Correlation Analysis Between the Growth of Wild-Simulated Ginseng and the Soil Bacterial Community in the Central Region of South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting Wild-Simulated Ginseng and Soil Samples
2.2. Investigating Soil Chemistry in Wild-Simulated Ginseng Plantations
2.3. Collecting Wild-Simulated Ginseng Samples and Investigating Growth Characteristics
2.4. Soil Microbiome Analysis
2.5. Statistical and Correlation Analysis
3. Results and Discussion
3.1. Soil Chemistry of Wild-Simulated Ginseng Cultivation Sites
3.2. Growth Characteristics of 7- and 13-Year-Old Wild-Simulated Ginseng
3.3. Microbial Communities in the Soil of 7- and 13-Year-Old Wild-Simulated Ginseng Plantations
3.4. Correlation Between Soil Chemical Properties and Wild-Simulated Ginseng Growth Characteristics at the Growing Site
3.5. Correlation Between Wild-Simulated Ginseng Growth Characteristics and Soil Microbial Communities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potenza, M.A.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herval therapy: A brief history of Panax ginseng. J. Ginseng Res. 2022, 47, 359–365. [Google Scholar] [PubMed]
- Santacroce, L.; Bottalico, L.; Haxhirexha, K.; Topi, S.; Charitos, I.A. Prechemistry concepts and medical therapy among ancient physicians through the pre-socratic philosophers. Endoc. Metab. Immune Disord. Drug Targets 2020, 20, 1470–1477. [Google Scholar]
- Zhang, H.; Abid, S.; Ahn, J.C.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C.; Wang, Y. Characteristics of Panax ginseng cultivars in Korea and China. Molecules 2020, 25, 2635. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yi, M.J.; You, K.H.; Bae, K.H.; Han, J.Y.; Yi, J.S. Depletion of phosphorus in mountain soil and growth stimulation of Panax ginseng by phosphorus enrichment. J. Korean Soc. For. Sci. 2009, 98, 170–177. [Google Scholar]
- Larsen, J.; Jaramillo-Lopez, P.; Najera-Rincon, M.; Gonzalez-Esquivel, C.E. Biotic interactions in the rhizosphere in relation to plant and soil nutrient dynamics. J. Soil Sci. Plant Nut. 2015, 15, 449–463. [Google Scholar]
- Glick, B. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of development and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar]
- Lee, Y.M.; Ahn, J.H.; Choi, Y.M.; Weon, H.Y.; Yoon, J.H.; Song, J.K. Bacterial core community in soybean rhizosphere. Korean J. Microbiol. 2015, 51, 347–354. [Google Scholar]
- Pathan, S.I.; Ceccherini, M.T.; Sunseri, F.; Lupini, A. Rhizosphere as hotspot for plant-soil-microbe interaction. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R.S., Pathan, S.I., Ceccherini, M.T., Eds.; Springer Nature Singapore Pte.: Singapore, 2020; pp. 17–44. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kumar, M.; Varma, A. Role of PGPR in soil fertility and plant health. In Plant Growth Promoting Rhizobacteria (PGPR) and Medicinal Plant; Egamberdieva, D., Shrivastava, S., Varma, A., Eds.; Springer Nature: Basel, Switzerland, 2015; pp. 247–260. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; Teng, D.; Zhang, X.; He, X.; Zhang, Y.; Lv, G. Rhizobacterial communities of five co-occurring desert halophytes. Peer J. 2018, 6, e5508. [Google Scholar] [CrossRef]
- Matthews, A.; Pierce, S.; Hipperson, H.; Raymond, B. Rhizobacterial community assembly patterns vary between crop species. Front. Microbiol. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mo. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhang, P.; Trivedi, P.; Riera, N.; Wang, Y.; Liu, X.; Fan, G.; Tang, J.; Coletta-Filho, H.D.; et al. The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 2018, 9, 4894. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Bang, K.H.; Jo, I.H. Metagenomic analysis of bacterial endophyte community structure and functions in Panax ginseng at different ages. 3 Biotech 2019, 9, 300. [Google Scholar] [CrossRef]
- Vendan, R.T.; Yu, Y.J.; Lee, S.H.; Rhee, Y.H. Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J. Microbiol. 2010, 48, 559–565. [Google Scholar]
- Chowdhury, E.K.; Jeon, J.; Rim, S.O.; Park, Y.H.; Lee, S.K.; Bae, H. Composition, diversity and bioactivity of culturable bacterial endophytes in mountain-cultivated ginseng in Korea. Sci. Rep. 2017, 7, 10098. [Google Scholar]
- Kim, K.Y.; Kim, H.J.; Um, Y.; Jeon, K.S. Effect of soil properties and soil bacterial community on early growth characteristics of wild-simulated ginseng (Panax ginseng C.A. Meyer) in coniferous and mixed forest. Korean J. Med. Crop Sci. 2020, 28, 183–194. [Google Scholar]
- Nacke, H.; Thurmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schoning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-based assessment of bacterial community structure along different management types in german forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar]
- Yun, C.W.; Moon, H.S. Classification of forest vegetation type and environmental properties in Limestone area of Korea. J. Agric. Life Sci. 2009, 43, 1–8. [Google Scholar]
- Rural Development Administration (RDA). Analysis Manual of Comprehensive Examination Laboratory (Soil, Plant, Water and Liquid manure); Rural Development Administration: Suwon, Republic of Korea, 2013; pp. 31–53. [Google Scholar]
- Korea Seed and Variety Service (KSVS). Know-How of Characteristics Investigation of The Crops: Ginseng (Panax ginseng Meyer); Korea Seed and Variety Service: Gimcheon, Republic of Korea, 2014. [Google Scholar]
- Schloss, P.D. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS ONE 2009, 9, e8230. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. J. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar]
- Kim, K.Y.; Um, Y.; Jeong, D.H.; Kim, H.J.; Kim, M.J.; Jeon, K.S. Study on the correlation between the soil bacterial community and growth characteristics of wild-simulated ginseng (Panax ginseng C.A. Meyer). Korean J. Environ. Biol. 2019, 37, 380–388. [Google Scholar]
- NIFOS (National Institute of Forest Science). Wild-Simulated Ginseng Eco-Friendly Cultivation Manual; National Institute of Forest Science: Seoul, Republic of Korea, 2021; pp. 15–16. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorous availability to plants. Agriculture 2019, 9, 120. [Google Scholar]
- Choi, Y.E.; Kim, Y.S.; Yi, M.J.; Park, W.G.; Yi, J.S.; Chun, S.R.; Han, S.S.; Lee, S.J. Physiological and chemical characteristics of field- and mountain-cultivated ginseng roots. J. Plant Biol. 2007, 50, 198–205. [Google Scholar]
- Topa, M.A.; Cheeseman, J.M. Carbon and phosphorus partitioning in Pinus serotine seedlings growing under hypoxic and low-phosphorus conditions. Tree Physiol. 1992, 10, 195–207. [Google Scholar] [PubMed]
- Cheng, H.T.; Zhang, Y.Y.; Zhang, L.X.; Sun, H.; Gao, M. Changes of soil nutrients in ginseng under forest at different growth stages. Chin. Agric. Sci. Bull. 2011, 27, 47–52. [Google Scholar]
- Tsukui, M. Temporal variation in chemical composition of phenocrysts and magmatic temperature at Daisen volcano, southwest Japan. J. Volcanol. Geotherm. Res. 1985, 26, 317–336. [Google Scholar]
- Matsumoto, S.; Doi, H.; Kasuga, J. Changes over the years in soil chemical properties associated with the cultivation of ginseng (Panax ginseng Meyer) on andosol soil. Agriculture 2022, 12, 1223. [Google Scholar] [CrossRef]
- Li, N.; Sheng, K.; Zheng, Q.; Hu, D.; Zhang, L.; Wang, J.; Zhang, W. Inoculation with phosphate-solubilizing bacteria alters microbial community and activates soil phosphorous supply to promote maize growth. Land Degrad. Dev. 2023, 34, 777–788. [Google Scholar]
- Mu, P.; Ding, G.; Zhang, Y.; Jin, Q.; Liu, Z.; Guan, Y.; Zhang, L.; Liang, C.; Zhou, F.; Liu, N. Interactions between arbuscular mycorrhizal fungi and phosphate-soluble bacteria affect ginsenoside compositions by modulating the C:N:P stoichiometry in Panax ginseng. Front. Microbiol. 2024, 15, 1426440. [Google Scholar]
- Jeong, B.G.; Jung, G.R.; Kim, M.S.; Moon, H.G.; Park, S.J.; Chun, J. Ginsenoside contents and antioxidant activities of cultiva ted mountain ginseng (Panax ginseng C.A. Meyer) with different ages. Korean J. Food Preserv. 2019, 26, 90–100. [Google Scholar]
- Kim, K.Y.; Eo, H.J.; Kim, H.J.; Um, Y.; Jeong, D.H.; Huh, J.H.; Jeon, K.S. The Growth Characteristics and Ginsenoside Contents of Wild-simulated Ginseng (Panax ginseng C.A. Meyer) with Different Years by Rusty Roots. Korean J. Plant Res. 2021, 34, 403–410. [Google Scholar]
- Kim, K.; Um, Y.; Eo, H.J.; Park, H.W.; Jeon, K.S.; Kim, H.J. Study on the Correlation between the Ginsenoside Contents and Growth Characteristics of Wild-simulated Ginseng with Different Year-Roots (Panax ginseng C.A. Meyer). Korean J. Plant Res. 2020, 33, 255–262. [Google Scholar]
- Jin, H.O.; Kim, U.J.; Yang, D.C. Effect of nutritional environment in ginseng field on the plant growth of ginseng (Panax ginseng C.A. Meyer). J. Ginseng Res. 2009, 33, 234–239. [Google Scholar]
- Yun, Y.B.; Huh, J.H.; Jeong, D.H.; Kim, J.; Um, Y. Correlation analysis between growth characteristics and ginsenoside contents of 4-year-old wild-simulated ginseng (Panax ginseng C.A. Meyer) with different cultivation sites. J. Appl. Biol. Chem. 2022, 65, 253–259. [Google Scholar]
- Cao, T.; Luo, Y.; Shi, M.; Tian, X.; Kuzyakov, Y. Microbial interactions for nutrient acquisition in soil: Miners, scavengers, and carriers. Soil Biol. Biochem. 2024, 188, 109215. [Google Scholar]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agriculture practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [PubMed]
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C.S. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review. Sci. Total Environ. 2020, 746, 140967. [Google Scholar] [PubMed]
- Song, Y.; Liu, C.; Song, C.; Wang, X.; Ma, X.; Gao, J.; Gao, S.; Wang, L. Linking soil organic carbon mineralization with soil microbial land substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Zhu, P.; Xu, M.; Lu, C. Fertilization enhances mineralization of soil carbon and nitrogen pools by regulating the bacterial community and biomass. J. Soils Sediments 2021, 21, 1633–1643. [Google Scholar]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Flores-Felix, J.D.; Rivas, R. Overview of the role of rhizobacteria in plant salt stress tolerance. Agronomy 2021, 11, 1759. [Google Scholar] [CrossRef]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Kiraly, L.; Kunstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants-influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Verma, J.P.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar]
- Buresova, A.; Tejnecky, V.; Kopecky, J.; Drabek, O.; Madrova, P.; Rerichova, N.; Omelka, M.; Krizova, P.; Nemecek, K.; Parr, T.B.; et al. Litter chemical quality and bacterial community structure influenced decomposition in acidic forest soil. Eur. J. Soil Biol. 2021, 103, 103271. [Google Scholar]
- Llado, S.; Zifcakova, L.; Vetrovsky, T.; Eichlerova, I.; Baldiran, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar]
- Miyashita, N.T. Contrasting soil bacterial community structure between the phyla Acidobacteria and Proteobacteria in tropical Southeast Asian and temperate Japanese forests. Genes Genet. Syst. 2015, 90, 61–77. [Google Scholar]
- Chen, G.; Xue, Y.; Yu, X.; Li, C.; Hou, Y.; Zhu, H.; Jiang, L.; Zheng, W.; Feng, Z.; Li, Y.; et al. The structure and function of microbial community in rhizosphere soil of American ginseng (Panax quinquefolius L.) changed with planting years. Curr. Microbiol. 2022, 79, 281. [Google Scholar]
- Fang, X.; Wang, H.; Zhao, L.; Wang, M.; Sun, M. Diversity and structure of the rhizosphere microbial communities of wild and cultivated ginseng. BMC Microbiol. 2022, 22, 2. [Google Scholar]
- Goodwin, P.H. The endosphere microbiome of ginseng. Plants 2022, 11, 415. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, M.; Han, M.; Yang, L. Differences in the quality, yield, and soil microbiology of ginseng in different planting environments. Horticulture 2023, 9, 520. [Google Scholar]
- Li, X.; Liu, Q.; Gao, Y.; Zang, P.; Zheng, T. Effects of a co-bacterial agent on the growth, disease control, and quality of ginseng based on rhizosphere microbial diversity. BMC Microbiol. 2024, 24, 647. [Google Scholar]
- Zhang, J.; Liu, P.; Nie, B.; Liu, X.; Zhang, Z.; He, R.; Dong, W.; Ji, W. Effects of genotype and ecological environment on the community structure and function of symbiotic bacteria in rhizosphere of ginseng. BMC Microbiol. 2022, 22, 235. [Google Scholar]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020, 11, 752. [Google Scholar]
- Doherty, J.R.; Crouch, J.A.; Roberts, J.A. Plant age influences microbiome communities more than plant compartment in greenhouse-grown creeping bentgrass. Phytobiomes J. 2021, 5, 373–381. [Google Scholar]
- Wang, Q.; Sun, H.; Li, M.; Xu, C.; Zhang, Y. Different age-induced changes in rhizosphere microbial composition and function of Panax ginseng in transplatation mode. Front. Plant Sci. 2020, 11, 563240. [Google Scholar]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [PubMed]
- Pan, L.; Cai, B. Phosphate-solubilizing bacteria: Advances in their physiology, molecular mechanisms and microbial community effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, S.; Umbreen, S.; Zhou, C. Soil phosphorus fractionation and its association with soil phosphate-solubilizing bacteria in a chronosequence of vegetation restoration. Ecol. Eng. 2021, 164, 106208. [Google Scholar]
- Damo, J.L.C.; Ramirez, M.D.A.; Agake, S.; Pedro, M.; Brown, M.; Sekimoto, H.; Yokoyama, T.; Sugihara, S.; Okazaki, S.; Ohkama-Ohtsu, N. Isolation and characterization of phosphate solubilizing bacteria from paddy field soils in Japan. Microbes. Environ. 2022, 37, ME21085. [Google Scholar]
- Jiang, H.; Li, S.; Wang, T.; Chi, X.; Qi, P.; Chen, G. Interaction between halotolerant phosphate-solubilizing bacteria (Providencia rettgeri strain TPM23) and rock phosphate improves soil biochemical properties and peanut growth in saline soil. Front. Microbiol. 2021, 12, 777351. [Google Scholar] [CrossRef]
- Saadouli, I.; Mosbah, A.; Ferjani, R.; Stathopoulou, P.; Galiatsatos, I.; Asimakis, E.; Marasco, R.; Daffonchio, D.; Tsiamis, G.; Ouzari, H.I. The impact of the inoculation of phosphate-solubilizing bacteria Pantoea agglomerans on phosphorus availability and bacterial community dynamics of a semi-arid soil. Microorganisms 2021, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Yilmaz, P. Refining the taxonomic structure of the phylum Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 3796–3806. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Kurahashi, M.; Yanagi, K.; Yokota, A.; Harayama, S. Acanthopleuribacteria pedis gen. nov., sp. Nov., a marine bacterium isolated from a chiton, and description of Acanthopleuribacteraceae fam. nov., Acanthopleuribacterales ord. nov., Holophagaceae fam. nov., in the phylum ‘Acidobacteria’. Int. J. Syst. Evol. Microbiol. 2008, 58, 2597–2601. [Google Scholar] [CrossRef]
- Thrash, J.C.; Coated, J.D. Class II. Holophagae classis nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kampfer, P., Rainey, F.A., Whitman, W.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 4, p. 731. [Google Scholar]
- Oren, A.; Garrity, G.M. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2016, 66, 1603–1606. [Google Scholar] [CrossRef]
- Hackl, E.; Zechmeister-Boltenstern, S.; Bodrossy, L.; Sessitsch, A. Comparison of diversities and compositions of bacterial populations inhabiting natural forest soils. J. Appl. Environ. Microbiol. 2004, 70, 5057–5065. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Zhelezova, A.D.; Chernov, T.I.; Dadysh, S.N. Linking ecology and systematics of Acidobacteria: Distinct habitat preference of the Acidobacteria and Blasocatellia in tundra soils. PLoS ONE 2020, 15, e0230157. [Google Scholar] [CrossRef]
- Lejon, D.P.H.; Chaussod, R.; Ranger, J.; Ranjard, L. Microbial community structure and density under different tree species in an acid forest soils (morvan, France). Microb. Ecol. 2005, 50, 614–625. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, U.N.; van Elsas, J.D.; van Overbeek, L.S. Real-time PCR detection of Holophagae (Acidabacteria) and Verrucomicrobia subdivision 1 groups in bulk and leek (Allium porrum) rhizosphere soils. J. Microbiol. Methods 2010, 83, 141–148. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Zhang, J.; Qiao, Y.; Xu, C.; Liu, Y.; Qian, L.; Li, W.; Dong, B. Community shift of biofilms developed in a full-scale drinking water distribution system switching from different water sources. Sci. Total Environ. 2016, 544, 499–506. [Google Scholar] [CrossRef]
- Wang, X.; Liu, E.; Tian, L. Impact of soil bacteria on amylose synthesis in maize kernels under the substitution of manure nitrogen for mineral fertilizer nitrogen. Int. J. Biol. Macromol. 2024, 282, 136705. [Google Scholar]
- Zechmeister-Boltenstern, S.; Hackl, E.; Bachmann, G.; Pfeffer, M.; Englisch, M. Nurtient turnover, greenhouse gas exchange and biodiversity in natural forests of central Europe. In Tree Species Effects on Soils: Implications for Global Change; Binkely, D., Menyailo, O., Eds.; IV. Earth and Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 2005; Volume 55, pp. 31–50. [Google Scholar]
- Nguyen, N.L.; Kim, Y.J.; Hoang, V.A.; Subramaniyam, S.; Kang, J.P.; Kang, C.H.; Yang, D.C. Bacterial diversity and community structure in Korean ginseng field soil are shifted by cultivation time. PLoS ONE 2016, 11, e0155055. [Google Scholar]
- Sun, H.; Wang, Q.X.; Liu, N.; Li, L.; Zhang, C.G.; Liu, Z.B.; Zhang, Y.Y. Effects of different leaf litters on the physicochemical properties and bacterial communities in Panax ginseng-growing soil. Appl. Soil Ecol. 2017, 111, 17–24. [Google Scholar]
- Kim, K.; Kim, H.J.; Jeong, D.H.; Huh, J.H.; Jeon, K.S.; Um, Y. Correlation between soil bacterial community structure and soil properties in cultivation sites of 13-year-old Wild-simulated ginseng (Panax ginseng C.A. Meyer). Appl. Sci. 2021, 11, 937. [Google Scholar] [CrossRef]
- Akbarlou, M.; Nodehi, N. Relationship between some environmental factors with distribution of medicinal plants in ghorkhud protected region, Northern Khorasan Province, Iran. J. Rangel. Sci. 2016, 6, 63–72. [Google Scholar]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3662. [Google Scholar]
- Mansinhos, I.; Goncalves, S.; Romano, A. How climate change-related abiotic factors affect the production of industrial valuable compounds in Lamiaceae plant species: A review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar]
- Yuan, Y.; Tang, X.; Jia, Z.; Li, C.; Ma, J.; Zhang, J. The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests 2020, 11, 94. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Hu, M.F.; Tian, C.H. Biogeographical distribution of bacterial communities in saline agricultural soil. Geoderma 2020, 361, 114095. [Google Scholar] [CrossRef]
- Larsbrink, J.; McKee, L.S. Bacteroidetes bacteria in the soil: Glycan acquisition, enzyme secretion, and gliding motility. Adv. Appl. Microbiol. 2020, 110, 63–98. [Google Scholar]
- Pan, X.; Raaijmakers, J.M.; Carrion, V.J. Importance of Bacteroidetes in host-microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar]
- Shen, C.; Ge, Y.; Yang, T.; Chu, H. Verrucomicrobial elevational distribution was strongly influenced by soil pH and carbon/nitrogen ratio. J. Soils Sediments 2017, 18, 2449–2456. [Google Scholar] [CrossRef]
- Tsitko, I.; Lusa, M.; Lehto, J.; Parviainen, L.; Ikonen, A.T.K.; Lahdenpera, A.M.; Bonberg, M. The variation of microbial communities in a depth profile of an acidic, nutrient-poor boreal bog in Southwestern Finland. Open J. Ecol. 2014, 4, 832–859. [Google Scholar]
- Mujakic, I.; Piwosz, K.; Koblizek, M. Phylum gemmatimonnadota and its role in the environment. Microorganisms 2022, 10, 151. [Google Scholar]
- Zhang, B.; Wu, X.; Tai, X.; Sun, L.; Wu, M.; Zhang, W.; Chen, X.; Zhang, G.; Chen, T.; Liu, G.; et al. Variation in actinobacterial community composition and potential function in different soil ecosystems belonging to the arid heihe river basin of Northwest China. Front. Microbiol. 2019, 10, 2209. [Google Scholar] [CrossRef]
- Kim, K.Y.; Um, Y.; Kim, H.J.; Jeong, D.H.; Huh, J.H.; Jeon, K.S. The growth characteristics of wild-simulated ginseng (Panax ginseng C.A. Meyer) seedling according to the shrub layer. Korean J. Wild Ginseng 2020, 14, 1–7. [Google Scholar]
- Kim, J.D.; Lee, D.W.; Lee, K.S.; Choi, C.H.; Kang, K.H. Distribution and antimicrobial susceptibility of Clostridium species in soil contaminated with domestic livestock feces of Korea. J. Microbiol. Biotechnol. 2004, 14, 401–410. [Google Scholar]
- Kuhner, C.; Matthies, C.; Acker, G.; Schmittroth, M.; Gobner, A.S.; Drake, H.L. Clostridium akagii sp. nov. and Clostridium acidisoli sp. nov.: Acid-tolerant, N2-fixing clostridia isolated from acidic forest soil and litter. Int. J. Syst. Evol. Microbiol. 2000, 50, 873–881. [Google Scholar]
- Mowlick, S.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Proliferation of diversified clostridial species during biological soil disinfestation incorporated with plant biomass under various conditions. Appl. Microbiol. Biotechnol. 2013, 97, 8365–8379. [Google Scholar]
- Palmer, J.S.; Hough, R.L.; West, H.M.; Avery, L.M. A review of the abundance, behavior and detection of clostridial pathogens in agricultural soils. Eur. J. Soil Sci. 2019, 70, 911–929. [Google Scholar] [CrossRef]
- Wong, P.Y.; Cheng, K.Y.; Kaksonen, A.H.; Sutton, D.C.; Ginige, M.P. Enrichment of anodophilic nitrogen fixing bacteria in a bioelectrochemical system. Water Res. 2014, 64, 73–81. [Google Scholar] [CrossRef]
- Bao, P.; Huang, H.; Hu, Z.Y.; Haggblom, M.M.; Zhu, Y.G. Impact of temperature, CO2 fixation and nitrate reduction on selenium reduction, by a paddy soil Clostridium strain. J. Appl. Microbiol. 2012, 114, 703–712. [Google Scholar] [CrossRef]
- Verbon, E.H.; Liberman, L.M. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2000, 26, 227–242. [Google Scholar] [CrossRef]
- Doornbos, R.F.; vanLoon, L.C.; Bakker, P.A.H.M. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Haas, D.; Defago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef]
- Garbena, P.; Van Veen, J.A.; Van Elsas, J.D. Assessment of the diversity, and antagonism towards Rhizoctonis solani AG3, of Pseudomonas species in soil from different agricultural regimes. FEMS Microbiol. Ecol. 2004, 47, 51–64. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; Van Loon, L.C. Induced systematic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef]
- Van Wees, S.C.M.; Van der Ent, S.; Pieterse, C.M.J. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar]
- Kil, Y.J.; Eo, J.K.; Eom, A.H. Effect of arbuscular mycorrhizal fungi on growth of Korean ginseng (Panax ginseng C. A. Mey.) seedlings. Korean J. Mycol. 2013, 41, 81–84. [Google Scholar]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar]

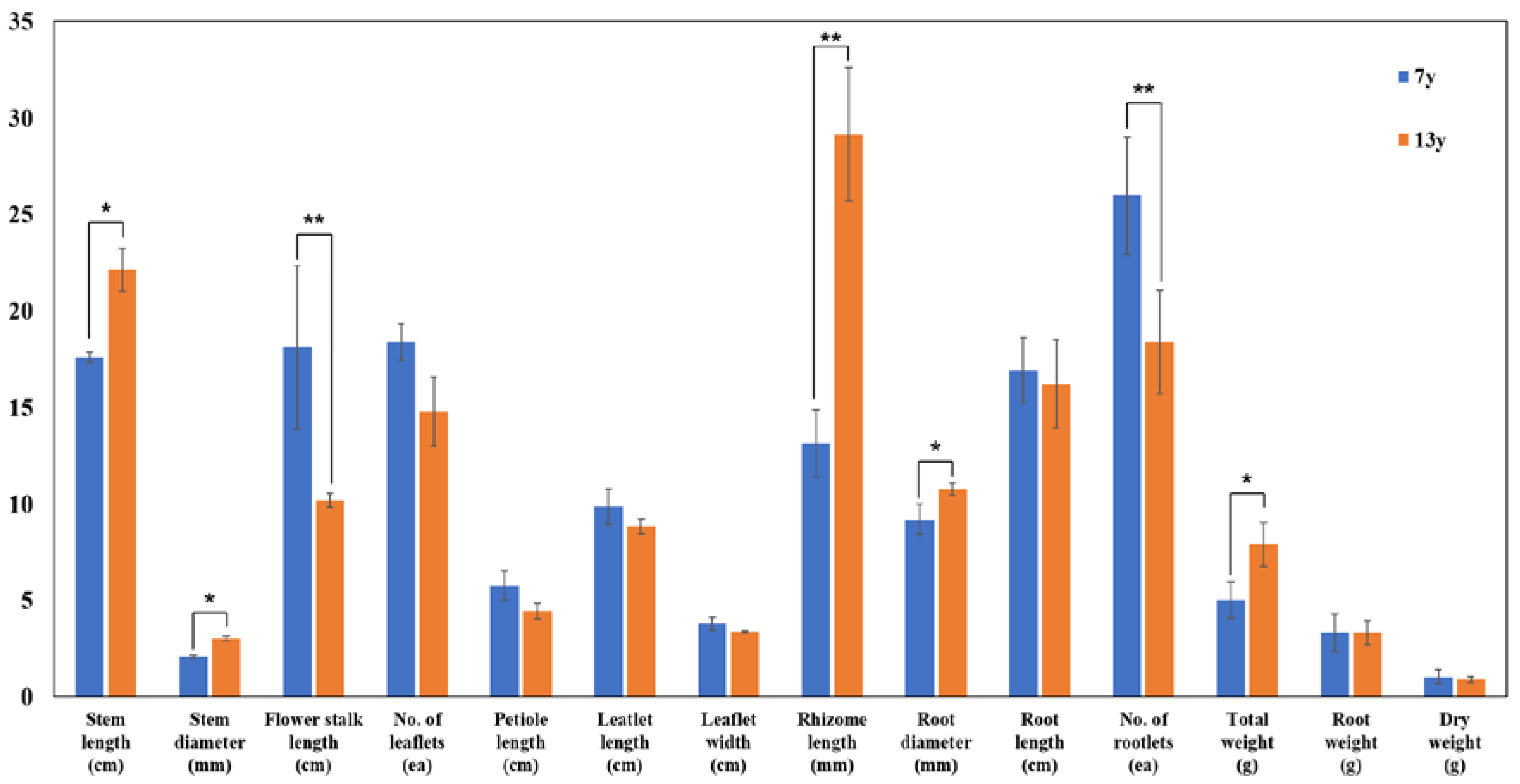
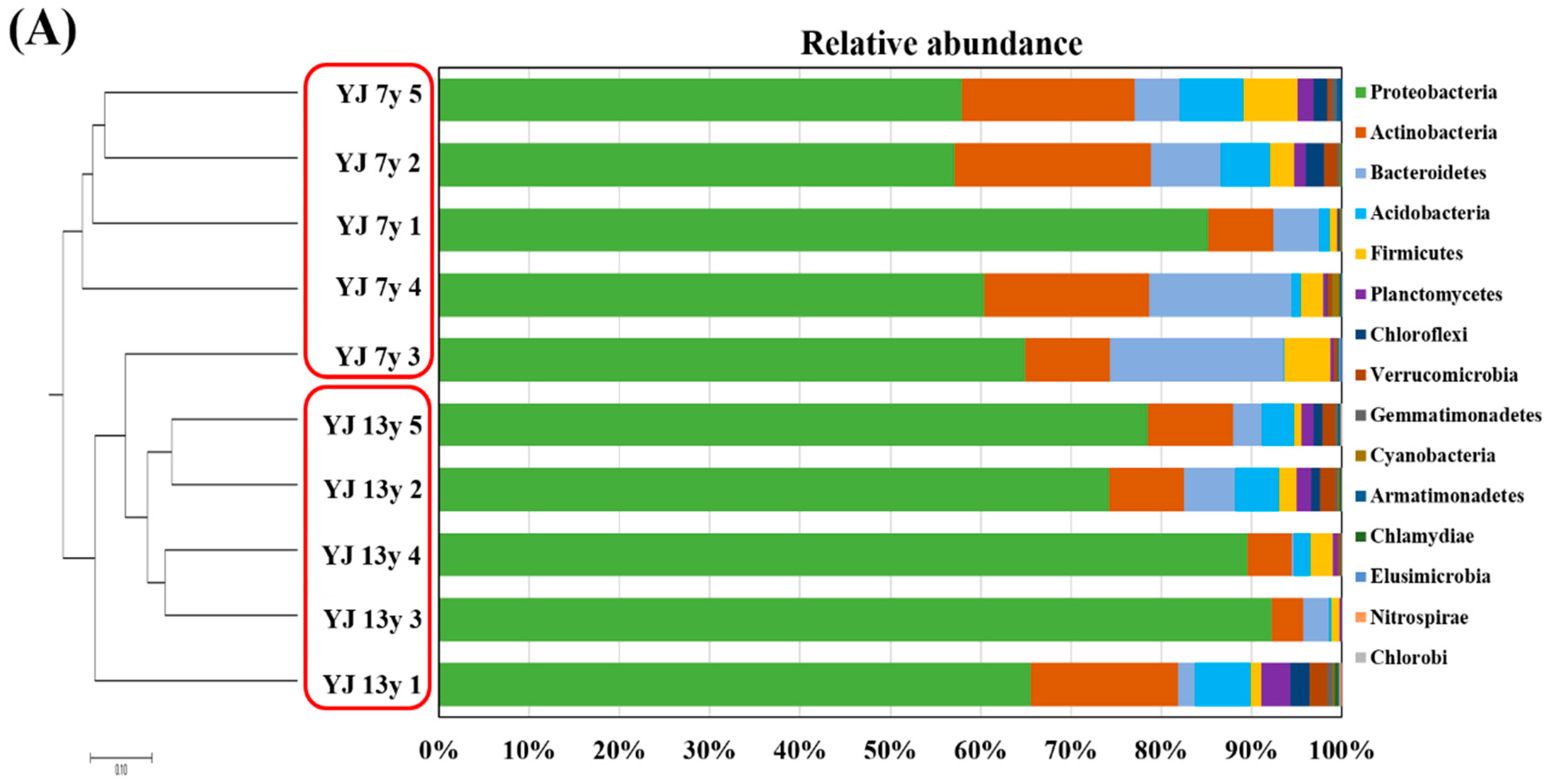
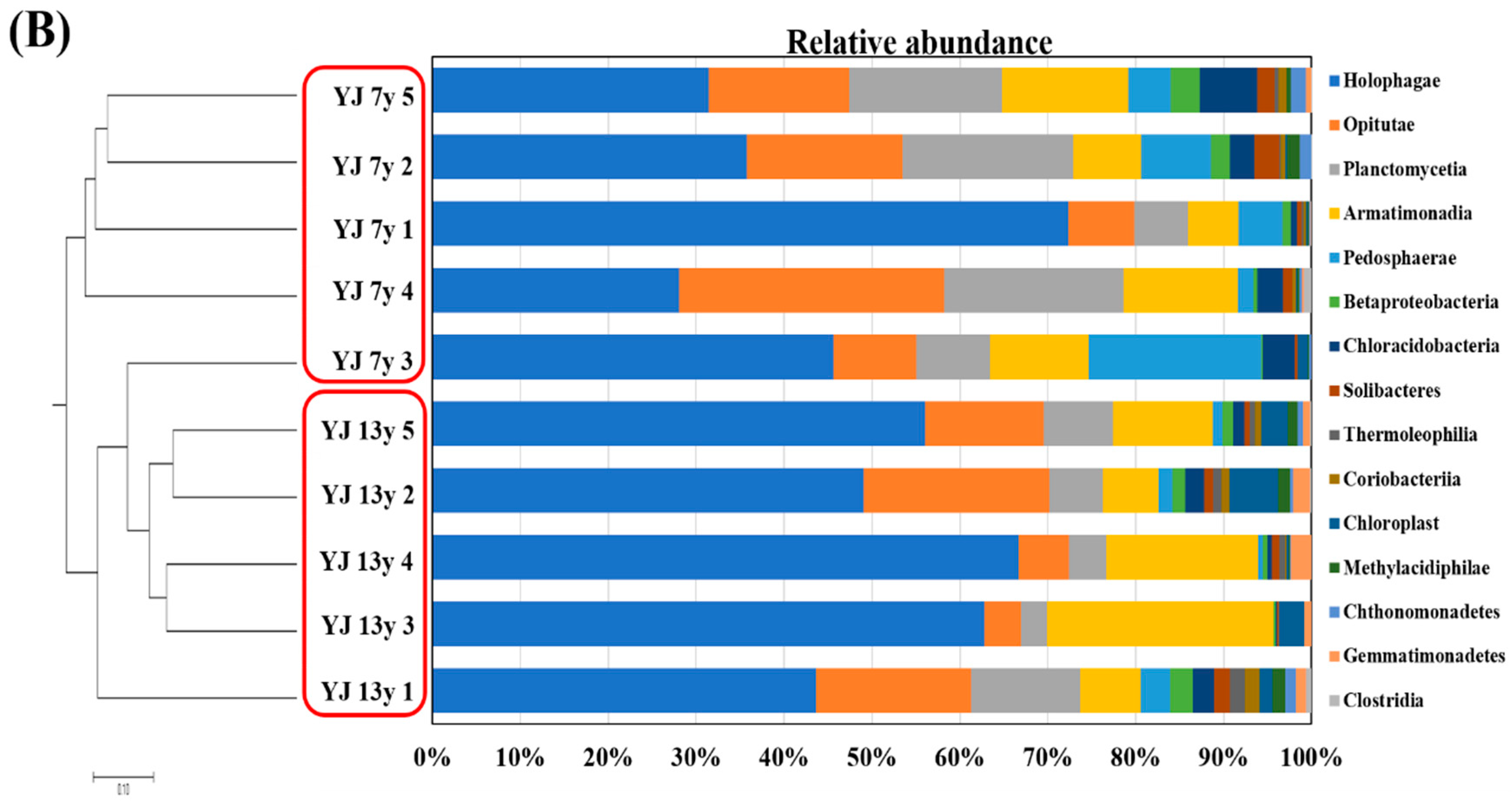
| Soil Properties | Growth Characteristics | ||||
|---|---|---|---|---|---|
| Stem Length | Flower Stalk Length | Rhizome Length | No. of Rootlets | Total Weight | |
| pH | 0.429 (0.397) | 0.200 (0.747) | 0.418 (0.229) | 0.079 (0.828) | 0.857 (0.014) * |
| EC | 0.370 (0.470) | −0.949 (0.014) * | −0.219 (0.544) | −0.157 (0.665) | −0.337 (0.460) |
| OM | 0.493 (0.321) | −0.718 (0.172) | 0.237 (0.510) | −0.585 (0.155) | −0.180 (0.699) |
| TN | 0.371 (0.468) | −0.500 (0.391) | 0.006 (0.987) | −0.492 (0.148) | 0.029 (0.961) |
| Avail. P | −0.086 (0.872) | −0.600 (0.285) | −0.636 (0.048) * | 0.243 (0.498) | −0.464 (0.294) |
| Ex. K | −0.086 (0.872) | −0.400 (0.505) | 0.335 (0.343) | −0.670 (0.034) * | 0.114 (0.758) |
| Ex. Ca | −0.829 (0.042) * | −0.100 (0.873) | −0.381 (0.352) | 0.024 (0.955) | −0.500 (0.253) |
| Ex. Mg | −0.116 (0.827) | −0.359 (0.553) | −0.170 (0.638) | −0.210 (0.560) | 0.030 (0.954) |
| Ex. Na | −0.145 (0.784) | −0.308 (0.614) | −0.351 (0.320) | 0.049 (0.892) | −0.704 (0.077) |
| CEC | 0.200 (0.704) | −0.500 (0.391) | 0.164 (0.651) | −0.377 (0.283) | 0.214 (0.645) |
| Growth Characteristics | Bacterial Communities | ||||
|---|---|---|---|---|---|
| Phylum | Class | ||||
| Bacterioidetes | Pedosphaerae | Thermoleophilia | Gemmatimonadetes | Clostridia | |
| Stem length | −0.164 (0.651) | −0.491 (0.150) | −0.091 (0.803) | 0.127 (0.726) | 0.288 (0.419) |
| Stem diameter | 0.236 (0.511) | −0.176 (0.627) | −0.358 (0.310) | −0.285 (0.425) | 0.276 (0.440) |
| Flower stalk length | −0.029 (0.957) | −0.543 (0.266) | −0.600 (0.208) | −0.429 (0.397) | −0.429 (0.397) |
| Number of leaflets | −0.449 (0.193) | −0.739 (0.015) * | 0.209 (0.562) | 0.548 (0.101) | 0.118 (0.745) |
| Petiole length | −0.448 (0.194) | −0.730 (0.017) * | 0.129 (0.723) | 0.448 (0.194) | 0.267 (0.456) |
| Leaflet length | 0.018 (0.960) | −0.322 (0.364) | −0.097 (0.789) | 0.036 (0.920) | 0.135 (0.709) |
| Leaflet width | −0.098 (0.789) | −0.366 (0.298) | 0.104 (0.776) | 0.171 (0.637) | 0.228 (0.526) |
| Rhizome length | 0.673 (0.033) * | 0.624 (0.054) | −0.855 (0.002) ** | −0.891 (0.001) ** | −0.239 (0.506) |
| Root diameter | −0.152 (0.676) | −0.382 (0.276) | 0.224 (0.533) | 0.200 (0.580) | 0.301 (0.399) |
| Root length | 0.109 (0.763) | −0.231 (0.521) | 0.164 (0.650) | 0.097 (0.789) | 0.517 (0.126) |
| Number of rootlets | −0.280 (0.434) | −0.584 (0.077 ) | 0.626 (0.053) | 0.608 (0.062) | 0.634 (0.049) * |
| Total weight | 0.067 (0.855) | −0.285 (0.425) | −0.248 (0.489) | −0.103 (0.777) | −0.018 (0.960) |
| Root weight | 0.115 (0.751) | −0.079 (0.829) | −0.152 (0.676) | −0.200 (0.580) | −0.006 (0.987) |
| Dry weight | 0.085 (0.815) | −0.146 (0.688) | −0.164 (0.650) | −0.158 (0.663) | 0.080 (0.826) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Yun, Y.-B.; Park, M.; Um, Y. Correlation Analysis Between the Growth of Wild-Simulated Ginseng and the Soil Bacterial Community in the Central Region of South Korea. Appl. Sci. 2025, 15, 3465. https://doi.org/10.3390/app15073465
Kim K, Yun Y-B, Park M, Um Y. Correlation Analysis Between the Growth of Wild-Simulated Ginseng and the Soil Bacterial Community in the Central Region of South Korea. Applied Sciences. 2025; 15(7):3465. https://doi.org/10.3390/app15073465
Chicago/Turabian StyleKim, Kiyoon, Yeong-Bae Yun, Myeongbin Park, and Yurry Um. 2025. "Correlation Analysis Between the Growth of Wild-Simulated Ginseng and the Soil Bacterial Community in the Central Region of South Korea" Applied Sciences 15, no. 7: 3465. https://doi.org/10.3390/app15073465
APA StyleKim, K., Yun, Y.-B., Park, M., & Um, Y. (2025). Correlation Analysis Between the Growth of Wild-Simulated Ginseng and the Soil Bacterial Community in the Central Region of South Korea. Applied Sciences, 15(7), 3465. https://doi.org/10.3390/app15073465






