Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Defining Materials
2.2. Numerical Simulation Setup
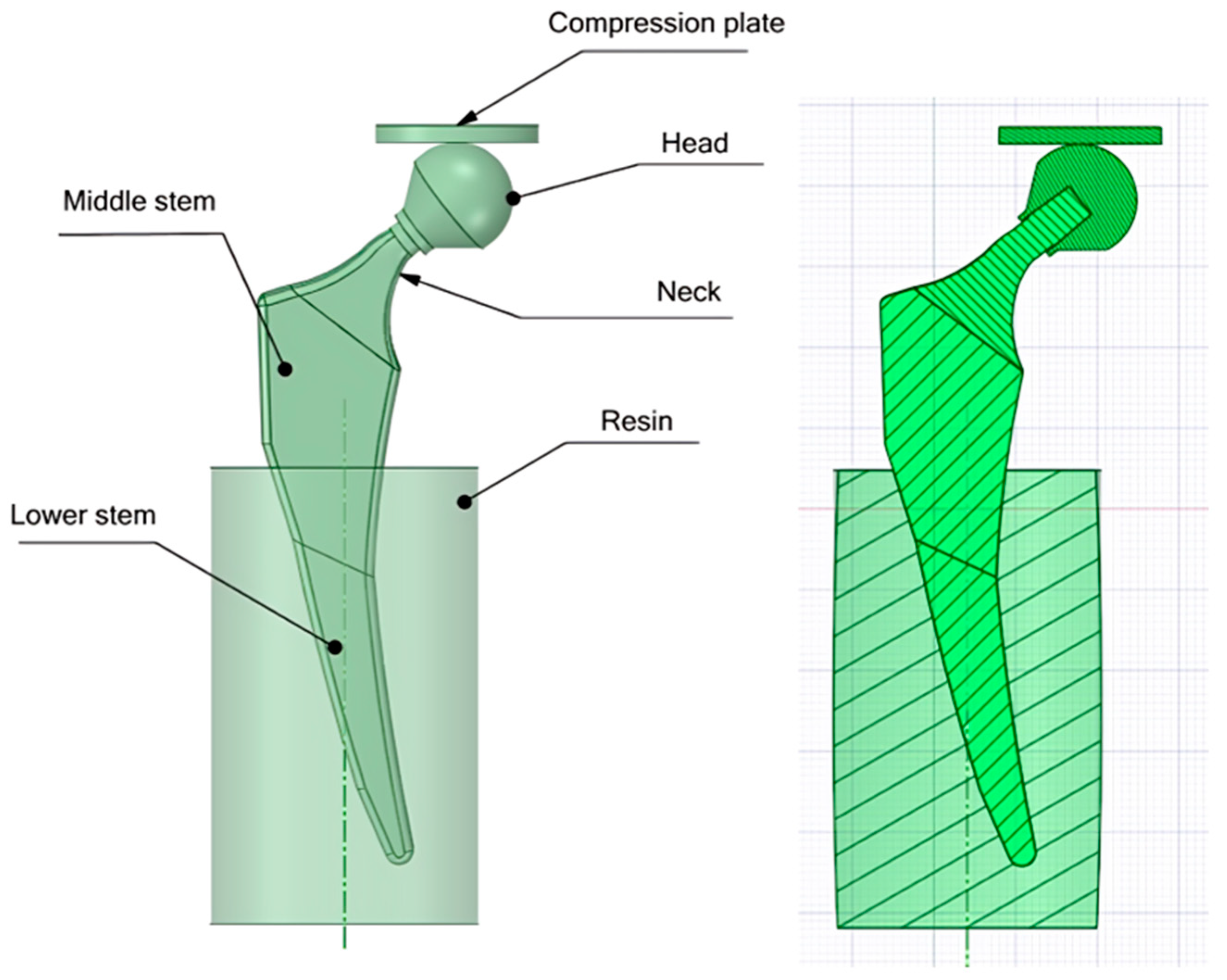
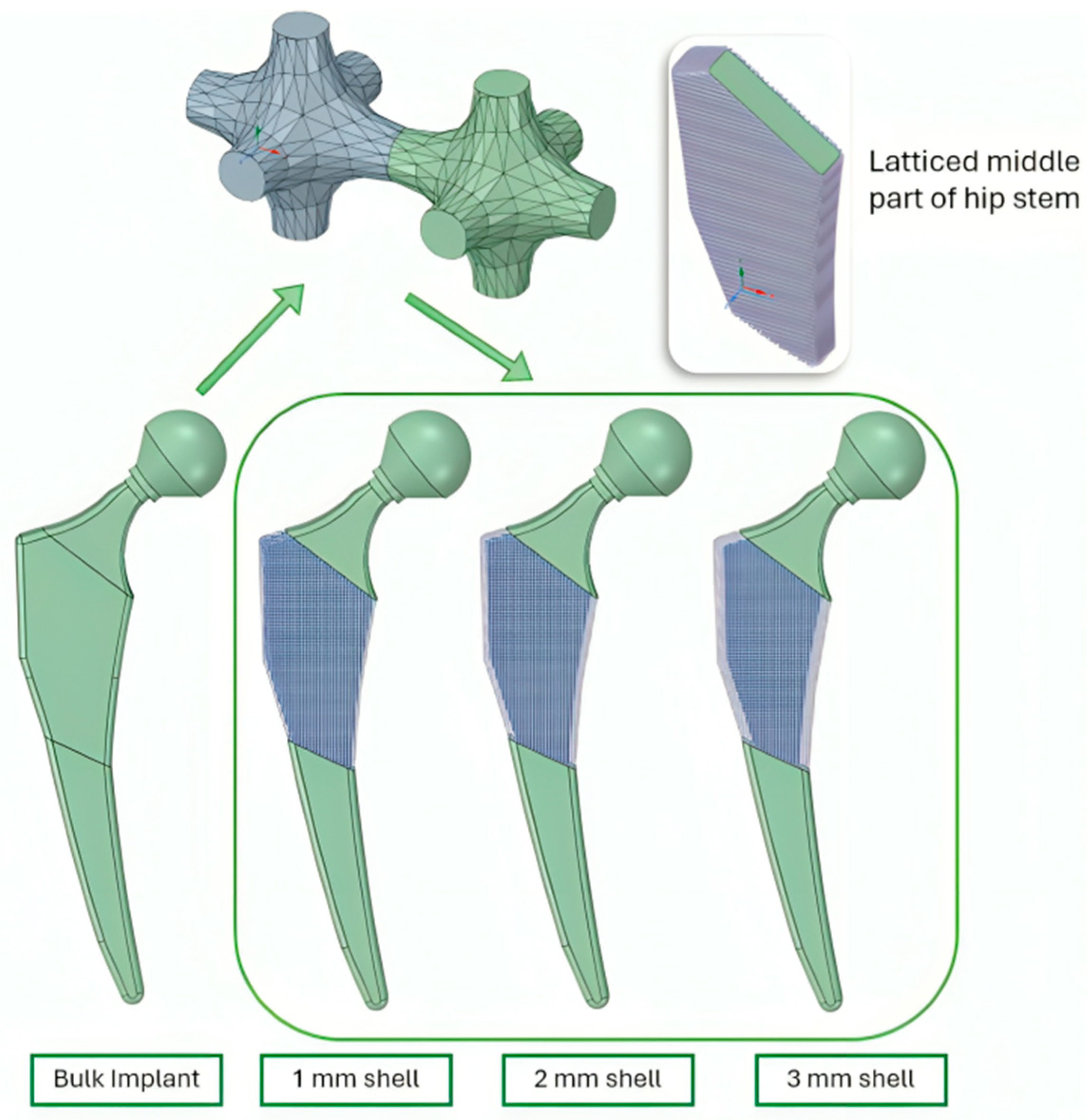
2.2.1. Designing CAD Files
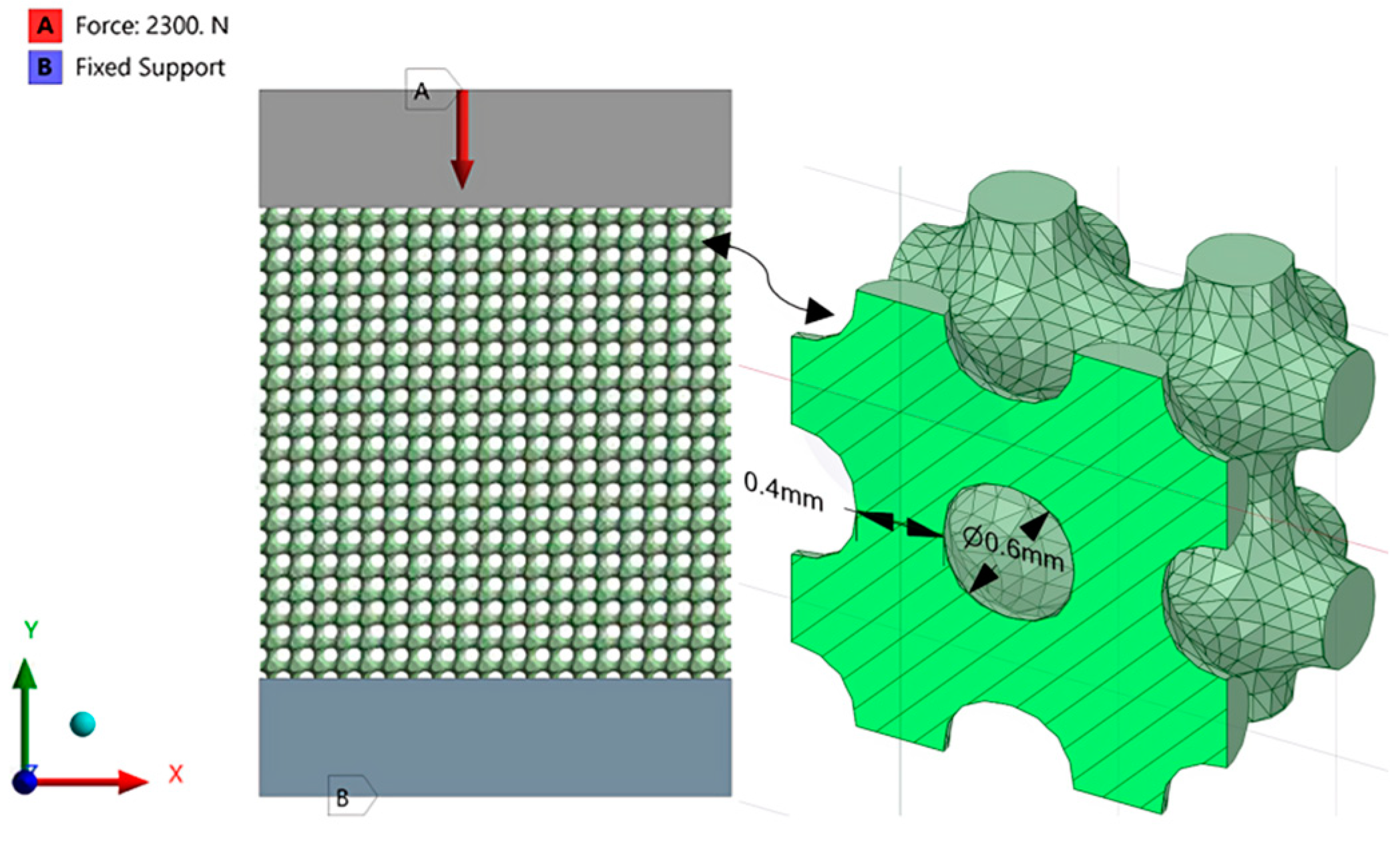
2.2.2. Applying Lattice Structures
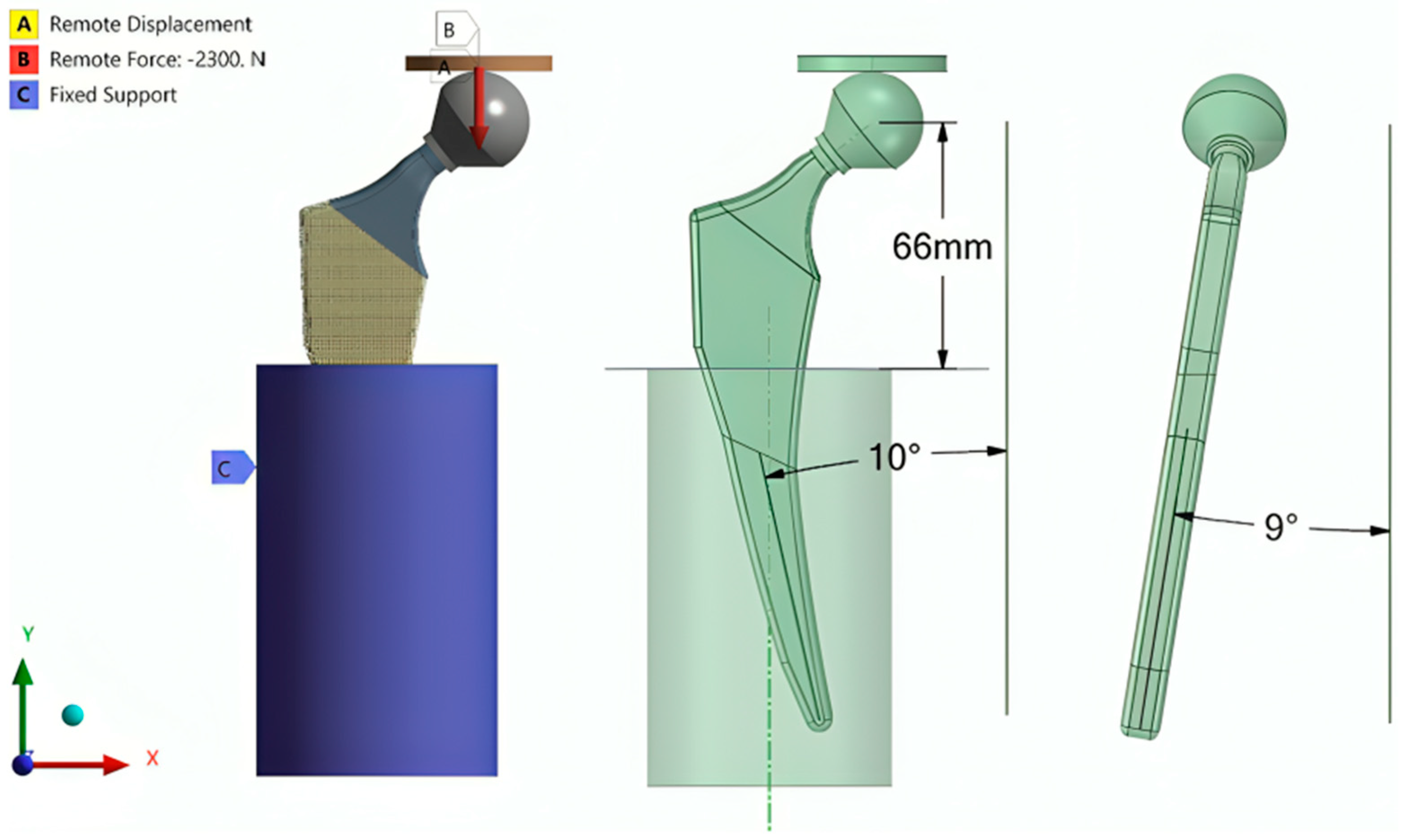
2.2.3. Finite Element Analysis (Latticed Model)
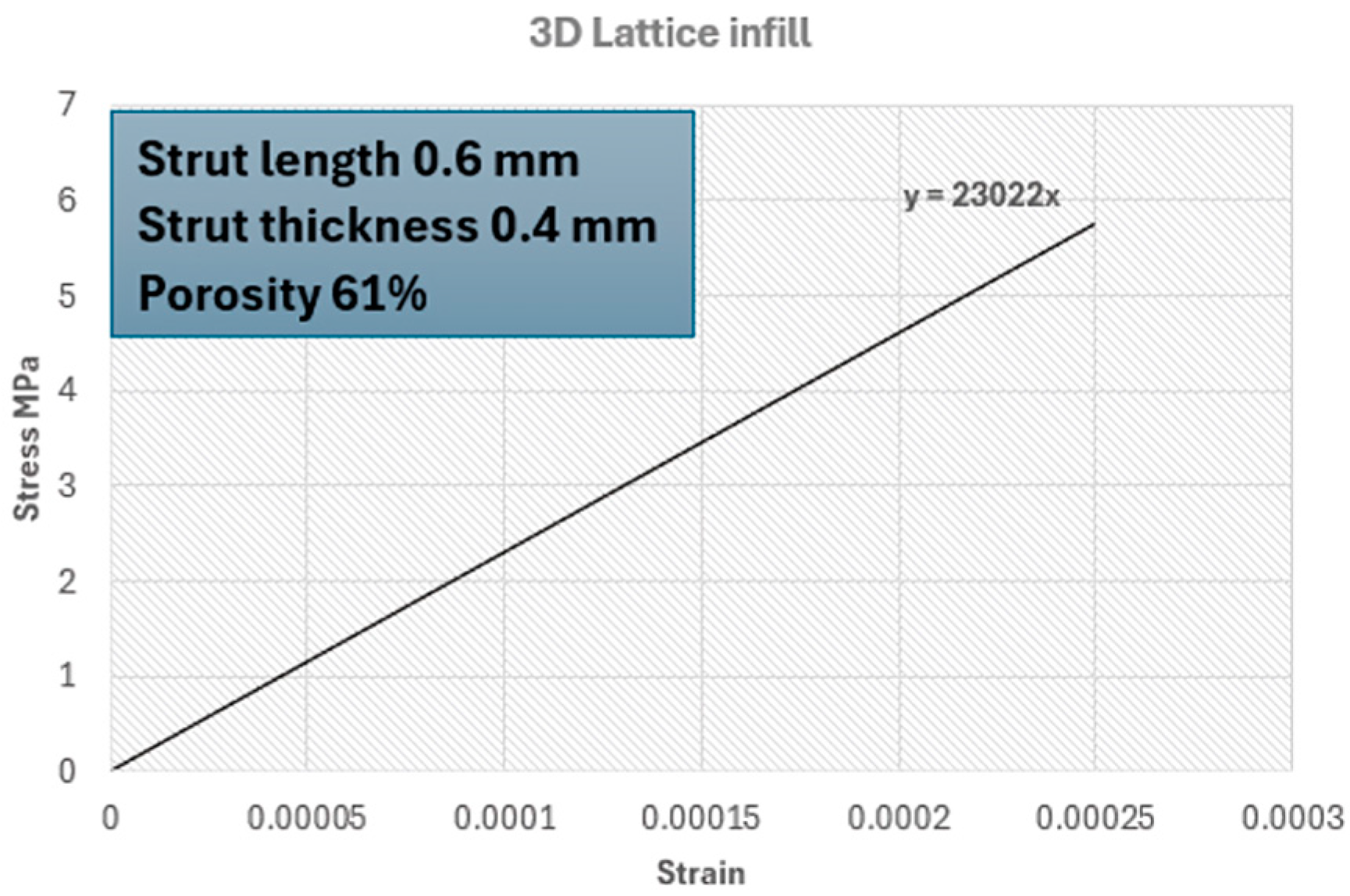
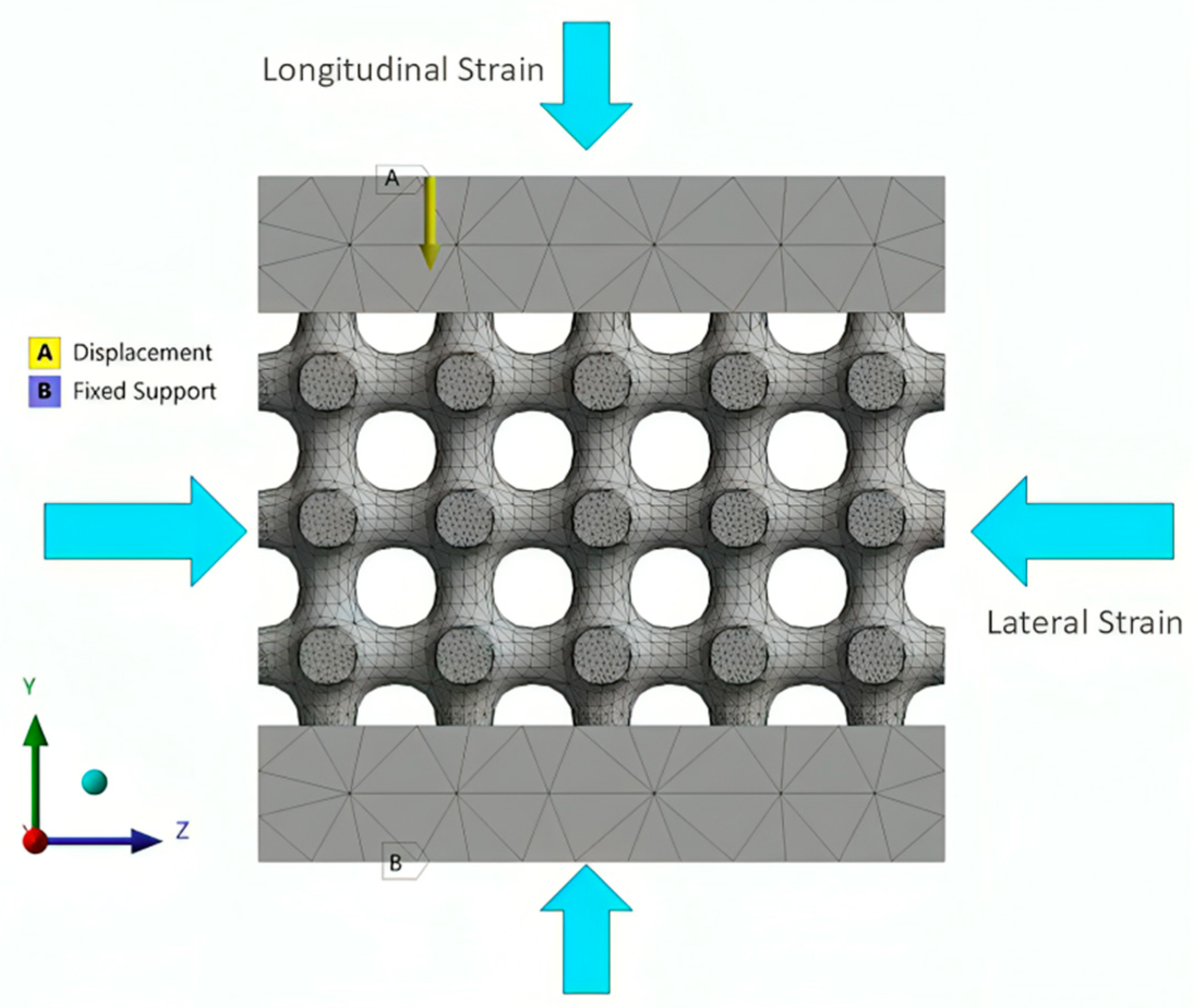
2.2.4. Setting the Surrogate Model
3. Results
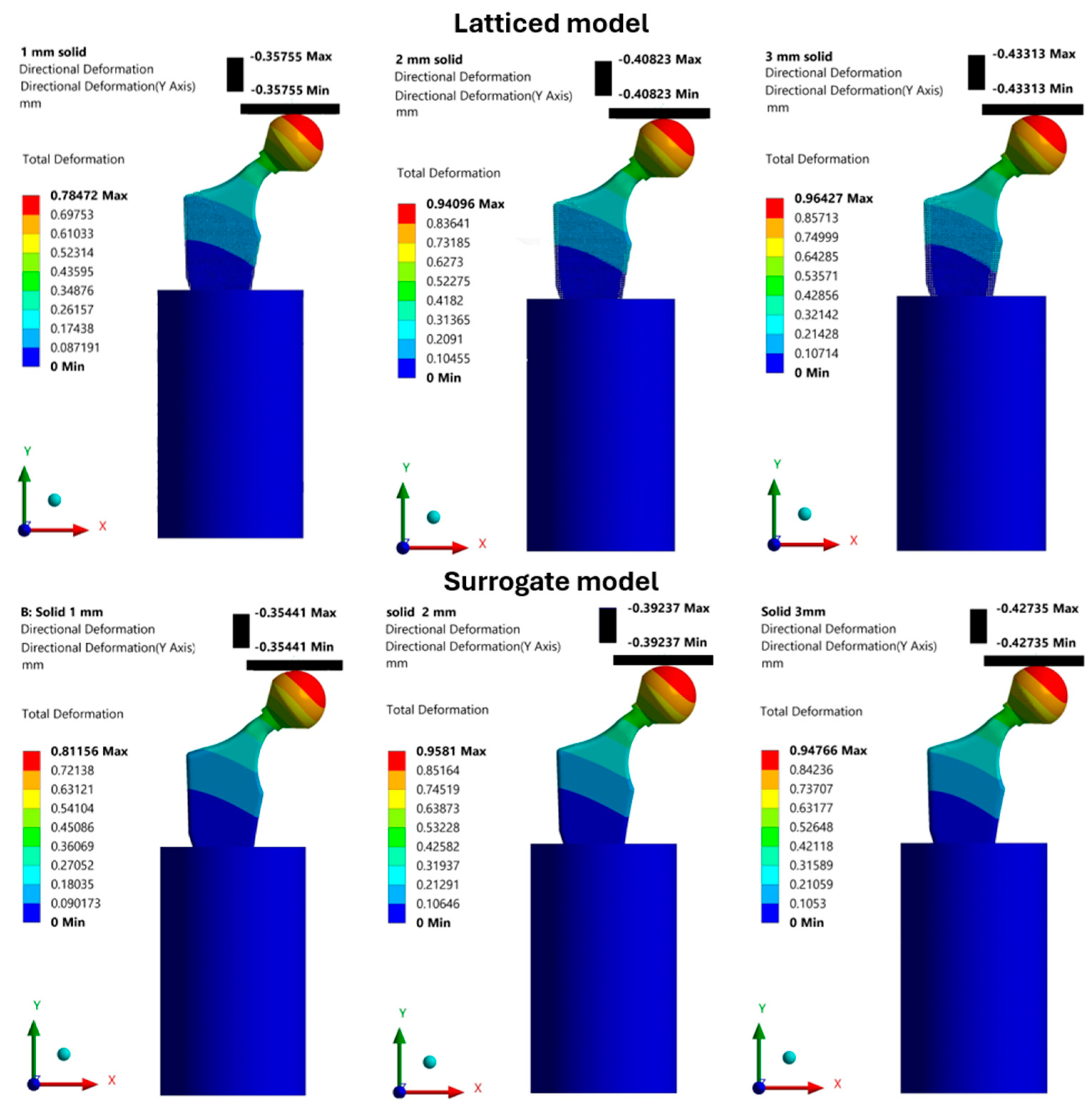
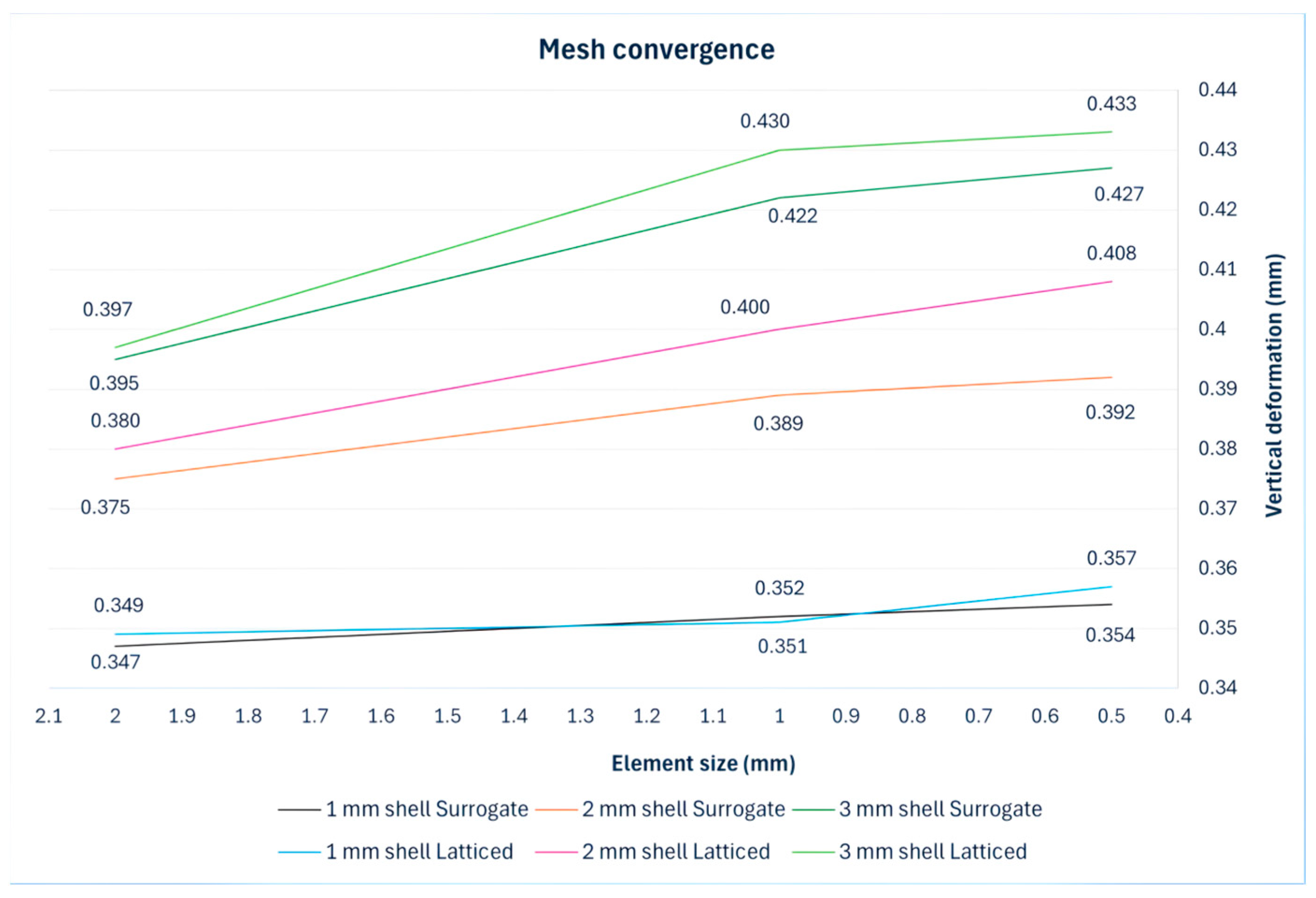
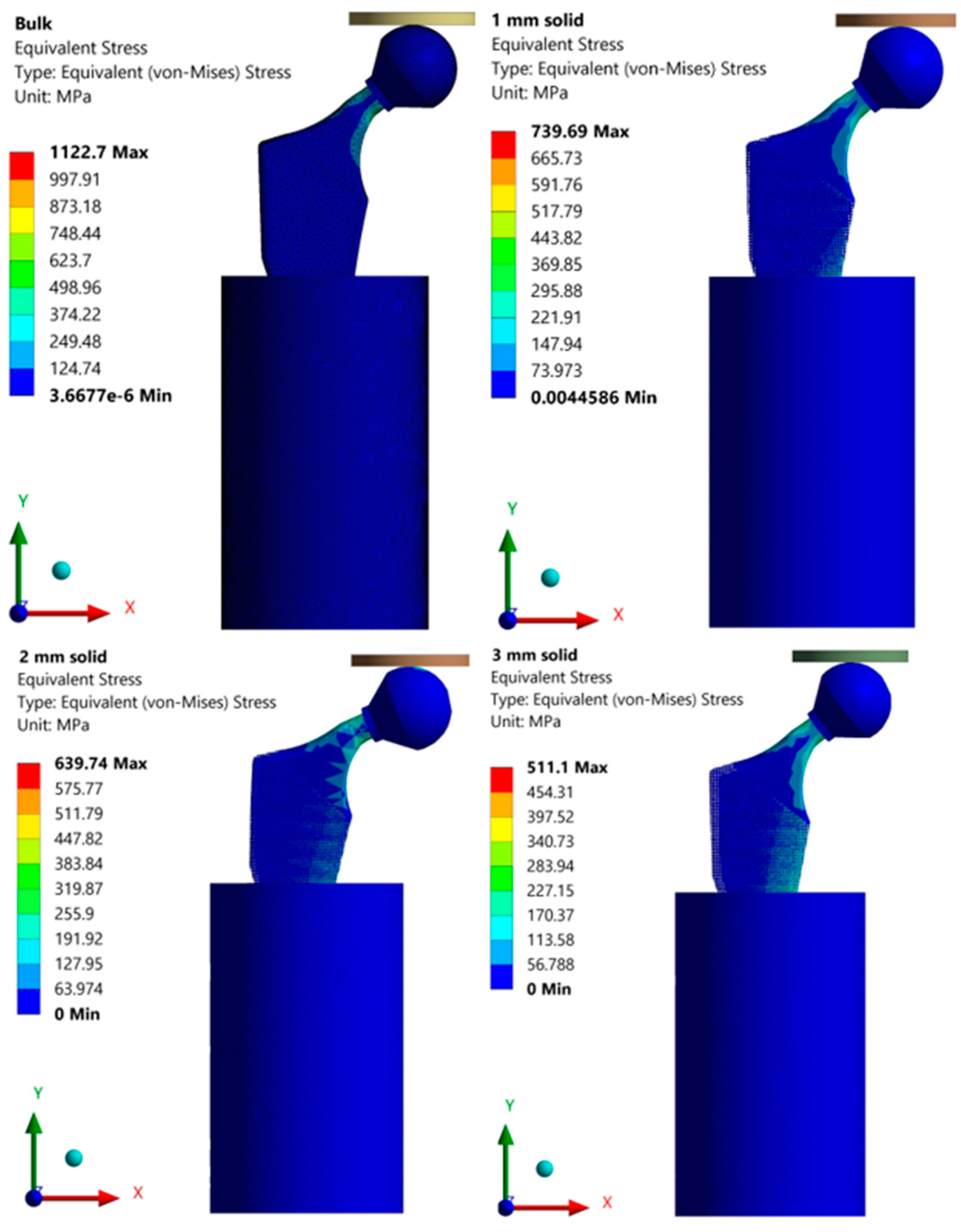
Finite Element Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Park, J.O.; Bronzino, J.D. Biomaterials; Informa: London, UK, 2002. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Capek, J.; Machova, M.; Fousova, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonská, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C 2016, 69, 631–639. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.-H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.-H.; Kwon, T.-Y. Microstructures and Mechanical Properties of Co-Cr Dental Alloys Fabricated by Three CAD/CAM-Based Processing Techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.H.; Beck, M.; Scheffer, C. Microstructure and mechanical properties of direct metal laser sintered TI-6AL-4V. S. Afr. J. Ind. Eng. 2015, 26, 1–10. [Google Scholar] [CrossRef]
- Bari, K. Design, Simulation, and Mechanical Testing of 3D-Printed Titanium Lattice Structures. J. Compos. Sci. 2023, 7, 32. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti–6Al–4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater. 2015, 51, 61–73. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; VAN Rietbergen, B. The relationship between stress shielding and bone resorption around total Hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D. Effect of sintering conditions on the microstructural and mechanical characteristics of porous magnesium materials prepared by powder metallurgy. Mater. Sci. Eng. C 2014, 35, 21–28. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D.; Oborná, A. Microstructural and mechanical properties of biodegradable iron foam prepared by powder metallurgy. Mater. Des. 2015, 83, 468–482. [Google Scholar] [CrossRef]
- Caravaggi, P.; Liverani, E.; Leardini, A.; Fortunato, A.; Belvedere, C.; Baruffaldi, F.; Fini, M.; Parrilli, A.; Mattioli-Belmonte, M.; Tomesani, L.; et al. CoCr porous scaffolds manufactured via selective laser melting in orthopedics: Topographical, mechanical, and biological characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2343–2353. [Google Scholar] [CrossRef]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef]
- Bosco, R.; Van Den Beucken, J.V.D.; Leeuwenburgh, S.; Jansen, J. Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Distefano, F.; Pasta, S.; Epasto, G. Titanium Lattice Structures Produced via Additive Manufacturing for a Bone Scaffold: A Review. J. Funct. Biomater. 2023, 14, 125. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Nogueira, P.; Lopes, P.; Oliveira, L.; Alves, J.L.; Magrinho, J.P.G.; de Deus, A.M.; Vaz, M.F.; Silva, M.B. Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types. Metals 2024, 14, 780. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Kadir, M.R.A.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Liu, S. Finite-Element-Mesh Based Method for Modeling and Optimization of Lattice Structures for Additive Manufacturing. Materials 2018, 11, 2073. [Google Scholar] [CrossRef]
- Syam, W.P.; Jianwei, W.; Zhao, B.; Maskery, I.; Elmadih, W.; Leach, R. Design and analysis of strut-based lattice structures for vibration isolation. Precis. Eng. 2018, 52, 494–506. [Google Scholar] [CrossRef]
- Cantaboni, F.; Ginestra, P.; Tocci, M.; Colpani, A.; Avanzini, A.; Pola, A.; Ceretti, E. Modelling and FE simulation of 3D printed Co-Cr Lattice Structures for biomedical applications. Procedia CIRP 2022, 110, 372–377. [Google Scholar] [CrossRef]
- Paik, J.; Kim, D.; Kim, H.; Kim, H.-S. Numerical analysis of thermal damage by changing irradiation angle for peri-implantitis using photothermal therapy. J. Radiat. Res. Appl. Sci. 2024, 17, 101054. [Google Scholar] [CrossRef]
- Hoffer, J.G.; Geiger, B.C.; Ofner, P.; Kern, R. Mesh-Free Surrogate Models for Structural Mechanic FEM Simulation: A Comparative Study of Approaches. Appl. Sci. 2021, 11, 9411. [Google Scholar] [CrossRef]
- Suttakul, P.; Vo, D.; Fongsamootr, T.; Wanison, R.; Mona, Y.; Katongtung, T.; Tippayawong, N.; Thawon, I. The role of machine learning for insight into the material behavior of lattices: A surrogate model based on data from finite element simulation. Results Eng. 2024, 23, 102547. [Google Scholar] [CrossRef]
- Gongora, A.E.; Friedman, C.; Newton, D.K.; Yee, T.D.; Doorenbos, Z.; Giera, B.; Duoss, E.B.; Han, T.Y.-J.; Sullivan, K.; Rodriguez, J.N. Accelerating the design of lattice structures using machine learning. Sci. Rep. 2024, 14, 13703. [Google Scholar] [CrossRef]
- Bai, J.; Li, M.; Shen, J. Prediction of Mechanical Properties of Lattice Structures: An Application of Artificial Neural Networks Algorithms. Materials 2024, 17, 4222. [Google Scholar] [CrossRef]
- Liu, C.; Li, S. High-resolution topology optimization method of multi-morphology lattice structures based on three-dimensional convolutional neural networks (3D-CNN). Struct. Multidiscip. Optim. 2023, 66, 235. [Google Scholar] [CrossRef]
- Fongsamootr, T.; Suttakul, P.; Tippayawong, N.; Nanakorn, P.; Cappellini, C. Bending behavior of 2D periodic plates with different unit cells: Numerical and experimental investigations. Mater. Today Commun. 2022, 31, 103774. [Google Scholar] [CrossRef]
- Kamranfard, M.R.; Darijani, H.; Rokhgireh, H.; Khademzadeh, S. Analysis and optimization of strut-based lattice structures by simplified finite element method. Acta Mech. 2023, 234, 1381–1408. [Google Scholar] [CrossRef]
- Müller, P.; Synek, A.; Stauß, T.; Steinnagel, C.; Ehlers, T.; Gembarski, P.C.; Pahr, D.; Lachmayer, R. Development of a density-based topology optimization of homogenized lattice structures for individualized hip endoprostheses and validation using micro-FE. Sci. Rep. 2024, 14, 5719. [Google Scholar] [CrossRef]
- Sam, P.; Nanakorn, P.; Theerakittayakorn, K.; Suttakul, P. Closed-form effective elastic constants of frame-like periodic cellular solids by a symbolic object-oriented finite element program. Int. J. Mech. Mater. Des. 2017, 13, 363–383. [Google Scholar] [CrossRef]
- Suttakul, P.; Nanakorn, P.; Vo, D. Effective out-of-plane rigidities of 2D lattices with different unit cell topologies. Arch. Appl. Mech. 2019, 89, 1837–1860. [Google Scholar] [CrossRef]
- Theerakittayakorn, K.; Nanakorn, P.; Sam, P.; Suttakul, P. Exact forms of effective elastic properties of frame-like periodic cellular solids. Arch. Appl. Mech. 2016, 86, 1465–1482. [Google Scholar] [CrossRef]
- Wang, A.-J.; McDowell, D.L. In-Plane Stiffness and Yield Strength of Periodic Metal Honeycombs. J. Eng. Mater. Technol. 2004, 126, 137–156. [Google Scholar] [CrossRef]
- Alkentar, R.; File, M.; Mankovits, T. Use of compression test to determine the Young’s modulus of the titanium alloy Ti6Al4V manufactured via direct metal laser sintering. Int. Rev. Appl. Sci. Eng. 2023, 14, 256–262. [Google Scholar] [CrossRef]
- Alkentar, R.; Kladovasilakis, N.; Tzetzis, D.; Mankovits, T. Effects of Pore Size Parameters of Titanium Additively Manufactured Lattice Structures on the Osseointegration Process in Orthopedic Applications: A Comprehensive Review. Crystals 2023, 13, 113. [Google Scholar] [CrossRef]
- Alkentar, R.; Máté, F.; Mankovits, T. Investigation of the Performance of Ti6Al4V Lattice Structures Designed for Biomedical Implants Using the Finite Element Method. Materials 2022, 15, 6335. [Google Scholar] [CrossRef]
- ISO 13314:2011; Mechanical Testing of Metals—Ductility Testing—Compression Test for Porous and Cellular Metals. ISO: Geneva, Switzerland, 2011.
- Standard Specification for Titanium and Titanium Alloy Bars and Billets. 2019. Available online: https://store.astm.org/standards/b348 (accessed on 21 March 2025).
- U.S Titanium Industry. Titanium Alloys Ti6Al4V. Available online: https://www.azom.com/article.aspx?ArticleID=1547 (accessed on 9 February 2025).
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- ISO 7206-4:2010; Partial and Total Hip Joint Prostheses—Part 4: Determination of Endurance Properties and Performance of Stemmed Femoral Components 2010. ISO: Geneva, Switzerland, 2010.
- ASTM F2033-12; Standard Specification for Total Hip Joint Prosthesis and Hip Endoprosthesis Bearing Surfaces Made of Metallic, Ceramic, and Polymeric Materials. ASTM International: West Conshohocken, PA, USA, 2012.

| Element | Chemical Composition Percentage% |
|---|---|
| Al | 5.50–6.50 |
| V | 3.50–4.50 |
| O | 0.13 |
| N | 0.05 |
| C | 0.08 |
| H | 0.012 |
| Fe | 0.25 |
| Y | 0.005 |
| Other elements each | 0.1 |
| Other elements total | 0.4 |
| Property | Value | Unit |
|---|---|---|
| Elastic modulus | 106,247 | MPa |
| Mass density | 4.4 | g/cm3 |
| Poisson’s ratio | 0.34 | - |
| Lattice Type | Longitudinal Strain | Lateral Strain | Poisson’s Ratio |
|---|---|---|---|
| 3D lattice infill type | 0.1001 | 0.0384 | 0.384 |
| Material | Young’s Modulus MPa | Poisson’s Ratio | Applied to |
|---|---|---|---|
| Ti6Al4V (Ti64 grade 23) | 106,247 | 0.34 | Hip implant’s parts except for the latticed part |
| Lattice structure surrogate part (measured) | 23,022 | 0.384 | Latticed part |
| CoCrMo | 220,000 | 0.35 | Femoral head |
| 316Steel | 190,000 | 0.26 | Pressure plate |
| Resin | 3100 | 0.31 | Base |
| Model | 1 mm Lattice | 2 mm Lattice | 3 mm Lattice |
|---|---|---|---|
| Vertical Deformation mm | |||
| Geometrically latticed model | 0.357 | 0.408 | 0.433 |
| Surrogate model | 0.354 | 0.392 | 0.427 |
| Deviation % | 1 | 4 | 1 |
| 1 mm Lattice | 2 mm Lattice | 3 mm Lattice | ||||
|---|---|---|---|---|---|---|
| Model | Normal Model | Surrogate Model | Normal Model | Surrogate Model | Normal Model | Surrogate Model |
| Time (s) | 1580 | 17 | 7363 | 20 | 9683 | 24 |
| Maximum memory used (MB) | 1878 | 116 | 1924 | 124 | 2293 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkentar, R.; Mankovits, T. Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis. Appl. Sci. 2025, 15, 3522. https://doi.org/10.3390/app15073522
Alkentar R, Mankovits T. Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis. Applied Sciences. 2025; 15(7):3522. https://doi.org/10.3390/app15073522
Chicago/Turabian StyleAlkentar, Rashwan, and Tamás Mankovits. 2025. "Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis" Applied Sciences 15, no. 7: 3522. https://doi.org/10.3390/app15073522
APA StyleAlkentar, R., & Mankovits, T. (2025). Development of Surrogate Model for Patient-Specific Lattice-Structured Hip Implant Design via Finite Element Analysis. Applied Sciences, 15(7), 3522. https://doi.org/10.3390/app15073522






