Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace
Abstract
1. Introduction
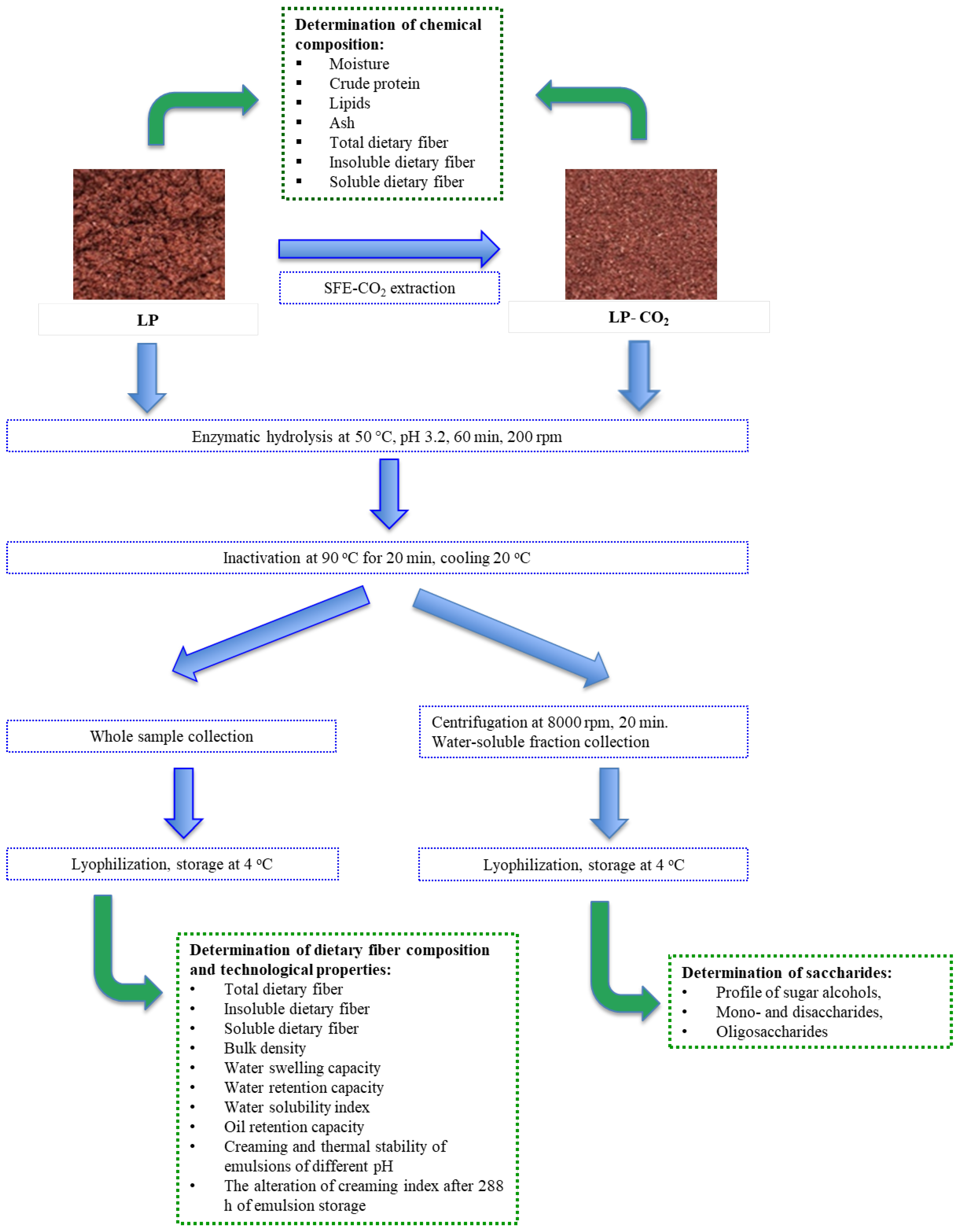
2. Materials and Methods
2.1. Lingonberry Pomace Characteristics and Enzymatic Modification
2.2. Chemical Composition Determination
2.3. Saccharide Analysis by High-Pressure Liquid Chromatography with Refractive Index Detector
2.4. The Analysis of Technological Properties
2.5. Emulsion Preparation and Analysis of Stability
2.6. Statistical Analyses
3. Results
3.1. Chemical Composition of LP and LP-CO2
3.2. Chemical Composition of Enzymatically Treated LP
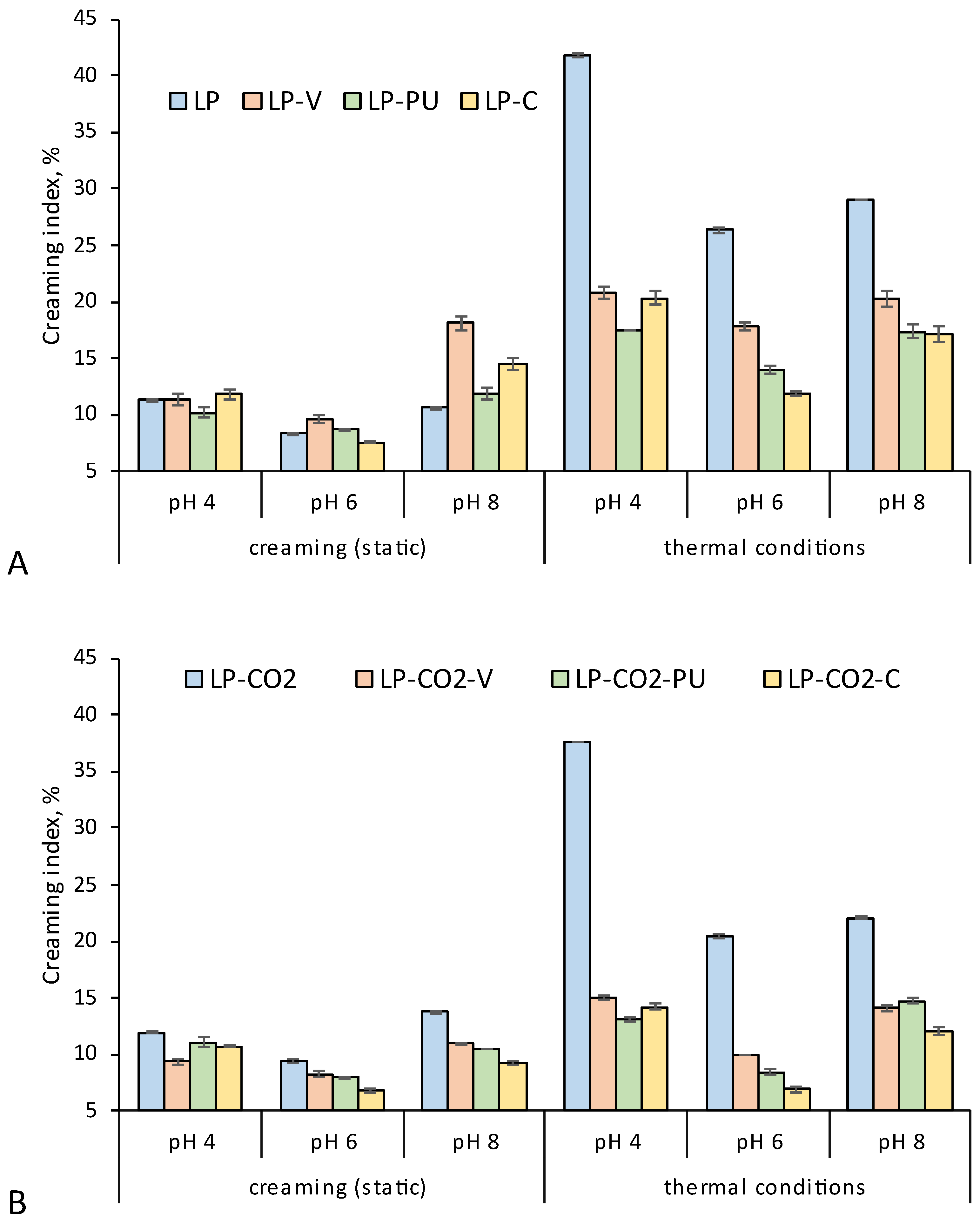
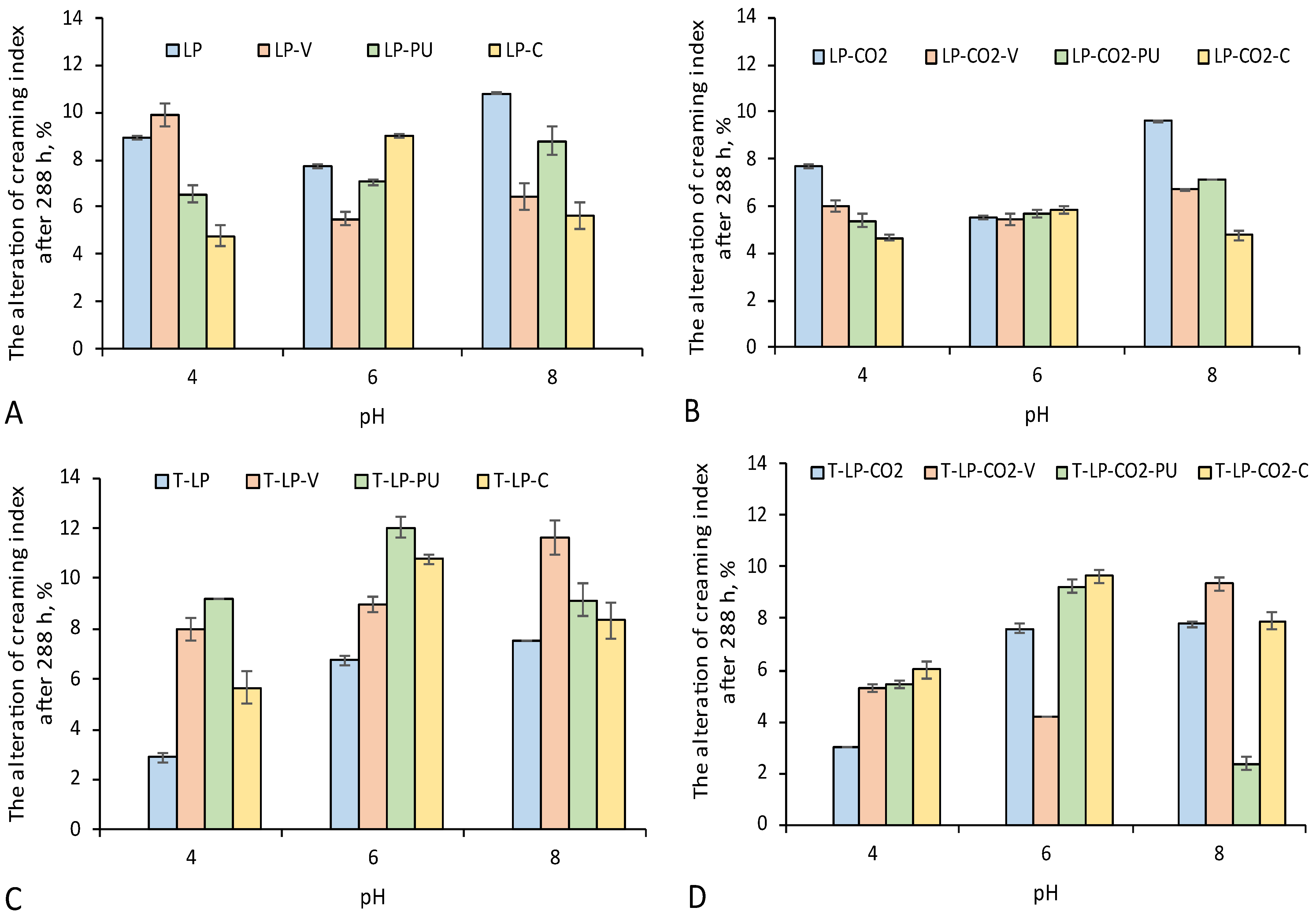
3.3. Technological Properties of Modified LP
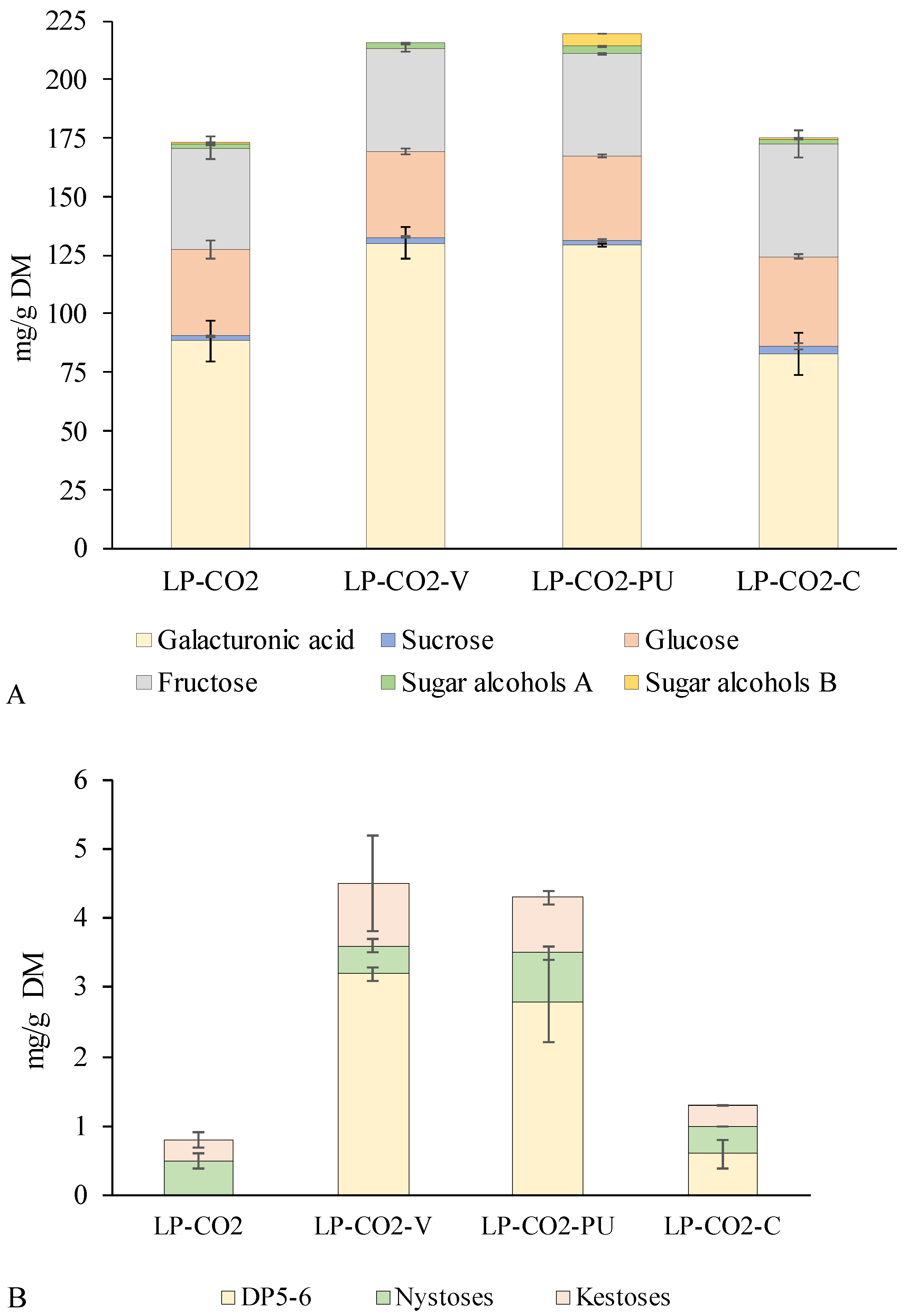
3.4. Mono- and Oligosaccharides of Modified LP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SFE-CO2 | Supercritical carbon dioxide |
| TDF | Total dietary fiber |
| SDS | Soluble dietary fiber |
| IDF | Insoluble dietary fiber |
| WSC | Water swelling capacity |
| WRC | Water retention capacity |
| WSI | Solubility index |
| ORC | Oil retention capacity |
| BD | Bulk density |
| LP | Lingonberry pomace |
| LP-CO2 | Lingonberry pomace modified using supercritical carbon dioxide extraction |
| LP-V | Lingonberry pomace after hydrolysis with Viscozyme® L |
| LP-PU | Lingonberry pomace after hydrolysis with Pectinex® Ultra Tropical |
| LP-C | Lingonberry pomace after hydrolysis with Celluclast® 1.5 L |
| LP-CO2-V | Lingonberry pomace after CO2 extraction and hydrolysis with Viscozyme® L |
| LP-CO2-PU | Lingonberry pomace after CO2 extraction and hydrolysis with Pectinex® Ultra Tropical |
| LP-CO2-C | Lingonberry pomace after CO2 extraction and hydrolysis with Celluclast® 1.5 L |
References
- Ross, K.A.; Godfrey, D.; Fukumoto, L. The chemical composition, antioxidant activity and α-glucosidase inhibitory activity of water-extractable polysaccharide conjugates from northern Manitoba lingonberry. Cogent Food Agric. 2015, 1, 1109781. [Google Scholar] [CrossRef]
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food grade lingonberry extract: Polyphenolic composition and in vivo protective effect against oxidative stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, K.; Heiniö, R.L.; Juvonen, R.; Kössö, T.; Puupponen-Pimiä, R. Relation of sensory perception with chemical composition of bioprocessed lingonberry. Food Chem. 2014, 157, 148–156. [Google Scholar] [CrossRef]
- Marsol-Vall, A.; Kelanne, N.; Nuutinen, A.; Yang, B.; Laaksonen, O. Influence of enzymatic treatment on the chemical composition of lingonberry (Vaccinium vitis-idaea) juice. Food Chem. 2021, 339, 128052. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of berry pomace to design functional foods. Food Rev. Int. 2021, 39, 3204–3224. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Serna-Saldivar, S.O.; Welti-Chanes, J. Dietary Fiber Concentrates from Fruit and Vegetable By-products: Processing, Modification, and Application as Functional Ingredients. Food Bioprocess Technol. 2018, 11, 1439–1463. [Google Scholar] [CrossRef]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding Value to Fruit Processing Waste: Innovative Ways to Incorporate Fibers from Berry Pomace in Baked and Extruded Cereal-based Foods—A SUSFOOD Project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef]
- Jagelaviciute, J.; Basinskiene, L.; Cizeikiene, D.; Syrpas, M. Technological Properties and Composition of Enzymatically Modified Cranberry Pomace. Foods 2022, 11, 2321. [Google Scholar] [CrossRef]
- Amundsen, M.; Hykkerud, A.L.; Kelanne, N.; Tuominen, S.; Schmidt, G.; Laaksonen, O.; Yang, B.; Martinussen, I.; Jaakola, L.; Aaby, K. Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages. Foods 2023, 12, 2154. [Google Scholar] [CrossRef]
- Schmid, V.; Trabert, A.; Schäfer, J.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Modification of Apple Pomace by Extrusion Processing: Studies on the Composition, Polymer Structures, and Functional Properties. Foods 2020, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Tamkutė, L.; Pukalskas, A.; Syrpas, M.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Fractionation of cranberry pomace lipids by supercritical carbon dioxide extraction and on-line separation of extracts at low temperatures. J. Supercrit. Fluids 2020, 163, 104884. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kavaliauskaitė, A.; Tamkutė, L.; Pukalskienė, M.; Syrpas, M.; Venskutonis, P.R. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef]
- Total Dietary Fiber Assay Procedure. Megazyme 2017; pp. 1–19. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 1 May 2022).
- Syrpas, M.; Valanciene, E.; Augustiniene, E.; Malys, N. Valorization of Bilberry (Vaccinium myrtillus L.) Pomace by Enzyme-Assisted Extraction: Process Optimization and Comparison with Conventional Solid-Liquid Extraction. Antioxidants 2021, 10, 773. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of Carrot (Daucus carota Linn. Var. Sativa Hoffm.) Pomace Insoluble Dietary Fiber with Complex Enzyme Method, Ultrafine Comminution, and High Hydrostatic Pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef]
- Jagelavičiutė, J.; Čižeikienė, D.; Bašinskienė, L. Enzymatic Modification of Apple Pomace and Its Application in Conjunction with Probiotics for Jelly Candy Production. Appl. Sci. 2025, 15, 599. [Google Scholar] [CrossRef]
- Keršienė, M.; Jasutienė, I.; Eisinaitė, V.; Venskutonis, P.R.; Leskauskaitė, D. Designing multiple bioactives loaded emulsions for the formulations for diets of elderly. Food Funct. 2020, 11, 2195–2207. [Google Scholar] [CrossRef]
- Alba, K.; Campbell, G.M.; Kontogiorgos, V. Dietary fibre from berry-processing waste and its impact on bread structure: A review. J. Sci. Food Agric. 2019, 99, 4189–4199. [Google Scholar] [CrossRef]
- Nguyen, C.L.; Nguyen, H.V.H. The Quality of Mulberry Juice as Affected by Enzyme Treatments. Beverages 2018, 4, 41. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Iqbal, S.; Tirpanalan-Staben, Ö.; Franke, K. Modification of Dietary Fibers to Valorize the By-Products of Cereal, Fruit and Vegetable Industry-A Review on Treatment Methods. Plants 2022, 11, 3466. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Fang, H.; Gao, Y.; Su, T.; Niu, Y.; Yu, L. Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic Conversion of Date Fruit Fiber Concentrates into a New Product Enriched in Antioxidant Soluble Fiber. LWT 2017, 75, 727–734. [Google Scholar]
- Yoon, K.Y.; Cha, M.; Shin, S.R.; Kim, K.S. Enzymatic Production of a Soluble-Fibre Hydrolyzate from Carrot Pomace and Its Sugar Composition. Food Chem. 2005, 92, 151–157. [Google Scholar]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and characterization of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef]
- Čechovičienė, I.; Šlepetienė, A.; Gumbytė, M.; Paulauskienė, A.; Tarasevičienė, Ž. Composition and Physicochemical Properties of Pomace of Various Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2024, 10, 38. [Google Scholar] [CrossRef]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Witczak, T.; Witczak, M.; Socha, R.; StĘPień, A.; Grzesik, M. Candied orange peel produced in solutions with various sugar compositions: Sugar composition and sorption properties of the product. J. Food Process Eng. 2017, 40, e12367. [Google Scholar] [CrossRef]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/ functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT Food Sci. Technol. 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Yang, G.; Sun, L.; Song, X.; Chen, Q.; Bao, Y.; Luo, T.; Wang, J. Microstructure, physicochemical properties, and adsorption capacity of deoiled red raspberry pomace and its total dietary fiber. LWT Food Sci. Technol. 2022, 153, 112478. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Gieteling, J.; Nikiforidis, C.V.; Boom, R.M.; van der Goot, A.J. Interfacial properties of green leaf cellulosic particles. Food Hydrocoll. 2017, 71, 8–16. [Google Scholar] [CrossRef]
- Wallecan, J.; McCrae, C.; Debon, S.J.J.; Dong, J.; Mazoyer, J. Emulsifying and stabilizing properties of functionalized orange pulp fibers. Food Hydrocoll. 2015, 47, 115–123. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Huc-Mathis, D.; Almeida, G.; Michon, C. Pickering emulsions based on food by-products: A comprehensive study of soluble and insoluble contents. J. Colloid. Interface Sci. 2021, 581, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of total dietary fiber using AOAC Method 2009.01 (AACC International Approved Method 32-45.01): Evaluation and updates. Cereal Chem. 2013, 90, 396–414. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2015, 36, 594–606. [Google Scholar] [CrossRef]
| Parameter | LP | LP-CO2 |
|---|---|---|
| Moisture, % | 3.41 ± 0.04 a | 3.58 ± 0.09 b |
| Crude protein | 8.60 ± 0.28 a | 9.03 ± 0.01 b |
| Lipids | 12.68 ± 0.39 a | 7.60 ± 0.04 b |
| Ash | 1.18 ± 0.01 a | 1.24 ± 0.01 b |
| TDF | 73.85 ± 0.79 a | 77.20 ± 1.62 b |
| IDF | 65.36 ± 0.69 a | 68.26 ± 1.47 b |
| SDF | 8.49 ± 0.1 a | 8.94 ± 0.15 b |
| IDF/SDF ratio | 7.698 | 7.635 |
| 1 Mono- and oligosaccharides | 3.68 ± 1.22 a | 4.93 a ± 1.20 b |
| LP Modification | LP-V | LP-PU | LP-C | LP-CO2-V | LP-CO2-PU | LP-CO2-C |
|---|---|---|---|---|---|---|
| TDF | 59.21 ± 0.31 a | 59.9 ± 1.0 a | 68.82 ± 0.05 c | 62.6 ± 0.12 b | 63.86 ± 0.55 b | 72.1 ± 0.09 d |
| IDF | 55.22 ± 0.38 a | 55.97 ± 0.97 ab | 59.21 ± 0.26 c | 58.49 ± 0.18 bc | 59.33 ± 0.42 c | 62.12 ± 0.12 d |
| SDF | 3.99 ± 0.07 a | 3.93 ± 0.03 a | 9.62 ± 0.21 c | 4.11 ± 0.06 a | 4.53 ± 0.13 b | 9.98 ± 0.03 c |
| IDF/SDF ratio | 13.840 b | 14.242 b | 6.155 a | 14.231 b | 13.097 c | 6.224 a |
| BD, g/mL | WSC, mL/g | WRC, g/g | WSI, % | ORC, g/g | |
|---|---|---|---|---|---|
| LP | 0.30 ± 0 d | 1.298 ± 0.004 c | 5.03 ± 0.20 a | 19.20 ± 0.05 b | 2.27 ± 0.02 a |
| LP-CO2 | 0.30 ± 0.001 d | 1.296 ± 0.006 c | 4.88 ± 0.09 a | 18.62 ± 0.05 a | 2.30 ± 0.01 a |
| LP-V | 0.26 ± 0.001 c | 1.075 ± 0.005 b | 6.08 ± 0.11 d | 23.33 ± 0.33 e | 3.27 ± 0.03 b |
| LP-PU | 0.25 ± 0.001 b | 1.073 ± 0.004 b | 6.12 ± 0.01 d | 26.28 ± 0.08 g | 3.35 ± 0.05 b |
| LP-C | 0.23 ± 0.001 a | 0.538 ± 0.003 a | 5.68 ± 0.01 c | 22.53 ± 0.06 d | 3.75 ± 0.02 c |
| LP-CO2-V | 0.26 ± 0 c | 1.078 ± 0 b | 5.91 ± 0.01 d | 23.26 ± 0.01 e | 3.34 ± 0.01 b |
| LP-CO2-PU | 0.25 ± 0.001 b | 1.075 ± 0.003 b | 5.61 ± 0.02 c | 24.47 ± 0.21 f | 3.69 ± 0.01 c |
| LP-CO2-C | 0.23 ± 0 a | 0.537 ± 0 a | 5.35 ± 0.01 b | 19.78 ± 0 c | 3.86 ± 0.05 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimkutė, S.; Bašinskienė, L.; Syrpas, M.; Čižeikienė, D. Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace. Appl. Sci. 2025, 15, 3661. https://doi.org/10.3390/app15073661
Šimkutė S, Bašinskienė L, Syrpas M, Čižeikienė D. Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace. Applied Sciences. 2025; 15(7):3661. https://doi.org/10.3390/app15073661
Chicago/Turabian StyleŠimkutė, Simona, Loreta Bašinskienė, Michail Syrpas, and Dalia Čižeikienė. 2025. "Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace" Applied Sciences 15, no. 7: 3661. https://doi.org/10.3390/app15073661
APA StyleŠimkutė, S., Bašinskienė, L., Syrpas, M., & Čižeikienė, D. (2025). Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace. Applied Sciences, 15(7), 3661. https://doi.org/10.3390/app15073661






