Wearable 256-Element MUX-Based Linear Array Transducer for Monitoring of Deep Abdominal Muscles
Abstract
1. Introduction
2. Materials and Methods
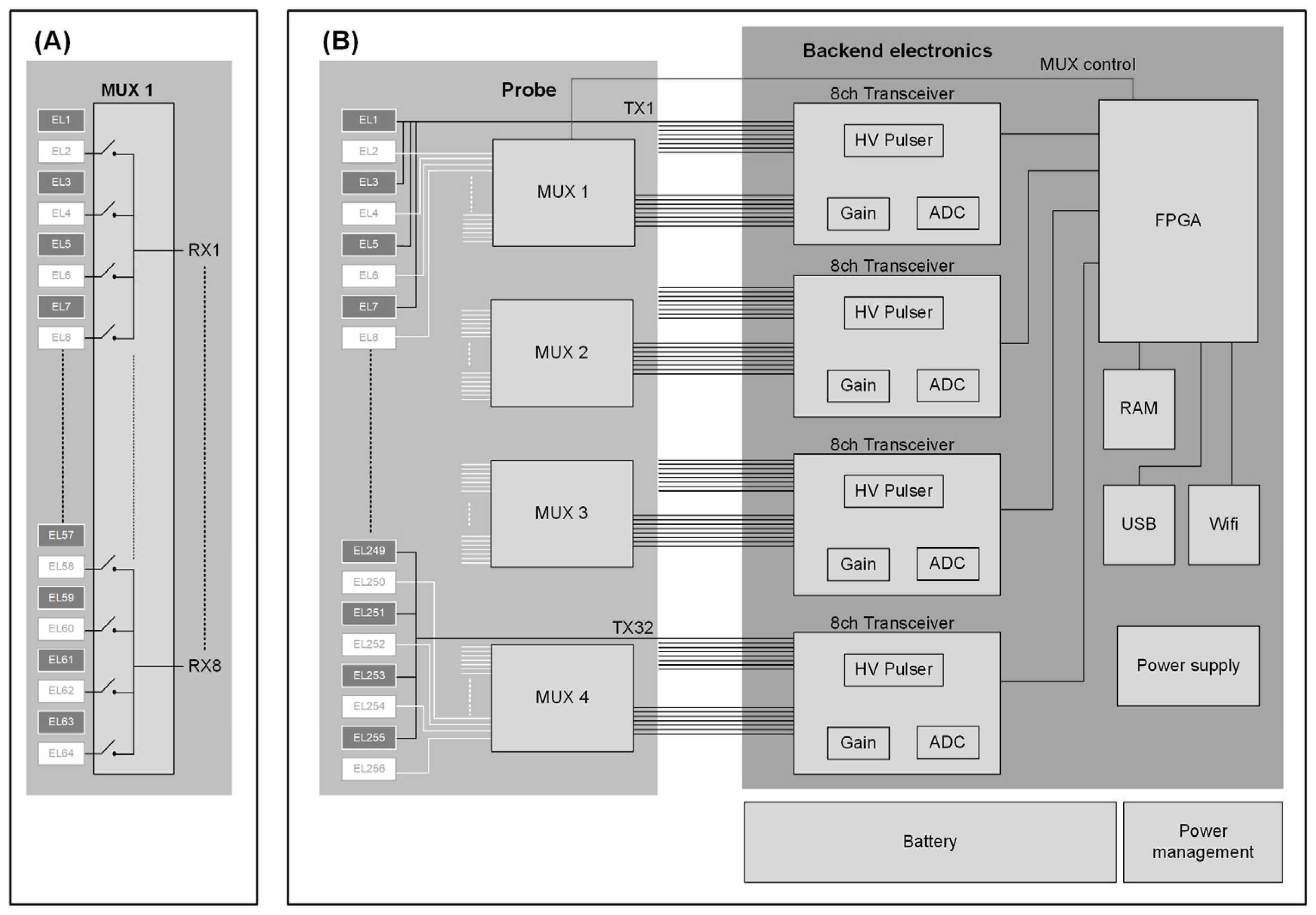
2.1. System Design
2.2. Multiplexer Development
2.3. Multichannel Backend Electronics
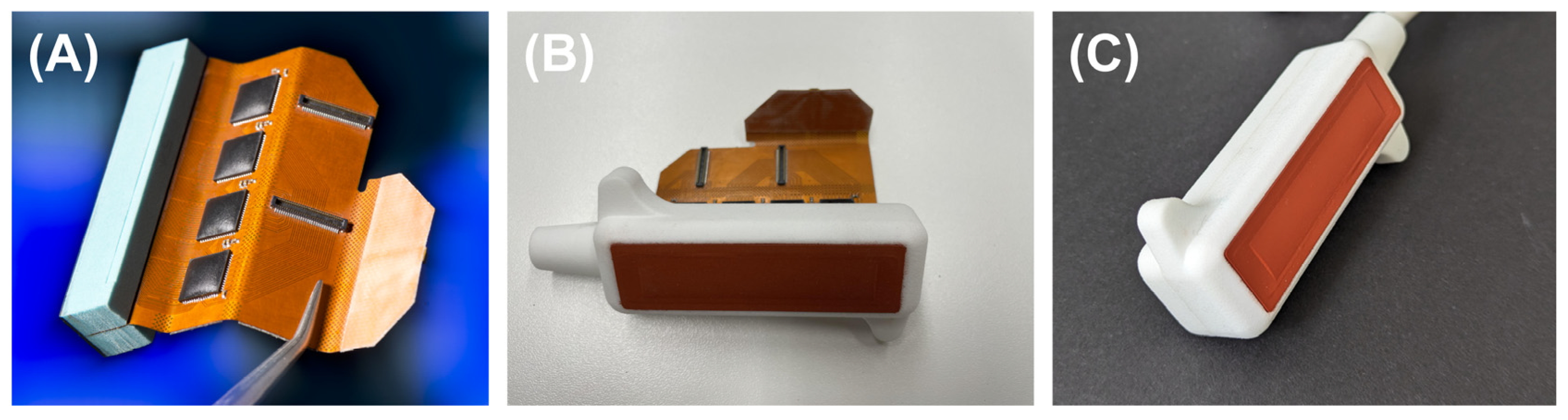
2.4. Transducer Development
2.5. Beamforming Algorithm and Image Processing
3. Results
3.1. System Characterization
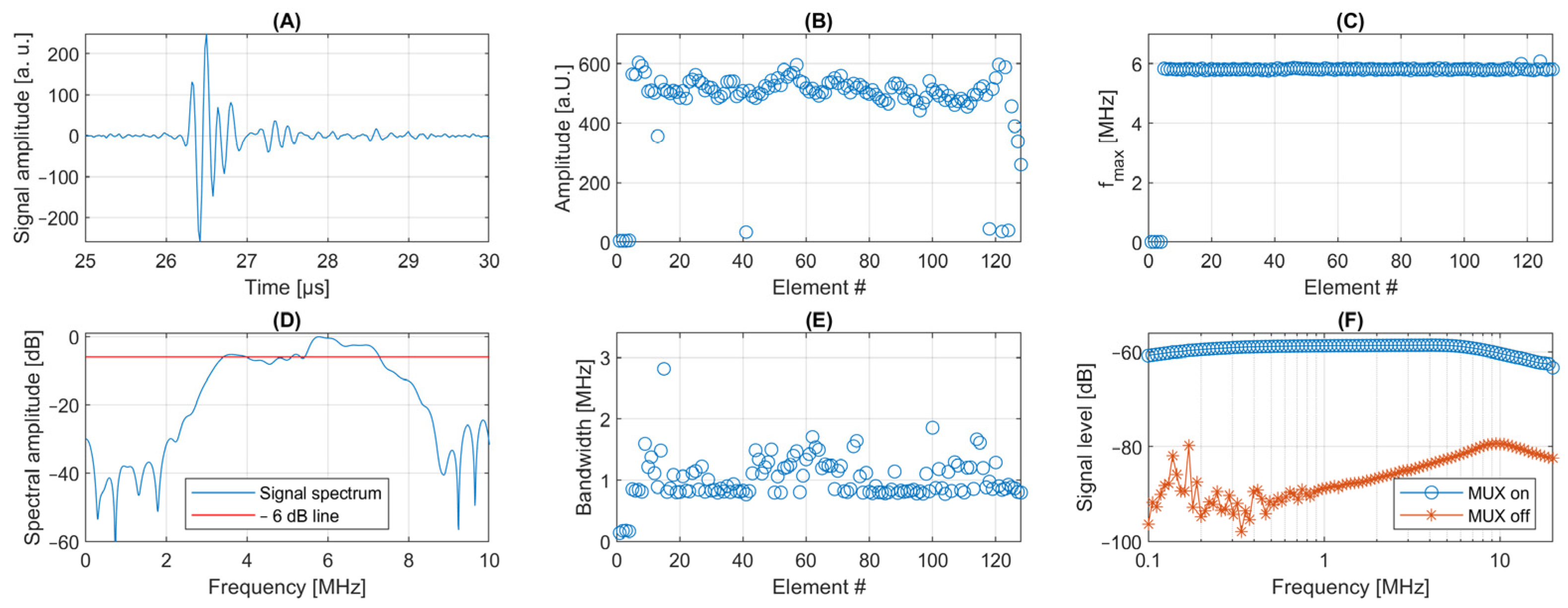
3.1.1. Multiplexer Characterization
3.1.2. Transducer Characterization
3.1.3. Data Transfer
3.1.4. System Imaging Performance—Phantom Study
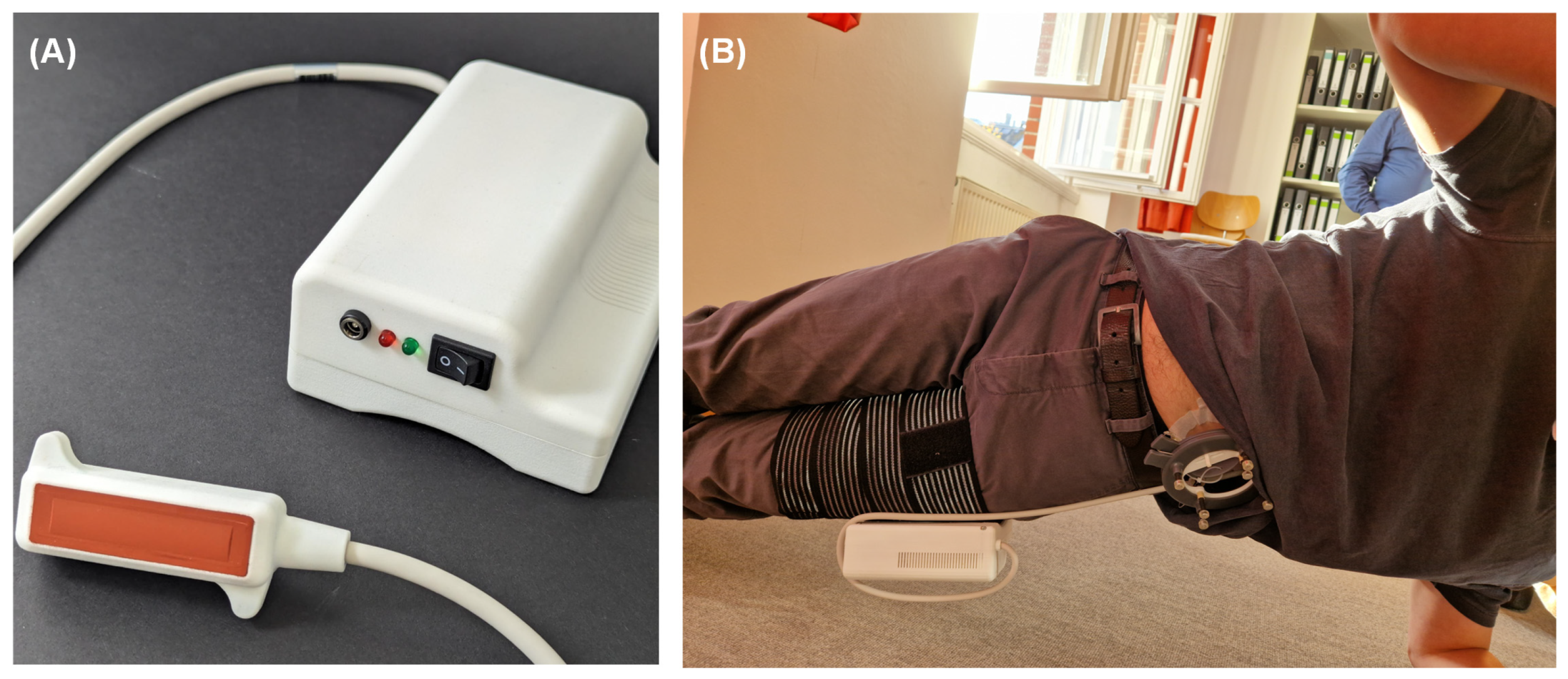
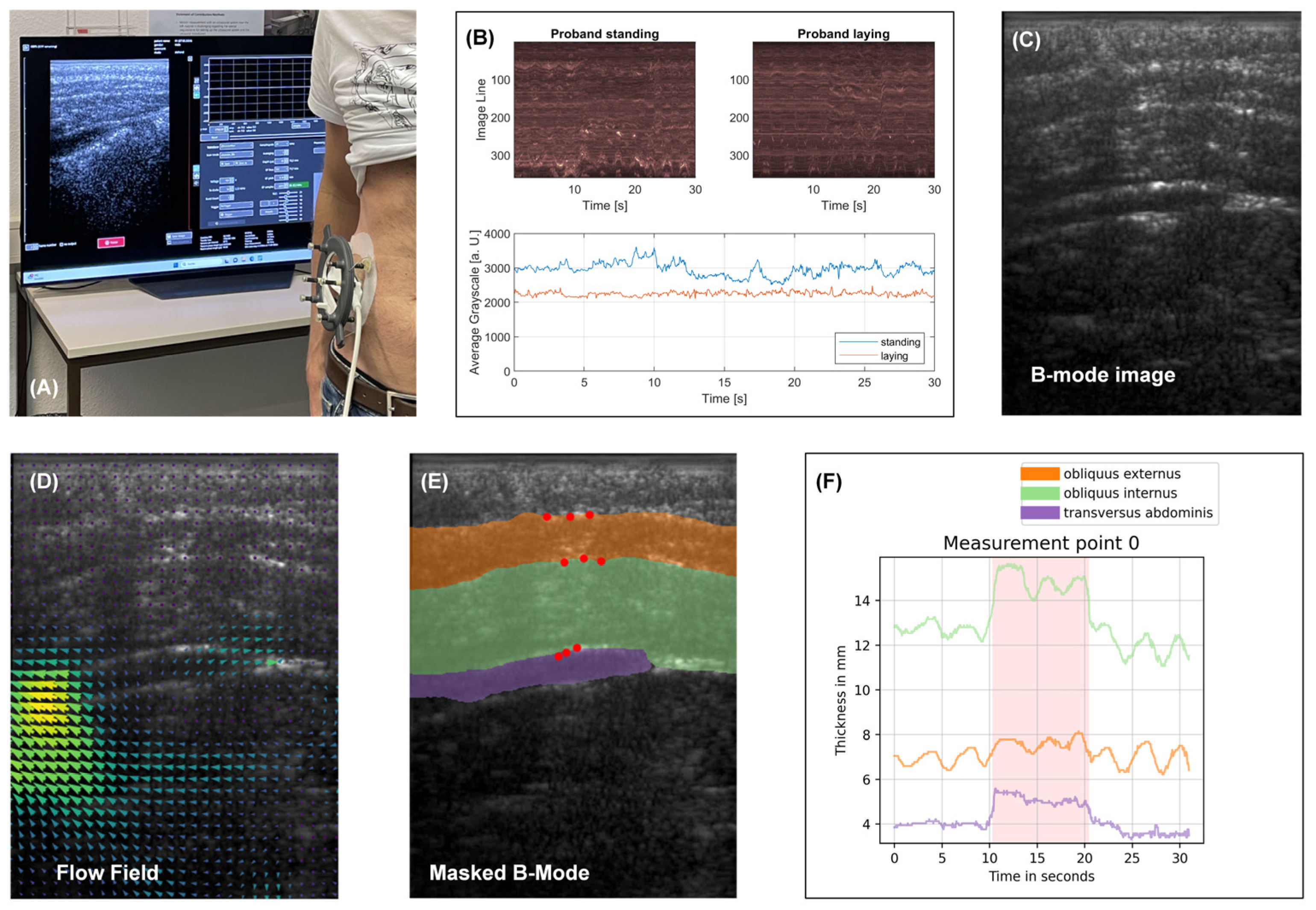
3.2. In-Vivo Testing
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Der Lippe, E.; Krause, L.; Prost, M.; Wengler, A.; Leddin, J.; Müller, A.; Zeisler, M.-L.; Anton, A.; Rommel, A.; BURDEN 2020 Study Group Prävalenz von Rücken- und Nackenschmerzen in Deutschland. Ergebnisse der Krankheitslast-Studie BURDEN 2020. J. Health Monit. 2021, 6, 2–14. [Google Scholar] [PubMed]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; van Tulder, M.W. Exercise Therapy for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2021, 9, CD009790. [Google Scholar] [CrossRef] [PubMed]
- França, F.R.; Burke, T.N.; Hanada, E.S.; Marques, A.P. Segmental Stabilization and Muscular Strengthening in Chronic Low Back Pain—A Comparative Study. Clinics 2010, 65, 1013–1017. [Google Scholar] [CrossRef]
- Shaughnessy, M.; Caulfield, B.A. Pilot Study to Investigate the Effect of Lumbar Stabilisation Exercise Training on Functional Ability and Quality of Life in Patients with Chronic Low Back Pain. Int. J. Rehabil. Res. 2004, 27, 297–301. [Google Scholar] [PubMed]
- Richardson, C. Therapeutic Exercise for Spinal Segmental Stabilization. In Lower Back Pain; Churchill Livingstone: Edinburgh, UK, 1998; ISBN 978-0-443-05802-8. [Google Scholar]
- Mödinger, M.; Woll, A.; Wagner, I. Video-Based Visual Feedback to Enhance Motor Learning in Physical Education—A Systematic Review. Ger. J. Exerc. Sport Res. 2022, 52, 447–460. [Google Scholar]
- Van Hides, J.A.; Richardson, C.A. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J. Orthop. Sports Phys. Ther. 2006, 36, 920–925. [Google Scholar] [CrossRef]
- Mannion, A.F.; Pulkovski, N.; Gubler, D.; Gorelick, M.; O’Riordan, D.; Loupas, T.; Schenk, P.; Gerber, H.; Sprott, H. Muscle Thickness Changes during Abdominal Hollowing: An Assessment of between-Day Measurement Error in Controls and Patients with Chronic Low Back Pain. Eur. Spine J. 2008, 17, 494–501. [Google Scholar]
- Lee, D.H.; Hong, S.K.; Lee, Y.S.; Kim, C.H.; Hwang, J.M.; Lee, Z.; Kim, J.M.; Park, D. Is abdominal hollowing exercise using real-time ultrasound imaging feedback helpful for selective strengthening of the transversus abdominis muscle?: A prospective, randomized, parallel-group, comparative study. Medicine 2018, 97, e11369. [Google Scholar] [CrossRef]
- Shamsi, M.; Sarrafzadeh, J.; Jamshidi, A.; Zarabi, V.; Pourahmadi, M.R. The effect of core stability and general exercise on abdominal muscle thickness in non-specific chronic low back pain using ultrasound imaging. Physiother. Theory Pract. 2016, 32, 277–283. [Google Scholar] [CrossRef]
- Zhang, L.; Marcus, C.; Lin, D.; Mejorado, D.; Schoen, S.J., Jr.; Pierce, T.T.; Kumar, V.; Fernandez, S.V.; Hunt, D.; Li, Q.; et al. A conformable phased-array ultrasound patch for bladder volume monitoring. Nat. Electron. 2024, 7, 77–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, T.; Deng, Y.; Zhao, Z.; Wang, R.; He, Q.; Luo, J.; Li, J.; Du, K.; Wu, T.; et al. A low-voltage-driven MEMS ultrasonic phased-array transducer for fast 3D volumetric imaging. Microsyst. Nanoeng. 2024, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Elloian, J.; Jadwiszczak, J.; Arslan, V.; Sherman, J.D.; Kessler, D.O.; Shepard, K.L. Flexible ultrasound transceiver array for non-invasive surface-conformable imaging enabled by geometric phase correction. Sci. Rep. 2022, 12, 16184. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, Z.; Gao, X.; Bian, Y.; Wu, R.S.; Park, G.; Lou, Z.; Zhang, Z.; Xu, X.; Chen, X.; et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat. Biotechnol. 2024, 42, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Vostrikov, S.; Benini, L.; Cossettini, A. WULPUS: A Wearable Ultra Low-Power Ultrasound probe for multi-day monitoring of carotid artery and muscle activity. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 1 December 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Fournelle, M.; Grün, T.; Speicher, D.; Weber, S.; Yilmaz, M.; Schoeb, D.; Miernik, A.; Reis, G.; Tretbar, S.; Hewener, H. Portable Ultrasound Research System for Use in Automated Bladder Monitoring with Machine-Learning-Based Segmentation. Sensors 2021, 21, 6481. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Hettiarachchi, N.; Yan, J.; Liu, H. A wearable ultrasound system for sensing muscular morphological deformations. IEEE Trans. Syst. Man Cybern. Syst. 2021, 51, 3370–3379. [Google Scholar] [CrossRef]
- Xu, X.; Wala, S.A.; Vishwa, A.; Shen, J.; K, D.; Devi, S.; Chandak, A.; Dixit, S.; Granata, E.; Pithadia, S.; et al. A programmable platform for accelerating the development of smart ultrasound transducer probe. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Vostrikov, S.; Tille, J.; Benini, L.; Cossetini, A. TinyProbe: A wearable 32-chanel multi-modal wireless ultrasound probe. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 72, 64–76. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.; Hassan, R.; Ren, H. Review on Wearable System for Positioning Ultrasound Scanner. Machines 2023, 11, 325. [Google Scholar] [CrossRef]
- Liu, J.Y.; Xu, J.; Forsberg, F.; Liu, J.-B. CMUT/CMOS-based Butterfly iQ—A portable sonoscope. Adv. Ultrasound Diagn. Ther. 2019, 3, 115–118. [Google Scholar]
- Salvi, M.; Caresio, C.; Meiburger, K.M.; De Santi, B.; Molinari, F.; Minetto, M.A. Transverse Muscle Ultrasound Analysis (TRAMA): Robust and Accurate Segmentation of Muscle Cross-Sectional Area. Ultrasound Med. Biol. 2019, 45, 672–683. [Google Scholar] [CrossRef]
- Ardhianto, P.; Tsai, J.-Y.; Lin, C.-Y.; Liau, B.-Y.; Jan, Y.-K.; Akbari, V.B.H.; Lung, C.-W. A Review of the Challenges in Deep Learning for Skeletal and Smooth Muscle Ultrasound Images. Appl. Sci. 2021, 11, 4021. [Google Scholar] [CrossRef]
- Marzola, F.; van Alfen, N.; Doorduin, J.; Meiburger, K.M. Deep learning segmentation of transverse musculoskeletal ultrasound images for neuromuscular disease assessment. Comput. Biol. Med. 2021, 135, 104623. [Google Scholar] [CrossRef] [PubMed]
- Strohm, H.; Rothluebbers, S.; Perotti, L.; Stamm, O.; Fournelle, M.; Jenne, J.; Guenther, M. Contraction assessment of abdominal muscles using automated segmentation designed for wearable ultrasound applications. Int. J. CARS 2024, 19, 1607–1614. [Google Scholar] [CrossRef]
- Perotti, L.; Stamm, O.; Strohm, H.; Jenne, J.; Fournelle, M.; Lahmann, N.; Müller-Werdan, U. Learning Transversus Abdominis Activation in Older Adults with Chronic Low Back Pain Using an Ultrasound-Based Wearable: A Randomized Controlled Pilot Study. J. Funct. Morphol. Kinesiol. 2025, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Hollman, K.W.; Rigby, K.W.; O’Donnell, M. Coherence factor of speckle from a multi-row probe. In Proceedings of the IEEE Ultrasonics Symposium. Proceedings. International Symposium (Cat. No.99CH37027), Tahoe, NV, USA, 17–20 October 1999; pp. 1257–1260. [Google Scholar] [CrossRef]
- Fournelle, M.; Bost, W. Wave front analysis for enhanced time-domain beamforming of point-like targets in optoacoustic imaging using a linear array. Photoacoustics 2019, 14, 67–76. [Google Scholar] [CrossRef]
- Forney, G.D. The Viterbi Algorithm. Proc. IEEE 1973, 61, 268–278. [Google Scholar] [CrossRef]
- Bouguet, J.-Y. Pyramidal Implementation of the Lucas Kanade Feature Tracker Description of the Algorithm; Technical Report; Microprocessor Research Labs, Intel Corporation: Santa Clara, CA, USA, 1999. [Google Scholar]
- Bradski, G. OpenCV Library. Dr. Dobb’s J. Softw. Tools 2000, 120, 122–125. [Google Scholar]
- Liebgott, H.; Rodriguez-Molares, A.; Cervenansky, F.; Jensen, J.A.; Bernard, O. Plane-wave imaging challenge in medical ultrasound. In Proceedings of the IEEE Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speicher, D.; Grün, T.; Weber, S.; Hewener, H.; Klesy, S.; Rumanus, S.; Strohm, H.; Stamm, O.; Perotti, L.; Tretbar, S.H.; et al. Wearable 256-Element MUX-Based Linear Array Transducer for Monitoring of Deep Abdominal Muscles. Appl. Sci. 2025, 15, 3600. https://doi.org/10.3390/app15073600
Speicher D, Grün T, Weber S, Hewener H, Klesy S, Rumanus S, Strohm H, Stamm O, Perotti L, Tretbar SH, et al. Wearable 256-Element MUX-Based Linear Array Transducer for Monitoring of Deep Abdominal Muscles. Applied Sciences. 2025; 15(7):3600. https://doi.org/10.3390/app15073600
Chicago/Turabian StyleSpeicher, Daniel, Tobias Grün, Steffen Weber, Holger Hewener, Stephan Klesy, Schabo Rumanus, Hannah Strohm, Oskar Stamm, Luis Perotti, Steffen H. Tretbar, and et al. 2025. "Wearable 256-Element MUX-Based Linear Array Transducer for Monitoring of Deep Abdominal Muscles" Applied Sciences 15, no. 7: 3600. https://doi.org/10.3390/app15073600
APA StyleSpeicher, D., Grün, T., Weber, S., Hewener, H., Klesy, S., Rumanus, S., Strohm, H., Stamm, O., Perotti, L., Tretbar, S. H., & Fournelle, M. (2025). Wearable 256-Element MUX-Based Linear Array Transducer for Monitoring of Deep Abdominal Muscles. Applied Sciences, 15(7), 3600. https://doi.org/10.3390/app15073600








