Abstract
The present study evaluated the effect of two inoculum concentrations on the degradation of crude oil by Corynebacterium stationis CsPe-1. To this end, two treatment systems were utilized, each containing Davies Minimum Medium, 1% crude oil, and bacterial inoculum at concentrations of 10% and 15%, respectively. The degree of oil biodegradation was determined by evaluating the biochemical oxygen demand (BOD5), the chemical oxygen demand (COD), the concentration and fractions of oil and grease, and the total petroleum hydrocarbons (TPH). The results indicated that both BOD5 and COD exhibited an increase after a 20-day treatment period. For the 10% and 15% inoculum concentrations, a statistically significant difference was observed between the initial and final values of oils and fats (p < 0.05). In both systems, the levels of oils and fats decreased by 61%, contrasting with the control system, which exhibited minimal variation. A significant difference (p < 0.05) was observed in the degradation of TPH at the two inoculum concentrations. The findings indicated that the biodegradation of TPH was more efficient with an inoculum of 15%, resulting in a 79.94% reduction in fraction 3 (28–40 carbon chains). Fraction 1 exhibited less degradation, attributable to the toxicity of short-chain n-alkanes. Genomic analysis identified the pcaG and pcaH genes, which have been linked to the degradation of polycyclic aromatic hydrocarbons. This study underscores the biotechnological potential of strain CsPe-1 for the remediation of hydrocarbon-contaminated environments, thereby contributing to the realization of Sustainable Development Goals 14 and 15.
1. Introduction
Currently, 80% of energy demand is met by fossil fuels, and this demand is expected to increase by 48% over the next 20 years [1]. Part of these fossil fuels are hydrocarbons, which are organic compounds with hydrogen and carbon in their chemical structure, forming various raw materials that are important energy sources for industry and are present in urban life as part of products such as petrol, solvents, and paints [2,3,4,5]. Some hydrocarbons are found in nature and are chemical energy carriers, such as petroleum, which is a derivative of fossil fuels with a liquid consistency ranging from yellowish to black in color and is naturally formed in underground geological formations [3,6]. Crude oil is composed of cycloalkanes (about 45%), alkanes (about 30%) and aromatic hydrocarbons (about 15%) [6].
Although petroleum hydrocarbons are important energy resources, they are the major source of organic pollutants, are highly toxic, and are in high demand, which has led to increased environmental pollution from exploration, production, maintenance, transport, storage, and accidental spills [7,8]. In the case of accidental oil spills, these hydrocarbons reach the groundwater through the mechanism of percolation through the soil and can also reach the oceans directly, negatively affecting the ecosystem and threatening the life that inhabits it [9,10,11]. Hydrocarbon compounds are also known to be carcinogenic and neurotoxic organic pollutants [12].
Unfortunately, petroleum hydrocarbons are difficult to degrade, so more attention has been paid to biodegradation methods that are part of bioremediation mechanisms, which is a promising technology and an alternative to physical and chemical methods that have become ineffective and costly in the removal of pollutants produced by anthropogenic activities and rapid industrialization [12,13,14]. On the other hand, the biodegradation of petroleum hydrocarbons is a technique that can be economical and environmentally friendly because it uses the same microbiota isolated from petroleum [15]. This microbiota is able to utilize oil as a carbon and energy source, making these microorganisms potential bioremediation agents for oil-contaminated environments [16]. On the other hand, studies to biodegrade oil from contaminated environments are in line with Sustainable Development Goals 14 and 15, Life Below Water and Life on Land, respectively. Hydrocarbon bioremediation studies involve a practice that promotes the preservation of ecosystems [17,18].
To date, 70 genera and 200 species of microorganisms capable of degrading oil have been reported, including bacteria, fungi and cyanobacteria, which are considered to be oleophilic microorganisms [15,19,20,21]. Among the bacteria, the genus Bacillus stands out [22], as well as Burkholderia, Mycobacterium, Polaromonas, and Pseudomonas, which have been identified in oil-contaminated soils [18]. Fungi of the genera Candida, Rhodotorula, and Aspergillus have shown potential for the biodegradation of petroleum hydrocarbons [23,24,25]. Moreover, fungal–bacterial consortia have been tested to improve oil biodegradation, such as the oil-degrading fungus Scedosporium sp. ZYY and the surfactant-producing bacterium Acinetobacter sp. Y2 [26]. All of these microorganisms can degrade petroleum hydrocarbons by producing hydrolase enzymes such as monooxygenases, dioxygenases, alkane hydroxylase, and catechol dioxygenase, which can degrade aromatic hydrocarbon alkanes to simpler computations that can then enter the tricarboxylic acid cycle [27]. It has also been reported that the Gram-negative bacterium Stenotrophomonas maltophilia can survive in a wide range of environments and use many complex substances for its growth and development [28]. It can also use polycyclic aromatic hydrocarbons as its sole source of carbon and energy [29], and is capable of degrading petroleum either alone or in consortium with other bacteria [11,30,31], as it produces various enzymes for degrading keratin, atrazine, trichloroethylene, and dichlorodiphenyltrichloroethane (DDT), and even acts in the bioremediation of metals [32].
Corynebacterium stationis is a Gram-positive, rod-shaped bacterium, 0.6–1 mm in diameter, without spores, occurring as single cells, in pairs or in clusters, and also in angular, V-shaped, or occasionally palisaded arrangements. They are facultative anaerobes, but develop best under aerobic conditions [33,34]. These bacteria can be isolated from a variety of environments including animal skin and mucous membranes, foliage, soil, some dairy products, fresh water, salt water, and sewage. Bacteria from soil are considered GRAS (generally recognized as safe) organisms, have genetic stability so they do not mutate easily, and can withstand changes in environmental factors such as pH, osmotic pressure, and temperature [35]. Corynebacterium species are lipophilic, and can assimilate carbon from various sources and have the ability to produce biosurfactants, which allows them to degrade oils and hydrocarbons and grow efficiently, so they have the potential to be used in the in situ bioremediation of environments contaminated with petroleum or petroleum hydrocarbons [36,37,38]. Studies have been conducted using these free and immobilized bacteria and it has been observed that when the free bacteria are used, 71.9% degradation of total petroleum hydrocarbons (TPH) is obtained, while when the bacteria are immobilized on a support with biocarbon, 78.9% degradation is obtained [38].
Some Corynebactrium species also have the ability to degrade a wide range of aromatic compounds, including inorganic species of arsenic (As), a toxic metalloid that is ubiquitously found in nature and has caused serious health problems worldwide. Corynebactrium is one of the most arsenic-resistant bacteria and has been investigated and enhanced by metabolic engineering to be used as an efficient bio-tool for bio-bioaccumulation of arsenic and for the sequestration of metals such as cadmium (Cd) and lead (Pb) [35].
Genomic techniques, such as 16s rDNA sequencing, are important for the study of species with bioremediation potential, as they can identify possible genes involved in the translation of enzymes responsible for hydrocarbon degradation [39], such as alkane monooxygenase LadAβ genes [40]. On the other hand, when applying in situ bioremediation techniques to oil-contaminated soils, it is important to control the proportion and ratio of native species, soil conditioning, and the introduction of oil-degrading strains [41].
Petroleum is a persistent pollutant with significant adverse effects on ecosystems and human health. Conventional physical–chemical remediation methods are costly and often cause collateral environmental damage. In this context, the use of C. stationis for the bioremediation of hydrocarbons has emerged as a sustainable and cost-effective strategy, with the additional advantage of eliminating the generation of additional toxic waste. This approach has potential for future studies on a larger scale or under real conditions. The present study therefore sought to evaluate the impact of two inoculum concentrations on crude oil degradation by the Peruvian strain C. stationis CsPe-1. The use of native strains is particularly promising for the bioremediation of oil-contaminated environments, since these strains show adaptability to the environmental conditions of their origin. It is noteworthy that this research is aligned with Sustainable Development Goals (SDGs) 14 (Life Below Water) and 15 (Life on Land), underscoring its significance for global sustainability initiatives.
2. Materials and Methods
2.1. Culture of the C. stationis CsPe-1 Strain
The C. stationis CsPe-1 strain was donated by the laboratory of the Instituto y Centro de Investigación de la Universidad Cesar Vallejo (Trujillo, Peru). This strain was used because of its potential to biodegrade petroleum, as mentioned in some studies [36,42,43]. The strain was reactivated to assess substrate degradation in cells. A suspension of the culture was transferred to an Erlenmeyer flask containing 200 mL of sterile tryptic soy broth. It was incubated at 30 °C with magnetic stirring at 60 rpm for 48 h. Then, 0.1 mL per surface was inoculated onto glucose agar plates and incubated at 30 °C for 24–48 h. After the incubation period, Gram staining was performed to check the purity of the culture.
2.2. Substrate
Crude oil samples were collected from the Peña Negra area (northeastern Peru) of the Talara-Piura refinery (Peru). An approximate volume of 500 mL was collected in sterile, airtight glass bottles. Date, temperature, and GPS coordinates were recorded. The samples were transferred to the Laboratory of the Institute for Research in Science and Technology of the César Vallejo University, where they were kept refrigerated until processing.
2.3. Adaptation of the Bacteria to Crude Oil Medium
A culture suspension was prepared in sterile physiological saline until it reached the turbidity of the Mac Farland nephelometer tube N° 1, and 10 mL was seeded in 190 mL of minimal salt medium with 1% crude oil as the sole source of carbon. It was incubated at 30 °C, with magnetic stirring at 60 RPM for 24 h. Bacteria were counted at 0 and 24 h, by the pour plate technique using nutrient agar, to verify the survival of the bacteria in the oil medium and the ability to use oil as the sole source of carbon for growth. Work was carried out in triplicate. This procedure was carried out based on the methodology of Otiniano et al. (2022) with some variations [44].
2.4. Preparation of Systems to Assess the Crude Oil Degradation
To evaluate the degradation of crude oil by C. stationis CsPe-1, the following systems were prepared in triplicate: System 1: 10% inoculum and 445 mL of Davis Minimum Medium (DMM), 50 mL of C. stationis CsPe-1 inoculum (3 × 108 cell.mL−1) and 5 mL of crude oil (1%); System 2: 15% inoculum, 420 mL of DMM, 75 mL of C. stationis CsPe-1 inoculum (3 × 108 cell.mL−1) and 5 mL of crude oil (1%) and for Control: 495 mL of DMM and 5 mL of crude oil (1%).
In all systems, the pH was adjusted to 5 and incubated under agitation at 70 RPM at room temperature (20 ± 2 °C), and with 2 VVM of aeration for 15 to 20 days [45]. Samples were taken at 0, 24, 48, 96, 192, and 384 h to monitor crude oil degradation by indirect and direct methods.
2.5. Evaluation of the Oil Degradation
Evaluation of the variation of biochemical oxygen demand (BOD5) and chemical oxygen demand (COD) during crude oil degradation was performed using the Winkler technique modified by Alsterberg [46,47]. This technique was used to determine the oxygen consumption required by C. stationis to degrade crude oil. It was carried out at 0 and 384 h.
2.5.1. Determination of Chemical Oxygen Demand (COD)
The COD measure how much oxygen is required to oxidize organic (oil) in the medium liquid. Dissolved oxygen was calculated using the following formulae:
mg O2 dissolved.Liter−1 = (A × N × 8 × 1000)/(V × Vfco − 2Vfco)
In the given equation, A denotes the titration rate, N is the sodium thiosulphate normal (1/40), V is the volume of the titrated sample, and Vfco is the volume of the BOD flask.
2.5.2. Determination of Biochemical Oxygen Demand (BOD5)
This test measures the amount of oxygen used by microorganisms to break down organic matter (oil) in water. The BOD5 was determined using the following formula:
where the OD1 is the initial dissolved oxygen content, OD2 is the final dissolved oxygen content, and P is the dilution factor, which is the ratio of sample volume to diluted sample volu.
BOD5 (mg O2 dissolved.liter−1) = (OD1 − OD2)/P
2.6. TPH Analysis by Gas Chromatography and Percentage of Reduction (%)
Total petroleum hydrocarbons (TPH), fractions 1, 2, and 3 were analyzed by gas chromatography using 500 mL of each sample, both initially contaminated with oil and after oil degradation by C. stationis bacteria: EPA Method 8015 C.
The percentage reduction of total petroleum hydrocarbons (TPH) and each petroleum hydrocarbon fraction was calculated by utilizing the initial and final concentrations of each component analyzed. The following formula was employed for this calculation:
Percentage of reduction (%) = (Initial Conc.-Final Conc.)/(Initial concentration) × 100
2.7. Genomic Sequencing and Characterization Analysis
The C. stationis CsPe-1 strain was reactivated on nutrient agar to obtain a pure culture and sent to the Ecobiotechnology S.A.C. laboratory for whole genome sequencing using Illumina PE 2 × 300 bp. Quality control of genomic sequencing was performed using FastQC v0.12.1 and Fastp v0.23.4 software. Genome assembly was performed using the Unicycler tool. Taxonomic identification of the species was performed by Sanger sequencing of the 16S ribosomal rRNA gene followed by genomic taxonomy using the TYGS (Type Genome Server) platform. The PROKKA program was used for genomic annotation and the KEGG database was used to identify genes related to oil degradation.
2.8. Statistical Analysis
The arithmetic means of the results obtained in the 3 replicates of the experiment were calculated. Analysis of variance was applied at 95% significance level to determine if there was a significant difference between the means of specific growth rate, BOD5, total hydrocarbons and biodegradation efficiency of crude oil. Analyses were performed using SPPS version 26 statistical software.
3. Results and Discussion
Table 1 shows the BOD5 values that increased after 20 days of oil treatment with C. stationis CsPe-1. This result is very different from the value obtained with another C. stationis CsPe-1 strain, which was able to reduce BOD5 by 98.4% and COD by 97.5% in oil-contaminated soils after 40 days [48]. This could be explained by the fact that after 20 days, C. stationis CsPe-1 is already in its stationary phase of growth, and oxygen consumption by the surviving population has been reported to increase during this phase [49].

Table 1.
Analysis of BOD5 and COD in oil degradation systems inoculated with C. stationis CsPe-1 contained in a stirred bioreactor.
In Table 2, it was observed that at both 10% and 15% inoculum concentrations, the initial and final values of oils and fats showed significant differences (p < 0.05). However, no significant differences were found when comparing these values between the two concentrations (p > 0.05). In this case, an aerobic degradation process was carried out, in which the biodegradation of PAHs is initiated by the enzyme oxygenase, which, in the presence of oxygen, catalyzes the addition of oxygen atoms to aromatic compounds. The result is the formation of catechol or protocatechol with hydroxyl groups. Then, alcohol dehydrogenase enzymes convert alcohol to aldehyde and then to fatty acids. These enter the β-oxidation process and acetyl CoA is produced, which reacts with fatty acids in the cytosol of prokaryotic cells or can also enter the citric acid (TCA) cycle [16,50,51,52]. Similar results have been reported with other bacterial strains of the genera Pseudomonas and Rhodococcus [53,54,55]. However, Corynebacterium stand out for their ability to adapt to a wide range of environmental conditions, which makes them particularly useful for bioremediation in various contaminated environments [56,57]. The effectiveness of C. stationis CsPe-1 can be attributed to its ability to produce biosurfactants, which enhance the solubility of hydrocarbon molecules in the aqueous medium, facilitating their access to bacterial cells [58]. These biosurfactants allow for the emulsification of oils, favoring the decomposition of hydrocarbons into more manageable units for the action of microbial enzymes. Notably, in this study, the 15% inoculum concentration exhibited a more substantial reduction in oils and fats compared to the 10% treatment, which can be attributed to the enhanced biosurfactant production, thereby increasing the bioavailability of hydrocarbons for degradation.

Table 2.
Analysis of the variation of the concentration of oils and fats in oil degradation systems inoculated with a strain of C. stationis CsPe-1.
In Figure 1a, a significant reduction in the concentration of the TPH and petroleum fractions due to the action of C. stationis CsPe-1 is evident at both the 10 and 15% inoculum concentrations. The observed variations in the reduced concentration may be attributable to the structural characteristics inherent to each fraction. At a particular inoculum concentration, a lower concentration is observed, with the exception of fraction 2. As shown in Figure 1b, the highest percentage reductions were achieved in all fractions when the 15% inoculum of C. stationis CsPe-1was used. Fraction 3 exhibited a notably higher percentage of reduction, reaching a value of 79.94% (127.4 mg L−1) with the 15% inoculum. The degradation of petroleum hydrocarbons has been linked to the presence of certain enzymes, such as oxygenase and peroxidase, as well as the production of biosurfactants, as evidenced in the case of Corynebacterium aurimucosum [37,59]. Furthermore, the genus Corynebacterium has demonstrated a remarkable capacity for hydrocarbon degradation, as evidenced by studies on Corynebacterium sp. strain GS5-66 [60,61]. Conversely, the propensity of C. stationis to degrade medium- and long-chain hydrocarbons (F2 and F3) has been observed in Corynebacterium variabile HRJ4, a salt-tolerant strain capable of degrading n-alkanes with chains ranging from 16 to 28 carbons [61]. The lowest percentage of reduction occurred in fraction 1, at both low and high concentrations of initial inoculum, reaching values of 47.03% and 65.07%, respectively. This outcome can be attributed, potentially, to the toxicity of short-chain n-alkanes due to their capacity to disrupt bacterial membranes. These findings are significant because they demonstrate the potential for experimental optimization of the hydrocarbon degradation process using this strain [36].
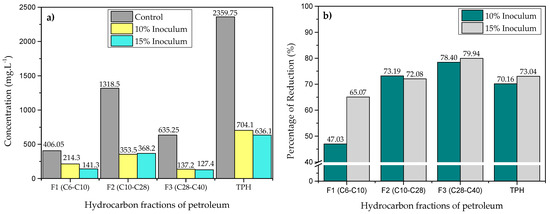
Figure 1.
(a) Concentrations and (b) percentage reduction of the petroleum and TPH fractions analyzed by gas chromatography.
Genomic Characterization of C. stationis CsPe-1
The sequencing report indicated that 11,013,512 raw reads were generated, with ap-proximately 92.5% exhibiting a Phred-score greater than 30 (Q30), indicating good sequencing depth and quality. Downstream analyses included de novo assembly using Unicycler, resulting in a 2.8 Mb assembled genome comprising 24 contigs greater than 200 bp, with a 54.8% GC content. Genome annotation using PROKKA produced 2566 CDS, two ribosomal operons, and 51 tRNAs across the 24 annotated contigs. These results are consistent with other C. stationis genomes deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/datasets/genome/?taxon=1705, accessed on 1 January 2025). Genomic taxonomy analysis using TYGS (Type Genome Server) suggested that, based on the 16S rRNA gene, strain CsPe-1 belongs to Corynebacterium ammoniagenes, clustering with C. ammoniagenes DSM 20306. However, whole-genome analysis revealed higher similarity to C. stationis, with a dDDH value of 85.1% (values ≥ 70% indicate the same species), supporting the classification of CsPe-1 as C. stationis (Figure 2).
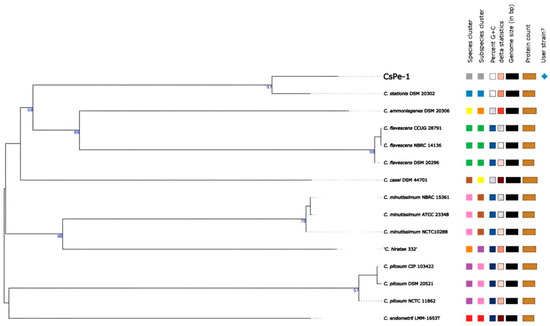
Figure 2.
Phylogenetic tree of the CsPe-1 strain based on the complete genome. The tree was generated using the TYGS tool (tygs.dsmz.de), using the orthologous sequences of the CsPe-1 genome (assembly) and other reference species strains.
In order to understand the oil-degrading capacity of the CsPe-1 strain and the potential to degrade other xenobiotics, the presence of genes associated with the metabolism and degradation of xenobiotics was evaluated according to their functional category from the KEGG (Kyoto Encyclopedia of Genes and Genomes) database. This is an invaluable tool for exploring metabolic pathways and genes involved in various biological processes, including the degradation of compounds such as oil [61]. Although oil is not considered a xenobiotic (due to its natural origin and biodegradability), according to the KEGG database, the potential genes associated with oil degradation fall under the category of metabolism and metabolic pathway xenobiotic biodegradation and metabolism.
The analyses identified the potential action in oil degradation of two genes associated with the degradation of polycyclic aromatic hydrocarbons: pcaG and pcaH. The degradation of aromatic compounds and petroleum in bacteria is a crucial process for the bioremediation of contaminated environments. The pcaG and pcaH genes, which code for the enzyme protocatechuate 3,4-dioxygenase, play an important role in the degradation of aromatic compounds via the β-ketoadipate pathway. The pcaG and pcaH genes were identified in six isolates of the Roseobacter lineage, a major taxon of marine α-proteobacteria. These genes enable the degradation of aromatic compounds through the β-ketoadipate pathway [62]. On the other hand, in Agrobacterium radiobacter, two sets of pcaH and pcaG genes were identified that code for two different protocatechuate 3,4-dioxygenases. One of these sets appears to have been acquired by lateral gene transfer, indicating significant genetic diversity and adaptability in the degradation of aromatic compounds [63].
4. Conclusions
The Peruvian strain C. stationis CsPe-1 demonstrated remarkable efficacy in the degradation of petroleum hydrocarbons, achieving a maximum reduction of 73.04% of the total petroleum hydrocarbons (TPHs), with an exceptional 79.94% in fraction 3 (carbon chains from 28 to 40). This efficiency was most evident at an inoculum concentration of 15%, where an increase in the BOD5 and COD was also observed, as well as in the reduction of acids and fats, key indicators of biological and chemical activity during the biodegradation process. From genomic analysis, we identified the presence of pcaG and pcaH genes associated with polycyclic aromatic hydrocarbon degradation. The role of these genes could be due to other bacterial species with the capacity to degrade crude oil, so it is necessary to investigate the genomic determinants in other bacterial species that share this capacity. These findings underscore the promise of C. stationis CsPe-1 as a biotechnological instrument in the remediation of hydrocarbon-contaminated environments, with the potential to enhance its efficacy through the optimization of experimental conditions and inoculum concentrations.
Author Contributions
Conceptualization, M.D.L.C.-N.; methodology, N.M.O. and L.C.-C.; software, W.R.-V.; validation, S.R.-F. and M.D.L.C.-N.; formal analysis, N.M.O. and W.R.-V.; investigation, M.D.L.C.-N.; data curation, W.R.-V. and N.M.O.; writing—original draft preparation, W.R.-V., N.M.O. and M.A.M.; writing—review and editing, L.C.-C. and M.A.M.; supervision, M.D.L.C.-N.; project administration, M.D.L.C.-N. and S.R.-F.; funding acquisition, M.D.L.C.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The research was funded by the Universidad Cesar Vallejo, with resolution code P-2022-177.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moodley, P.; Trois, C. Lignocellulosic Biorefineries: The Path Forward. In Sustainable Biofuels; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–42. ISBN 9780128202975. [Google Scholar]
- Olah, G.A.; Molnar, A.; Surya Prakash, G.K. Hydrocarbon Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781119390534. [Google Scholar]
- Rosen, M.A. Energy Sustainability with a Focus on Environmental Perspectives. Earth Syst. Environ. 2021, 5, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Enerijiofi, K.E. Bioremediation of Environmental Contaminants: A Sustainable Alternative to Environmental Management. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 461–480. ISBN 9780128205242. [Google Scholar]
- Bharadwaj, P.; Tripathi, D.; Pandey, S.; Tapadar, S.; Bhattacharjee, A.; Das, D.; Palwan, E.; Rani, M.; Kumar, A. Molecular Biology Techniques for the Detection of Contaminants in Wastewater. In Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 217–235. ISBN 9780128218815. [Google Scholar]
- Maria-Valeria, K.; Stylianos, T.; Vasiliki, P.; Doxakis, A.; Nikolaos, R.; Maria, L.; Pavlos, P. Petroleum Intoxication: Literature Review and Case Report on Poisoning by Gasoline. Soud. Lek. 2020, 65, 16–21. [Google Scholar] [PubMed]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Vandana; Priyadarshanee, M.; Mahto, U.; Das, S. Mechanism of Toxicity and Adverse Health Effects of Environmental Pollutants. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–53. ISBN 9780323854559. [Google Scholar]
- Ruberg, E.J.; Williams, T.D.; Elliott, J.E. Review of Petroleum Toxicity in Marine Reptiles. Ecotoxicology 2021, 30, 525–536. [Google Scholar] [CrossRef]
- Turner, N.R.; Renegar, D.A. Petroleum Hydrocarbon Toxicity to Corals: A Review. Mar. Pollut. Bull. 2017, 119, 1–16. [Google Scholar] [CrossRef]
- Gupta, P.K.; Mustapha, H.I.; Singh, B.; Sharma, Y.C. Bioremediation of Petroleum Contaminated Soil-Water Resources Using Neat Biodiesel: A Review. Sustain. Energy Technol. Assess. 2022, 53, 102703. [Google Scholar] [CrossRef]
- Ravi, A.; Ravuri, M.; Krishnan, R.; Narenkumar, J.; Anu, K.; Alsalhi, M.S.; Devanesan, S.; Kamala-Kannan, S.; Rajasekar, A. Characterization of Petroleum Degrading Bacteria and Its Optimization Conditions on Effective Utilization of Petroleum Hydrocarbons. Microbiol. Res. 2022, 265, 127184. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, H.; Zeng, F.; Jiang, L.; Atakpa, E.O.; Chen, G.; Zhang, C.; Xie, Q. A Biosurfactant-Producing Yeast Rhodotorula sp.CC01 Utilizing Landfill Leachate as Nitrogen Source and Its Broad Degradation Spectra of Petroleum Hydrocarbons. World J. Microbiol. Biotechnol. 2022, 38, 68. [Google Scholar] [CrossRef]
- Singh, M.; Jayant, K.; Mehra, A.; Bhutani, S.; Kaur, T.; Kour, D.; Suyal, D.C.; Singh, S.; Rai, A.K.; Yadav, A.N. Bioremediation—Sustainable Tool for Diverse Contaminants Management: Current Scenario and Future Aspects. J. Appl. Biol. Biotechnol. 2022, 48–63. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial Degradation of Petroleum Hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Osabohien, R. Editorial: Nutrition and Sustainable Development Goal 15: Life on Land. Front. Nutr. 2024, 11, 1453607. [Google Scholar] [CrossRef]
- Neumann, B.; Ott, K.; Kenchington, R. Strong Sustainability in Coastal Areas: A Conceptual Interpretation of SDG 14. Sustain. Sci. 2017, 12, 1019–1035. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, F.; Li, G.; Ruan, H.; Li, X.; Zhong, L.; Chen, G.; Rui, Y. Falsochrobactrum tianjinense sp. Nov., a New Petroleum-Degrading Bacteria Isolated from Oily Soils. Int. J. Environ. Res. Public Health 2022, 19, 11833. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Tripathi, V.; Manickam, N. Bacterial- and Fungal-Mediated Biodegradation of Petroleum Hydrocarbons in Soil. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–427. ISBN 9780323858397. [Google Scholar]
- Masika, W.S.; Moonsamy, G.; Mandree, P.; Ramchuran, S.; Lalloo, R.; Kudanga, T. Biodegradation of Petroleum Hydrocarbon Waste Using Consortia of Bacillus sp. Bioremediat. J. 2021, 25, 72–79. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Al-Zahrani, R.M.; Marraiki, N. The Crude Oil Biodegradation Activity of Candida Strains Isolated from Oil-Reservoirs Soils in Saudi Arabia. Sci. Rep. 2022, 12, 10708. [Google Scholar] [CrossRef]
- Benmessaoud, S.; Anissi, J.; Kara, M.; Assouguem, A.; AL-Huqail, A.A.; Germoush, M.O.; Ullah, R.; Ercisli, S.; Bahhou, J. Isolation and Characterization of Three New Crude Oil Degrading Yeast Strains, Candida Parapsilosis SK1, Rhodotorula Mucilaginosa SK2 and SK3. Sustainability 2022, 14, 3465. [Google Scholar] [CrossRef]
- Othman, A.R.; Ismail, N.S.; Abdullah, S.R.S.; Hasan, H.A.; Kurniawan, S.B.; Sharuddin, S.S.N.; Ismail, N.‘i. Potential of Indigenous Biosurfactant-Producing Fungi from Real Crude Oil Sludge in Total Petroleum Hydrocarbon Degradation and Its Future Research Prospects. J. Environ. Chem. Eng. 2022, 10, 107621. [Google Scholar] [CrossRef]
- Atakpa, E.O.; Zhou, H.; Jiang, L.; Ma, Y.; Liang, Y.; Li, Y.; Zhang, D.; Zhang, C. Improved Degradation of Petroleum Hydrocarbons by Co-Culture of Fungi and Biosurfactant-Producing Bacteria. Chemosphere 2022, 290, 133337. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Vermelho, A.B.; Rosado, A.S. Petroleum-Degrading Enzymes: Bioremediation and New Prospects. Enzym. Res. 2011, 2011, 475193. [Google Scholar] [CrossRef] [PubMed]
- Elufisan, T.O.; Rodríguez-Luna, I.C.; Oyedara, O.O.; Sánchez-Varela, A.; Hernández-Mendoza, A.; Dantán Gonzalez, E.; Paz-González, A.D.; Muhammad, K.; Rivera, G.; Villalobos-Lopez, M.A.; et al. The Polycyclic Aromatic Hydrocarbon (PAH) Degradation Activities and Genome Analysis of a Novel Strain Stenotrophomonas sp. Pemsol Isolated from Mexico. PeerJ 2020, 8, e8102. [Google Scholar] [CrossRef] [PubMed]
- Arulazhagan, P.; Al-Shekri, K.; Huda, Q.; Godon, J.J.; Basahi, J.M.; Jeyakumar, D. Biodegradation of Polycyclic Aromatic Hydrocarbons by an Acidophilic Stenotrophomonas maltophilia Strain AJH1 Isolated from a Mineral Mining Site in Saudi Arabia. Extremophiles 2017, 21, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Gosai, H.B.; Panseriya, H.Z.; Patel, P.G.; Patel, A.C.; Shankar, A.; Varjani, S.; Dave, B.P. Exploring Bacterial Communities through Metagenomics during Bioremediation of Polycyclic Aromatic Hydrocarbons from Contaminated Sediments. Sci. Total Environ. 2022, 842, 156794. [Google Scholar] [CrossRef]
- Muangchinda, C.; Rungsihiranrut, A.; Prombutara, P.; Soonglerdsongpha, S.; Pinyakong, O. 16S Metagenomic Analysis Reveals Adaptability of a Mixed-PAH-Degrading Consortium Isolated from Crude Oil-Contaminated Seawater to Changing Environmental Conditions. J. Hazard. Mater. 2018, 357, 119–127. [Google Scholar] [CrossRef]
- Brooke, J.S.; Di Bonaventura, G.; Berg, G.; Martinez, J.-L. Editorial: A Multidisciplinary Look at Stenotrophomonas maltophilia: An Emerging Multi-Drug-Resistant Global Opportunistic Pathogen. Front. Microbiol. 2017, 8, 1511. [Google Scholar] [CrossRef]
- Bernard, K.A.; Wiebe, D.; Burdz, T.; Reimer, A.; Ng, B.; Singh, C.; Schindle, S.; Pacheco, A.L. Assignment of Brevibacterium stationis (ZoBell and Upham 1944) Breed 1953 to the Genus Corynebacterium, as Corynebacterium stationis Comb. Nov., and Emended Description of the Genus Corynebacterium to Include Isolates That Can Alkalinize Citrate. Int. J. Syst. Evol. Microbiol. 2010, 60, 874–879. [Google Scholar] [CrossRef]
- Bernard, K. The Genus Corynebacterium. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128012383. [Google Scholar]
- Ray, D.; Anand, U.; Jha, N.K.; Korzeniewska, E.; Bontempi, E.; Proćków, J.; Dey, A. The Soil Bacterium, Corynebacterium glutamicum, from Biosynthesis of Value-Added Products to Bioremediation: A Master of Many Trades. Environ. Res. 2022, 213, 113622. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Zeynalipour, M.S.; Musa, F.H. Isolation and Characterization of Crude Oil Degrading Bacteria from the Persian Gulf (Khorramshahr Provenance). Mar. Pollut. Bull. 2014, 82, 39–44. [Google Scholar] [CrossRef]
- Dwivedi, A.; Kumar, A.; Bhat, J.L. Production and Characterization of Biosurfactant from Corynebacterium Species and Its Effect on the Growth of Petroleum Degrading Bacteria. Microbiology 2019, 88, 87–93. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Wang, L.; Liu, J.; Gurav, R.G.; Sun, K. A Novel Bioremediation Strategy for Petroleum Hydrocarbon Pollutants Using Salt Tolerant Corynebacterium Variabile HRJ4 and Biochar. J. Environ. Sci. 2016, 47, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, P. Genomic Potential of Stenotrophomonas maltophilia in Bioremediation with an Assessment of Its Multifaceted Role in Our Environment. Front. Microbiol. 2016, 7, 967. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, X.; Guo, J.; Liang, X.; Xie, Q.; Chen, S. Bioremediation of Oil-Contaminated Soil by Combination of Soil Conditioner and Microorganism. J. Soils Sediments 2020, 20, 2121–2129. [Google Scholar] [CrossRef]
- Semai, A.; Plewniak, F.; Lledo, J.; Annonay, G.; Vandecasteele, C.; Lopez-Roques, C.; Bertin, P.N. Complete Genome Sequence of Stenotrophomonas maltophilia 1800, a New Bacterial Strain with Potential for Bioremediation of Oil-Contaminated Environments. Microbiol. Resour. Announc. 2022, 11, e0111621. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Rahman, T.; Lakshmanaperumalsamy, P.; Banat, I.M. Occurrence of Crude Oil Degrading Bacteria in Gasoline and Diesel Station Soils. J. Basic Microbiol. 2002, 42, 284–291. [Google Scholar] [CrossRef]
- Niño Camacho, L.; Torres Sáenz, R. Implementación de diferentes técnicas analíticas para la determinación de biomasa bacteriana de cepas Pseudomonas Putida biodegradadoras de fenol. Rev. Ion Investig. Optim. Nuevos Procesos Ing. 2010, 23, 41–46. [Google Scholar]
- Otiniano, N.M.; Rojas-Villacorta, W.; De La Cruz-Noriega, M.; Lora-Cahuas, C.; Mendoza-Villanueva, K.; Benites, S.M.; Gallozzo-Cardenas, M.; Rojas-Flores, S. Effect of Inoculum Concentration on the Degradation of Diesel 2 by a Microbial Consortium. Sustainability 2022, 14, 16750. [Google Scholar] [CrossRef]
- Hernández castellanos, N.D. Establecimiento de Un Proceso de Biorremediación Usando Stenotrophomonas maltophilia, Universidad Veracruzana: México. 2016. Available online: https://www.uv.mx/pozarica/mca/general-3/generaciones/ (accessed on 15 March 2025).
- Olga, N.M. Demanda Bioquimica de Oxìgeno—5 Días, Incubaciòn Y Electrometrìa. Available online: http://www.ideam.gov.co/documents/14691/38155/Demanda+Bioquímica+de+Oxígeno..pdf/ca6e1594-4217-4aa3-9627-d60e5c077dfa (accessed on 19 November 2024).
- Jáuregui, S.; Robles, H. Effect of Inoculum Concentration of a Native Bacterial Consortium on the Degradation of Vinasse Nitrates from an Alcohol Distillery. Agroindustrial Sci. 2017, 7, 57–66. [Google Scholar] [CrossRef]
- Zafra, G.; Regino, R.; Agualimpia, B.; Aguilar, F. Molecular Characterization and Evaluation of Oil-Degrading Native Bacteria Isolated from Automotive Service Station Oil-Contaminated Soils. Chem. Eng. Trans. 2016, 49, 511–516. [Google Scholar] [CrossRef]
- Riedel, T.E.; Berelson, W.M.; Nealson, K.H.; Finkel, S.E. Oxygen Consumption Rates of Bacteria under Nutrient-Limited Conditions. Appl. Environ. Microbiol. 2013, 79, 4921–4931. [Google Scholar] [CrossRef]
- Bekele, G.K.; Gebrie, S.A.; Mekonen, E.; Fida, T.T.; Woldesemayat, A.A.; Abda, E.M.; Tafesse, M.; Assefa, F. Isolation and Characterization of Diesel-Degrading Bacteria from Hydrocarbon-Contaminated Sites, Flower Farms, and Soda Lakes. Int. J. Microbiol. 2022, 2022, 5655767. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, R.M.; Al-Otibi, F.; Marraiki, N.; Alharbi, R.I.; Aldehaish, H.A. Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia. Molecules 2022, 27, 6450. [Google Scholar] [CrossRef] [PubMed]
- Hazaimeh, M.D.; Ahmed, E.S. Bioremediation Perspectives and Progress in Petroleum Pollution in the Marine Environment: A Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 54238–54259. [Google Scholar] [CrossRef] [PubMed]
- Enerijiofi, K.E.; Ahonsi, C.O.; Ajao, E.K. Biodegradation Potentials of Waste Engine Oil by Three Bacterial Isolates. J. Appl. Sci. Environ. Manag. 2020, 24, 489–493. [Google Scholar] [CrossRef]
- Astashkina, A.P.; Bakibayev, A.A.; Plotnikov, E.V.; Kolbysheva, Y.V.; Mukashev, A.B. Study of the Hydrocarbon-Oxidizing Activity of Bacteria of the Genera Pseudomonas and Rhodococcus. Procedia Chem. 2015, 15, 90–96. [Google Scholar] [CrossRef]
- Auffret, M.; Labbé, D.; Thouand, G.; Greer, C.W.; Fayolle-Guichard, F. Degradation of a Mixture of Hydrocarbons, Gasoline, and Diesel Oil Additives by Rhodococcus aetherivorans and Rhodococcus wratislaviensis. Appl. Environ. Microbiol. 2009, 75, 7774–7782. [Google Scholar] [CrossRef]
- Abbas, A.F.; Muhsen, M.A. Molecular Characterization of Some Lead Resistant Bacteria Isolated from Polluted Environments. AIP Conf. Proc. 2023, 2834, 020018. [Google Scholar]
- Saha, R.; Banerjee, D.B.; Manna, S.; Banerjee, S. Microbial Bioremediation: A Promising Approach to Withstand Heavy Metal Contamination in Soil and Its Future Possibilities. In Synergistic Approaches for Bioremediation of Environmental Pollutants: Recent Advances and Challenges; Elsevier: Amsterdam, The Netherlands, 2022; pp. 227–262. ISBN 9780323918602. [Google Scholar]
- Souza, E.C.; Vessoni-Penna, T.C.; de Souza Oliveira, R.P. Biosurfactant-Enhanced Hydrocarbon Bioremediation: An Overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Muthukamalam, S.; Sivagangavathi, S.; Dhrishya, D.; Sudha Rani, S. Characterization of Dioxygenases and Biosurfactants Produced by Crude Oil Degrading Soil Bacteria. Braz. J. Microbiol. 2017, 48, 637–647. [Google Scholar] [CrossRef]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent Advances in Bacterial Degradation of Hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Collier, L.S.; Neidle, E.L.; Moran, M.A. Key Aromatic-Ring-Cleaving Enzyme, Protocatechuate 3,4-Dioxygenase, in the Ecologically Important Marine Roseobacter Lineage. Appl. Environ. Microbiol. 2000, 66, 4662–4672. [Google Scholar] [CrossRef] [PubMed]
- Contzen, M.; Stolz, A. Characterization of the Genes for Two Protocatechuate 3,4-Dioxygenases from the 4-Sulfocatechol-Degrading Bacterium Agrobacterium Radiobacter Strain S2. J. Bacteriol. 2000, 182, 6123–6129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).