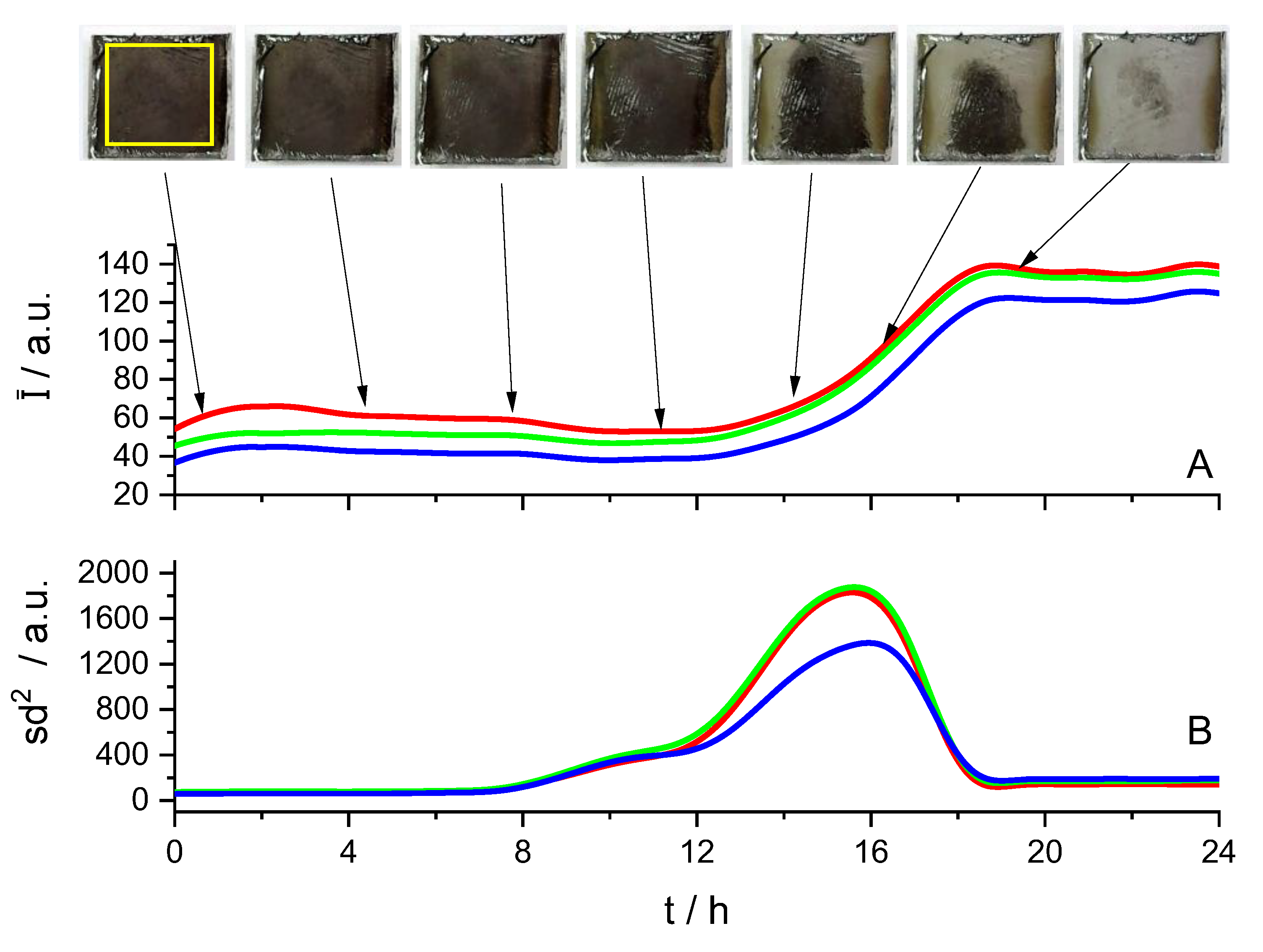
3.1. Cleaning by Dissolution
3.2. Peeling off Cleaning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pontefract, R. Bacterial Adherence—Its Consequences in Food-Processing. Can. Inst. Food Sci. Technol. J. 1991, 24, 113–117. [Google Scholar] [CrossRef]
- Boulané-Petermann, L. Processes of Bioadhesion on Stainless Steel Surfaces and Cleanability: A Review with Special Reference to the Food Industry. Biofouling 1996, 10, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.S.; Al-Taher, F.M.; Moorman, M.; DeVries, J.W.; Tippett, R.; Swanson, K.M.J.; Fu, T.-J.; Salter, R.; Dunaif, G.; Estes, S.; et al. Cleaning and Other Control and Validation Strategies to Prevent Allergen Cross-Contact in Food-Processing Operations. J. Food Prot. 2008, 71, 445–458. [Google Scholar] [CrossRef]
- Rossi, S.; Leso, S.M.; Calovi, M. Study of the Corrosion Behavior of Stainless Steel in Food Industry. Materials 2024, 17, 1617. [Google Scholar] [CrossRef]
- Chung, M.M.S.; Tsai, J.-H.; Lu, J.; Padilla Chevez, M.; Huang, J.-Y. Microbubble-Assisted Cleaning To Enhance the Removal of Milk Deposits from the Heat Transfer Surface. ACS Sustain. Chem. Eng. 2022, 10, 8380–8387. [Google Scholar] [CrossRef]
- Dallagi, H.; Jha, P.K.; Faille, C.; Le-Bail, A.; Rawson, A.; Benezech, T. Removal of Biocontamination in the Food Industry Using Physical Methods: An Overview. Food Control 2023, 148, 109645. [Google Scholar] [CrossRef]
- Elsayed, S.; Galal, H.; Allam, A.; Schmidhalter, U. Passive Reflectance Sensing and Digital Image Analysis for Assessing Quality Parameters of Mango Fruits. Sci. Hortic. 2016, 212, 136–147. [Google Scholar]
- Alonso, V.P.P.; Lemos, J.G.; Nascimento, M.d.S.d. Yeast Biofilms on Abiotic Surfaces: Adhesion Factors and Control Methods. Int. J. Food Microbiol. 2023, 400, 110265. [Google Scholar] [CrossRef]
- Crandall, P.G.; O’Bryan, C.A.; Wang, D.; Gibson, K.E.; Obe, T. Environmental Monitoring in Food Manufacturing: Current Perspectives and Emerging Frontiers. Food Control 2024, 159, 110269. [Google Scholar] [CrossRef]
- Gomes, L.C.; Saubade, F.; Amin, M.; Spall, J.; Liauw, C.M.; Mergulhão, F.; Whitehead, K.A. A Comparison of Vegetable Leaves and Replicated Biomimetic Surfaces on the Binding of Escherichia coli and Listeria monocytogenes. Food Bioprod. Process. 2023, 137, 99–112. [Google Scholar] [CrossRef]
- Wan Omar, W.H.; Mahyudin, N.A.; Azmi, N.N.; Mahmud Ab Rashid, N.-K.; Ismail, R.; Mohd Yusoff, M.H.Y.; Khairil Mokhtar, N.F.; Sharples, G.J. Effect of Natural Antibacterial Clays against Single Biofilm Formation by Staphylococcus Aureus and Salmonella Typhimurium Bacteria on a Stainless-Steel Surface. Int. J. Food Microbiol. 2023, 394, 110184. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Orihuel Iranzo, E.; Bertó Navarro, R.; Lorenzo Cartón, F.; López Tormo, C.; San José Serran, C.; Orgaz Martín, B. Biofilm-Marking Composition and Method for Detection of Same on Surfaces. European Patent EP2634260A1, 4 November 2013. [Google Scholar]
- Cappitelli, F.; Polo, A.; Villa, F. Biofilm Formation in Food Processing Environments Is Still Poorly Understood and Controlled. Food Eng. Rev. 2014, 6, 29–42. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Kabwanga, I.T.; Yetişemiyen, A.; Nankya, S. Dairy Industrial Hygiene: A Review on Biofilms Challenges and Control. Int. J. Res. Granthaalayah 2018, 6, 268–273. [Google Scholar] [CrossRef]
- Puga, C.H.; Dahdouh, E.; SanJose, C.; Orgaz, B. Listeria Monocytogenes Colonizes Pseudomonas Fluorescens Biofilms and Induces Matrix Over-Production. Front. Microbiol. 2018, 9, 1706. [Google Scholar] [CrossRef]
- Lorenzo, F.; Sanz-Puig, M.; Bertó, R.; Orihuel, E. Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination. Appl. Sci. 2020, 10, 744. [Google Scholar] [CrossRef]
- Brozkova, I.; Cervenka, L.; Motkova, P.; Fruhbauerova, M.; Metelka, R.; Svancara, I.; Sys, M. Electrochemical Control of Biofilm Formation and Approaches to Biofilm Removal. Appl. Sci.-Basel 2022, 12, 6320. [Google Scholar] [CrossRef]
- Sha, J.; Xu, C.; Xu, K. Progress of Research on the Application of Nanoelectronic Smelling in the Field of Food. Micromachines 2022, 13, 789. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Yang, K. Novel Antibacterial Metals as Food Contact Materials: A Review. Materials 2023, 16, 3029. [Google Scholar] [CrossRef]
- de Larios, J.M.; Zhang, J.; Zhao, E.; Gockel, T.; Ravkin, M. Evaluating Chemical Mechanical Cleaning Technology for Post-CMP Applications. Micro 1997, 15, 61. [Google Scholar]
- Toure, Y.; Mabon, N.; Sindic, M. Soil Model Systems Used to Assess Fouling, Soil Adherence and Surface Cleanability in the Laboratory: A Review. Biotechnol. Agron. Société Environ. 2013, 17, 527–539. [Google Scholar]
- Chen, Y.L.; Zhu, S.M.; Lee, S.J.; Wang, J.C. The Technology Combined Electrochemical Mechanical Polishing. J. Mater. Process. Technol. 2003, 140, 203–205. [Google Scholar] [CrossRef]
- Memisi, N.; Moracanin, S.V.; Milijasevic, M.; Babic, J.; Djukic, D. CIP Cleaning Processes in the Dairy Industry. Procedia Food Sci. 2015, 5, 184–186. [Google Scholar] [CrossRef]
- Fryer, P.J.; Christian, G.K.; Liu, W. How Hygiene Happens: Physics and Chemistry of Cleaning. Int. J. Dairy Technol. 2006, 59, 76–84. [Google Scholar] [CrossRef]
- Orihuel, E.; Bertó, R.; Lorenzo, F.; López, C. Mechanical Energy Balance in Surface Cleaning by Pressurised Water Spray: A Simplified Model. Ehedg Yearb. 2017, 2018, 32–37. [Google Scholar]
- Padmanabhan, N.T.; John, H. Titanium Dioxide Based Self-Cleaning Smart Surfaces: A Short Review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Leclercq-Perlat, M.-N.; Lalande, M. Cleanability in Relation to Surface Chemical Composition and Surface Finishing of Some Materials Commonly Used in Food Industries. J. Food Eng. 1994, 23, 501–517. [Google Scholar] [CrossRef]
- Köhler, H.; Stoye, H.; Mauermann, M.; Weyrauch, T.; Majschak, J.-P. How to Assess Cleaning? Evaluating the Cleaning Performance of Moving Impinging Jets. Food Bioprod. Process. 2015, 93, 327–332. [Google Scholar] [CrossRef]
- Lang, M.P.; Kocaoglu-Vurma, N.A.; Harper, W.J.; Rodriguez-Saona, L.E. Multicomponent Cleaning Verification of Stainless Steel Surfaces for the Removal of Dairy Residues Using Infrared Microspectroscopy. J. Food Sci. 2011, 76, C303–C308. [Google Scholar] [CrossRef]
- Schöler, M.; Fuchs, T.; Helbig, M.; Augustin, W.; Scholl, S.; Majschak, J.-P. Monitoring of the Local Cleaning Efficiency of Pulsed Flow Cleaning Procedures. In Proceedings of the International Conference on Heat Exchanger Fouling and Cleaning, Schladming, Austria, 14–19 June 2009. [Google Scholar]
- Detry, J.G.; Deroanne, C.; Sindic, M. Hydrodynamic Systems for Assessing Surface Fouling, Soil Adherence and Cleaning in Laboratory Installations. Biotechnol. Agron. Soc. Environ. 2009, 13, 427–439. [Google Scholar]
- Davidson, C.A.; Griffith, C.J.; Peters, A.C.; Fielding, L.M. Evaluation of Two Methods for Monitoring Surface Cleanliness—ATP Bioluminescence and Traditional Hygiene Swabbing. Luminescence 1999, 14, 33–38. [Google Scholar] [CrossRef]
- Rizzotto, F.; Khalife, M.; Hou, Y.; Chaix, C.; Lagarde, F.; Scaramozzino, N.; Vidic, J. Recent Advances in Electrochemical Biosensors for Food Control. Micromachines 2023, 14, 1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gao, S.; Zhuang, Y.; Hu, W.; Zhao, J.; Yi, Z. Non-Destructive Sensor for Glucose Solution Concentration Detection Using Electromagnetic Technology. Micromachines 2024, 15, 758. [Google Scholar] [CrossRef]
- Assaifan, A.K. Thiol-SAM Concentration Effect on the Performance of Interdigitated Electrode-Based Redox-Free Biosensors. Micromachines 2024, 15, 1254. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Krambeck Franco, M.; Suarez, W.T.; Santos, V.B. dos Digital Image Method Smartphone-Based for Furfural Determination in Sugarcane Spirits. Food Anal. Methods 2017, 10, 508–515. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Perianes, E.; Vicente, F. Use of RGB Digital Video Analysis to Study Electrochemical Processes Involving Color Changes. Electrochem. Commun. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Minz, P.S.; Sawhney, I.K.; Saini, C.S. Algorithm for Processing High Definition Images for Food Colourimetry. Measurement 2020, 158, 107670. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Vicente, F. Quantification of Electrochromic Kinetics by Analysis of RGB Digital Video Images. Electrochem. Commun. 2018, 93, 86–90. [Google Scholar] [CrossRef]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone Based Bioanalytical and Diagnosis Applications: A Review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Guillén, E.; Vicente, F. RGB Video Electrochemistry of Copper Electrodeposition/Electrodissolution in Acid Media on a Ternary Graphite:Copper:Polypropylene Composite Electrode. Electrochim. Acta 2019, 305, 72–80. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Guillén, E.; Vicente, F. Kinetics of Surface Chemical Reactions from a Digital Video. J. Phys. Chem. C 2020, 124, 2050–2059. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital Image Colorimetry on Smartphone for Chemical Analysis: A Review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Vicente, F. A Statistical Interpretation of the Voltammetry of Adsorbed Substances under the Perspective View of the Digital Video Electrochemistry. Microchem. J. 2022, 181, 107844. [Google Scholar] [CrossRef]
- Cánovas-Saura, A.; Serrano-Luján, L.; Beltrán, V.; Padilla, J. Neural Network-Based Digital Camera Spectrophotometer: Application to Chromogenic Technologies Characterization. ACS Appl. Opt. Mater. 2024, 2, 1403–1412. [Google Scholar] [CrossRef]
- García-Jareño, J.J.; Agrisuelas, J.; Vargas, Z.; Vicente, F. Electrogeneration and Characterization of Poly(Methylene Blue) Thin Films on Stainless Steel 316 Electrodes—Effect of pH. Molecules 2024, 29, 3752. [Google Scholar] [CrossRef]
- García-Jareño, J.J.; Agrisuelas, J.; Vicente, F. Overview and Recent Advances in Hyphenated Electrochemical Techniques for the Characterization of Electroactive Materials. Materials 2023, 16, 4226. [Google Scholar] [CrossRef]
- Agrisuelas, J.; García-Jareño, J.J.; Piedras, M.; Vicente, F. Quality and Efficiency Control of Electrodeposition and Electrodissolution of Ni on a Ternary Composite Electrode Followed by Digital Video Electrochemistry. J. Electrochem. Soc. 2023, 170, 072505. [Google Scholar] [CrossRef]
- Christeyns—Striving for a Cleaner Future. Available online: https://www.christeyns.com/ (accessed on 3 November 2023).



| Nutritional Information | Per 100 g |
|---|---|
| Fats | 51 g |
| Saturated fats | 11 g |
| Carbohydrates | 15 g |
| Sugars | 8 g |
| Proteins | 25 g |
| Salt (NaCl) | 1.1 g |
| Order | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | |||||
| 1 | 2.3 | |||||
| 2 | 5.8 |
| Region | /h | /h | /h | /h | /h | |
|---|---|---|---|---|---|---|
| Upper left | 12.4 | 14.2 | 16 | 0.9 | 0.8 | 0.95 |
| Lower right | 12.5 | 15.4 | 16.5 | 1.3 | 1.2 | 0.92 |
| Global | 11.5 | 15.6 | 18.7 | 2.5 | 1.7 | 0.70 |
| Conditions for the Cleaning Process | 1st Process (Whitening) | 2nd Process (Peeling off) | ||||||
|---|---|---|---|---|---|---|---|---|
| T/K | Vibration | Conc. (%) | /s | /s | /s | /s | /s | /s |
| 293 | NO | 1% | 160 | 2255 | ||||
| 293 | NO | 3% | 20 | 1280 | ||||
| 293 | YES | 1% | 810 | 1240 | 2030 | |||
| 293 | YES | 3% | 0 | 500 | 1270 | |||
| 313 | NO | 1% | 0 | 4000 | ||||
| 313 | NO | 3% | 902 | 2058 | 2556 | |||
| 313 | YES | 1% | 460 | 750 | 1040 | |||
| 313 | YES | 3% | 0 | 177 | 414 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Jareño, J.J.; Agrisuelas, J.; López, C.; Lorenzo, F.; Vicente, F. Monitorization of Cleaning Processes from Digital Video Spatiotemporal Analysis: Cleaning Kinetics from Characteristic Times. Appl. Sci. 2025, 15, 3745. https://doi.org/10.3390/app15073745
García-Jareño JJ, Agrisuelas J, López C, Lorenzo F, Vicente F. Monitorization of Cleaning Processes from Digital Video Spatiotemporal Analysis: Cleaning Kinetics from Characteristic Times. Applied Sciences. 2025; 15(7):3745. https://doi.org/10.3390/app15073745
Chicago/Turabian StyleGarcía-Jareño, José J., Jerónimo Agrisuelas, Celia López, Fernando Lorenzo, and Francisco Vicente. 2025. "Monitorization of Cleaning Processes from Digital Video Spatiotemporal Analysis: Cleaning Kinetics from Characteristic Times" Applied Sciences 15, no. 7: 3745. https://doi.org/10.3390/app15073745
APA StyleGarcía-Jareño, J. J., Agrisuelas, J., López, C., Lorenzo, F., & Vicente, F. (2025). Monitorization of Cleaning Processes from Digital Video Spatiotemporal Analysis: Cleaning Kinetics from Characteristic Times. Applied Sciences, 15(7), 3745. https://doi.org/10.3390/app15073745






