Abstract
The need to reduce greenhouse gas emissions and guarantee a stable and reliable energy supply has resulted in an increase in the demand for sustainable energy storage solutions over the last decade. Rechargeable batteries with solid-state electrolytes (SSE) have become a focus area due to their potential for increased energy density, longer cycle life, and safety over conventional liquid electrolytic batteries. The superionic sodium conductor (NASICON) Na3Zr2Si2PO12 has gained a lot of attention among ESS because of its exceptional electrochemical properties, which make it a promising candidate for solid-state sodium-ion batteries. NASICON’s open frame structure makes it possible to transport sodium ions efficiently even at room temperature, while its wide electrochemical window enables high-voltage operation and reduces side reactions, resulting in safer battery performance. Furthermore, NASICON is more compatible with sodium ion systems, can help with electrode interface issues, and is simple to process. The characteristics of NASICON make it a highly desirable and vital material for solid-state sodium-ion batteries. The aim of this study is to prepare and characterize ceramic membranes that contain Na3.06Zr2Si2PO12 and Na3.18Zr2Si2PO12, and measure their stability in seawater batteries that serve as solid electrolytes. The surface analysis revealed that the Na3.06Zr2Si2PO12 powder has a specific surface area of 7.17 m2 g−1, which is more than the Na3.18Zr2Si2PO12 powder’s 6.61 m2 g−1. During measurement, the NASICON samples showed ionic conductivities of 8.5 × 10−5 and 6.19 × 10−4 S cm−1. Using platinum/carbon (Pt/C) as a catalyst and seawater as a source of cathodes with sodium ions (Na+), batteries were charged and discharged using different current values (50 and 100 µA) for testing. In an electrochemical cell, a battery with a NASICON membrane and Pt/C catalysts with 0.00033 g platinum content was used to assess reproducibility at a constant current of 2 h. After 100 h of operation, charging and discharging voltage efficiency was 71% (50/100 µA) and 83.5% (100 µA). The electric power level is observed to increase with the number of operating cycles.
1. Introduction
The major contribution to climate change due to carbon emissions from fossil fuels necessitates the development of sustainable and clean energy sources and the decarbonization of existing ones [1,2,3]. Significant environmental degradation is the result of global dependence on non-renewable fossil fuels. To address this pressing global energy problem, a transformative strategy is necessary. Despite their benefits, renewable energy sources are restricted by natural constraints. Therefore, a solution for energy storage that is characterized by simplicity, efficiency, resource abundance, and environmental friendliness is crucial [4]. The grid’s flexibility and efficiency are enhanced by energy storage through the provision of rapid response and balancing services, which ultimately improves its overall stability and reliability. Furthermore, these technologies are crucial in reducing greenhouse gas emissions by enabling the use of renewable energy sources and optimizing energy use, thereby assisting in global efforts to combat climate change [1,5,6]. Using rechargeable batteries is a cost-effective method of storing electricity, resulting in a significant decrease in global carbon emissions and a delay in the onset of an energy crisis [7,8]. Energy storage is a process that stores energy produced at one time for later use. Energy storage technologies that can store electricity, thermal energy, or mechanical energy in different forms include batteries, pumped hydro storage, compressed air energy storage, flywheels, and thermal energy storage systems [1]. The development of new materials and technologies, along with constant improvement and creative redesign, has been intensified due to the global shift toward sustainability. In this context, electrochemical energy storage (EES) is crucial, from mobile computing and communication activation to large-scale intermittent energy storage [9]. The popularity of renewable energy sources is leading to the need for high-performance and sustainable electrochemical energy storage and conversion systems, such as lithium-ion batteries (LIBs). The rising demand for LIBs in commerce, as well as the limited availability and uneven geographic distribution of lithium resources, have led to a steady increase in raw material prices [4,8]. Solid sodium-ion batteries, which have high energy density and good safety, are viewed as promising candidates for the next generation of energy storage systems [10]. However, the considerable interfacial resistance and dendrite growth between sodium electrolytes and solids, especially at high current densities, severely limits their development [11,12].
The success of alkali-ion intercalation in solid materials was the basis for the development of modern Na-ion batteries, which is attributed to initial research. In 1976, it was noteworthy to publish the first papers on Na-ion and Li-ion intercalation in successive months. Shacklette and his colleagues demonstrated a Na-ion battery that had specific components for the cathode, electrolyte, and anode in 1987. Shishikura et al. exhibited remarkable cycle stability in their system by filing a patent for an alternative battery design. Between 1990 and 2000, research on sodium-ion batteries declined as lithium-ion batteries dominated the market after Sony’s commercialization [4].
The competitiveness of sodium-ion batteries (SIBs) over lithium-ion batteries (LIBs) can be attributed to the fact that sodium is abundantly available and cheap for fixed land-based power applications and grid stabilization [4,8,11]. The energy storage requirements of the metal sodium battery can be met with the high specific capacity (1165 mAh g−1) and a relatively low electrochemical potential (−2.7 V) of Na metal. Low thermal stability, flammability, and electrolyte leakage are common issues experienced by conventional SIBs that are derived from organic liquid electrolytes. To solve this problem, mechanically stable solid-state electrolytes (SSEs) can replace traditional liquid electrolytes. Recent research has revealed that solid-state sodium-ion batteries can utilize non-combustible solid-state electrolytes to achieve a higher energy density and enhance safety. The group of inorganic solid electrolytes (ISEs) comprises Na3Zr2Si2PO12, sulfide Na3SbS4 and halide Na2.25Y0.25Zr0.75Cl6 [11].
Efforts to improve the performance of electrode materials are essential due to the impact of low temperatures (LT) performance on SIBs [4]. Although liquid electrolytes are widely used because of their low cost, high ionic conductivity, and ease of use, solid electrolytes are often preferred because they are nontoxic, nonflammable, noncorrosive, nonvolatile, and thermally stable over a wide temperature range. One of the most promising candidates for sodium storage electrodes in SIB materials is electrode materials that are based on sodium superionic conductors (NASICON), which have significant structural stability and high ionic conductivity [2,5,13]. Among all the inorganic fillers, active NASICON material (such as Na3Zr2Si2PO12, Na3.4Zr1.9Zn0.1 Si2.2P0.8O12) due to its high ionic conductivity and excellent chemical stability and attention [4]. NASICON-type materials with the general formula AxM2 (XO4)3 possess interesting properties such as high thermal and chemical stability, high ionic conductivity, and low thermal expansion. Li-ion and Na-ion batteries can use NASICON-type materials as cathodes, anodes, and electrolytes, depending on their A cations and M transition metals. Solid state Na-ion batteries use Na1+xZr2P3−xSixO12 (0 ≤ x ≤ 3), (NZSP), which has a difficult to reduce from its stable oxidation state + 4, as their electrolyte. The R3C (Rhombohedral) space group is maintained for all components in NASICON materials, except for those in the range 1.8 ≤ x ≤ 2.2 that have monoclinic structures (C2/c groups). Monoclinic Na1+xZr2P3−xSixO12 (1.8 ≤ x ≤ 2.2), especially when x = 2, has the largest ionic conductivity, which can reach 2.0 × 10−1 S cm−1 at 300 °C and 6.7 × 10−4 S cm −1 at room temperature (RT). Modified Na3Zr2Si2PO12 with sintering additives has a conductivity of 1.63 × 10−3 S cm−1 at room temperature. The versatility and robustness of sodium-ion battery systems are due to NASICON ceramics’ wide electrochemical stability window, which can be compatible with different electrode materials and operating voltages [1]. Additionally, NASICON materials are resistant to humidity [11].
Advanced battery technologies can take advantage of the rising demand for energy storage solutions, fueled by the increasing adoption of electric vehicles, renewable energy integration, and grid modernization efforts. Solid-state sodium batteries are designed to address critical issues in energy storage technology by taking advantage of the abundant sodium and its potential advantages in cost, safety, and performance as an alternative to lithium. Solid-state sodium batteries can be more recyclable than lithium-ion batteries, resulting in better durability [14].
The use of rechargeable seawater batteries (SWBs) is attracting attention as a potential solution for marine environment batteries. As the cathode material, SWBs utilize Na+ ions that are dissolved in seawater or saltwater. Seawater is being investigated for its use as a cathode material or electrolyte. Flooding is avoided because these SWBs operate while submerged in seawater. The safety of the operation is maintained by the absence of hazardous components and an explosion risk. The development of SWBs has occurred between coin cells and prismatic cells. Despite their significant contributions to the development of materials and platforms suitable for operating in seawater, the unit cell performance only reached 2.6 V and 2 W, resulting in limited applications. SWB modules with both series and parallel connections necessitate research for applications with high operating voltage and high-power driving conditions [15].
The storage of electrochemical energy can be achieved by using conventional seawater batteries that combine a sodiation/desodiation anode and an electrolysis cathode. The design of a seawater battery consists of an anode in an organic electrolyte and a seawater cathode with a current collector, which can be utilized for energy storage and water desalination. The optimal anode compartment for a high-performance seawater battery must include electrolyte and electrode material. The positive electrode was made up of an organic electrolyte and an electrode material used for the anode of the cell. Anode materials and their associated anode compartments must meet multiple criteria in addition to their reversible ion absorption ability. Elemental sodium is often used as an electrode material due to its abundant supply and theoretical capacity of 1166 mAh per kilogram. In the end, lightweight metals or alloy materials such as magnesium or aluminum offer the possibility of a high theoretical specific capacity (Mg: 2200 mAh g−1, Al: 2980 mAh g−1) and can even serve as electrodes. Various carbon materials are being utilized to explore the cathode side. Carbon felt has demonstrated mechanical stability, high flexibility, and conductivity. Carbon sponge electrolytic is another electrolyte in the cathode that is openly surrounded by a highly interconnected and macroporous framework. Low discharge voltage gaps, high voltage efficiency, high power density, and long-term cycling stability were achieved thanks to the bifunctional electrocatalytic OER and ORR activities. In another study, the performance of an activated carbon cloth as a current collector on the cathode side was assessed against that of a reduced carbon felt surface. The carbon cloth electrode’s electrical formation of double layers is aided by the electrolytic OER/ORR activity [16].
This study aims to produce and examine ceramic membranes that contain Na3.06Zr2Si2PO12 and Na3.18Zr2Si2PO12. Furthermore, we will examine their resilience in solid electrolytes used in seawater batteries. Our aim is to conduct electrochemical testing on NASICON ceramic membrane batteries in an electrochemical cell with seawater as the aqueous electrolyte.
2. Materials and Methods
2.1. Making the Na3.06Zr2Si2PO12 (NAS-2%) and Na3.18Zr2Si2PO12 (NAS-6%) Membranes
The creation of the NAS-2% and NAS-6% membranes involved a solid-state reaction process. This involved mixing Na2CO3 (Alfa Aesar, Haverhill, MA, USA), SiO2 (Umicore, Brussels, Belgium), ZrO2 (Sigma-Aldrich, St. Louis, MO, USA), and NH4H2PO4 (Scharlab, Barcelona, Spania) in the stoichiometric ratio of 3.06:2.0:2.0:1, and 3.18:2.0:2.0:1, respectively. The raw materials were mixed for 12 h using a ZrO2 container, with ZrO2 balls, 10 mm (4 h) diameter, 5 mm diameter (4 h) and 3 mm diameter (4 h), at a rotation speed of 300 rpm. After being obtained, the mixture was dried at 80 °C. For 4 h, the powder was preheated at 600 °C and then calcinated at 1100 °C for another 4 h. The calcined powder was ground for 4 h using 5 mm diameter ZrO2 balls while grinding at 300 rpm, and the mixture was dried at 80 °C. The NASICON powder was pressed into a hydraulic press by cold isostatic pressing at various pressing forces (between 3300 and 4200 KgF) for 5 min to form pellets with a diameter of about 20 mm and a thickness of 1.3 mm. To avoid contamination, the pellets and their powder were placed in an alumina crucible and then heated in the furnace before sintering. To remove volatile species, the fabricated pellets were sintered in a tube furnace at a heating rate of 5 °C per minute for 10 h at different temperatures (1150 °C, 1175 °C, 1200 °C) and in nitrogen gas. Laboratory pellets were compared with a pre-established commercial NASICON membrane (NZSP, 4TOONE Ltd., Ulsan, Republic of Korea) [17].
2.2. Methods and Equipment for Characterization of Membranes
The Autosorb IQ analyzer (Quantachrome, Boynton Beach, FL, USA) was utilized to determine the exact surface area by employing the BET (Brunauer-Emett-Teller) method. Nitrogen was dissolving at 77 K, and the samples were degassed at 115 °C for 4–6 h prior to analysis. A study was conducted to evaluate the distribution of the pores using the Barrett-Joyner-Halenda (BJH) method and the desorption isotherm equations. The NASICON samples that were pre-sintered at 1100 °C were examined for their morphology with the FESEM VP variable pressure of Carl Zeiss (Jena, Germany). Electrochemical impedance spectroscopy (potentiostatic) was used to measure conductivity using Versa STAT 3F (Chicago, IL, USA) equipment. The interfaces between the electrodes and the electrolyte can be identified through the difference in amplitude and phase angle. The Alpha-A modular measurement system from NOVOCONTROL (Montbauer, Germany) was used for broadband dielectric spectroscopy (BDS) to measure the frequency of the dielectric atoms; it has a temperature controller from Quattro Cryosystem that has a stability of greater than 0.1 K. Isothermal scanning was carried out on the samples at a frequency range of 10−1 to 107 Hz by using an AC signal with an amplitude of 0.1 V.
2.3. Getting Batteries with a Ceramic Membrane as Their Solid Electrolyte
The battery was assembled in a glove box with a highly purified Ar atmosphere, with low oxygen and water levels at 1.0 ppm. The solid electrolyte was produced by using a NASICON selective membrane that has a thickness of about 1.3 mm and a diameter of about 20 mm. The anode compartment is held by the round, perforated top cover, which is coated with this membrane. An additional element was added to the process, which involved the addition of sodium biphenyl (Na-BP) in diethylene glycol dimethyl ether (DEGDME), an organic electrolyte 1 M trifluoromethansulfonate sodium (NaCF3SO3, Sigma-Aldrich Co., St. Louis, MO, USA), in tetraethylene glycol (TEGDME, Sigma-Aldrich Co., St. Louis, MO, USA) and a stainless steel mesh of 0.122 cm. The separation of Na from the NASICON membrane was performed using a glass membrane with a thickness of 16 mm and a diameter of about 19 mm. After inserting the stainless-steel spacer, which measures 19 mm in diameter, a metal spring was used to push the compartment tightly into the SWB. Using a metal spring, the compartment inside the SWB was secured. The battery was secured using an adjustable assembly/seal mechanism to avoid organic electrolyte leakage and water infiltration (see Figure 1).
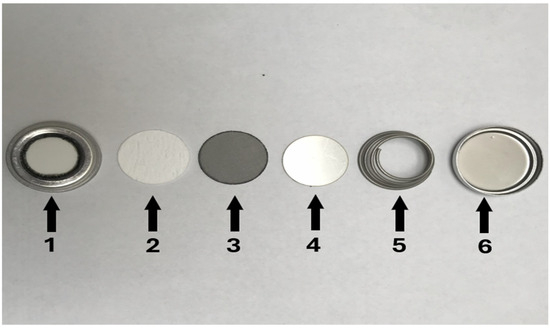
Figure 1.
The NASICON ceramic membrane battery has a top that contains the NASICON membrane (1), glass paper membrane (2), stainless steel mesh (3), stainless steel separator (4), compression spring (5), and the bottom (6).
2.4. The Process of Preparing Both the Catalytic Mixture and the Cathode
The catalyst mixture for ink was prepared by dispensing 1.3 mL of binder solution with 0.025 g of 40% Pt/C catalyst (Thermo Scientific, Ward Hill, MA, USA). The binder solution contained a dispersion of Nafion D-521 with a water content of 5%, distilled water of 0.25 mL, and 99% pure 2-propanol. Two hours were spent sonicating the mixture. Preparation of the cathode involved spraying 50 µL of catalytic ink onto a carbon felt electrode and 0.00033 g Pt. After adding catalyst ink to the carbon felt, it was placed in an oven at 80 °C for three hours to dry.
3. Results
3.1. Analyzing the Structure of NASICON Membranes (2% and 6% Excess Na)
The properties of particulate matter can be determined with the help of particle size analysis and its distribution. The conductivity of crystalline electrolytes is influenced by particle size, and generally, reducing particle size leads to better ionic conductivity. A rigorous and very energetic ball grinding procedure is used to obtain a significantly finer degree of particles after starting with initial macro precursors (0.0055 mm for ZrO2 and 0.2 mm to 0.5 mm for SiO2). The precursor mixture is preserved after sintering with an average particle size of ~1.0 µm in the small micrometric range after ball milling. Table 1 shows that 10% of particles are below 1000 nm for NASICON powder with an excess of 2% and 6% respectively (523.8 nm and 910.5 nm respectively), while for NASICON membranes sintered at 1150 °C the particles have a size between 1056.8 (NAS-2%) and 1517.0 nm (NAS-6%). Of NASICON membranes sintered at 1175 °C the particles have a size between 1161.7 nm (NAS-2%) and 679.5 nm (NAS-6%); in time for sintered NASICON membranes at 1200 °C the particles have a size between 1010.8 nm (NAS-2%) and 833.6 nm (NAS-6%). From the data obtained, it is noted that during sintering, there is a change in the texture of the composition of NASICON as a direct consequence of the high sintering temperature. The higher the sintering temperature, a decrease in the particle size is observed.

Table 1.
Particle size distribution for NASICON powder and membranes with excess of 2% and 6% Na.
The microstructural and elemental investigations were carried out using a field emission scanning electron microscope with auto electronic emission at variable pressure (field emission scanning electron microscope variable pressure—FESEM VP) from the brand Carl Zeiss. The microscope is equipped with an EDX (energy dispersive X-ray spectroscopy) system that allows the quantitative and qualitative analysis of all chemical elements up to B (Z = 5). Figure 2 presents the surface images of the crack in the sample powder of Na3.06Zr2Si2PO12 and Na3.18Zr2Si2PO12. As shown in Figure 2, the micrographs show the close arrangement and packaging of NASICON particles with smoothed granules and diameters in the micron range to the submicron, as well as the appearance of network mesopores, through the unoccupied spaces between the particle assemblies. In order to investigate the chemical composition of the NASICON samples, energy spectrum tests were performed on samples of Na3.06Zr2Si2PO12 and Na3.18Zr2Si2PO12 that had been calcined at 1100 °C. For the two NASICON 2% and 6% powders, the chemical composition is composed of the elements Na, Zr, Si, P and O, and the calculated atomic ratios of Na, Si and O were 13.54:9.96:59.22 for Na3.06Zr2Si2PO12 and 14.24:9.78:58.99 for Na3.18Zr2Si2PO12, which are close to the theoretical atomic ratio (15:10:60). The homogeneous distribution of elements was observed in EDX mappings through EDX analysis. The content of Zr and P for the two samples is approximately constant, based on EDS analysis. It has been established that the total molar ratios of Zr and P are almost identical to the total theoretical molar ratios (15%). Studies have shown that during conventional sintering (at about 1000 °C), there is a saturated vapor pressure of the elements Na and P above the sample that prevents further vaporization and also maintains the stability of the solid electrolyte NZSP [18].
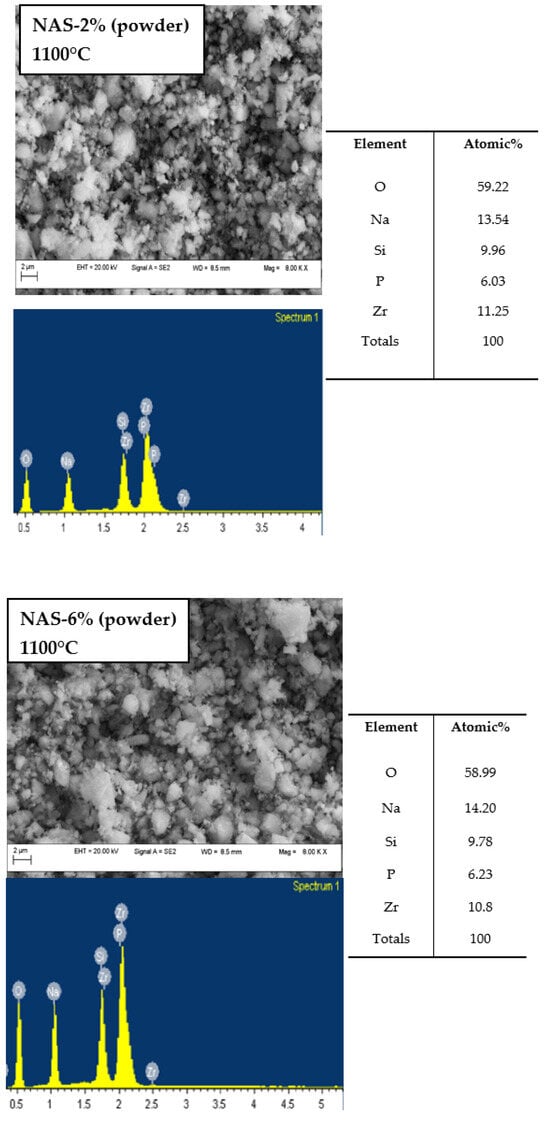
Figure 2.
EDX examination of powders NAS-2% and NAS-6% that were calcined at 1100 °C (Atomic %).
The membrane samples were examined using BET and BJH analysis to examine their surface and micropores. Figure 3 provides access to the results. To determine the specific surface area, one must use the static (volumetric) manometric method to determine the amount of gas N2 that has adsorbed or de-sorbed from the surface of porous solids. Both adsorption and desorption isotherms show a greater presence of a mesoporous structure in the hysteresis loop. Hysteresis arises due to differences in interface geometry between adsorption and desorption. The NASICON 2% powder indicated an area of 7.17 m2 g−1 and a volume of pores of 0.024 cm3 g−1, while the powder with an excess of 6% indicated an area of 6.61 m2 g−1 and a volume of pores of 0.093 cm3 g−1. The measurement of nitrogen adsorption/desorption isotherms was carried out at 77 K. According to the obtained curves; it was found that there is a hysteresis loop in adsorption-desorption isotherms, which implies that there is a mesoporous structure mainly present.
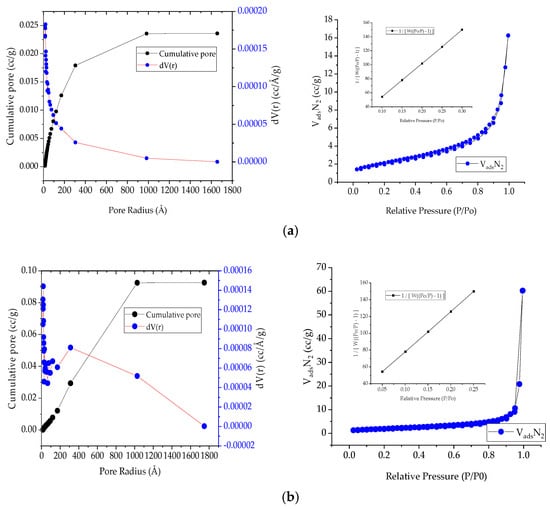
Figure 3.
BET adsorption and desorption isotherms for NASICON powder: (a) 2% excess Na; (b) 6% excess Na.
3.2. Investigating the Electrical Conductivity of the NASICON Ceramic Membrane
Ionic conductivity is the capacity for ions to move through the electrolyte. The ionic conductivity is increased by the faster the ionic movement. The importance of ion conductivity cannot be overstated because, in a rechargeable energy storage device, ions move from the anode to the cathode during discharge and vice versa during charging due to their movement from the anode to the cathode. In order to store and release energy, these devices are dependent on the movement of ions between the electrodes through the electrolyte. As the ions move through the electrolyte at a faster pace, the device improves its energy storage and release efficiency. The power and energy density of the device can be increased by fast charging and discharging due to high ionic conductivity. As a result of lower ionic conductivity, the electrodes may decay over time due to the slow movement of ions. Due to this degradation, the device’s service life can be reduced, and performance can be decreased. By increasing ion conduction, the device can increase its power and high energy density, which allows it to store more energy and deliver it more quickly [15].
Different electrolytes have chemical and physical properties that can influence the conduction mechanism of ions, including composition, concentration, and structure. In a powerful electrolytic solution, it is mainly through a migration process, in which the ions move through the electrolyte in response to an electric field, is, while the migraines of ions are facilitated by solvent molecules, which help stabilize the ions and reduce interactions between the two. The mechanism of conduction of ions in ceramic, composite, and crystalline electrolytes is mainly through a process mediated by the vacancy, in which ions move through the network by exchanging places with vacancies from the crystal structure. Defects in the crystalline structure, such as vacancies or dislocations, facilitate the process mediated by the vacancy and allow ions to move through the lattice [19].
Figure 4 and Figure 5 show Nyquist diagrams for NAS-2% ceramic membranes and NAS-6%, respectively, where the real and imaginary components of the measured conductivity are represented in the broadband range from 10−1 to 106 Hz at room temperature. Electrochemical impedance spectroscopy (EIS) (potentiostatic) was used to measure conductivity using Versa STAT 3F (UK) equipment. Information about the interfaces between the electrodes and the electrolyte is provided by the difference in amplitude and phase angle as part of an electrochemical experiment.
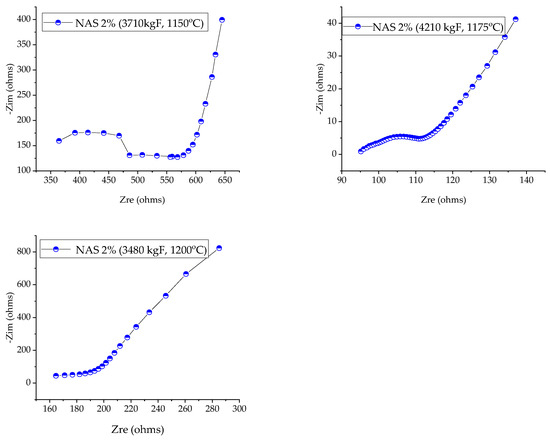
Figure 4.
Nyquist diagrams for NAS-2% ceramic membranes prepared at different sintering temperatures (1150, 1175 and 1200 °C), analyzed at room temperature (RT).
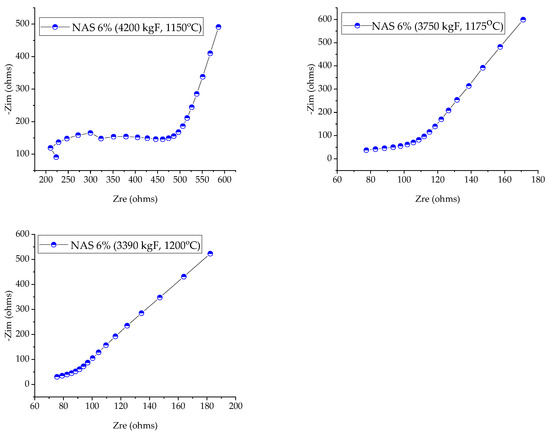
Figure 5.
Nyquist diagrams for NAS-6% ceramic membranes prepared at different sintering temperatures (1150, 1175 and 1200 °C), analyzed at room temperature (RT).
The Nyquist diagram was utilized to represent EIS data, which shows the real and imaginary parts of the impedance at various frequencies. Two semicircles were present in the high-frequency region, while an oblique line was present in the low-frequency region in Nyquist plots. It is demonstrated that the Na+ transport is present in the electrolyte and that blocking ions at the electrode–electrode–electrolyte interface has an impact.
Information on molecular relaxation at different temperatures and the impact of variable electric fields (variable frequencies) is obtained through dielectric characterization. Specific parameters, such as relative permittivity (also known as the dielectric constant), are recorded in this regard. When the sample is swept repeatedly over a wide range (10−1–107 Hz) at different temperatures, the two components of reality (ε′) and imaginary (ε″) are observed.
It is possible to express the total dielectric constant (εr) as a function of the capacitance of a capacitor (Equation (1)) or as a multi-component parameter consisting of a real and imaginary component (ε′r and ε″r) (Equations (2a) and (2b)):
Cp is the capacitance taken on the parallel faces, and C0 is the capacitance of the vacuum, where C0 = ε0A/d, and where ε0 is the vacuum’s permeability, the capacitor’s transverse surface area is defined by A between the plates where the dielectric material is located, thickness of the dielectric layer can be measured by d;ω = 2πf is the angular frequency of the applied field, and δ = 90 − θ, where θ is the phase angle.
In Figure 6 and Figure 7, it can be seen how the dielectric constant (DC) changes with the frequency for samples analyzed at room temperature. It has been noted that the dielectric constant values decrease with an increase in frequency. Imperfections, such as vacancies or concentrated electronic fields, can lead to dielectric relaxation in materials. The increase in dielectric constants in low frequencies can be explained by the mechanisms responsible for spatial load polarization. Load carriers follow the applied field at low frequencies according to this model, which leads to increased polarization and, therefore, high permittivity values. On the other hand, at high frequencies, load carriers cannot track the applied field because the period is too short, and the field changes direction before the tasks align with the direction of the field. Thus, the electrical constant decreases, as does the net trend.
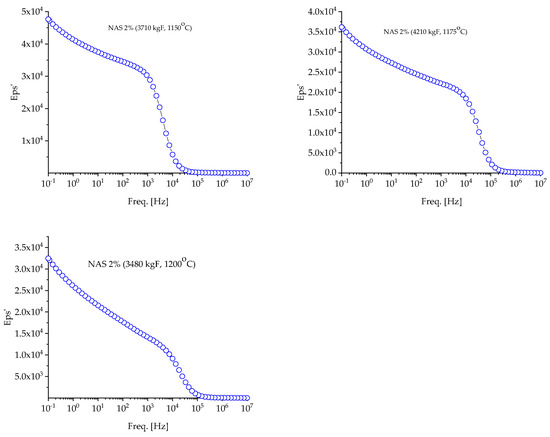
Figure 6.
Evolution of the dielectric constant of NASICON 2% samples with frequency at room temperature (RT).
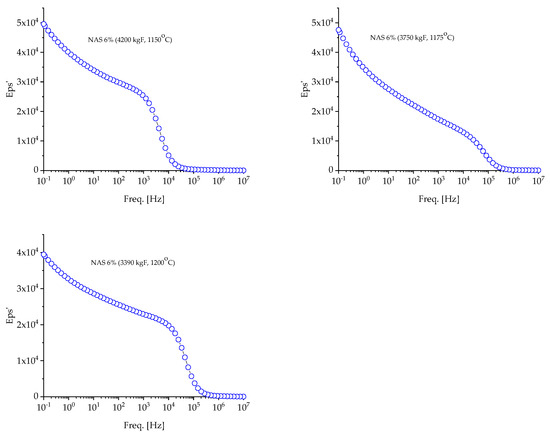
Figure 7.
Evolution of the dielectric constant of NASICON 6% samples with frequency at room temperature (RT).
The DC ion conductivities (σ) of the analyzed samples were calculated at room temperature after analyzing the data from wideband dielectric spectroscopy (Figure 8 and Figure 9). The conductivity values measured are compared with the ones obtained for commercial NASICON in a magnitude-wise comparison.
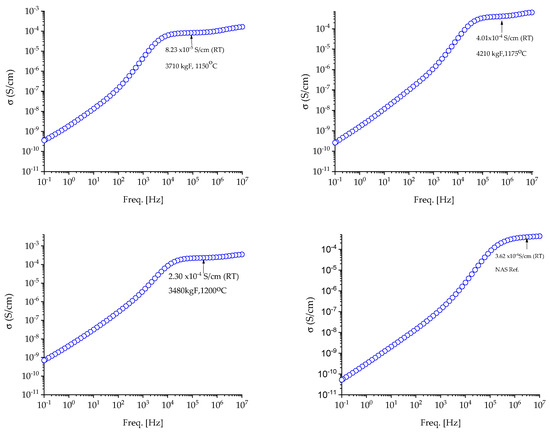
Figure 8.
Variation in conductivity of NASICON samples by 2% excess sodium and NAS Ref. at room temperature (RT).
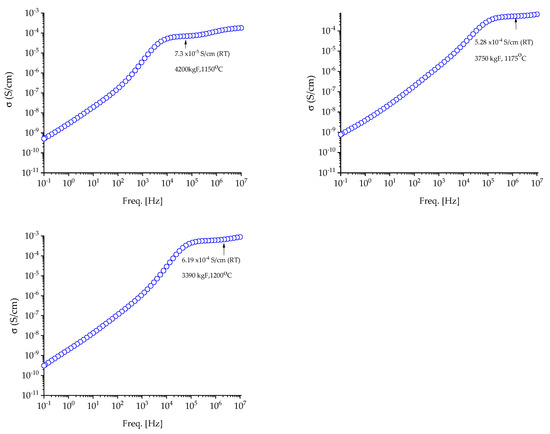
Figure 9.
Variation in conductivity of NASICON samples by 6% excess sodium at room temperature (RT).
The measured NASICON samples reached ionic conductivities ranging from 8.5 × 10−5 (1150 °C) to 4.0 × 10−4 S cm−1 (1175 °C) for NAS-2% samples and values ranging from 7.3 × 10−5 (1150 °C) to 6.19 × 10−4 S cm−1 (1200 °C) for NAS 6%. For comparison, a commercial NASICON membrane with an ionic conductivity of 3.62 × 10−4 S cm−1. Experimental data analysis revealed that NASICON samples sintered at 1175 °C and 1200 °C had higher ion conductivity values than commercial ones. It should be noted that the higher the sintering temperature, the greater the ionic conductivity of the membranes.
4. Discussion
4.1. The NASICON Principles Used in Seawater Electrolytic Processes
Just like Li+ in lithium-ion batteries and Pb2+ in lead-acid batteries, seawater can act as an energy carrier. NASICON is the crucial component of the rechargeable seawater battery (SWB) that selectively transfers Na+ from seawater, and it exemplifies this potential. SWBs are specifically designed for water, particularly saltwater environments, unlike many conventional batteries that are not. The battery structure has three electrodes: one positive electrode (seawater) and one negative electrode (NASICON). When the solid electrolyte is in direct contact with the seawater, Na+ can pass between the electrodes. To transfer Na+, a buffer layer is created with a non-aqueous electrolyte, while the negative electrode’s active material is Na metal. In comparison to other aqueous or underwater batteries, this battery has the advantage of heat control and explosion prevention while operating in seawater conditions.
NASICON-based seawater electrolytic technologies are based on the selective exclusion of the majority of seawater components, making it possible for only Na+ to pass through. The NASICON solid electrolyte, which acts as a ceramic separator, can help facilitate sustainable redox reactions in seawater, such as sodium ions reacting with water electrolysis and chloride oxidation. Using sodium as a charge carrier, NASICON-based seawater electrolytic technologies aim to store energy. The negative electrode undergoes various redox reactions, including Na deposition/dissolution, while the positive electrode is where oxygen evolution and reduction reactions typically occur. The utilization of abundant seawater and air as open cathodes enhances the economic feasibility of this system (Figure 10) [20].
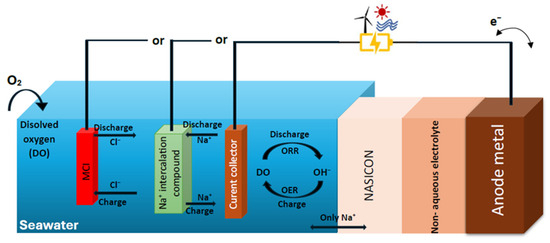
Figure 10.
Schematic of NASICON seawater battery.
Ceramic membranes are becoming more and more popular due to their exceptional mechanical stability and high selectivity, but their chemical stability in seawater is limited. NASICON is a ceramic material that conducts sodium ions and has been shown to be stable in seawater while showing high selectivity and conductivity of sodium ions. The most ionic conductivity among the materials of Na1+xZr2Si2P3−xO12, with a x = 2, was discovered by Hong and Goodenough in 1976 with Na3Zr2Si2PO12 (NZSP) [21,22,23,24,25,26].
The structure of Na1+xSixZr2P3−xO12 is composed of ZrO6 octahedra that connect at corners with PO4/SiO4 tetrahedra. Na+ ions are present in the interstitial zones of this framework. The ZrO6 octahedral structure has six oxygen molecules, while the oxygen corners of silicate or phosphate tetrahedral structures have oxygen molecules that connect them. Along the axis, repeating structures are formed in this manner. The main way to transport sodium ions in the 3D structure, which is located in the interstitial sites between octahedral and tetrahedral structures, is through migration through holes [27,28,29,30].
The ionic conductivity of NASICON at room temperature (RT) is comparable to that of solid organic electrolytes (10−6–10−3 Scm−1), with a range of 10−4–10−3 Scm−1. By controlling the Na+ sites (Na1, Na2, and Na3) and the barriers that ions traverse, the ion-conducting mechanism of the NASICON is governed. The NASICON formula unit has four locations, but two-thirds of them are currently in use. The interstitial site is filled with three oxygen atoms, which create a triangular barrier with a cross-sectional area of 5.223 Å [13].
Seawater batteries require NASICON to be stable in seawater. NASICON membranes have been found to exhibit virtually no increase in ionic conductivity after being immersed in seawater for 150 days, as evidenced by studies. The conductivity values of the blank sample and the sample submerged in seawater were from 3.8 × 10−4 to 3.1 × 10−4 S cm−1 [13]. During 1440 h of submersion in seawater, both the blank sample and the sample submerged in it did not exhibit significant changes in the phase structure of XRD peaks, as noted [3,17]. NASICON proved its chemical compatibility with seawater during extensive testing, demonstrating electrochemical stability for more than a year [13].
Redox reactions are initiated by Na+ (Equation (3)) at the negative electrode and seawater at the positive electrode during SWB’s operations. The positive electrode releases electrical energy through the use of ambient oxygen gases, which results in a reduction reaction called the oxygen reduction reaction (ORR) (Equation (4)). Chlorine evolution is outnumbered by the thermodynamically favored oxygen evolution reaction (OER) (Equation (5)), or the charging cycle could result in the formation of chlorine oxyacids (Equations (6)–(8)). Overall reactions are expressed as (Equation (9)) [20]:
The negative electrode is essential in the capacity of a SWB. Na metallic is the most promising material because it can provide high specific capacity (1.166 mAh g−1) and enable the construction of anode SWBs that can utilize more efficient seawater as an active component [20]. Reversibility of deposition/stripping and safety of cell operation is hindered by the higher reactiveness of Na compared to Li metal. To address these worries, it is necessary to implement an effective strategy that involves forming a stable passivation layer (SEI) on the surface and engineering the anodic components to ensure reversible behavior. The use of Na metal presents challenges, leading to the investigation of various alternatives for negative electrodes.
NASICON’s ability to effectively block electron transfer between the negative electrode and seawater prevents short circuits while incorporating anolyte into SWB, even with the electronic conductivity of the anolyte. Anolytes, such as sodium biphenyl (Na-BP in DEGDME), are represented by sodium biphenyl. Na-BP is proven to effectively prevent problems like gas evolution, sodium dendrite growth, and dead capacitance formation, which are common concerns with conventional electrodes and liquid electrolytes, according to research. The specific capacity of 17 mAh g−1 at 0.25 mA cm−2 was demonstrated, which provides a significant advantage by complementing the current electrode capacity [20,31,32].
Alkaline metals such as Na are capable of effectively storing hydrogen under normal temperature and pressure conditions, as they generate hydrogen gas when interacting with water. Without any interference from water splitting, NASICON can harvest Na metals from seawater. An electrochemical bridge is made between seawater (source Na+) and the electrode (Na metal) to achieve this, which gives Na+ a pathway and prevents water from passing through the electrode. Using a SWB platform, it is possible to create a hydrogen storage system based on NASICON. Na metal is formed during the charging process (Equation (10)) as Na+ moves from seawater to the negative electrode. During the charging process, electrodeposition occurs on the current collector at the negative electrode during the selective extraction of Na metal from seawater, as previously confirmed by previous research. The positive electrode may simultaneously experience different oxidation reactions, such as OER or Na deintercalation. The sodium that is deposited by the negative electrode is subject to oxidation and released into the water by NASICON during subsequent discharge.
As shown in Equation (9), hydrogen is stored and collected while charging, and when it is discharged, it is produced, and energy is recovered. The SWB is a good option for hydrogen storage as it has a remarkable volumetric capacity of 42 to 218 g L−1, even with standard atmospheric pressure (1 atm) and ambient temperature (25 °C). The volumetric energy density of hydrogen at ambient conditions is approximately 0.0824 kgm3, which means that this exceeds the inherently low volumetric energy density, which is approximately 0.01 MJ L−1 H2. Both methane and gasoline have a volumetric energy density of 0.04 MJ L−1 H2, which is much higher than hydrogen’s, which is a challenge to their commercial viability [20].
4.2. Testing the Performance of Seawater Batteries Containing the NASICON Membrane
At the beginning of our battery testing experiments containing the NASICON selective membrane, we conducted membrane stability tests (degradation) in seawater at different pH levels (5.1, 6.8 and 8.5) for 90 days. The NASICON membrane degradation over time was achieved with an excess of 6% with the best ionic conductivity (NAS 6-1200) through an immersion test in a concentration solution ~25 g NaCl L−1. Each membrane was submerged in 70 mL of NaCl solution and put in bottles with the lid to prevent it from penetrating the air. In addition to measuring the pH and ionic conductivity of the solution, X-ray diffraction (XRD) measurements were also made to see if changes in the chemical composition of the membrane take place over time. A Rigaku MiniFlex 600 X-Ray (Tokyo, Japonia) speaker was employed for performing XRD measurements, which utilized a CuK-a X-ray source with a wavelength of 1.541838°. A monochromator was used to scan from 10° to 60° at high resolution, with a step scan of 0.01° and a scan speed of 1.0° min−1, with a goal to reduce noise levels. Thus, XRD measurements were made on NASICON membranes immersed in NaCl solution of different pH levels: 5.1, 6.8 and 8.5, after 60 and 90 days. XRD spectra (Figure 11) were obtained through long scans and with the addition of a monochromator to suppress noise levels. The NASICON samples were converted into suspension using ethanol to allow deposition by evaporation and sedimentation. The spectra were smoothed and corrected according to the baseline. It was observed that there were no differences between the samples in terms of spectra—the apparent differences in intensity between the profiles being the result of scaling. It was found that the stability of the NASICON membrane is determined according to the pH of the NaCl solution and the sinking time. Thus, after 90 days, NaCl solutions had pH values of 7.65, 7.81 and 7.85, while the ion conductivity of the three solutions had values of 6.0 mS cm−1, 6.13 mS cm−1, respectively 6.35 mS cm−1 [33].
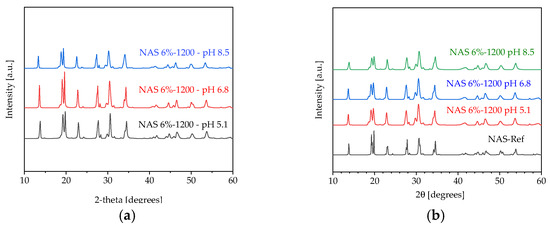
Figure 11.
XRD measurements on NAS 6%-1200 at different pH levels (a) 60 days; (b) 90 days.
The NASICON ceramic membranes with the highest conductivity were chosen for the battery’s fabrication. The high conductivity membrane we chose had excess sodium that was sintered at 1200 °C (6.19 × 10−4 S cm−1). The electrochemical cell with a capacity of 2.0 L was tested for reproducibility using a Pt/C catalyst with a concentration of platinum of 0.00033 g at different currents (0.05 and 0.1 mA). The loading/unloading tests were carried out with seawater with a concentration of ~25 g NaCl L−1 and CO2 flowing at a speed of 0.5 L min −1. The duration of a cycle was 2 h.
Chemical reactions [34,35] are used to describe the mechanism of CO2 dissolution (Equations (11) and (12)].
Both the anodic oxidation reaction (Equation (13)) and the cathodic hydrogen release reaction (Equation (14)) of metallic sodium are electrochemical reactions (Equation (15)).
Figure 12, Figure 13, Figure 14 and Figure 15 show the charge/discharge curves for potential, power, energy, and capacity, which have currents of 50 and 100 µA. The total operating time was approximately 100 h, as each cycle lasted for 2 h. From the potential curves, at a current of 50 µA per charge, it was observed that the discharge/charge potential was 2.18 V and 3.06 V with a potential difference of 0.88 V while for applying a current of 100 µA was 2.73 V, respectively, 3.27 V with a potential difference of 0.54 V. The voltage efficiency during charge and discharge was 71% (50/100 µA) and 83.5% (100/100 µA) after around 100 h of operation. It is found that at a constant value of the discharge current and at the constant temperature of the electrolyte, the discharge voltage decreases over time due to the increase of the potential, electromotive voltage, and resistance of the electrodes and electrolytes. When the value of the charge current is constant as temperature, the charge voltage increases over time due to the increase of the potential electromotive energy. The power of the battery when applying a current of 50 µA per discharge and 100 µA per charge was 0.153 mW and 0.2177 mW, respectively, while for a current of 100 µA per charge, the discharge and the load were 0.273 mW and 0.327 mW, respectively. The energy obtained for a current of 100 mA was 32.2 mWh per charge and 22.98 mWh per discharge at a current of 50 µA, while for a current of 100 µA, it was 45.98 mWh per charge loading and 37.82 mWh per unloading (Table 2). The applied current is responsible for increasing the accumulated energy, as shown by the analysis of energy curves. Using a current of 50/100 µA (discharge/charging) was obtained a capacity of 0.10 mAh per charge and 0.19 mAh per discharge while a current of 100 µA was obtained capacity of 0.033 mAh per charge and 0.04 mAh per discharge.
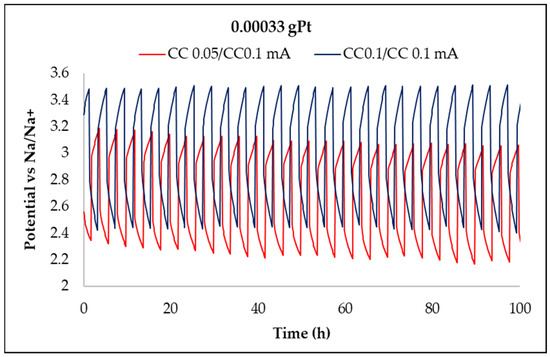
Figure 12.
Time evolution of the NASICON membrane battery potential at currents of 0.05 and 0.1 mA.
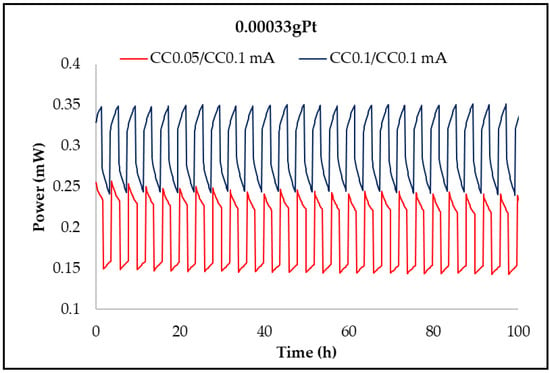
Figure 13.
Time evolution of the NASICON membrane battery power at currents of 0.05 and 0.1 mA.
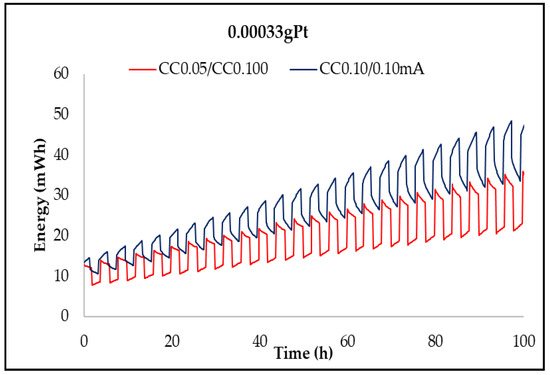
Figure 14.
Time evolution of the energy accumulated by the NASICON membrane battery at currents of 0.05 and 0.1 mA.
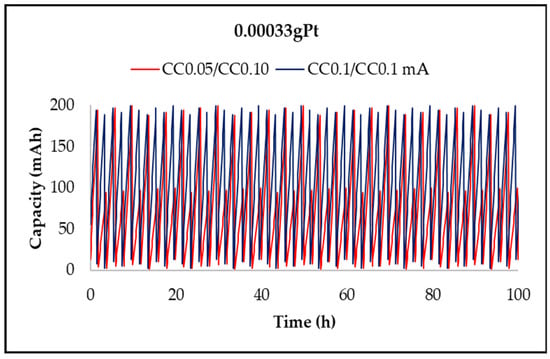
Figure 15.
Time evolution of the NASICON membrane battery capacity at currents of 0.05 and 0.1 mA.

Table 2.
Technical properties of the NASICON ceramic membrane battery after operation for about 100 h with Pt/C catalyst (0.00033 gPt).
5. Conclusions
This study aims to study the production and identification of ceramic membranes that contain Na3.06Zr2Si2PO12 and Na3.18Zr2Si2PO12, and to evaluate their stability in seawater batteries that are solid electrolytes. The specific surface area of 7.17 m2 g−1 for Na3.06Zr2Si2PO12 powder was discovered by the surface analysis to be greater than the Na3.18Zr2Si2PO12 powder’s 6.61 m2 g−1. The measurement revealed that the NASICON samples had ionic conductivities of 8.5 × 10−5 and 6.19 × 10−4 S cm−1. Charge and discharge batteries with different current values (50–100 µA) using Pt/C as a catalyst and seawater as an analyzer were the tasks of testing. A NASICON membrane and Pt/C catalysts with 0.00033 g of platinum content in an electrochemical cell were employed to evaluate reproducibility for 2 h, and the total operating time was approximately 100 h. Charge and discharge voltage efficiency was 71% (50/100 µA) and 83.5% (100 µA) after 100 h of operation. The battery’s power was 0.153 mW and 0.2177 mW when it was charged with a current of 100 µA and discharged with 50 µA, respectively. Downloading and loading were 0.273 mW and 0.327 mW, respectively, at a current of 100 µA on both download and load. The energy obtained for a current of 0.1 mA was 32.2 mWh per charge and 22.98 mWh per discharge at a current of 50 µA, while for a current of 100 µA, it was 45.98 mWh per charge loading and 37.82 mWh per unloading. The applied current causes the accumulated energy to increase, as evidenced by the analysis of energy curves. Using a current of 50/100 µA (discharge/charging) was obtained a capacity of 100 µAh per charge and 0.19 mAh per discharge while a current of 100 µA was obtained capacity of 0.033 mAh per charge and 0.04 mAh per discharge.
Author Contributions
Conceptualization, M.I. and M.B.; methodology, M.I. and M.B.; software, G.E.U.; validation, M.I., A.M. and A.O.; formal analysis, M.B. and S.E.B.; investigation, M.I., M.B., A.O., S.E.B. and G.E.U.; writing—original draft preparation, M.I., M.B. and A.O.; supervision, A.M.; project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
The Ministry of Research, Innovation, and Digitization of Romania provided financial support for this work through the projects PN 23 15 01 03 and PN 23 15 01 04, Contract No. 20N/2023, project no. 345/2021, 4C-ICSI project, SMIS 125119; project no. 308/2020, HyRo 2.0 project, SMIS 127318.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
No conflicts of interest have been declared by the authors.
References
- Randhawa, K.S. Advanced ceramics in energy storage applications: Batteries to hydrogen energy. J. Energy Storage 2024, 98, 113122. [Google Scholar] [CrossRef]
- Pandit, B.; Johansen, M.; Andersen, B.P.; Martínez-Cisneros, C.S.; Levenfeld, B.; Ravnsbæk, D.B.; Varez, A. All-solid-state sodium-ion batteries operating at room temperature based on NASICON-type NaTi2(PO4)3 cathode and ceramic NASICON solid electrolyte: A complete in situ synchrotron X-ray study. Chem. Eng. J. 2023, 472, 144509. [Google Scholar] [CrossRef]
- Cai, X.; Yue, Y.; Yi, Z.; Liu, J.; Sheng, Y.; Lu, Y. Challenges and industrial perspectives on the development of sodium ion batteries. Nano Energy 2024, 129, 110052. [Google Scholar] [CrossRef]
- Rostami, H.; Valio, J.; Suominen, P.; Tynjälä, P.; Lassi, U. Advancements in cathode technology, recycling strategies, and market dynamics: A comprehensive review of sodium ion batteries. Chem. Eng. J. 2024, 495, 153471. [Google Scholar] [CrossRef]
- Cheng, E.J.; Yang, T.; Liu, Y.; Chai, L.; Garcia-Mendez, R.; Kazyak, E.; Fu, Z.; Luo, G.; Chen, F.; Inada, R.; et al. Correlation between mechanical properties and ionic conductivity of polycrystalline sodium superionic conductors: A relative density-dominant relationship. Mater. Today Energy 2024, 44, 101644. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Zhang, J.; Zhang, J.; Wang, X.; Wen, L.; Tang, G.; Hu, M.; Wang, E.; Chen, W. Critical review on cathode electrolyte interphase towards stabilization for sodium-ion batteries. Nano Energy 2024, 128, 109814. [Google Scholar]
- Wang, C.; Wang, C.; Li, M.; Zhang, S.; Zhang, C.; Chou, S.; Mao, J.; Guo, Z. Design of thin solid-state electrolyte films for safe and energy-dense batteries. Mater. Today 2023, 72, 235–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, G.; Lin, J.; Zhu, J.; Pan, J.; Fang, G.; Liang, S.; Cao, X. A Multicationic-Substituted Configurational Entropy-Enabled NASICON Cathode for High-Power Sodium-Ion Batteries. Nano Energy 2024, 128, 109812. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, D.; Verma, D.K.; Parwati, K.; Ranjan, P.; Rai, R.; Krishnamoorthi, S.; Khan, R. Fundamental chemical and physical properties of electrolytes in energy storage devices: A review. J. Energy Storage 2024, 81, 110361. [Google Scholar] [CrossRef]
- Cai, S.; Meng, W.; Tian, H.; Luo, T.; Wang, L.; Li, M.; Luo, J.; Liu, S. Artificial porous heterogeneous interface for all-solid-state sodium ion battery. J. Colloid Interface Sci. 2022, 632, 179–185. [Google Scholar] [CrossRef]
- Ruan, Y.; Huang, X.; Lei, H.; Liu, Y.; Wang, J.; Sun, W.; Song, S. A flexible and free-standing composite solid electrolyte with a pomegranate-like structure for solid-state sodium-ion batteries. Solid State Ion. 2023, 403, 116406. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.-Y.; Zhang, N.; Wang, P.-F.; Liu, Z.-L.; Zhang, J.-H.; Shu, J.; Sun, Y.; Li, C.-S.; Yi, T.-F. A high-entropy strategy for stable structure of sodium ion batteries: From fundamentals to applications. Chem. Eng. J. 2024, 496, 153743. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, X.; Ao, H.; He, G.; Xiao, H.; Chen, Y. Advances in sodium-ion batteries at low-temperature: Challenges and strategies. J. Energy Chem. 2023, 90, 518–539. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, B.; Ren, D.; Yu, X.; Gao, Y.; Wu, Y.; Yang, Y.; Chen, Z.; Zhou, Z. Advancing solid-state sodium batteries: Status quo of sulfide-based solid electrolytes. Mater. Today 2024, 80, 429–449. [Google Scholar] [CrossRef]
- Kim, D. Development of Rechargeable Seawater Battery Module: Design of Cathode Current Collector to reduce Resistance Imbalance for Long Cycle Life. Ph.D. Dissertation, Ulsan National Institute of Science and Technology (UNIST), Ulsan, Republic of Korea, 2023. [Google Scholar]
- Arnold, S.; Wang, L.; Presser, V. Dual-use of seawater batteries for energy storage and water desalination. Small 2022, 18, e2107913. [Google Scholar] [CrossRef] [PubMed]
- Iordache, M.; Oubraham, A.; Petreanu, I.; Sisu, C.; Borta, S.; Capris, C.; Soare, A.; Marinoiu, A. NASICON Membrane with High Ionic Conductivity Synthesized by High-Temperature Solid-State Reaction. Materials 2024, 17, 823. [Google Scholar] [CrossRef]
- Niazmand, M.; Khakpour, Z.; Mortazavi, A. Electrochemical properties of nanostructure NASICON synthesized by chemical routes: A comparison between coprecipitation and sol-gel. J. Alloys Compd. 2019, 798, 311–319. [Google Scholar] [CrossRef]
- Miao, X.; Di, H.; Ge, X.; Zhao, D.; Wang, P.; Wang, R.; Wang, C.; Yin, L. AlF3-modified anode-electrolyte interface for effective Na dendrites restriction in NASICON-based solid-state electrolyte. Energy Storage Mater. 2020, 30, 170–178. [Google Scholar] [CrossRef]
- Suntharam, N.M.; Bashir, S.; Vengadaesvaran, B.; Abd Rahim, N.; Reasmyraj, S.; Ramesh, S.; Ramesh, K.; Prasankumar, T. Fundamentals and key components of sodium-ion batteries: Challenges and future perspectives. Mater. Today Chem. 2024, 42, 102350. [Google Scholar] [CrossRef]
- Fuentes, R.; Figueiredo, F.; Marques, F.; Franco, J. Influence of microstructure on the electrical properties of NASICON materials. Solid State Ion. 2001, 140, 173–179. [Google Scholar] [CrossRef]
- Jolley, A.G.; Cohn, G.; Hitz, G.T.; Wachsman, E.D. Improving the ionic conductivity of NASICON through aliovalent cation substitution of Na3Zr2Si2PO12. Ionics 2015, 21, 3031–3038. [Google Scholar] [CrossRef]
- Guin, M.; Tietz, F.; Guillon, O. New promising NASICON material as solid electrolyte for sodium-ion batteries: Correlation between composition, crystal structure and ionic conductivity of Na3+xSc2SixP3−xO12. Solid State Ion. 2016, 293, 18–26. [Google Scholar] [CrossRef]
- Park, H.; Jung, K.; Nezafati, M.; Kim, C.S.; Kang, B. Sodium ion diffusion in Nasicon (Na3Zr2Si2PO12) solid electro-lytes: Effects of excess sodium. ACS Appl. Mater. Interfaces 2016, 8, 27814–27824. [Google Scholar]
- Naqash, S.; Ma, Q.; Tietz, F.; Guillon, O. Na3Zr2(SiO4)2(PO4) prepared by a solution-assisted solid state reaction. Solid State Ion. 2017, 302, 83–91. [Google Scholar] [CrossRef]
- Jalalian-Khakshour, A.; Phillips, C.O.; Jackson, L.; Dunlop, T.O.; Margadonna, S.; Deganello, D. Solid-state synthesis of NASICON (Na3Zr2Si2PO12) using nanoparticle precursors for optimisation of ionic conductivity. J. Mater. Sci. 2019, 55, 2291–2302. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Sun, Z.; Ni, Q.; Jin, H.; Zhao, Y. Recent advance on NASICON electrolyte in solid-state sodium metal batteries. Energy Storage Mater. 2023, 56, 582–599. [Google Scholar] [CrossRef]
- Oueldna, N.; Sabi, N.; Ben Youcef, H. Correlation between physical properties and the electrochemical behavior in inorganic solid-state electrolytes for lithium and sodium batteries: A comprehensive review. J. Energy Storage 2024, 86, 111254. [Google Scholar] [CrossRef]
- Liu, G.; Yang, J.; Wu, J.; Peng, Z.; Yao, X. Inorganic Sodium Solid Electrolytes: Structure Design, Interface Engineering and Application. Adv. Mater. 2024, 36, e2311475. [Google Scholar] [CrossRef]
- Wang, D.; Moreno, S.; Gao, M.; Guo, J.; Xu, B.; Voigt, D.; Voit, B.; Appelhans, D. Protocells Capable of Generating a Cytoskeleton-Like Structure from Intracellular Membrane-Active Artificial Organelles. Adv. Funct. Mater. 2023, 33, 2306904. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Avdeev, M.; Wan, H.; Han, F.; Shen, L.; Zou, Z.; Shi, S.; Hu, Y.-S.; Wang, C.; et al. Ultrastable All-Solid-State Sodium Rechargeable Batteries. ACS Energy Lett. 2020, 5, 2835–2841. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Guo, Q.; Yu, X.; Cen, S.; Ma, H.; Chen, J.; Wang, D.; Mao, Z.; Dong, C. Atomically bonding Na anodes with metallized ceramic electrolytes by ultrasound welding for high-energy/power solid-state sodium metal batteries. Carbon Energy 2022, 5, e299. [Google Scholar] [CrossRef]
- Iordache, M.; Oubraham, A.; Borta, S.; Marinoiu, A. The Chemical Stability of NASICON Solid Electrolyte for Seawater Batteries. In Proceedings of the 2024 16th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Iasi, Romania, 27–28 June 2024; pp. 1–6. [Google Scholar]
- Iordache, M.; Oubraham, A.; Borta, S.; Ungureanu, G.; Marinoiu, A. Testing the Stability of NASICON Solid Elec-trolyte in Seawater Batteries. Energies 2024, 17, 5241. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Liu, S.; Chen, Z.; Yang, J.; Chen, Z.; Li, A.; Wen, Q.; Wang, L.; Qiu, S.; et al. Application of solid electrolytes in electrochemical reduction of CO2 or O2. Chem. Eng. J. 2024, 481, 148452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).