Effect of Thermal and Non-Thermal Pretreatments and Fermentation on the Amino Acid and Biogenic Amine Content of Oyster Mushroom
Abstract
1. Introduction
1.1. Importance of Proteins, Amino Acids, and Biogenic Amines to Food Quality
1.2. Oyster Mushroom (Pleurotus ostreatus)
1.3. Pretreatment of Mushrooms
1.3.1. Blanching (In Water)
1.3.2. Steaming
1.3.3. Oven Pretreatment
1.3.4. Microwave Pretreatment
1.3.5. Pretreatment with High Hydrostatic Pressure
1.3.6. Ultraviolet Light (UV) Pretreatment
1.4. Fermentation of Mushrooms
2. Materials and Methods
2.1. Mushroom Pretreatments and Fermentation
2.2. Mushroom Fermentation
2.3. Determination of the Essential Amino Acids and Proteinogenic (Total) Amino Acids
2.4. Free Amino Acids and Biogenic Amines
2.5. Statistical Evaluation of the Data
3. Results
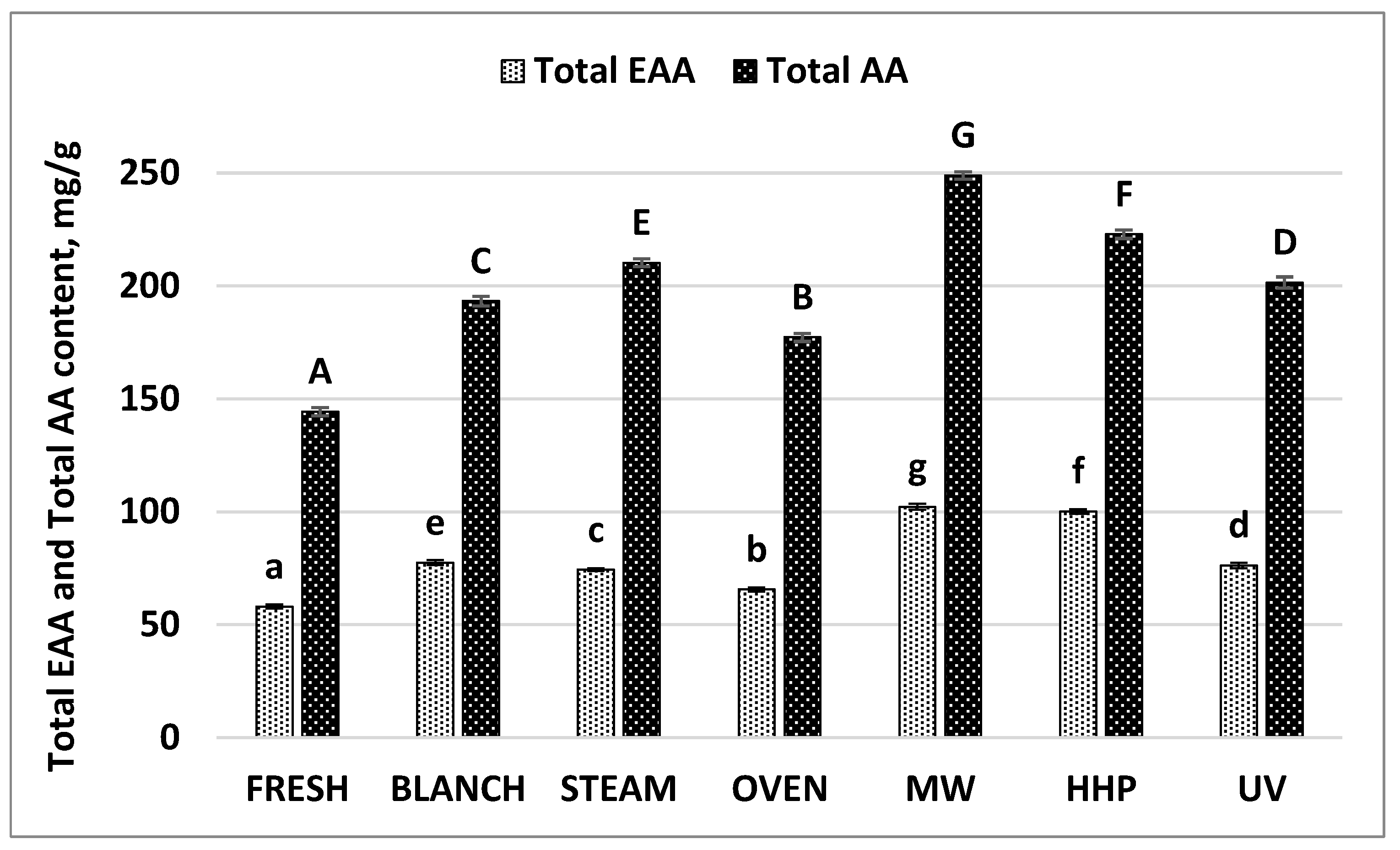
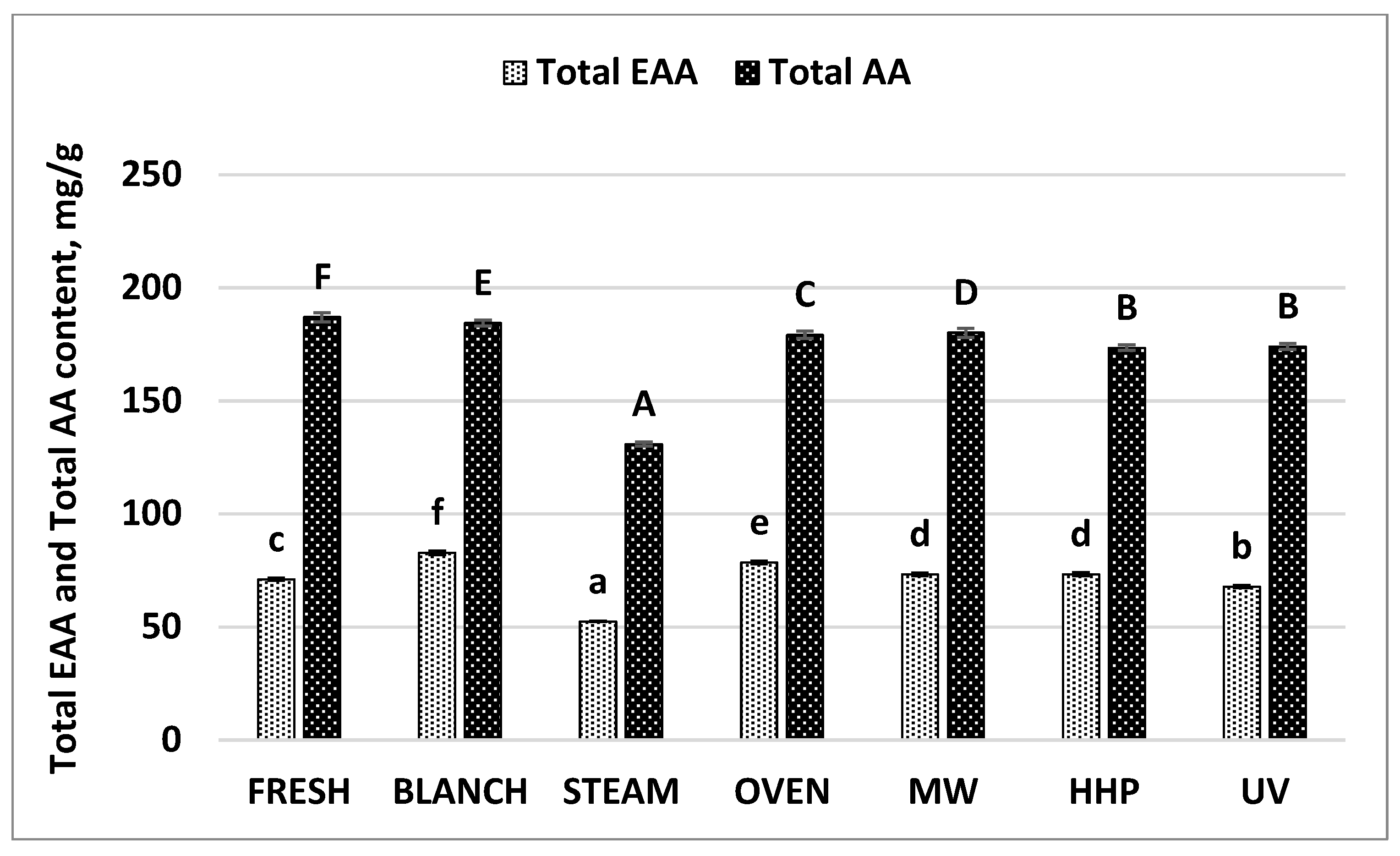
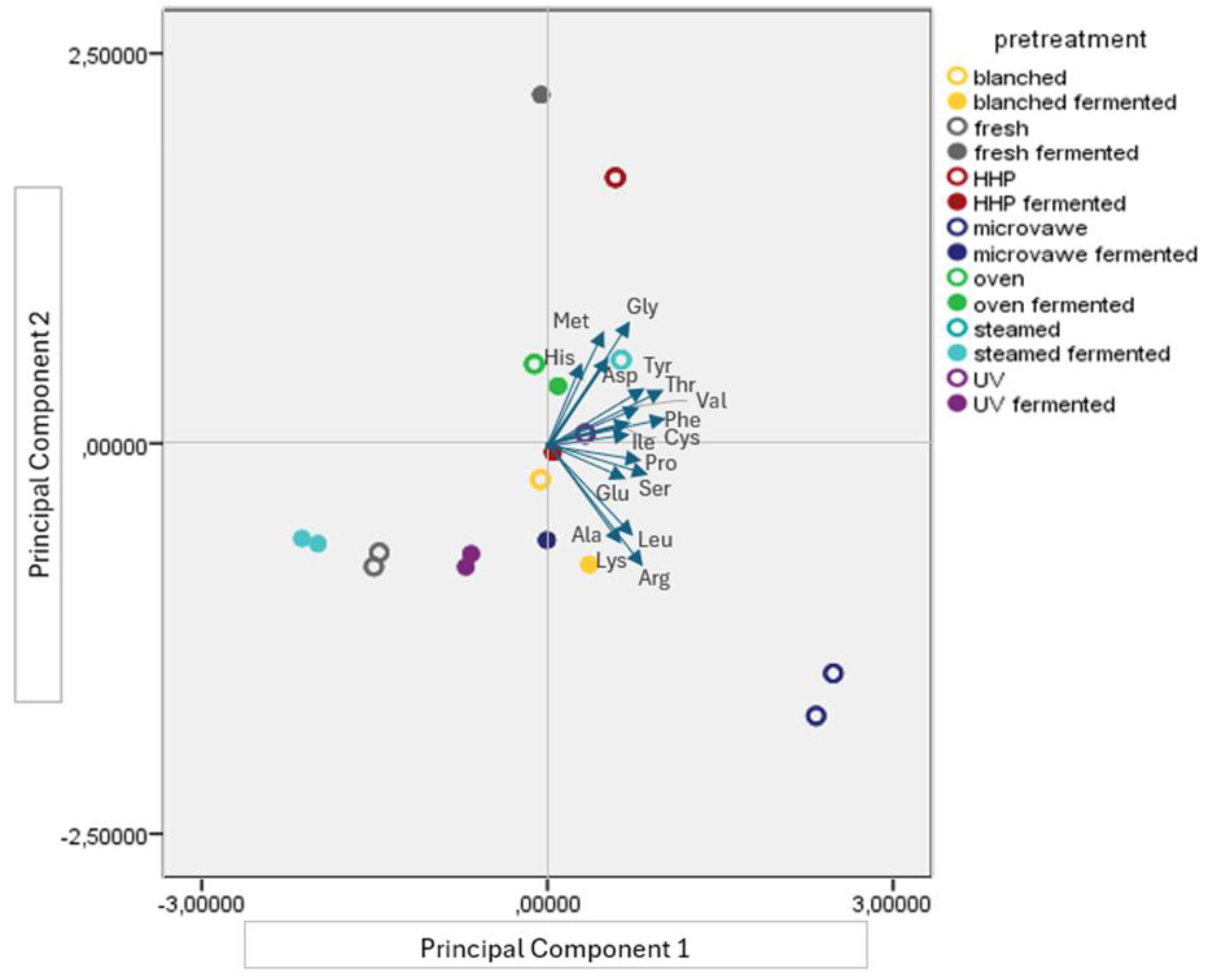
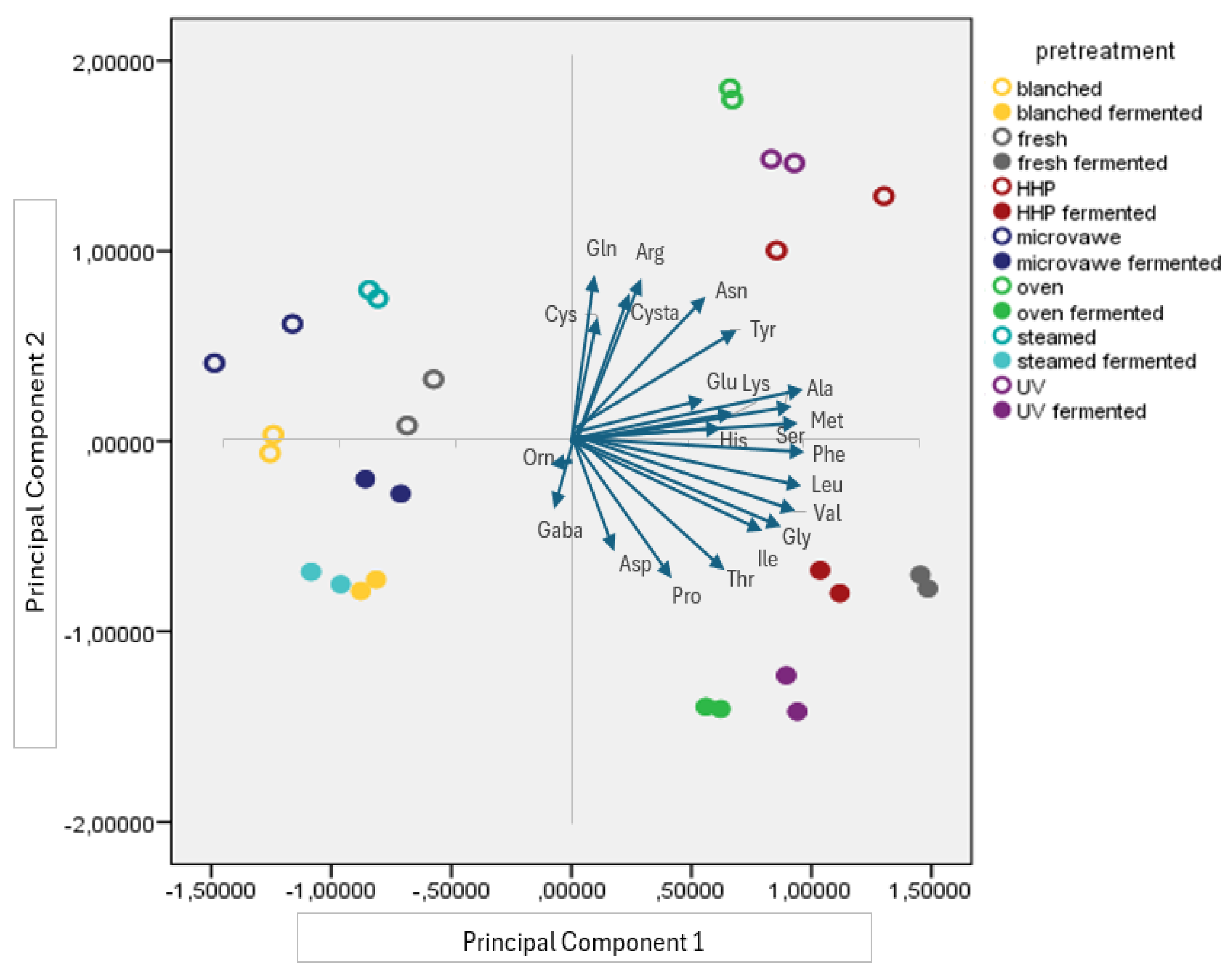
3.1. Essential Amino Acid and Proteinogenic Amino Acid Profile of the Mushroom Samples
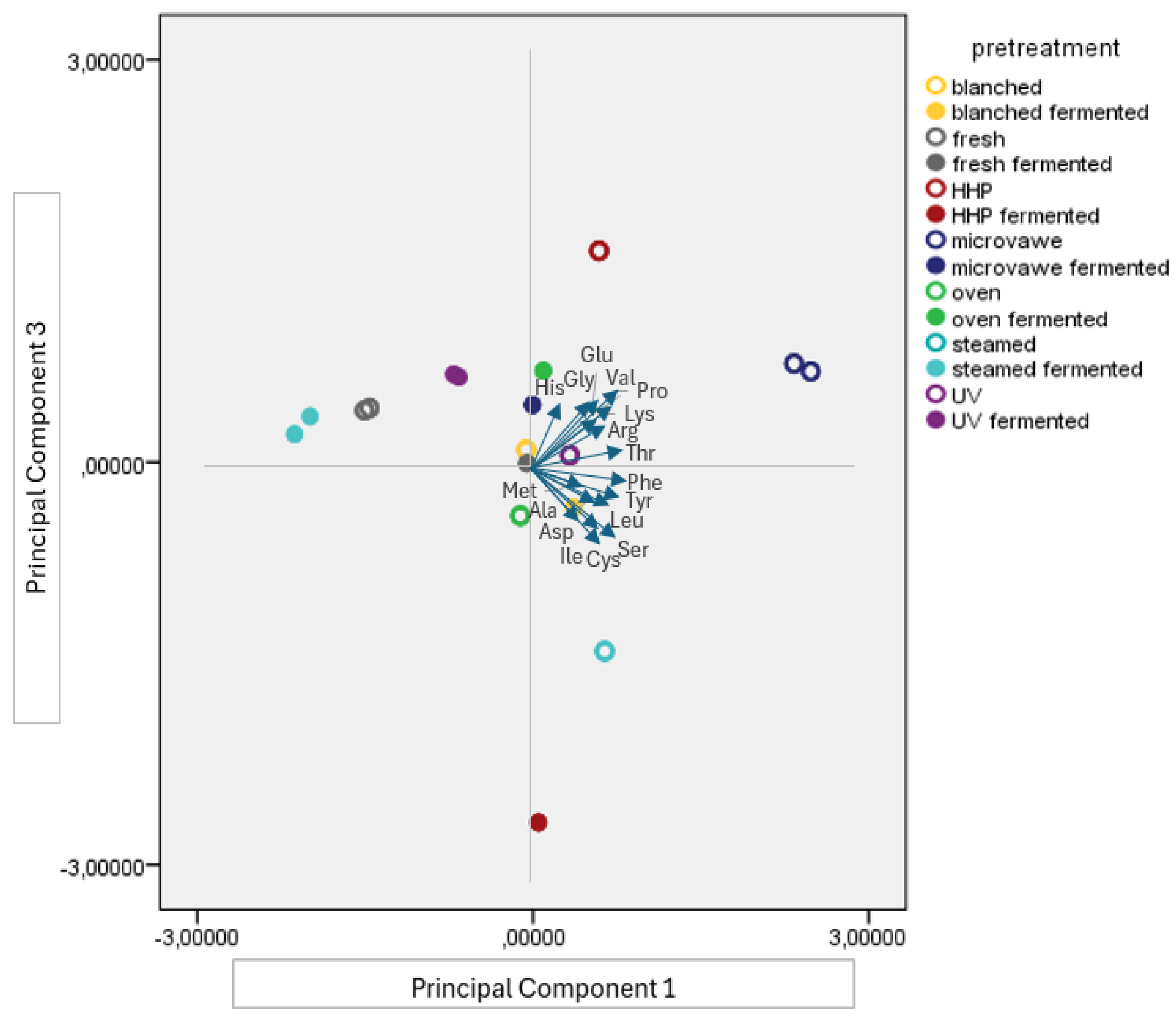
3.2. Free Amino Acid Profile of the Mushroom Samples
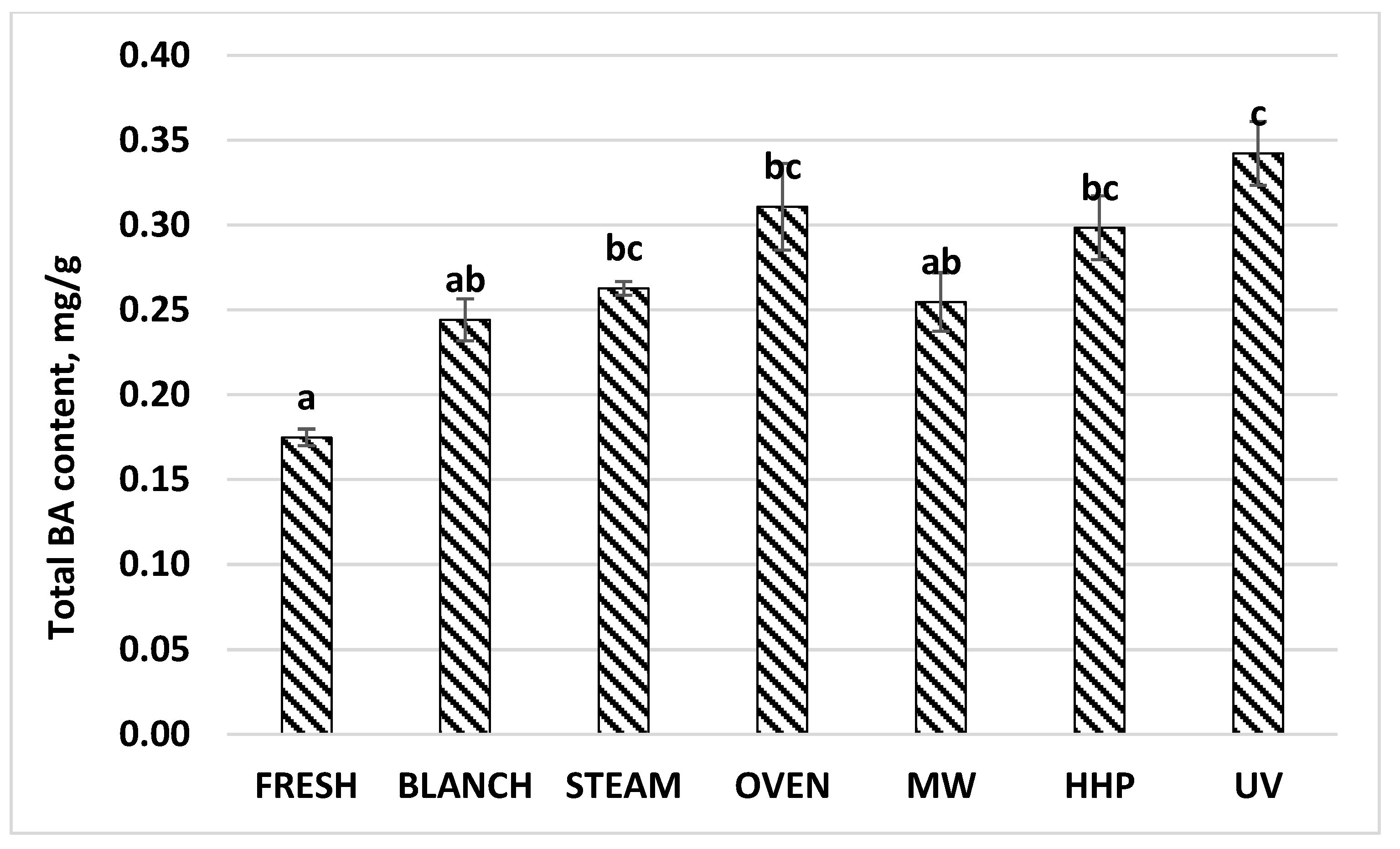
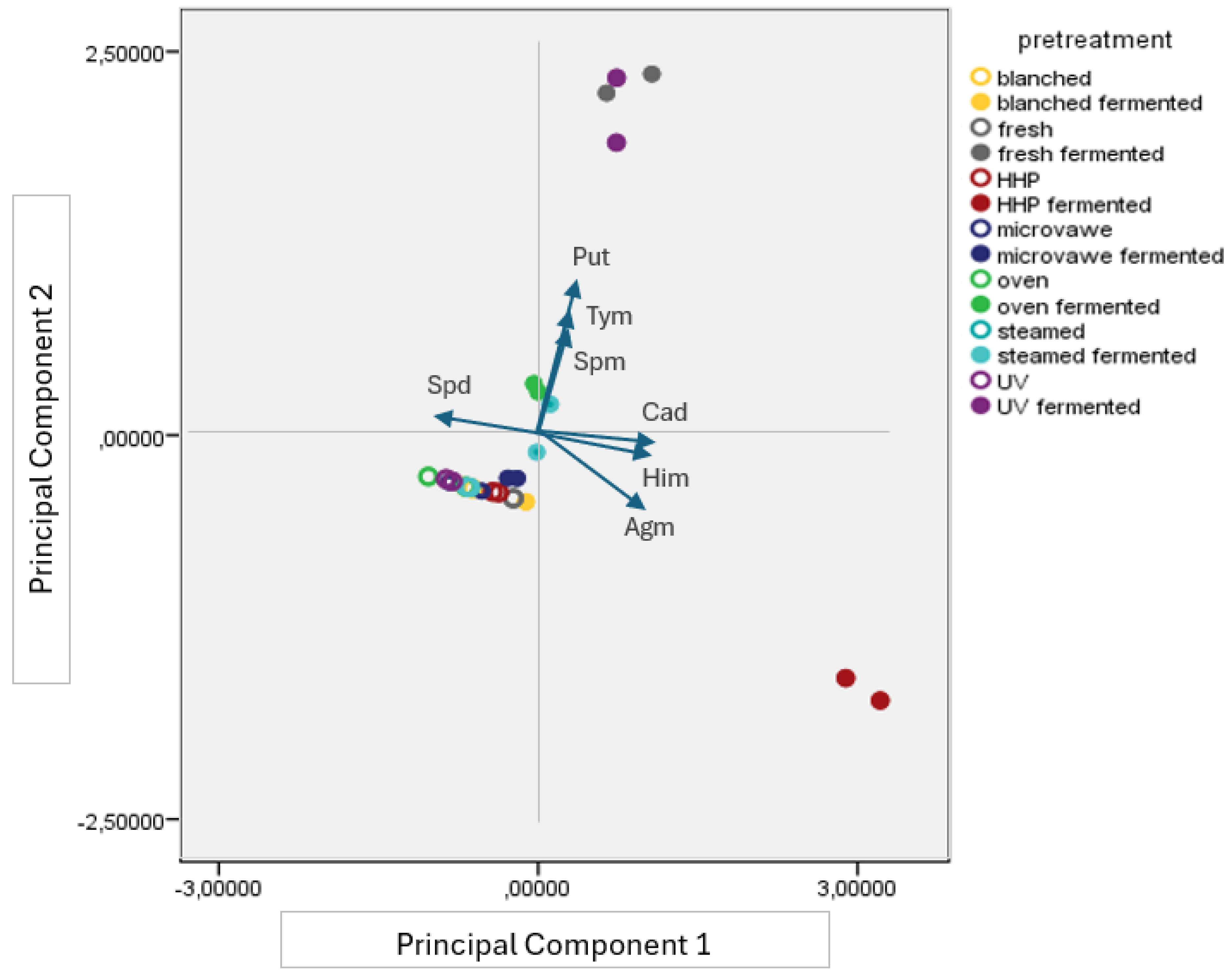
3.3. Biogenic Amine Profile of the Mushroom Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Proteinogenic Amino Acid Profile of Pretreated Mushroom Samples (Mean ± SE, n = 4) (*: Essential Amino Acid)
| Amino Acid (mg/g) | Fresh | Blanched | Steamed | Oven | Microwave | HHP | UV | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Asp | 24.37 | 0.80 | 36.27 | 0.04 | 51.90 | 0.01 | 26.63 | 0.00 | 37.50 | 1.06 | 30.91 | 0.00 | 34.17 | 0.04 |
| Thr * | 6.70 | 0.12 | 8.34 | 0.00 | 8.91 | 0.01 | 6.04 | 0.01 | 11.07 | 0.48 | 9.27 | 0.01 | 7.94 | 0.00 |
| Ser | 1.47 | 0.03 | 2.13 | 0.02 | 3.16 | 0.01 | 1.93 | 0.00 | 3.15 | 0.10 | 2.31 | 0.00 | 2.55 | 0.01 |
| Glu | 29.16 | 0.00 | 43.48 | 0.02 | 41.12 | 0.00 | 39.44 | 0.00 | 52.45 | 0.39 | 45.76 | 0.00 | 51.48 | 0.00 |
| Pro | 5.19 | 0.07 | 4.84 | 0.02 | 7.47 | 0.01 | 6.46 | 0.00 | 8.38 | 0.06 | 7.65 | 0.00 | 5.90 | 0.00 |
| Gly | 8.36 | 0.21 | 10.43 | 0.06 | 11.21 | 0.00 | 13.66 | 0.01 | 14.46 | 0.10 | 15.70 | 0.00 | 11.41 | 0.02 |
| Ala | 12.53 | 0.22 | 12.26 | 0.04 | 12.35 | 0.00 | 14.67 | 0.00 | 22.36 | 0.24 | 12.45 | 0.00 | 10.51 | 0.01 |
| Val * | 7.22 | 0.34 | 11.94 | 0.00 | 9.71 | 0.03 | 8.53 | 0.01 | 13.47 | 0.36 | 14.80 | 0.01 | 13.82 | 0.00 |
| Cys | 1.43 | 0.03 | 1.95 | 0.01 | 3.17 | 0.00 | 2.13 | 0.00 | 2.33 | 0.02 | 2.32 | 0.00 | 2.49 | 0.00 |
| Met * | 1.98 | 0.09 | 3.68 | 0.00 | 2.83 | 0.01 | 3.68 | 0.00 | 3.29 | 0.11 | 3.83 | 0.00 | 2.30 | 0.03 |
| Ile * | 4.22 | 0.04 | 4.96 | 0.00 | 8.48 | 0.02 | 8.94 | 0.00 | 8.10 | 0.25 | 6.36 | 0.00 | 4.46 | 0.04 |
| Leu * | 11.83 | 0.15 | 18.58 | 0.00 | 15.26 | 0.03 | 10.41 | 0.01 | 21.15 | 0.13 | 11.22 | 0.00 | 18.04 | 0.07 |
| Tyr | 3.78 | 0.20 | 4.50 | 0.00 | 5.55 | 0.02 | 6.78 | 0.00 | 6.08 | 0.05 | 5.65 | 0.00 | 6.79 | 0.01 |
| Phe * | 5.81 | 0.00 | 7.20 | 0.00 | 7.94 | 0.01 | 7.47 | 0.01 | 10.43 | 0.24 | 7.69 | 0.00 | 8.82 | 0.00 |
| Lys * | 8.38 | 0.01 | 9.16 | 0.00 | 7.36 | 0.02 | 7.65 | 0.00 | 14.80 | 0.22 | 8.17 | 0.00 | 8.94 | 0.00 |
| His * | 3.66 | 0.19 | 4.28 | 0.04 | 5.56 | 0.03 | 4.50 | 0.00 | 6.57 | 0.06 | 31.50 | 0.01 | 4.74 | 0.01 |
| Arg * | 8.25 | 0.05 | 9.32 | 0.05 | 8.30 | 0.00 | 8.34 | 0.00 | 13.34 | 0.13 | 7.31 | 0.00 | 7.21 | 0.00 |
| Total AA | 144.34 | 1.82 | 193.32 | 0.09 | 210.29 | 0.02 | 177.26 | 0.2 | 248.91 | 1.55 | 222.90 | 0.00 | 201.57 | 0.02 |
| Total EAA * | 58.05 | 0.86 | 77.46 | 0.01 | 74.35 | 0.00 | 65.56 | 0.00 | 102.22 | 0.79 | 100.15 | 0.00 | 76.27 | 0.00 |
Appendix B. The Proteinogenic Amino Acid Profile of Pretreated Fermented Mushroom Samples (Mean ± SE, n = 4) (*: Essential Amino Acid)
| Amino Acid (mg/g) | Fresh Fermented | Blanched Fermented | Steamed Fermented | Oven Fermented | Microwave Fermented | HHP Fermented | UV Fermented | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Asp | 53.79 | 0.16 | 28.45 | 0.10 | 22.22 | 0.48 | 27.72 | 0.00 | 27.33 | 0.01 | 36.27 | 0.06 | 32.89 | 0.74 |
| Thr * | 10.36 | 0.01 | 7.30 | 0.01 | 5.04 | 0.11 | 7.99 | 0.00 | 8.03 | 0.00 | 7.65 | 0.02 | 6.37 | 0.21 |
| Ser | 1.07 | 0.00 | 1.92 | 0.03 | 1.30 | 0.11 | 1.89 | 0.01 | 1.90 | 0.00 | 3.17 | 0.03 | 1.33 | 0.03 |
| Glu | 20.40 | 0.08 | 27.59 | 0.00 | 27.15 | 0.23 | 32.46 | 0.12 | 38.02 | 0.00 | 22.30 | 0.08 | 35.61 | 0.26 |
| Pro | 5.18 | 0.00 | 6.81 | 0.00 | 4.53 | 0.35 | 7.01 | 0.05 | 7.67 | 0.00 | 4.38 | 0.03 | 6.53 | 0.08 |
| Gly | 16.82 | 0.00 | 9.69 | 0.01 | 7.57 | 0.13 | 13.11 | 0.01 | 8.87 | 0.00 | 7.02 | 0.03 | 9.75 | 0.16 |
| Ala | 11.24 | 0.01 | 19.29 | 0.00 | 11.32 | 0.08 | 12.71 | 0.00 | 15.34 | 0.01 | 18.78 | 0.09 | 14.88 | 0.04 |
| Val * | 11.95 | 0.00 | 14.52 | 0.01 | 7.15 | 0.27 | 15.64 | 0.00 | 11.61 | 0.00 | 8.76 | 0.03 | 9.44 | 0.00 |
| Cys | 1.43 | 0.00 | 1.67 | 0.00 | 1.16 | 0.01 | 1.71 | 0.00 | 2.38 | 0.00 | 2.82 | 0.02 | 1.47 | 0.00 |
| Met * | 3.87 | 0.00 | 2.37 | 0.00 | 2.00 | 0.09 | 3.79 | 0.00 | 2.05 | 0.00 | 3.81 | 0.03 | 2.58 | 0.04 |
| Ile * | 6.20 | 0.00 | 9.12 | 0.02 | 4.26 | 0.22 | 7.21 | 0.00 | 5.26 | 0.00 | 8.01 | 0.01 | 5.58 | 0.10 |
| Leu * | 10.56 | 0.00 | 17.78 | 0.00 | 11.05 | 0.00 | 14.62 | 0.00 | 13.07 | 0.02 | 17.18 | 0.05 | 13.68 | 0.06 |
| Tyr | 5.90 | 0.02 | 6.13 | 0.00 | 3.09 | 0.10 | 4.08 | 0.00 | 5.33 | 0.00 | 5.42 | 0.03 | 3.70 | 0.16 |
| Phe * | 9.74 | 0.01 | 8.93 | 0.00 | 5.53 | 0.07 | 8.60 | 0.00 | 8.53 | 0.00 | 8.68 | 0.08 | 7.27 | 0.01 |
| Lys * | 7.54 | 0.02 | 8.83 | 0.00 | 7.83 | 0.13 | 8.56 | 0.02 | 8.76 | 0.01 | 7.67 | 0.01 | 9.72 | 0.07 |
| His * | 3.94 | 0.04 | 4.80 | 0.00 | 2.89 | 0.02 | 3.74 | 0.00 | 6.76 | 0.00 | 4.81 | 0.02 | 4.32 | 0.02 |
| Arg * | 6.97 | 0.12 | 9.16 | 0.07 | 6.75 | 0.10 | 8.37 | 0.00 | 9.16 | 0.00 | 6.77 | 0.04 | 8.89 | 0.11 |
| Total AA | 186.96 | 0.41 | 184.36 | 0.02 | 130.86 | 0.85 | 179.21 | 0.18 | 180.07 | 0.41 | 173.50 | 0.34 | 174.02 | 0.35 |
| Total EAA * | 71.13 | 0.30 | 82.81 | 0.21 | 52.50 | 0.31 | 78.52 | 0.22 | 73.23 | 0.34 | 73.34 | 0.25 | 67.87 | 0.40 |
Appendix C. The Free Amino Acid Profile of Pretreated Mushroom Samples (Mean ± SE, n = 4)
| Free Amino Acid (mg/g) | Fresh | Blanched | Steamed | Oven | Microwave | HHP | UV | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Asp | 1.36 | 0.02 | 1.43 | 0.02 | 2.77 | 0.03 | 1.60 | 0.01 | 1.52 | 0.19 | 1.79 | 0.09 | 1.48 | 0.03 |
| Thr | 0.71 | 0.00 | 0.60 | 0.01 | 0.77 | 0.03 | 0.70 | 0.02 | 0.53 | 0.04 | 0.87 | 0.01 | 1.00 | 0.05 |
| Ser | 1.73 | 0.27 | 1.39 | 0.02 | 1.50 | 0.22 | 2.66 | 0.04 | 1.13 | 0.31 | 2.46 | 0.36 | 2.82 | 0.07 |
| Asn | 3.64 | 0.04 | 2.10 | 0.08 | 2.69 | 0.41 | 8.06 | 0.27 | 2.60 | 0.09 | 6.56 | 0.14 | 6.04 | 0.16 |
| Glu | 3.62 | 0.02 | 3.11 | 0.11 | 5.14 | 0.12 | 4.22 | 0.17 | 4.64 | 0.10 | 4.44 | 0.11 | 7.68 | 0.22 |
| Gln | 2.53 | 0.09 | 2.16 | 0.08 | 5.89 | 0.00 | 6.59 | 0.08 | 3.59 | 0.12 | 4.39 | 0.14 | 3.46 | 0.12 |
| Pro | 0.62 | 0.03 | 0.57 | 0.05 | 0.54 | 0.05 | 0.54 | 0.01 | 0.61 | 0.03 | 0.71 | 0.00 | 0.59 | 0.04 |
| Gly | 0.94 | 0.05 | 0.50 | 0.02 | 0.71 | 0.01 | 0.97 | 0.05 | 0.56 | 0.07 | 1.20 | 0.24 | 1.60 | 0.06 |
| Ala | 3.80 | 0.15 | 1.95 | 0.13 | 2.63 | 0.05 | 5.43 | 0.40 | 3.21 | 0.31 | 6.84 | 0.22 | 6.67 | 0.11 |
| Val | 1.35 | 0.24 | 1.14 | 0.20 | 1.28 | 0.01 | 2.29 | 0.02 | 0.95 | 0.21 | 2.51 | 0.19 | 2.26 | 0.08 |
| Cys | 0.34 | 0.07 | 0.30 | 0.02 | 0.44 | 0.00 | 0.48 | 0.04 | 0.58 | 0.05 | 0.50 | 0.05 | 0.73 | 0.05 |
| Met | 0.74 | 0.03 | 0.23 | 0.02 | 0.34 | 0.00 | 0.89 | 0.03 | 0.19 | 0.05 | 1.29 | 0.16 | 1.06 | 0.08 |
| Cysta | 0.62 | 0.07 | 0.41 | 0.01 | 1.08 | 0.00 | 1.19 | 0.05 | 0.84 | 0.19 | 1.04 | 0.10 | 1.36 | 0.06 |
| Ile | 1.03 | 0.17 | 1.01 | 0.02 | 0.67 | 0.00 | 1.30 | 0.01 | 0.55 | 0.09 | 1.59 | 0.11 | 1.31 | 0.02 |
| Leu | 2.62 | 0.03 | 1.48 | 0.08 | 1.67 | 0.00 | 3.58 | 0.06 | 1.42 | 0.21 | 5.23 | 0.45 | 3.70 | 0.02 |
| Tyr | 1.52 | 0.09 | 0.89 | 0.02 | 1.24 | 0.07 | 2.37 | 0.02 | 0.92 | 0.13 | 2.79 | 0.28 | 2.48 | 0.05 |
| Phe | 1.30 | 0.04 | 1.32 | 0.05 | 1.09 | 0.08 | 1.98 | 0.04 | 0.89 | 0.11 | 2.83 | 0.21 | 2.14 | 0.04 |
| Gaba | 1.62 | 0.04 | 1.33 | 0.03 | 1.23 | 0.02 | 1.73 | 0.03 | 1.27 | 0.04 | 1.49 | 0.01 | 1.31 | 0.02 |
| Orn | 0.51 | 0.12 | 0.39 | 0.01 | 0.85 | 0.00 | 1.17 | 0.09 | 0.83 | 0.12 | 0.67 | 0.06 | 1.20 | 0.08 |
| Lys | 1.33 | 0.02 | 1.24 | 0.04 | 1.93 | 0.00 | 3.16 | 0.08 | 0.81 | 0.11 | 2.72 | 0.09 | 1.98 | 0.04 |
| His | 0.77 | 0.10 | 0.80 | 0.01 | 0.71 | 0.00 | 1.66 | 0.01 | 0.47 | 0.09 | 1.27 | 0.10 | 1.61 | 0.06 |
| Arg | 1.38 | 0.30 | 1.61 | 0.22 | 1.65 | 0.03 | 2.63 | 0.03 | 0.89 | 0.18 | 2.46 | 0.26 | 2.07 | 0.08 |
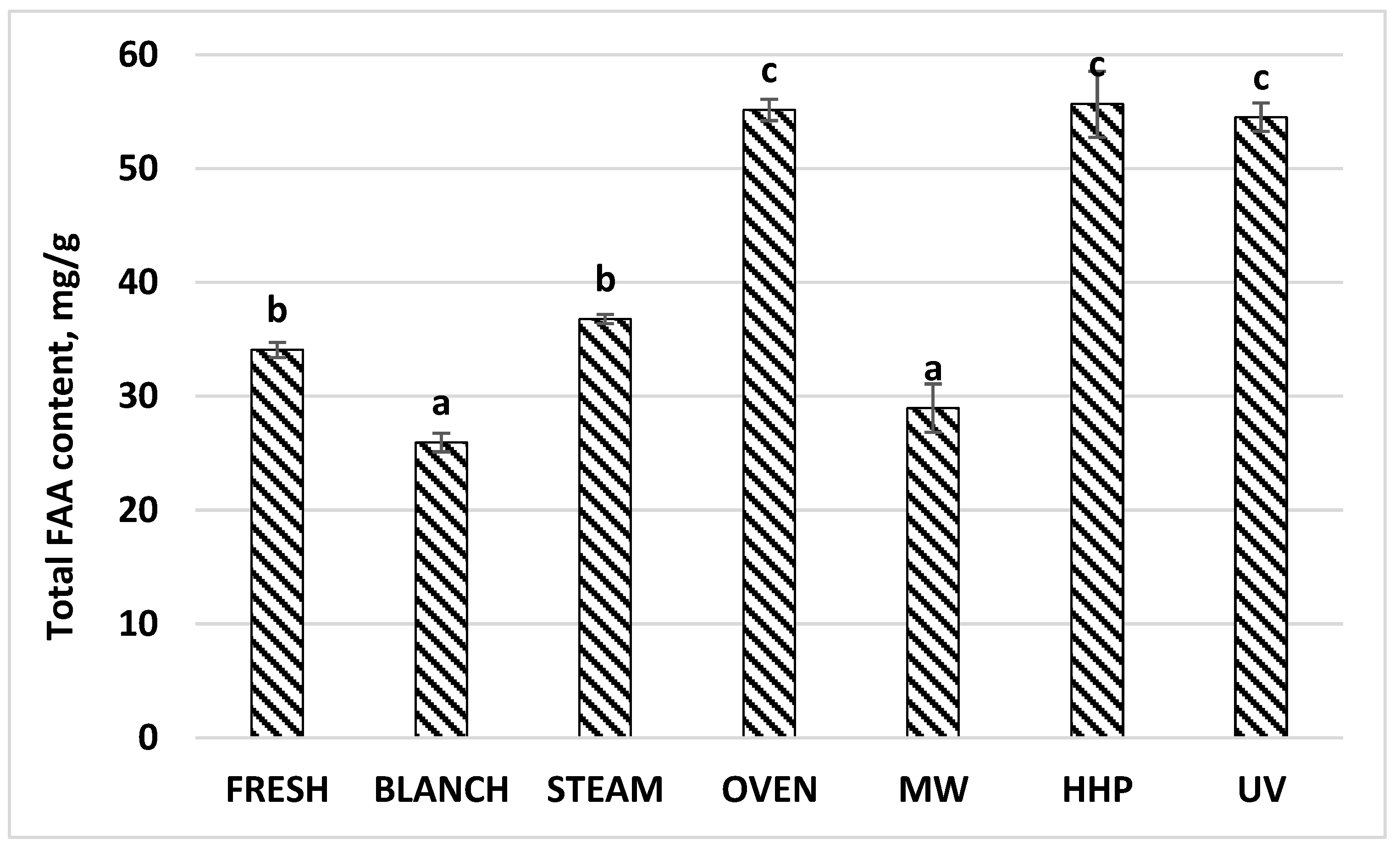
| Total FAA | 34.07 | 0.82 | 25.95 | 0.24 | 36.80 | 0.48 | 55.17 | 0.39 | 28.98 | 2.58 | 55.68 | 3.16 | 54.54 | 0.58 |
Appendix D. The Free Amino Acid Profile of Pretreated Fermented Mushroom Samples (Mean ± SE, n = 4)
| Free Amino Acid (mg/g) | Fresh Fermented | Blanched Fermented | Steamed Fermented | Oven Fermented | Microwave Fermented | HHP Fermented | UV Fermented | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Asp | 2.12 | 0.20 | 2.19 | 0.04 | 2.25 | 0.05 | 3.23 | 0.15 | 1.79 | 0.04 | 1.56 | 0.10 | 3.19 | 0.06 |
| Thr | 1.71 | 0.08 | 1.10 | 0.11 | 1.27 | 0.02 | 2.00 | 0.02 | 0.48 | 0.07 | 2.00 | 0.02 | 1.52 | 0.03 |
| Ser | 3.74 | 0.06 | 1.08 | 0.11 | 1.09 | 0.04 | 2.05 | 0.07 | 1.00 | 0.09 | 2.63 | 0.22 | 2.61 | 0.05 |
| Asn | 2.14 | 0.04 | 1.69 | 0.01 | 1.72 | 0.02 | 2.34 | 0.16 | 1.16 | 0.10 | 4.32 | 0.19 | 3.04 | 0.06 |
| Glu | 6.40 | 0.07 | 1.20 | 0.01 | 1.27 | 0.06 | 7.01 | 0.03 | 4.35 | 0.37 | 7.00 | 0.02 | 2.18 | 0.12 |
| Gln | 2.37 | 0.05 | 1.41 | 0.01 | 1.27 | 0.08 | 1.38 | 0.03 | 1.40 | 0.12 | 1.68 | 0.02 | 1.55 | 0.04 |
| Pro | 0.98 | 0.05 | 0.85 | 0.04 | 0.68 | 0.12 | 0.75 | 0.01 | 0.82 | 0.07 | 0.80 | 0.07 | 0.86 | 0.06 |
| Gly | 2.28 | 0.02 | 1.02 | 0.05 | 0.83 | 0.05 | 2.00 | 0.02 | 0.99 | 0.08 | 2.01 | 0.01 | 2.11 | 0.06 |
| Ala | 6.57 | 0.04 | 1.24 | 0.06 | 2.75 | 0.01 | 5.13 | 0.23 | 3.49 | 0.23 | 5.16 | 0.18 | 6.13 | 0.19 |
| Val | 3.20 | 0.03 | 1.69 | 0.04 | 1.42 | 0.01 | 3.31 | 0.05 | 1.59 | 0.07 | 3.22 | 0.11 | 3.17 | 0.06 |
| Cys | 0.47 | 0.03 | 0.40 | 0.01 | 0.33 | 0.01 | 0.29 | 0.02 | 0.53 | 0.01 | 0.26 | 0.05 | 0.39 | 0.02 |
| Met | 1.11 | 0.03 | 0.33 | 0.02 | 0.34 | 0.01 | 0.80 | 0.00 | 0.45 | 0.01 | 1.01 | 0.04 | 1.11 | 0.05 |
| Cysta | 0.80 | 0.01 | 0.79 | 0.02 | 0.76 | 0.07 | 0.45 | 0.01 | 0.72 | 0.03 | 0.48 | 0.02 | 0.79 | 0.02 |
| Ile | 2.10 | 0.02 | 1.73 | 0.04 | 0.68 | 0.03 | 2.20 | 0.07 | 0.72 | 0.17 | 2.20 | 0.01 | 2.12 | 0.10 |
| Leu | 5.36 | 0.33 | 1.87 | 0.04 | 1.69 | 0.06 | 5.35 | 0.08 | 2.32 | 0.10 | 5.01 | 0.04 | 5.57 | 0.16 |
| Tyr | 1.43 | 0.09 | 0.80 | 0.02 | 0.85 | 0.00 | 1.29 | 0.14 | 0.66 | 0.06 | 2.63 | 0.05 | 0.87 | 0.05 |
| Phe | 2.96 | 0.06 | 1.00 | 0.02 | 1.00 | 0.04 | 2.06 | 0.10 | 1.26 | 0.04 | 2.46 | 0.08 | 2.57 | 0.14 |
| Gaba | 1.11 | 0.17 | 1.12 | 0.06 | 4.85 | 0.09 | 1.68 | 0.04 | 2.29 | 0.18 | 1.24 | 0.03 | 4.26 | 0.14 |
| Orn | 0.59 | 0.02 | 0.70 | 0.00 | 1.42 | 0.16 | 1.50 | 0.03 | 1.86 | 0.11 | 0.89 | 0.02 | 0.65 | 0.05 |
| Lys | 3.52 | 0.08 | 1.34 | 0.27 | 2.09 | 0.13 | 1.30 | 0.04 | 2.60 | 0.20 | 2.15 | 0.06 | 2.74 | 0.14 |
| His | 1.59 | 0.03 | 1.73 | 0.04 | 1.10 | 0.08 | 0.78 | 0.09 | 0.99 | 0.07 | 1.39 | 0.10 | 1.39 | 0.01 |
| Arg | 1.11 | 0.03 | 0.96 | 0.09 | 0.98 | 0.06 | 1.10 | 0.00 | 1.19 | 0.07 | 1.04 | 0.01 | 0.84 | 0.17 |
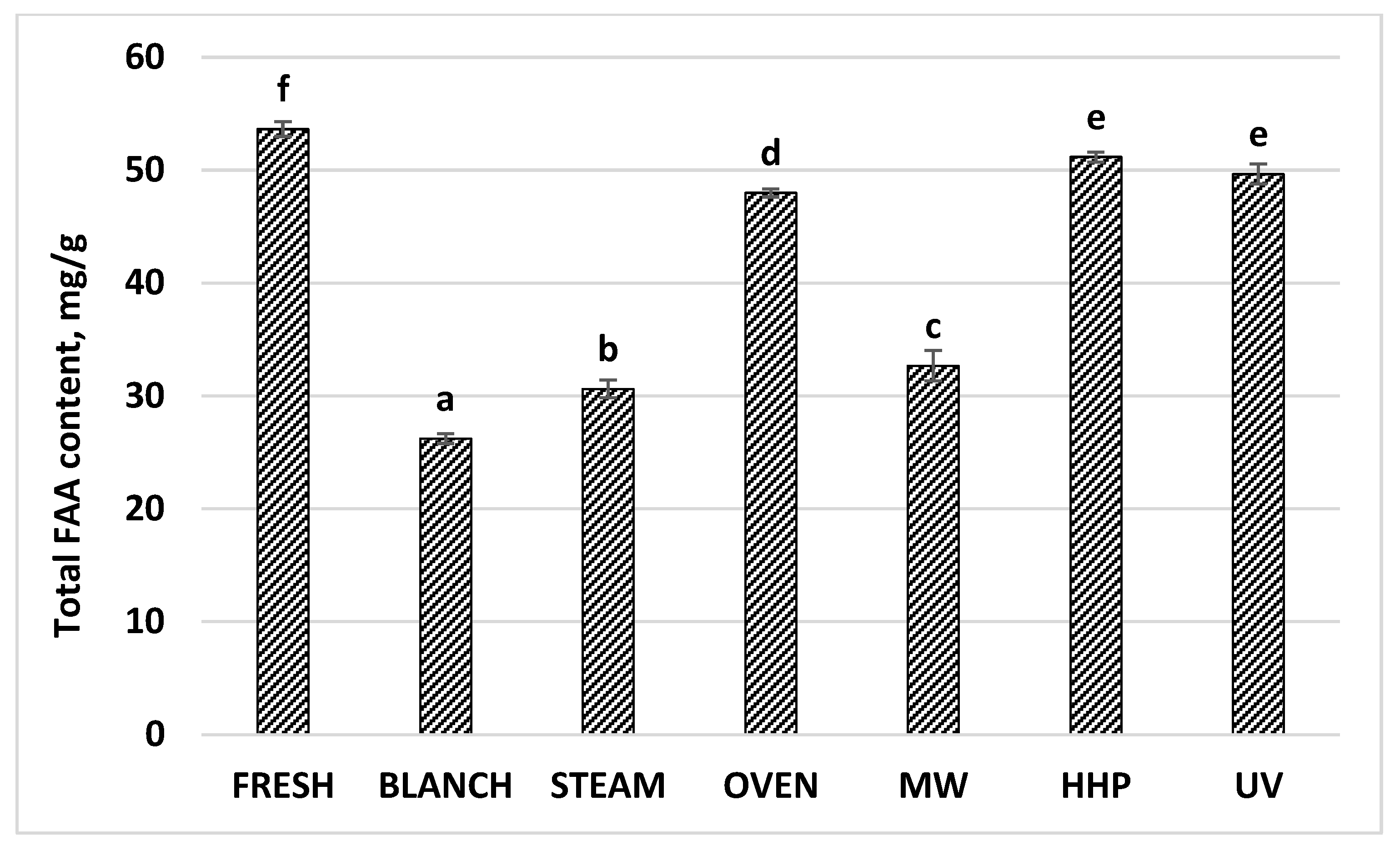
| Total FAA | 53.65 | 0.69 | 26.24 | 0.56 | 30.64 | 0.93 | 48.00 | 0.41 | 32.68 | 1.67 | 51.17 | 0.53 | 49.67 | 0.29 |
Appendix E. The Biogenic Amine Profile of Pretreated Mushroom Samples (Mean ± SE, n = 4) (n.d.: Not Detected)
| Biogenic Amine (mg/g) | Fresh | Blanched | Steamed | Oven | Microwave | HHP | UV | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Him | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Tym | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.04 | 0.01 |
| Put | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cad | 0.02 | 0.00 | n.d. | n.d. | 0.01 | 0.00 | n.d. | n.d. | n.d. | n.d. | 0.04 | 0.01 | n.d. | n.d. |
| Spd | 0.15 | 0.00 | 0.24 | 0.02 | 0.26 | 0.01 | 0.31 | 0.08 | 0.25 | 0.07 | 0.26 | 0.01 | 0.30 | 0.01 |
| Agm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Spm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total BA | 0.17 | 0.01 | 0.24 | 0.02 | 0.26 | 0.00 | 0.31 | 0.08 | 0.25 | 0.07 | 0.30 | 0.02 | 0.34 | 0.02 |
Appendix F. The Biogenic Amine Profile of Pretreated Fermented Mushroom Samples (Mean ± SE, n = 4) (n.d.: Not Detected)
| Biogenic Amine (mg/g) | Fresh Fermented | Blanched Fermented | Steamed Fermented | Oven Fermented | Microwave Fermented | HHP Fermented | UV Fermented | |||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | |
| Him | 0.15 | 0.02 | n.d. | n.d. | 0.46 | 0.07 | 0.44 | 0.00 | 0.24 | 0.03 | 1.23 | 0.12 | 0.50 | 0.07 |
| Tym | 0.76 | 0.19 | n.d. | n.d. | 0.08 | 0.01 | 0.11 | 0.00 | n.d. | n.d. | 0.10 | 0.02 | 4.10 | 0.44 |
| Put | 0.27 | 0.01 | n.d. | n.d. | 0.10 | 0.05 | 0.15 | 0.01 | 0.03 | 0.00 | 0.01 | 0.00 | 0.26 | 0.03 |
| Cad | 0.08 | 0.03 | 0.02 | 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.15 | 0.00 | 0.03 | 0.00 |
| Spd | 0.16 | 0.01 | 0.12 | 0.01 | 0.17 | 0.03 | 0.21 | 0.01 | 0.18 | 0.01 | n.d. | n.d. | 0.17 | 0.03 |
| Agm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.08 | 0.01 | n.d. | n.d. |
| Spm | 0.48 | 0.10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total BA | 1.90 | 0.06 | 0.14 | 0.01 | 0.81 | 0.05 | 0.91 | 0.02 | 0.45 | 0.02 | 1.56 | 0.15 | 5.05 | 0.57 |
References
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef]
- Elmadfa, I.; Meyer, A.L. Animal Proteins as Important Contributors to a Healthy Human Diet. Annu. Rev. Anim. Biosci. 2017, 5, 111–131. [Google Scholar] [PubMed]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative Protein Sources and Novel Foods: Benefits, Food Applications and Safety Issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar]
- Sarkadi, L.S. Amino acids and biogenic amines as food quality factors. Pure Appl. Chem. 2019, 91, 289–300. [Google Scholar] [CrossRef]
- Costa, M.P.; Rodrigues, B.L.; Frasao, B.S.; Conte-Junior, C.A. Chapter 2—Biogenic Amines as Food Quality Index and Chemical Risk for Human Consumption. In Food Quality: Balancing Health and Disease; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 75–108. [Google Scholar]
- Bartkiene, E.; Zokaityte, E.; Starkute, V.; Mockus, E.; Klupsaite, D.; Lukseviciute, J.; Bogomolova, A.; Streimikyte, A.; Ozogul, F. Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods 2022, 11, 1800. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- EU Commission Regulation. Commission Regulation (EU) No 1019/2013 of 23 October 2013 Amending Annex I to Regulation (EC) No 2073/2005 as Regards Histamine in Fishery Products Text with EEA Relevance; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Jabłońska-Ryś, E.; Sławińska, A.; Stachniuk, A.; Stadnik, J. Determination of biogenic amines in processed and unprocessed mushrooms from the Polish market. J. Food Compos. Anal. 2020, 92, 103492. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Tripathi, A. Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 2021, 45, e13748. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Esqueda, M.; Rodríguez-Carpena, J.G.; Fernández-López, J.; Perez-Alvarez, J.A.; Sánchez-Escalante, A. Pleurotus Genus as a Potential Ingredient for Meat Products. Foods 2022, 11, 779. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk CW, I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Techn. 2017, 52, 2382–2392. [Google Scholar]
- Mumpuni, A.; Ekowati, N.; Purnomowati, P.; Purwati, E.S. Growth and Protein Content Establishment of Pleurotus ostreatus on Liquid and Solid Medium. Biosaintifika J. Biol. Biol. Educ. 2017, 9, 572. [Google Scholar]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of Biogenic Amines in Food—Existing and Emerging Approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [PubMed]
- Sangeeta Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom processing: Innovative techniques and technologies. Food Chem. X 2024, 23, 101774. [Google Scholar]
- Ritota, M.; Manzi, P.; Ritota, M.; Manzi, P. Edible mushrooms: Functional foods or functional ingredients? A focus on Pleurotus spp. AIMS Agric. Food 2023, 8, 391–439. [Google Scholar]
- Jabłońska-Ryś, E.; Skrzypczak, K.; Sławińska, A.; Radzki, W.; Gustaw, W. Lactic Acid Fermentation of Edible Mushrooms: Tradition, Technology, Current State of Research: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 655–669. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Paniwnyk, L.; Lelas, V. Ultrasonic effect on pH, electric conductivity, and tissue surface of button mushrooms, Brussels sprouts and cauliflower. Czech J. Food Sci. 2007, 25, 90–100. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wang, A.; Chen, J.; Tian, G. Appropriate water blanching treatment to keep quality and color of Agaricus bisporus slices. Trans. Chin. Soc. Agric. Eng. 2014, 30, 268–273. [Google Scholar]
- Reis, F.R. (Ed.) New Perspectives on Food Blanching; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–48. ISBN 3319839896. [Google Scholar]
- Murphy, R.Y.; Johnson, E.R.; Duncan, L.K.; Clausen, E.C.; Davis, M.D.; March, J.A. Heat Transfer Properties, Moisture Loss, Product Yield, and Soluble Proteins in Chicken Breast Patties During Air Convection Cooking. Poult. Sci. 2001, 80, 508–514. [Google Scholar]
- Bernaś, E.; Jaworska, G. Effect of microwave blanching on the quality of frozen Agaricus bisporus. Food Sci. Technol. Int. 2015, 21, 245–255. [Google Scholar]
- Ranjan, S.; Dasgupta, N.; Walia, N.; Thara Chand, C.; Ramalingam, C. Microwave Blanching: An Emerging Trend in Food Engineering and its Effects on Capsicum annuum L. J. Food Process Eng. 2017, 40, e12411. [Google Scholar] [CrossRef]
- Kenesei, G.; Jónás, G.; Salamon, B.; Dalmadi, I. Thermograms of the combined High Hydrostatic Pressure and Sous-vide treated Longissimus dorsi of pork. J. Phys. Conf. Ser. 2017, 950, 042007. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar]
- Nketia, S.; Buckman, E.S.; Dzomeku, M.; Akonor, P.T. Effect of processing and storage on physical and texture qualities of oyster mushrooms canned in different media. Sci. Afr. 2020, 9, e00501. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [PubMed]
- Rhee, S.J.; Lee, J.-E.; Lee, C.-H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, H.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Mu, H. Effects of fermentation with different microbial species on the umami taste of Shiitake mushroom (Lentinus edodes). LWT-Food Sci. Technol. 2021, 141, 110889. [Google Scholar]
- Liu, Y.; Xie, X.; Ibrahim, S.A.; Khaskheli, S.G.; Yang, H.; Wang, Y.; Huang, W. Characterization of Lactobacillus pentosus as a starter culture for the fermentation of edible oyster mushrooms (Pleurotus spp.). LWT-Food Sci. Technol. 2016, 68, 21–26. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Vegetable and Fruit Fermentation by Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 216–230. [Google Scholar]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Boylu, M.; Hitka, G.; Kenesei, G. Sausage Quality during Storage under the Partial Substitution of Meat with Fermented Oyster Mushrooms. Foods 2024, 13, 2115. [Google Scholar] [CrossRef]
- Boylu, M.; Hitka, G.; Kenesei, G. Effect of alternative pre-treatments and fermentation on quality characteristics of oyster mushrooms. Prog. Agric. Eng. Sci. 2023, 19 (Suppl. S1), 35–45. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Kowalczyk, D.; Stadnik, J. Content of Biogenic Amines and Physical Properties of Lacto-Fermented Button Mushrooms. Appl. Sci. 2022, 12, 8957. [Google Scholar] [CrossRef]

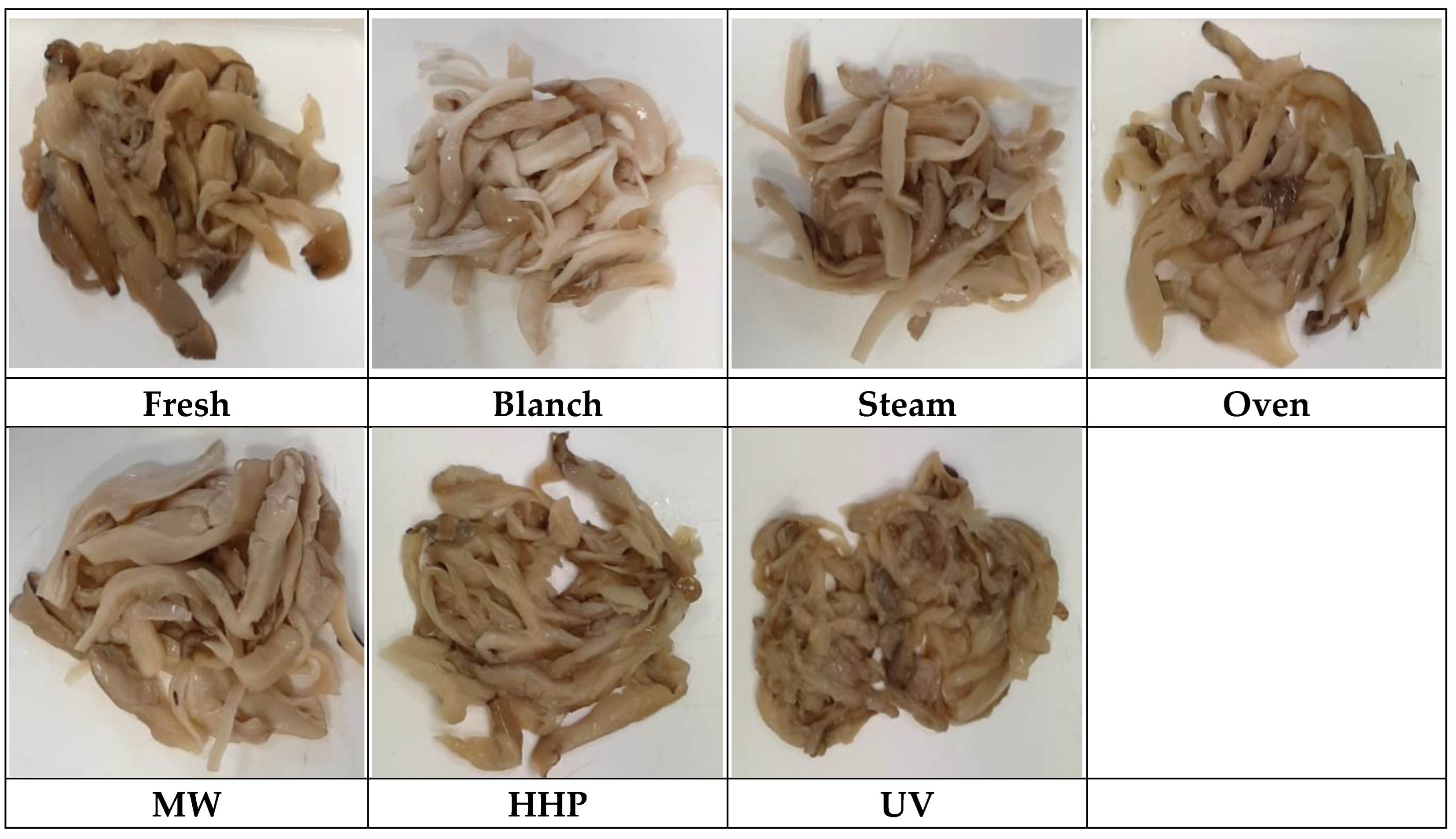

| Proteinogenic and Free Amino Acids | Biogenic Amines | |
|---|---|---|
| Cation exchange column type | IONEX OSTION LCP5020 | OSTION LG ANB |
| Column size | 200 mm × 3.7 mm | 60 mm × 3.7 mm |
| Column temperature | 55 °C and 65 °C | 55 °C and 65 °C |
| Reactor temperature | 121 °C | 121 °C |
| Eluent flow rate | 0.30 cm3/min | 0.30 cm3/min |
| Ninhydrin flow rate | 0.25 cm3/min | 0.25 cm3/min |
| Sample volume injected | 100 µL | 100 µL |
| Detection wavelength | 440 nm (proline) and 570 nm | 570 nm |
| Buffers | Li-citrate buffers | Na/K-citrate buffers |
| Analysis time | 200 min | 97 min |
| Detection limit | 0.5 µmol/dm3 | 0.5 µmol/dm3 |
| Standard deviation | 2–5% | 2–5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenesei, G.; Boylu-Kovács, M.; Gashi, A.; Mednyánszky, Z.; Takács, K.; Simon-Sarkadi, L. Effect of Thermal and Non-Thermal Pretreatments and Fermentation on the Amino Acid and Biogenic Amine Content of Oyster Mushroom. Appl. Sci. 2025, 15, 3509. https://doi.org/10.3390/app15073509
Kenesei G, Boylu-Kovács M, Gashi A, Mednyánszky Z, Takács K, Simon-Sarkadi L. Effect of Thermal and Non-Thermal Pretreatments and Fermentation on the Amino Acid and Biogenic Amine Content of Oyster Mushroom. Applied Sciences. 2025; 15(7):3509. https://doi.org/10.3390/app15073509
Chicago/Turabian StyleKenesei, György, Meltem Boylu-Kovács, Albert Gashi, Zsuzsanna Mednyánszky, Krisztina Takács, and Livia Simon-Sarkadi. 2025. "Effect of Thermal and Non-Thermal Pretreatments and Fermentation on the Amino Acid and Biogenic Amine Content of Oyster Mushroom" Applied Sciences 15, no. 7: 3509. https://doi.org/10.3390/app15073509
APA StyleKenesei, G., Boylu-Kovács, M., Gashi, A., Mednyánszky, Z., Takács, K., & Simon-Sarkadi, L. (2025). Effect of Thermal and Non-Thermal Pretreatments and Fermentation on the Amino Acid and Biogenic Amine Content of Oyster Mushroom. Applied Sciences, 15(7), 3509. https://doi.org/10.3390/app15073509









