Is IBS a Food Allergy? Confocal Laser Endomicroscopy Findings in Patients with IBS: A Narrative Review
Abstract
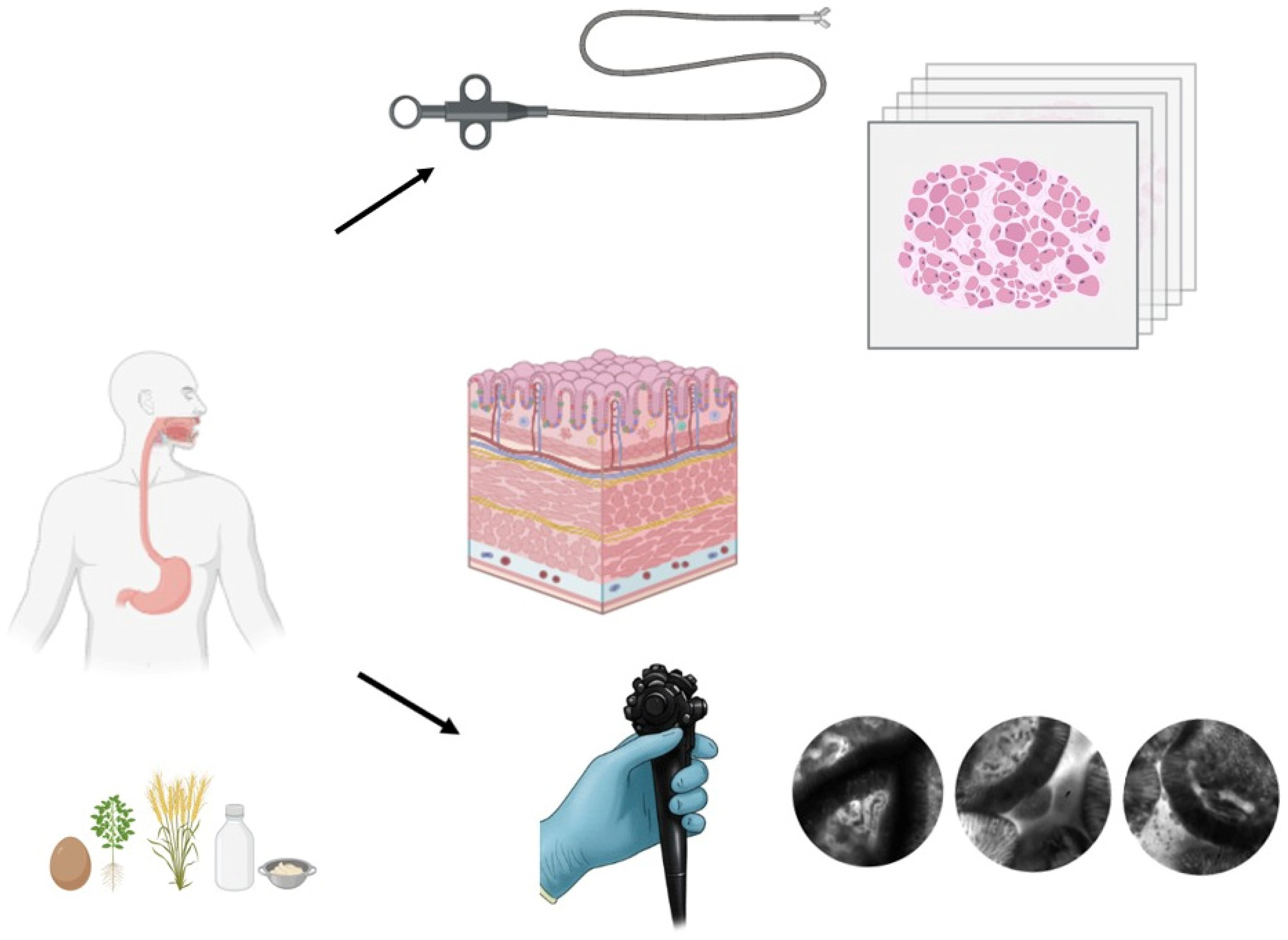
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
Mucosal Alterations and Food Allergy in IBS: Insights from Confocal Laser Endomicroscopy
| Autor | Population | Methods | CLE Results | Dietary Intervention |
|---|---|---|---|---|
| Turcotte et al., 2013 [37] | 35 patients. 17 IBS and 18 HC | CLE captured images post-fluorescein injection to examine epithelial gaps between villi | IBS patients had significantly higher epithelial gap density compared to controls | no |
| Fritscher-Ravens et al., 2014 [38] | 36 IBS and 10 HC | CLE for FCT in IBS and HS patients. Observed parameters: IELs, epithelial breaks, and intervillous space | 61.1% of IBS patients were CLE+. IELs increased from 18.7 ± 0.9 to 24.7 ± 1.4 (p = 0.0001). Not confirmed histologically. Epithelial breaks increased from 5.5 ± 0.6 to 11.9 ± 1.5 per 1000 epithelial cells (p < 0.001). Intervillous space widened from 8.1 ± 3.5 µm to 41.1 ± 2.5 µm (p = 0.0001) | After an exclusion diet, 74% of CLE-positive patients experienced symptom reduction over 12 months |
| Fritscher-Ravens et al., 2019 [39] | 155 IBS patients and 10 healthy controls | CLE was used for FCT in IBS and HC by evaluating immune activation, epithelial integrity, and barrier function. Duodenal biopsies samples were analyzed for claudin-2, occludin, ECP, tryptase and intraepithelial lymphocytes. | 70% of IBS patients were CLE positive, showing increased intraepithelial lymphocytes (p = 0.001) and tight junction alterations, including elevated claudin-2 and reduced occludin levels (p < 0.05) | After an exclusion diet, 73% of CLE-positive patients experienced symptom reduction over 6 months |
| Rath et al., 2021 [36] | 35 with FA and 27 non FA with GI symptoms | Patients with and without FA, both experiencing gastrointestinal symptoms triggered by food intake. CLE observations included fluorescein leakage, cell shedding, and microerosions | Barrier dysfunction in the terminal ileum detected in 96% of FA patients vs. 33% of non-FA patients (p < 0.0001). Microerosions significantly more frequent in FA (92.6% vs. 24.2%, p < 0.0001). Crypt architecture showed no differences. CLE proved highly effective in ruling out FA | no |
| Gjini et al., 2022 [40] | 34 patients with functional abdominal pain | CLE assessed spontaneous fluorescein leakage and DFC reactions. Duodenal biopsies analyzed inflammation and immune markers | 67.6% showed barrier dysfunction. Duodenal biopsies were normal, with no inflammation, atrophy, or structural abnormalities. Intraepithelial lymphocytes, mast cells, and dysfunction markers remained within normal limits, confirming no significant mucosal alterations | 69.5% reported pain relief after a four-week CLE-guided exclusion diet |
| Frieling et al., 2024 [41] | 71 patients with functional gastrointestinal symptoms. 52 patients with self-reported GARF and 19 patients without GARF | CLE assessed spontaneous fluorescein leakage and DFC reactions. Duodenal biopsies analyzed inflammation and immune markers | 74% of GARF− and 70% of GARF+ showed fluorescein leakage and cell shedding. Duodenal biopsies showed no significant inflammation | After four weeks of dietary changes, 79% of GARF+ patients experienced significant symptom reduction, compared to 14% of GARF−, (p < 0.05) |
| Grover et al., 2021 [42] | 26 IBS patients and 15 HC | Duodenal lipid infusion. Intestinal permeability evaluated via a lactulose/mannitol excretion test; mucosal structure analyzed using CLE. Transcriptomic analysis to assess TRPV channel expression | No change in intestinal permeability between groups. IBS patients experienced more severe symptoms. Increased TRPV1 and TRPV3 expression in the duodenum and jejunum correlated with symptoms. TRPV1 linked to pain and lower rectal sensitivity thresholds (r = −0.48, p < 0.05), while TRPV3 associated with bloating and urgency, especially in females | no |
| Heßler et al., 2023 [43] | 172 IBS patients | Selective diet based on CLE results compared to a restrictive diet. Symptom improvement assessed via FAQLQ-AF and IBS-SSS, with support from a mobile app | The 12-week protocol included microbiome monitoring, though results are still pending. | yes |
| Kiesslich et al., 2021 (Abstract) [44] | 256 IBS patients | A positive reaction was defined by increased fluorescein leakage and cell shedding after FCT and subsequent exclusion diet | 60% showed mucosal alteration. | 85% improved symptoms after 6 weeks. 74,6% after 6 months. |
| Kiesslich et al., 2020 (Abstract) [45] | 56 IBS patients | A positive reaction was defined by increased fluorescein leakage and cell shedding after FCT and subsequent exclusion diet | 58.9% showed mucosal alteration. | 84% improved symptoms after exclusion diet after six weeks |
| Blomsten et al., 2024 (Abstract) [46] | 43 IBS patients | A positive reaction was defined by increased fluorescein leakage and cell shedding after FCT, subsequent exclusion diet and reintroduction. | Half of the patients were CLE+ | Significant improvement after food elimination, and worsening after reintroduction, |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Pessarelli, T.; Sorge, A.; Elli, L.; Costantino, A. The low-FODMAP diet and the gluten-free diet in the management of functional abdominal bloating and distension. Front. Nutr. 2022, 9, 1007716. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Spencer, M.; Chey, W.D.; Eswaran, S. Dietary Renaissance in IBS: Has Food Replaced Medications as a Primary Treatment Strategy? Curr. Treat. Options Gastroenterol. 2014, 12, 424–440. [Google Scholar] [CrossRef]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef]
- Ostrowska, L.; Wasiluk, D.; Lieners, C.F.J.; Gałęcka, M.; Bartnicka, A.; Tveiten, D. Igg food antibody guided elimination-rotation diet was more effective than FODMAP diet and control diet in the treatment of women with mixed IBS—Results from an open label study. J. Clin. Med. 2021, 10, 4317. [Google Scholar] [CrossRef]
- Vita, A.A.; Zwickey, H.; Bradley, R. Associations between food-specific IgG antibodies and intestinal permeability biomarkers. Front. Nutr. 2022, 9, 962093. [Google Scholar] [CrossRef]
- Zar, S.; Mincher, L.; Benson, M.J.; Kumar, D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand. J. Gastroenterol. 2005, 40, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.L.; Li, Y.Q.; Li, W.J.; Guo, Y.T.; Lu, X.F.; Li, J.M.; Desmond, P.V. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy 2007, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Vara, E.J.; Valeur, J.; Hausken, T.; Lied, G.A. Extra-intestinal symptoms in patients with irritable bowel syndrome: Related to high total IgE levels and atopic sensitization? Scand. J. Gastroenterol. 2016, 51, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2024, 16, 92. [Google Scholar] [CrossRef]
- Abril, A.G.; Villa, T.G.; Sánchez-Pérez, Á.; Notario, V.; Carrera, M. The Role of the Gallbladder, the Intestinal Barrier and the Gut Microbiota in the Development of Food Allergies and Other Disorders. Int. J. Mol. Sci. 2022, 23, 14333. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for thought: A comprehensive review of food allergy 2021–2023. J. Allergy Clin. Immunol. 2024, 153, 576–594. [Google Scholar] [CrossRef]
- Jones, M.P.; Walker, M.M.; Ford, A.C.; Talley, N.J. The overlap of atopy and functional gastrointestinal disorders among 23 471 patients in primary care. Aliment. Pharmacol. Ther. 2014, 40, 382–391. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Furukawa, S.; Miyake, T.; Watanabe, J.; Nakamura, Y.; Taguchi, Y.; Yamamoto, T.; Kato, A.; Kusumoto, K.; Yoshida, O.; et al. Multimorbidity of Allergic Diseases Is Associated with Functional Gastrointestinal Disorders in a Young Japanese Population. J. Neurogastroenterol. Motil. 2024, 30, 229–235. [Google Scholar] [CrossRef]
- Stapel, S.O.; Asero, R.; Ballmer-Weber, B.K.; Knol, E.F.; Strobel, S.; Vieths, S.; Kleine-Tebbe, J. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Sumazaki, R.; Nagai, Y.; Shibasaki, M.; Takita, H. Immune response to food antigens: Kinetics of food-specific antibodies in the normal population. Pediatr. Int. 1997, 39, 322–328. [Google Scholar] [CrossRef]
- Whelan, K.; Ford, A.C.; Burton-Murray, H.; Staudacher, H.M. Dietary management of irritable bowel syndrome: Considerations, challenges, and solutions. Lancet Gastroenterol. Hepatol. 2024, 9, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.; Crowe, S.E. Gastrointestinal food allergy: New insights into pathophysiology and clinical perspectives. Gastroenterology 2005, 128, 1089–1113. [Google Scholar] [CrossRef]
- Zar, S.; Kumar, D.; Benson, M.J. Food hypersensitivity and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2001, 15, 439–449. [Google Scholar] [CrossRef]
- Stierstorfer, M.; Toro, B. Patch Test–Directed Dietary Avoidance in the Management of Irritable Bowel Syndrome. Cutis 2021, 108, 91–95. [Google Scholar] [CrossRef]
- Singh, P.; Chey, W.D.; Takakura, W.; Cash, B.D.; Lacy, B.E.; Quigley, E.M.; Randall, C.W.; Lembo, A. A novel, IBS-specific IgG ELISA-based elimination diet in irritable bowel syndrome: A randomized, sham-controlled trial. Gastroenterology 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Dunbar, K.; Canto, M. Confocal endomicroscopy. Curr. Opin. Gastroenterol. 2008, 24, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D. Confocal microscopy from the bench to the bedside. Gastrointest. Endosc. 2005, 62, 696–697. [Google Scholar] [CrossRef]
- Fugazza, A.; Gaiani, F.; Carra, M.C.; Brunetti, F.; Lévy, M.; Sobhani, I.; Azoulay, D.; Catena, F.; De’angelis, G.L.; De’angelis, N. Confocal Laser Endomicroscopy in Gastrointestinal and Pancreatobiliary Diseases: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 4638683. [Google Scholar] [CrossRef]
- Neumann, H.; Kiesslich, R.; Wallace, M.B.; Neurath, M.F. Confocal laser endomicroscopy: Technical advances and clinical applications. Gastroenterology 2010, 139, 388–392.e2. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, D.I.; Saftoiu, A.; Ciurea, T.; Popescu, C.; Georgescu, C.V.; Malos, A. Confocal laser endomicroscopy of the colon. J. Gastrointest. Liver Dis. 2010, 19, 207–211. [Google Scholar] [PubMed]
- Kiesslich, R.; Goetz, M.; Angus, E.M.; Hu, Q.; Guan, Y.; Potten, C.; Allen, T.; Neurath, M.F.; Shroyer, N.F.; Montrose, M.H.; et al. Identification of Epithelial Gaps in Human Small and Large Intestine by Confocal Endomicroscopy. Gastroenterology 2007, 133, 1769–1778. [Google Scholar] [CrossRef]
- De Palma, G.D. Confocal laser endomicroscopy in the “in vivo” histologicaldiagnosis of the gastrointestinal tract. World J. Gastroenterol. 2009, 15, 5770–5775. [Google Scholar] [CrossRef]
- Tontini, G.E.; Mudter, J.; Vieth, M.; Günther, C.; Milani, V.; Atreya, R.; Rath, T.; Nägel, A.; Hatem, G.; Sturniolo, G.C.; et al. Prediction of clinical outcomes in Crohn’s disease by using confocal laser endomicroscopy: Results from a prospective multicenter study. Gastrointest. Endosc. 2018, 87, 1505–1514.e3. [Google Scholar] [CrossRef]
- Rath, T.; Dieterich, W.; Kätscher-Murad, C.; Neurath, M.F.; Zopf, Y. Cross-sectional imaging of intestinal barrier dysfunction by confocal laser endomicroscopy can identify patients with food allergy in vivo with high sensitivity. Sci. Rep. 2021, 11, 12777. [Google Scholar] [CrossRef]
- Turcotte, J.-F.; Kao, D.; Mah, S.J.; Claggett, B.; Saltzman, J.R.; Fedorak, R.N.; Liu, J.J. Breaks in the wall: Increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest. Endosc. 2013, 77, 624–630. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020.e4. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients with Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated with Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e5. [Google Scholar] [CrossRef]
- Gjini, B.; Melchior, I.; Euler, P.; Kreysel, C.; Kalde, S.; Krummen, B.; Kiesslich, R.; Hemmerlein, B.; Frieling, T. Food intolerance in patients with functional abdominal pain: Evaluation through endoscopic confocal laser endomicroscopy. Endosc. Int. Open 2023, 11, E67–E71. [Google Scholar] [CrossRef]
- Frieling, T.; Gjini, B.; Melchior, I.; Euler, P.; Kreysel, C.; Kalde, S.; Krummen, B.; Kiesslich, R.; Hemmerlein, B. Gastrointestinal adverse reaction to food (GARF) and endoscopic confocal laser endomicroscopy (eCLE). Z. Gastroenterol. 2024, 62, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Berumen, A.; Peters, S.; Wei, T.; Breen-Lyles, M.; Harmsen, W.S.; Busciglio, I.; Burton, D.; Roque, M.V.; DeVault, K.R.; et al. Intestinal chemosensitivity in irritable bowel syndrome associates with small intestinal TRPV channel expression. Aliment. Pharmacol. Ther. 2021, 54, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Heßler, N.; Kordowski, A.; Sasse, J.; Ahlemann, G.; Schulz, F.; Schröder, T.; Exner, A.; Jablonski, L.; Jappe, U.; Bischoff, S.C.; et al. Study protocol to investigate the efficacy of confocal laser endomicroscopy-based selective single-elimination diet over standard fivefold elimination diet in patients with endomicroscopically proven food intolerance: App-assisted, monocentric, double-blind, randomised and controlled trial in Germany. BMJ Open 2023, 13, e072024. [Google Scholar] [CrossRef]
- Kiesslich, R.; Adib-Tezer, H.; Teubner, D.; Frieling, T.; Bayerl, C.; Wenda, N.K.; Gosepath, J. ID: 3526039 Food Allergy Sensitivity Test (Fast) with Endomicroscopy of the Duodenum Enables Tailored Exclusion Diet in Patients with Irritable Bowel Syndrome. Gastrointest. Endosc. 2021, 93, AB207. [Google Scholar] [CrossRef]
- Kiesslich, R.; Adib-Tezer, H.; Teubner, D.; Frieling, T.; Mudter, J.; Bayerl, C.; Wenda, N.K.; Gosepath, J. Su1344 Endomicroscopic Detection of Atypical Food Allergy in Patients with Irritable Bowel Syndrome—A New Diagnostic Era? Gastroenterology 2020, 158, S-558–S-559. [Google Scholar] [CrossRef]
- Blomsten, A.; van Gils, T.; Algera, J.P.; Josefsson, A.; Hreinsson, J.P.; Hedenström, P.; Störsrud, S.; Törnblom, H.; Simren, M. Tu1682 food-induced intestinal mucosal reactions in irritable bowel syndrome detected with confocal laser endomicroscopy. Gastroenterology 2024, 166, S-1377–S-1378. [Google Scholar] [CrossRef]
- Piche, T.; Barbara, G.; Aubert, P.; Varannes, S.B.D.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut 2009, 58, 196–201. [Google Scholar] [CrossRef]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef]
- Barone, M.; Gemello, E.; Viggiani, M.T.; Cristofori, F.; Renna, C.; Iannone, A.; Di Leo, A.; Francavilla, R. Evaluation of non-celiac gluten sensitivity in patients with previous diagnosis of irritable bowel syndrome: A randomized double-blind placebo-controlled crossover trial. Nutrients 2020, 12, 705. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Wrona, D.; Fuschi, D.; Marasco, G.; Stanghellini, V.; Barbara, G. Non-celiac gluten sensitivity in the context of functional gastrointestinal disorders. Nutrients 2020, 12, 3735. [Google Scholar] [CrossRef]
- Costantino, A.; Aversano, G.M.; Lasagni, G.; Smania, V.; Doneda, L.; Vecchi, M.; Roncoroni, L.; Pastorello, E.A.; Elli, L. Diagnostic management of patients reporting symptoms after wheat ingestion. Front. Nutr. 2022, 9, 1007007. [Google Scholar] [CrossRef]
- Bojarski, C.; Tangermann, P.; Barmeyer, C.; Buchkremer, J.; Kiesslich, R.; Ellrichmann, M.; Schreiber, S.; Schmidt, C.; Stallmach, A.; Roehle, R.; et al. Prospective, double-blind diagnostic multicentre study of confocal laser endomicroscopy for wheat sensitivity in patients with irritable bowel syndrome. Gut 2022, 71, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.M.; Aggarwal, K.R.; Shim, L.S.; Bassan, M.; Kalantar, J.S.; Weltman, M.D.; Jones, M.; Powell, N.; Talley, N.J. Duodenal eosinophilia and early satiety in functional dyspepsia: Confirmation of a positive association in an Australian cohort. J. Gastroenterol. Hepatol. 2014, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shen, J.; Kim, J.J.; Yu, Y.; Ma, L.; Dai, N. Increased Duodenal Eosinophil Degranulation in Patients with Functional Dyspepsia: A Prospective Study. Sci. Rep. 2016, 6, 34305. [Google Scholar] [CrossRef]
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.-J.; Castelain, M.-C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal permeability and fecal eosinophil-derived neurotoxin are the best diagnosis tools for digestive non-IgE-mediated cow’s milk allergy in toddlers. Clin. Chem. Lab. Med. 2013, 51, 351–361. [Google Scholar] [CrossRef]
- Saitoh, O.; Kojima, K.; Sugi, K.; Matsuse, R.; Uchida, K.; Tabata, K.; Nakagawa, K.; Kayazawa, M.; Hirata, I.; Katsu, K.-I. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am. J. Gastroenterol. 1999, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 2002, 51 (Suppl. S1), i41–i44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuentes, I.M.; Christianson, J.A. Ion channels, ion channel receptors, and visceral hypersensitivity in irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 1613–1618. [Google Scholar] [CrossRef]
- Azpiroz, F.; Bouin, M.; Camilleri, M.; Mayer, E.A.; Poitras, P.; Serra, J.; Spiller, R.C. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol. Motil. 2007, 19, 62–88. [Google Scholar] [CrossRef]
- Christianson, J.A.; Davis, B.M. The Role of Visceral Afferents in Disease. In Translational Pain Research: From Mouse to Man; Kruger, L., Light, A.R., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 3. [Google Scholar] [PubMed]
- Brierley, S.M.; Page, A.J.; Hughes, P.A.; Adam, B.; Liebregts, T.; Cooper, N.J.; Holtmann, G.; Liedtke, W.; Blackshaw, L.A. Selective Role for TRPV4 Ion Channels in Visceral Sensory Pathways. Gastroenterology 2008, 134, 2059–2069. [Google Scholar] [CrossRef]
- Cenac, N.; Bautzova, T.; Le Faouder, P.; Veldhuis, N.A.; Poole, D.P.; Rolland, C.; Bertrand, J.; Liedtke, W.; Dubourdeau, M.; Bertrand-Michel, J.; et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 2015, 149, 433–444.e7. [Google Scholar] [CrossRef] [PubMed]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M.V.; Jain, P.; Theofanous, S.A.; Hanning, N.; De Man, J.G.; Berg, M.; De Winter, B.; Alpizar, Y.A.; et al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1–Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients with Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W. Role of TRPV ion channels in sensory transduction of osmotic stimuli in mammals. Exp. Physiol. 2007, 92, 507–512. [Google Scholar] [CrossRef]
- Baker, M.G.; Sampson, H.A. Recent trends in food protein–induced enterocolitis syndrome (FPIES). J. Allergy Clin. Immunol. 2023, 151, 43–46. [Google Scholar] [CrossRef]
- Berin, M.C. Immunopathophysiology of food protein–induced enterocolitis syndrome. J. Allergy Clin. Immunol. 2015, 135, 1108–1113. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Martinez, C.; Gonzalez-Castro, A.M.; Vicario, M.; Santos, J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver 2012, 6, 305–315. [Google Scholar] [CrossRef]
- Nojkov, B.; Zhou, S.-Y.; Dolan, R.D.; Davis, E.M.; Appelman, H.D.; Guo, X.; Jackson, K.; Sturm, M.B.; Wang, T.D.; Owyang, C.; et al. Evidence of Duodenal Epithelial Barrier Impairment and Increased Pyroptosis in Patients with Functional Dyspepsia on Confocal Laser Endomicroscopy and “Ex Vivo” Mucosa Analysis. Am. J. Gastroenterol. 2020, 115, 1891–1901. [Google Scholar] [CrossRef]
- Schol, J.; Balsiger, L.M.; Toth, J.; Wauters, L.; Houte, K.V.D.; Huang, I.; Vanuytsel, T.; Carbone, F.; Tack, J.F. Tu1346: Role Of Atypical Food Allergies in Functional Dyspepsia: Evaluation by Six-Food Elimination Diet and Confocal Laser Endomicroscopy Food Allergy Testing. Gastroenterology 2022, 162, S926. [Google Scholar] [CrossRef]
- Langhorst, J.; Bittel, M.; Öznur, Ö.; Schnitker, J.; Buslei, R.; Förster, S.; Tannapfel, A. 1139: Primary and food-induced secondary barrier dysfunction in patients with crohns disease. Gastroenterology 2022, 162, S-267. [Google Scholar] [CrossRef]
- Frieling, T.; Gjini, B.; Melchior, I.; Hemmerlein, B.; Kiesslich, R.; Kuhlbusch-Zicklam, R. Eosinophilic esophagitis and duodenal food challenge—Evaluation through endoscopic confocal laser endomicroscopy. Z. Gastroenterol. 2023, 62, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Balsiger, L.M.; Rusticeanu, M.; Langhorst, J.; Sina, C.; Benamouzig, R.; Huang, C.; Tack, J.; Kiesslich, R. Review: Food-induced mucosal alterations visualized using endomicroscopy. Neurogastroenterol. Motil. 2024, 37, e14930. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Barrier dysfunction in the terminal ileum | 96.3 | 66.6 | 70.3 | 95.7 |
| Barrier dysfunction in the colon | 37.0 | 81.8 | 62.5 | 61.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, F.; Costantino, A.; Tontini, G.E.; Elli, L.; Siragusa, N.; Lasagni, G.; Dubini, M.; Scricciolo, A.; Vecchi, M. Is IBS a Food Allergy? Confocal Laser Endomicroscopy Findings in Patients with IBS: A Narrative Review. Appl. Sci. 2025, 15, 3717. https://doi.org/10.3390/app15073717
Pavan F, Costantino A, Tontini GE, Elli L, Siragusa N, Lasagni G, Dubini M, Scricciolo A, Vecchi M. Is IBS a Food Allergy? Confocal Laser Endomicroscopy Findings in Patients with IBS: A Narrative Review. Applied Sciences. 2025; 15(7):3717. https://doi.org/10.3390/app15073717
Chicago/Turabian StylePavan, Francesco, Andrea Costantino, Gian Eugenio Tontini, Luca Elli, Nicola Siragusa, Giovanni Lasagni, Marco Dubini, Alice Scricciolo, and Maurizio Vecchi. 2025. "Is IBS a Food Allergy? Confocal Laser Endomicroscopy Findings in Patients with IBS: A Narrative Review" Applied Sciences 15, no. 7: 3717. https://doi.org/10.3390/app15073717
APA StylePavan, F., Costantino, A., Tontini, G. E., Elli, L., Siragusa, N., Lasagni, G., Dubini, M., Scricciolo, A., & Vecchi, M. (2025). Is IBS a Food Allergy? Confocal Laser Endomicroscopy Findings in Patients with IBS: A Narrative Review. Applied Sciences, 15(7), 3717. https://doi.org/10.3390/app15073717








