Xylazine, a Drug Adulterant Whose Use Is Spreading in the Human Population from the U.S. to the U.K. and All Europe: An Updated Review
Abstract
1. Introduction
2. History and Chemistry of Xylazine
3. Pharmacokinetics and Pharmacodynamics of Xylazine
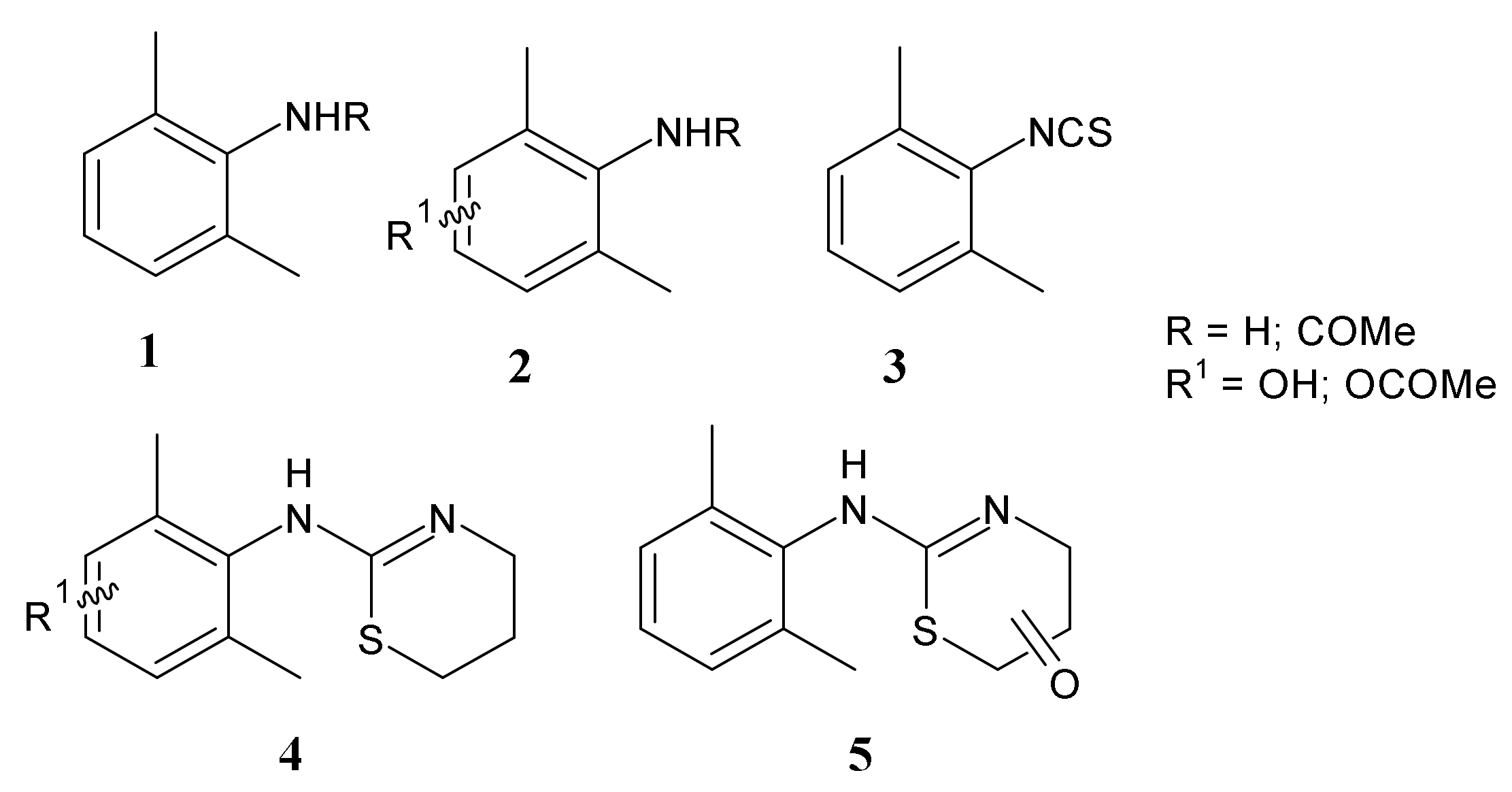
Studies on the Metabolism of Xylazine
4. Methods for the Detection of Xylazine and Its Metabolites
5. Combinations of Xylazine with Other Drugs
6. Toxicity of Xylazine
6.1. Adverse Effects
6.2. In Vivo Toxicity Studies in Animals
6.3. Toxicity Studies in Humans
7. Incidence of Xylazine Drug Overdose Events in the U.S.
8. Harm Reduction Initiatives for Xylazine
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard-Williams, E.; Tierney, A. Xylazine Induced Skin Necrosis. J. Gen. Int. Med. 2024, in press. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Sall, S.; Upshaw, W.C.; Spillers, N.J.; Vincik, L.Y.; De Witt, A.S.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Xylazine: A drug adulterant of clinical concern. Curr. Pain Headache Rep. 2024, 28, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. New Choking Epidemic Trends in Psychoactive Drugs: The Zombifying Combination of Fentanyl and Xylazine Cause Overdoses and Little Hope in Rehabilitation. Psychoactives 2024, 3, 132–136. [Google Scholar] [CrossRef]
- Hoffman, R.S. Closing the Xylazine Knowledge Gap. Clin. Toxicol. 2024, 61, 1013–1016. [Google Scholar] [CrossRef]
- Jain, L.; Kaur, J.; Ayub, S.; Ansari, D.; Ahmed, R.; Dada, A.Q.; Ahmed, S. Fentanyl and Xylazine Crisis: Crafting Coherent Strategies for Opioid Overdose Prevention. World J. Psych. 2024, 14, 760. [Google Scholar] [CrossRef]
- Salani, D.A.; Valdes, B.; Weidlich, C.; Zdanowicz, M.M. The New Street Adulterant Drug: What Clinicians Need to Know About Xylazine (Tranq). J. Emerg. Nurs. 2024, 50, 716–721. [Google Scholar] [CrossRef]
- Di Trana, A.; Berardinelli, D.; Montanari, E.; Berretta, P.; Basile, G.; Huestis, M.A.; Busardò, F.P. Molecular Insights and Clinical Outcomes of Drugs of Abuse Adulteration: New Trends and New Psychoactive Substances. Int. J. Mol. Sci. 2022, 23, 14619. [Google Scholar] [CrossRef]
- Wu, P.E.; Austin, E. Xylazine in the Illicit Opioid Supply. Can. Med. Assoc. J. 2024, 196, E133. [Google Scholar] [CrossRef]
- Marshall, S.A.; Nelson, L.A. Xylazine Adulteration in Illicit Fentanyl: A Threat to Public Health. J. Pharm. Pract. 2024, 38, 264–269. [Google Scholar] [CrossRef]
- Zhu, D.T. Public Health Impact and Harm Reduction Implications of Xylazine-involved Overdoses: A Narrative Review. Harm Reduct. J. 2023, 20, 131. [Google Scholar] [CrossRef]
- Alexander, R.S.; Canver, B.R.; Sue, K.L.; Morford, K.L. Xylazine and Overdoses: Trends, Concerns, and Recommendations. Am. J. Public Health 2022, 112, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.N.; Serafinski, R.; Ujeneza, M.; McKenzie, M.; Tardif, J.; Krotulski, A.J.; Badea, A.; Grossman, E.R.; Green, T.C. Xylazine Awareness, Desire, Use and Exposure: Preliminary Findings from the Rhode Island Community-Based Drug Checking Cohort Study. Drug Alcohol Depend. Rep. 2024, 11, 100247. [Google Scholar] [CrossRef] [PubMed]
- German, D.; Genberg, B.; Sugarman, O.; Saloner, B.; Sawyer, A.; Glick, J.L.; Gribbin, M.; Flynn, C. Reported Xylazine Exposure Highly Associated with Overdose Outcomes in a Rapid Community Assessment among People who Inject Drugs in Baltimore. Harm Reduct. J. 2024, 21, 18. [Google Scholar] [CrossRef]
- Copeland, C.S.; Rice, K.; Rock, K.L.; Hudson, S.; Streete, P.; Lawson, A.J.; Couchman, L.; Holland, A.; Morley, S. Broad Evidence of Xylazine in the UK Illicit Drug Market beyond Heroin Supplies: Triangulating from Toxicology, Drug-testing and Law Enforcement. Addiction 2024, 119, 1301–1309. [Google Scholar] [CrossRef]
- Habib, A.; Ali, T.; Fatima, L.; Nazir, Z.; Hafiz, A.I.; Haque, M.A. Xylazine in Illicit Drug Mixtures: A Growing Threat and Overlooked Danger. Ann. Med. Surg. 2024, 86, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Martín-Faivre, L.; Prince, L.; Cornu, C.; Villeret, B.; Sanchez-Guzman, D.; Rouzet, F.; Sallenave, J.M.; Garcia-Verdugo, I. Pulmonary Delivery of Silver Nanoparticles Prevents Influenza Infection by Recruiting and Activating Lymphoid Cells. Biomaterials 2025, 312, 122721. [Google Scholar] [CrossRef]
- Chung, J.; Kim, S.; Jeong, J.; Kim, D.; Jo, A.; Kim, H.Y.; Hwang, J.; Kweon, D.H.; Yoo, S.Y.; Chung, W.J. Preventive and Therapeutic Effects of a Super-multivalent Sialylated Filamentous Bacteriophage against the Influenza Virus. Biomaterials 2025, 312, 122736. [Google Scholar] [CrossRef]
- Ensandoust, T.; Khakpour-Taleghani, B.; Jafari, A.; Rostampour, M.; Rohampour, K.; Ch, M.H. Effect of Simultaneous Application of Adenosine A1 Receptor Agonist and A2A Receptor Antagonist on Memory, Inflammatory Factors, and PSD-95 in Lipopolysaccharide-induced Memory Impairment. Behav. Brain Res. 2025, 476, 115210. [Google Scholar] [CrossRef]
- Saniei, H.; Shirpoor, A.; Naderi, R. Cerebral Ischemia-Reperfusion Induced Neuronal Damage, Inflammation, miR-374a-5p, MAPK6, NLRP3, and Smad6 Alterations: Rescue Effect of N-acetylcysteine. Pharm. Sci. 2024, 30, 502–511. [Google Scholar] [CrossRef]
- Sheehy, E.J.; von Diemling, C.; Ryan, E.; Widaa, A.; O’Donnell, P.; Ryan, A.; Chen, G.; Brady, R.T.; López-Noriega, A.; Zeiter, S.; et al. Antibiotic-eluting Scaffolds with Responsive Dual-release Kinetics Facilitate Bone Healing and Eliminate S. aureus Infection. Biomaterials 2025, 313, 122774. [Google Scholar] [CrossRef]
- Liu, J.; Hanson, A.; Yin, W.; Wu, Q.; Wauthier, E.; Diao, J.; Dinh, T.; Macdonald, J.; Li, R.; Terajima, M.; et al. Decellularized Liver Scaffolds for Constructing Drug-metabolically Functional Ex Vivo Human Liver Models. Bioact. Mater. 2025, 43, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.D.C.J.; da Silva Nascimento, F.G.; Nonato, D.T.T.; Assreuy, A.M.S.; Chaves, E.M.C.; Aragão, G.F.; Soares, P.M.G.; Castro, R.R. Acute Toxicity Study of Bioactive Galactomannans from Seeds of Two Non-traditional Leguminosae. Rev. Caatinga 2025, 38, e12704. [Google Scholar] [CrossRef]
- Bangar, N.S.; Dixit, A.; Apte, M.M.; Tupe, R.S. Syzygium cumini (L.) Skeels Mitigate Diabetic Nephropathy by Regulating Nrf2 Pathway and Mitocyhondrial Dysfunction: In Vitro and In Vivo Studies. J. Ethnopharmacol. 2025, 336, 118684. [Google Scholar] [CrossRef] [PubMed]
- Habershon-Butcher, J.; Cutler, C.; Viljanto, M.; Hincks, P.R.; Biddle, S.; Paine, S.W. Re-evaluation of the Pharmacokinetics of Xylazine Administered to Thoroughbred Horses. J. Vet. Pharmacol. Ther. 2020, 43, 6–12. [Google Scholar] [CrossRef]
- Sellon, D.C.; Sanz, M.; Kopper, J.J. Acquisition and Use of Analgesic Drugs by Horse Owners in the United States. Equine Vet. J. 2023, 55, 69–77. [Google Scholar] [CrossRef]
- Teoh, W.K.; Muslim, N.Z.M.; Chang, K.H.; Abdullah, A.F. Abuse of Xylazine by Human and its Emerging Problems: A Review from Forensic Perspective. Mal. J. Med. Health Sci. 2022, 18, 190–201. [Google Scholar]
- Uniform Classification Guidelines for Foreign Substances and Recommended Penalties Model Rule. Association of Racing Commissioners International. Available online: http://tharacing.com/wp-content/uploads/2020/02/ARCI-Classification-Guidelines-and-Penalties.pdf (accessed on 4 August 2022).
- Knych, H.K.; Stanleya, S.D.; McKemiea, D.S.; Arthurc, R.M.; Kass, P.G. Pharmacokinetic and Pharmacodynamics of Xylazine Administered to Exercised Thoroughbred Horses. Drug Test. Anal. 2017, 9, 713–720. [Google Scholar] [CrossRef]
- JECFA. Residues of Some Veterinary Drugs in Animals and Foods. Abamectin, Chlortetracycline and Tetracycline, Clenbuterol, Cypermethrin, Moxidectin Neomycin Oxytetracycline, Spiramycin, Thiamphenicol, Tilmicosin, Xylazine. (JECFA 47). 1997. Available online: http://www.fao.org/docrep/W4601E/W4601E00.htm (accessed on 17 March 2025).
- Joint Expert Committee on Food Additives. Evaluation of Certain Veterinary Drug Residues in Food: Forty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organisation: Geneva, Switzerland, 1998; Volume 876. [Google Scholar]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 2,6-Xylidine (2,6-Dimethylaniline) (CAS No. 87-62-7) in Charles River CD Rats (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1990, 278, 1–138. [Google Scholar] [PubMed]
- IARC Report. Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines. IARC Monogr Eval Carcinog Risks Hum 57. 1993. Available online: http://monographs.iarc.fr/ENG/Monographs/vol57/index.php (accessed on 8 January 2025).
- Flajs, V.C.; MacNeil, J.D. Sedatives and Tranquilizers. In Chemical Analysis of Non-Antimicrobial Veterinary Drug Residues in Food; Wiley: Hoboken, NJ, USA, 2016; pp. 311–381. [Google Scholar] [CrossRef]
- Ćupić, V.; Čolić, M.; Pavičić, L.; Vučević, D.; Varagić, V.M. Immunomodulatory Effect of Xylazine, an α2 Adrenergic Agonist, on Rat Spleen Cells in Culture. J. Neuroimmunol. 2001, 113, 19–29. [Google Scholar] [CrossRef]
- Friedman, J.; Montero, F.; Bourgois, P.; Wahbi, R.; Dye, D.; Goodman-Meza, D.; Shover, C. Xylazine Spreads Across the US: A Growing Component of the Increasingly Synthetic and Polysubstance Overdose Crisis. Drug Alcohol. Depend. 2022, 233, 109380. [Google Scholar] [CrossRef]
- Debnath, R.; Chawla, P.A. Xylazine Addiction Turning Humans to Zombies: Fact or Myth? Health Sci. Rev. 2023, 9, 100132. [Google Scholar] [CrossRef]
- Meyer, G.M.; Maurer, H.H. Qualitative Metabolism Assessment and Toxicological Detection of Xylazine, a Veterinary Tranquilizer and Drug of Abuse, in Rat and Human Urine Using GC-MS, LC-MSn, and LC-HR-MSn. Anal. Bioanal. Chem. 2013, 405, 9779–9789. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.S.; Knable, B.M.; Relich, T.A.; Smathers, A.N.; Gionfriddo, M.R.; Nemecek, B.D.; Montepara, C.A.; Guarascio, A.J.; Covvey, J.R.; Zimmerman, D.E. Xylazine Poisoning: A Systematic Review. Clin. Toxicol. 2022, 60, 892–901. [Google Scholar] [CrossRef]
- Kyei, E.F.; Kyei, G.K.; Ansong, R.; Boakye, C.K.; Asamoah, E. Xylazine in the Unregulated Drug Market: An Integrative Review of Its Prevalence, Health Impacts, and Detection and Intervention Challenges in the United States. Policy Polit. Nurs. Pract. 2024, 25, 241–253. [Google Scholar]
- Krongvorakul, J.; Auparakkitanon, S.; Trakulsrichai, S.; Sanguanwit, P.; Sueajai, J.; Noumjad, N.; Wananukul, W. Use of Xylazine in Drug-Facilitated Crimes. J. Forensic Sci. 2018, 63, 1325–1330. [Google Scholar] [CrossRef]
- Bowles, J.M.; Copulsky, E.C.; Reed, M.K. Media Framing Xylazine as a “Zombie Drug” Is Amplifying Stigma onto People who Use Drugs. Int. J. Drug Pol. 2024, 125, 104338. [Google Scholar] [CrossRef]
- Lin, M.; Eubanks, L.M.; Zhou, B.; Janda, K.D. Evaluation of a Hapten Conjugate Vaccine against the “Zombie Drug” Xylazine. Chem. Commun. 2024, 60, 4711–4714. [Google Scholar] [CrossRef]
- Kacinko, S.L.; Mohr, A.L.A.; Logan, B.K.; Barbieri, E.J. Xylazine: Pharmacology Review and Prevalence and Drug Combinations in Forensic Toxicology Casework. J. Anal. Toxicol. 2022, 46, 911–917. [Google Scholar] [CrossRef]
- Alexander-Savino, C.V.; Mirowski, G.W.; Culton, D.A. Mucocutaneous Manifestations of Recreational Drug Use. Am. J. Clin. Dermatol. 2024, 25, 281–297. [Google Scholar] [CrossRef]
- Ruiz-Colón, K.; Chavez-Arias, C.; Díaz-Alcalá, J.E.; Martínez, M.A. Xylazine Intoxication in Humans and its Importance as an Emerging Adulterant in Abused Drugs: A Comprehensive Review of the Literature. Forensic Sci. Int. 2014, 240, 1–8. [Google Scholar] [CrossRef]
- Papudesi, B.N.; Malayala, S.V.; Regina, A.C. Xylazine Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Malaca, S.; Pesares, M.; Kapoor, A.; Berretta, P.; Busardò, F.P.; Pirani, F. Pharmacology and Toxicology of Xylazine: Quid Novum? Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7337–7345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, Y.; Tang, P. Competitive Antagonism of Xylazine on α7 Nicotinic Acetylcholine Receptors and Reversal by Curcuminoids. ACS Chem. Neurosci. 2024, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, S.G.; Nelson, M.; Wexler, H.R.; Stiller, C.R. Xylazine Hydrochloridine (Rompun) Overdose in Man. Clin. Toxicol. 1979, 15, 281–285. [Google Scholar] [CrossRef]
- Gallanosa, A.G.; Spyker, D.A.; Shipe, J.R.; Morris, D.L. Human Xylazine Overdose: A Comparative Review with Clonidine, Phenothiazines, and Tricyclic Antidepressants. Clin. Toxicol. 1981, 18, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Ikram, S.; Zainab, A.; Inam, M.; Azhar, A.; Saeed, A.S.E. Understanding the Rising Abuse of Veterinary Medicine Xylazine: A Review. IJS Glob. Health 2025, 8, e00513. [Google Scholar] [CrossRef]
- Kanske, P.; Heissler, J.; Schönfelder, S.; Forneck, J.; Wessa, M. Neural Correlates of Emotional Distractibility in Bipolar Disorder Patients, Unaffected Relatives, and Individuals with Hypomanic Personality. Am. J. Psych. 2013, 170, 1487–1496. [Google Scholar] [CrossRef]
- Nunez, J.; DeJoseph, M.E.; Gill, J.R. Xylazine, a Veterinary Tranquilizer, Detected in 42 Accidental Fentanyl Intoxication Deaths. Am. J. Forensic Med. Pathol. 2021, 42, 9–11. [Google Scholar] [CrossRef]
- Mai, T.; Zhang, Y.; Zhao, S. Xylazine Poisoning in Clinical and Forensic Practice: Analysis Method, Characteristics, Mechanism and Future Challenges. Toxics 2023, 11, 1012. [Google Scholar] [CrossRef]
- Lynch, K.L. New Insights into Xylazine Pharmacokinetics in Humans. Clin. Chem. 2024, 71, 230–231. [Google Scholar] [CrossRef]
- Jawa, R.; Ismail, S.; Shang, M.; Murray, S.; Murray-Krezan, C.; Zheng, Y.; Mackin, S.; Washington, K.; Alvarez, P.; Dillon, J.; et al. Drug Use Practices and Wound Care Experiences in the Age of Xylazine Adulteration. Drug Alcohol Depend. 2024, 263, 112390. [Google Scholar] [CrossRef]
- Bedard, M.L.; Huang, X.P.; Murray, J.G.; Nowlan, A.C.; Conley, S.Y.; Mott, S.E.; Loyack, S.J.; Cline, C.A.; Clodfelter, C.G.; Dasgupta, N.; et al. Xylazine is an Agonist at Kappa Opioid Receptors and Exhibits Sex-specific Responses to Opioid Antagonism. Addict. Neurosci. 2024, 11, 100155. [Google Scholar] [CrossRef] [PubMed]
- Oles, W.; Adeola, J.O.; Stone, A.B. Alpha-2 Antagonists Should Be Developed as Xylazine Antidotes in Humans. Reg Anesth Pain Med. 2024, in press. [Google Scholar] [CrossRef]
- Hopkins, T.J.H. The Clinical Pharmacology of Xylazine in Cattle. Aust. Veter. J. 1972, 48, 109–112. [Google Scholar] [CrossRef] [PubMed]
- EMEA Report. Committee for Veterinary Medicinal Products Xylazine Hydrochloride, Summary Report (1). The European Agency for the Evaluation of Medicinal Products, EMEA/MRL/611/99-Final-corrigendum. 1999. Available online: https://www.ema.europa.eu/en/search?search_api_fulltext=xylazine&f%5B0%5D=ema_search_entity_is_document%3ADocument (accessed on 17 March 2025).
- Brown, J.R. Use of Xylazine in Cattle. Mod. Vet. Pract. 1986, 67, 125–126. [Google Scholar]
- Guerri, G.; Cerasoli, I.; Straticò, P.; De Amicis, I.; Giangaspero, B.; Varasano, V.; Paolini, A.; Carluccio, A.; Petrizzi, L. The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia. Animals 2021, 11, 3433. [Google Scholar] [CrossRef]
- Eisenach, J.C.; De Kock, M.; Klimscha, W. α2-Adrenergic Agonists for Regional Anesthesia: A Clinical Review of Clonidine (1984–1995). J. Am. Soc. Anesthesiol. 1996, 85, 655–674. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, Y.; Hu, J.; Zhang, J.; Zhang, C.; Song, W.; Wang, X.; Zheng, B.; Zhou, X. Simultaneous Determination of Phenothiazine Drugs and their Metabolites Residues in Animal Derived Foods by High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Control 2025, 167, 110799. [Google Scholar] [CrossRef]
- Catalano, A. The 2,6-Xylyl Moiety as a Privileged Scaffold of Pharmaceutical Significance. Curr. Med. Chem. 2022, 29, 3984–3990. [Google Scholar] [CrossRef]
- Hoffman, G.R.; Giduturi, C.; Cordaro, N.J.; Yoshida, C.T.; Schoffstall, A.M.; Stabio, M.E.; Zuckerman, M.D. Classics in Chemical Neuroscience: Xylazine. ACS Chem. Neurosci. 2024, 15, 2091–2098. [Google Scholar] [CrossRef]
- Krūkle-Bērziņa, K.; Actiņš, A. Investigation of the Phase Transitions Occurring during and after the Dehydration of Xylazine Hydrochloride Monohydrate. Int. J. Pharm. 2014, 469, 40–49. [Google Scholar] [CrossRef]
- Abdulla, L.M.; Peach, A.A.; Holmes, S.T.; Dowdell, Z.T.; Watanabe, L.K.; Iacobelli, E.M.; Hirsh, D.A.; Rawson, J.M.; Schurko, R.W. Synthesis and Characterization of Xylazine Hydrochloride Polymorphs, Hydrates, and Cocrystals: A 35Cl Solid-State NMR and DFT Study. Cryst. Growth Des. 2023, 23, 3412–3426. [Google Scholar] [CrossRef]
- Choi, J.H.; Lamshöft, M.; Zühlke, S.; Abd El-Aty, A.M.; Rahman, M.M.; Kim, S.W.; Shim, J.H.; Spiteller, M. Analyses and Decreasing Patterns of Veterinary Antianxiety Medications in Soils. J. Hazard. Mater. 2014, 275, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Anban, J.D.; James, C.; Kumar, J.S.; Pradhan, S. Molecular Structure, Electronic Properties and Drug-Likeness of Xylazine by Quantum Methods and QSAR Analysis. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Timmermans, P.B.M.W.M.; De Jonge, A.; Thoolen, M.J.M.C.; Wilffert, B.; Batink, H.; Van Zwieten, P.A. Quantitative Relationships Between α-Adrenergic Activity and Binding Affinity of α-Adrenoceptor Agonists and Antagonists. J. Med. Chem. 1984, 27, 495–503. [Google Scholar] [CrossRef]
- Kawczak, P.; Bober, L.; Bączek, T. QSPR Analysis of Some Agonists and Antagonists of α-Adrenergic Receptors. Med. Chem. Res. 2015, 24, 372–382. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. (Eds.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publishing: London, UK, 1989; ISBN 91191028X. [Google Scholar]
- Hendawy, H.A.; Khaled, E. Novel Xylazine Voltammetric Sensors Based on Zinc Oxide Nanostructure. Egypt. J. Chem. 2023, 66, 673–683. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Promsuwan, K.; Saichanapan, J.; Phonchai, A.; Sadiq, N.S.M.; Teoh, W.K.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. Nanocoral-like Polyaniline-modified Graphene-based Electrochemical Paper-based Analytical Device for a Portable Electrochemical Sensor for Xylazine Detection. ACS Omega 2022, 7, 13913–13924. [Google Scholar] [CrossRef]
- Krūkle-Bērziņa, K.; Actiņš, A. The Effect of Excipients on the Stability and Phase Transition Rate of Xylazine Hydrochloride and Zopiclone. J. Pharm. Biomed. Anal. 2015, 107, 168–174. [Google Scholar] [CrossRef]
- Abdulla, L.M. 35CI Solid-State NMR Characterization of Polymorphs and Cocrystals of Xylazine HCI. ProQuest Dissertations & Theses, University of Windsor, Windsor, ON, Canada, 2022. Available online: https://www.proquest.com/openview/eac6973e9a01a09eb2cb499e7279997f/1?cbl=18750&diss=y&pq-origsite=gscholar (accessed on 17 March 2025).
- Spyridaki, M.H.; Lyris, E.; Georgoulakis, I.; Kouretas, D.; Konstantinidou, M.; Georgakopoulos, C.G. Determination of Xylazine and its Metabolites by GC-MS in Equine Urine for Doping Analysis. J. Pharm. Biomed. Anal. 2004, 35, 107–116. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Scali, E.; Scumaci, D.; Pellegrino, M.; Aquaro, S.; Saturnino, C.; Sinicropi, M.S. Impact of Cytochrome P450 Enzymes on the Phase I Metabolism of Drugs. Appl. Sci. 2023, 13, 6045. [Google Scholar] [CrossRef]
- Chamberlain, P.L.; Brynes, S.D. The Regulatory Status of Xylazine for Use in Food-producing Animals in the United States. J. Vet. Pharmacol. Ther. 1998, 21, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Delehant, T.M.; Denhart, J.W.; Lloyd, W.E.; Powell, J.D. Pharmacokinetics of Xylazine, 2,6-Dimethylaniline, and Tolazoline in Tissues from Yearling Cattle and Milk from Mature Dairy Cows after Sedation with Xylazine Hydrochloride and Reversal with Tolazoline Hydrochloride. Vet. Ther. 2003, 4, 128–134. [Google Scholar] [PubMed]
- Garcia-Villar, R.; Toutain, P.L.; Alvinerie, M.; Ruckebusch, Y. The Pharmacokinetics of Xylazine Hydrochloride: An Interspecific Study. J. Vet. Pharmacol. Ther. 1981, 4, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, A.; Barbarossa, A.; Ringer, S.K.; Jörger, F.B.; Bryner, M.; Bettschart-Wolfensberger, R. Clinical Randomized Comparison of Medetomidine and Xylazine for Isoflurane Balanced Anesthesia in Horses. Front. Vet. Sci. 2021, 8, 603695. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Khan, N.U.; Ali, H.; Gohar, A. Pharmacodynamics of Xylazine, Acepromazine and Diazepam on Various Physiological Parameter in Rabbits. Pak-Euro J. Med. Life Sci. 2022, 5, 147–154. [Google Scholar] [CrossRef]
- Latzel, S.T. Subspecies studies: Pharmacokinetics and Pharmacodynamics of a Single Intravenous Dose of Xylazine in Adult Mules and Adult Haflinger Horses. J. Equine Vet. Sci. 2012, 32, 816–826. [Google Scholar] [CrossRef]
- Veilleux-Lemieux, D.; Castel, A.; Carrier, D.; Beaudry, F.; Vachon, P. Pharmacokinetics of Ketamine and Xylazine in Young and Old Sprague–Dawley Rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 567–570. [Google Scholar]
- Tiantian, G.; Yongti, L.; Lulu, Z.; Yanan, L.; Hongri, R.; Chengwei, W.; Xiang, G. Pharmacokinetic Characterization of Xylazine in Goats with Simultaneous Anesthesia Studies. J. Northeast. Agric. Univ. 2024, 31, 86–96. [Google Scholar]
- Kästner, S.B.R. α2-Agonists in Sheep: A Review. Vet. Anaesth. Analg. 2006, 33, 79–96. [Google Scholar] [CrossRef]
- EMEA Report. Committee for Veterinary Medicinal Products Xylazine Hydrochloride (Extension to Dairy Cows), Summary Report (2). The European Agency for the Evaluation of Medicinal Products, EMEA/MRL/836/02-Final-Corrigendum. 2002. Available online: https://www.ema.europa.eu/en/search?search_api_fulltext=xylazine&f%5B0%5D=ema_search_entity_is_document%3ADocument (accessed on 17 March 2025).
- Elejalde, J.I.; Louis, C.J.; Elcuaz, R.; Pinillos, M.A. Drug Abuse with Inhalated Xylazine. Eur. J. Emerg. Med. 2003, 10, 252–253. [Google Scholar] [CrossRef]
- De Carvalho, L.L.; Nishimura, L.T.; Borges, L.P.; Cerejo, S.A.; Villela, I.O.; Auckburally, A.; de Mattos-Junior, E. Sedative and Cardiopulmonary Effects of Xylazine Alone or in Combination with Methadone, Morphine or Tramadol in Sheep. Vet. Anaesth. Analg. 2016, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Ramakrishnan, S.; Haridoss, P.; Arthanari, K.T. Effects of Intravenous Xylazine versus Dexmedetomidine Premedication with Ketamine-Midazolam-Isoflurane Anaesthesia for Castration in Horses. Ind. J. Anim. Sci. 2024, 94, 117–122. [Google Scholar] [CrossRef]
- Nev, T.O.; Orakpoghenor, O.; Aondowase, U.; Terfa, A.J. Comparative Haematological Responses in Rumenotomy: Impact of Diazepam and Xylazine Pre-medication in West African Dwarf Goats Undergoing Propofol Anaesthesia. J. Res. Vet. Sci. 2024, 2, 152–158. [Google Scholar] [CrossRef]
- Abouelfetouh, M.M.; Liu, L.; Salah, E.; Sun, R.; Nan, S.; Ding, M.; Ding, Y. The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats. Animals 2021, 11, 723. [Google Scholar] [CrossRef]
- Yayla, S.; Kılıç, E.; Ogün, M.; Catalkaya, E.; Ermutlu, C.; Aydın, U. Premedication for Intrathecal Anesthesia in Dogs: Xylazine versus Propofol. Iran. J. Vet. Sci. Technol. 2020, 12, 44–49. [Google Scholar] [CrossRef]
- Adam, M.; Lindén, J.; Raekallio, M.; Abu-Shahba, A.; Mannerström, B.; Seppänen-Kaijansinkko, R.; Meller, A.; Salla, K. Concentrations of Vatinoxan and Xylazine in Plasma, Cerebrospinal Fluid and Brain Tissue Following Intravenous Administration in Sheep. Vet. Anaesth. Analg. 2021, 48, 900–905. [Google Scholar] [CrossRef]
- DeRossi, R.; Gaspar, E.B.; Junqueira, A.L.; Beretta, M.P. A Comparison of Two Subarachnoid α2-Agonists, Xylazine and Clonidine, with Respect to Duration of Antinociception, and Hemodynamic Effects in Goats. Small Rumin. Res. 2003, 47, 103–111. [Google Scholar] [CrossRef]
- Pratt, S.; Jeong, S.; Ahern, B.; Goodwin, W. Adverse Reaction Following the Subarachnoid Injection of Xylazine in a Sheep. Veter. Sci. 2022, 9, 479. [Google Scholar] [CrossRef]
- Yaksh, T.L. Pharmacology of Spinal Adrenergic Systems Which Modulate Spinal Nociceptive Processing. Pharmacol. Biochem. Behav. 1985, 22, 845–858. [Google Scholar] [CrossRef]
- Fleetwood-Walker, S.M.; Mitchell, R.; Hope, P.J.; Molony, V.; Iggo, A. An α2 Receptor Mediates the Selective Inhibition by Noradrenaline of Nociceptive Responses of Identified Dorsal Horn Neurones. Brain Res. 1985, 334, 243–254. [Google Scholar] [CrossRef]
- Yu, X.; Franks, N.P.; Wisden, W. Sleep and Sedative States Induced by Targeting the Histamine and Noradrenergic Systems. Front. Neural Circuits 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus-Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2020, 11, 601519. [Google Scholar] [CrossRef] [PubMed]
- Gittel, C.; Brehm, W.; Wippern, M.; Roth, S.; Hillmann, A.; Michaele, A. Xylazine or Detomidine in Dairy Calves: A Comparison of Clinically Relevant Pharmacodynamic Parameters under Sedation. Tierärztl. Prax. Ausg. G Großtiere Nutztiere 2021, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Putter, J.; Sagner, G. Chemical Studies to Detect Residues of Xylazine Hydrochloride. Vet. Med. Rev. 1973, 2, 145–159. [Google Scholar]
- Mutlib, A.E.; Chui, Y.C.; Young, L.M.; Abbott, F.S. Characterization of Metabolites of Xylazine Produced In Vivo and In Vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos. 1992, 20, 840–848. [Google Scholar] [PubMed]
- Park Choo, H.Y.; Choi, S.O. The Metabolism of Xylazine in Rats. Arch. Pharm. Res. 1991, 14, 346–351. [Google Scholar] [CrossRef]
- Matos, R.R.; Martucci, M.E.P.; de Anselmo, C.S.; Alquino Neto, F.R.; Pereira, H.M.G.; Sardela, V.F. Pharmacokinetic Study of Xylazine in a Zebrafish Water Tank, a Human-like Surrogate, by Liquid Chromatography Q-Orbitrap Mass Spectrometry. Forensic Toxicol. 2020, 38, 108–121. [Google Scholar] [CrossRef]
- D’Orazio, J.; Nelson, L.; Perrone, J.; Wightman, R.; Haroz, R. Xylazine Adulteration of the Heroin–fentanyl Drug Supply: A Narrative Review. Ann. Intern. Med. 2023, 176, 1370–1376. [Google Scholar] [CrossRef]
- Gupta, R.; Holtgrave, D.R.; Ashburn, M.A. Xylazine—Medical and Public Health Imperatives. N. Engl. J. Med. 2023, 388, 2209–2212. [Google Scholar] [CrossRef]
- Pergolizzi, J., Jr.; LeQuang, J.A.K.; Magnusson, P.; Miller, T.L.; Breve, F.; Varrassi, G. The New Stealth Drug on the Street: A Narrative Review of Xylazine as a Street Drug. Cureus 2023, 15, e40983. [Google Scholar] [CrossRef]
- McLean, S.; Murphy, B.P.; Starmer, G.A.; Thomas, J. Methaemoglobin Formation Induced by Aromatic Amines and Amides. J. Pharm. Pharmacol. 1967, 19, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.W.; Yarbrough, J.W.; Woldemeskel, M. Toxicity to Tetrahymena and Abiotic Thiol Reactivity of Aromatic Isothiocyanates. Cell Biol. Toxicol. 2005, 21, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gayakwad, H.; Baggi, T.R. Analytical Methods for the Determination of Xylazine in Pharmaceutical, Clinical and Forensic Matrices—A Review. Med. Sci. Law 2024, in press. [Google Scholar] [CrossRef]
- Psomas, J.E.; Fletouris, D.J. Liquid Chromatographic Assay of Xylazine in Sheep and Cattle Plasma. J. Liq. Chromatogr. 1992, 1515, 1543–1551. [Google Scholar] [CrossRef]
- Donnell, T.M.; Kelly, M.T.; Smyth, M.R. HPLC Determination of Xylazine in Equine Plasma by High Performance Liquid Chromatography. Anal. Lett. 1993, 26, 1547–1556. [Google Scholar] [CrossRef]
- Li, P.; Han, H.; Zhai, X.; He, W.; Sun, L.; Hou, J. Simultaneous HPLC-UV Determination of Ketamine, Xylazine, and Midazolam in Canine Plasma. J. Chromatogr. Sci. 2012, 50, 108–113. [Google Scholar] [CrossRef]
- Poklis, A.; Mackell, M.A.; Case, M.E. Xylazine in Human Tissue and Fluids in a Case of Fatal Drug Abuse. J. Anal. Toxicol. 1985, 9, 234–236. [Google Scholar] [CrossRef]
- Stillwell, M.E. A reported Case Involving Impaired Driving Following Self-Administration of Xylazine. Forensic Sci. Int. 2003, 134, 25–28. [Google Scholar] [CrossRef]
- Zheng, J.X.; Randall, S.; Grimsrud, K.; Bainbridge, S.; Tran, N.K. Not Carfentanil—A Case of Unexpected Xylazine Detection. J. Appl. Lab. Med. 2024, 9, 629–634. [Google Scholar] [CrossRef]
- Barroso, M.; Gallardo, E.; Margalho, C.; Devesa, N.; Pimentel, J.; Vieira, D.N. Solid-phase Extraction and Gas Chromatographic-mass Spectrometric Determination of the Veterinary Drug Xylazine in Human Blood. J. Anal. Toxicol. 2007, 31, 165–169. [Google Scholar] [CrossRef]
- Delahaut, P.; Brasseur, P.Y.; Dubois, M. Multiresidue Method for the Detection of Tranquillisers, Xylazine, and a β-Blocker in Animal Production by Liquid Chromatography-tandem Mass Spectrometry. J. Chromatogr. A 2004, 1054, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Doran, G.S.; Bradbury, L.A. Quantitation of the Anaesthetic Xylazine in Ovine Plasma by LC–MS/MS. J. Chromatog. B 2015, 997, 81–84. [Google Scholar] [CrossRef]
- Holland, D.C.; Munns, R.K.; Roybal, J.E.; Hurlbut, J.A.; Long, A.R. Simultaneous Determination of Xylazine and its Major Metabolite, 2,6-Dimethylaniline, in Bovine and Swine Kidney by Liquid Chromatography. J AOAC Int. 1993, 76, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mi, X.; Li, S.; Chen, G. Determination of Xylazine and 2,6-Xylidine in Animal Tissues by Liquid Chromatography—Tandem Mass Spectrometry. J. Food Sci. 2013, 78, T955–T959. [Google Scholar] [CrossRef]
- Diekhans, K.; Yu, J.; Farley, M.; Rodda, L.N. Analysis of Over 250 Novel Synthetic Opioids and Xylazine by LC–MS-MS in Blood and Urine. J. Anal. Toxicol. 2024, 48, 150–164. [Google Scholar] [CrossRef]
- Mendes, L.F.; Souza e Silva, A.R.; Bacil, R.P.; Pires Serrano, S.H.; Angnes, L.; Longo Cesar Paixao, T.R.; de Araujo, W.R. Forensic Electrochemistry: Electrochemical Study and Quantification of Xylazine in Pharmaceutical and Urine Samples. Electrochim. Acta 2019, 295, 726–734. [Google Scholar] [CrossRef]
- De Lima, L.F.; De Araujo, W.R. Laser-scribed Graphene on Polyetherimide Substrate: An Electrochemical Sensor Platform for Forensic Determination of Xylazine in Urine and Beverage Samples. Microchim. Acta 2022, 189, 465. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Sun, Y.; Xing, Y.; Zhang, G. An Ultrasensitive Monoclonal Antibody-Based Lateral Flow Immunoassay for the Rapid Detection of Xylazine in Milk. Food Chem. 2022, 383, 132293. [Google Scholar] [CrossRef]
- Marroquin-Garcia, R.; van Wissen, G.; Cleij, T.J.; Eersels, K.; van Grinsven, B.; Diliën, H. Single-use Dye Displacement Colorimetry Assay Based on Molecularly Imprinted Polymers: Towards Fast and on-site Detection of Xylazine in Alcoholic Beverages. Food Control 2024, 161, 110403. [Google Scholar] [CrossRef]
- Levitas, M.; Thomas, C.; Widman, C.; DeColumna, J.; Allgaier, B.; Conley, E.; deHagen, T.; Freitas, I.; Horvath, H.; Lembert, B.; et al. Qualitative and Quantitative Determination of Xylazine in Oral Fluid. J. Anal. Toxicol. 2024, 48, 482–488. [Google Scholar] [CrossRef]
- Zheng, Q.; Cao, Y.; Chen, Y.; Zhu, W.; Zhang, S.M.; Ye, Q.; Zhou, C.; Liu, Y.; Jia, N. Portable Electrochemical Test Strip Based on Au/Graphene for Rapid On-site Detection of Xylazine in Raw Milk. Microchem. J. 2025, 212, 113205. [Google Scholar] [CrossRef]
- He, X. Hyperspectral Raman Imaging with Multivariate Curve Resolution-Alternating Least Square (MCR-ALS) Analysis for Xylazine-Containing Drug Mixtures. Forensic Sci. Int. 2025, 367, 112314. [Google Scholar] [CrossRef] [PubMed]
- Vinnikov, A.; Sheppard, C.W.; Wemple, A.H.; Stern, J.E.; Leopold, M.C. An Amperometric Sensor with Anti-Fouling Properties for Indicating Xylazine Adulterant in Beverages. Micromachines 2024, 15, 1340. [Google Scholar] [CrossRef] [PubMed]
- Kohtala, S. Ketamine-50 Years in Use: From Anesthesia to Rapid Antidepressant Effects and Neurobiological Mechanisms. Pharmacol. Rep. 2021, 73, 323–345. [Google Scholar] [CrossRef]
- Mees, L.; Fidler, J.; Kreuzer, M.; Fu, J.; Pardue, M.T.; Garcia, P.S. Faster Emergence Behavior from Ketamine/Xylazine Anesthesia with Atipamezole versus Yohimbine. PLoS ONE 2018, 13, e0199087. [Google Scholar] [CrossRef]
- Sandbaumhüter, F.A.; Theurillat, R.; Bettschart-Wolfensberger, R.; Thormann, W. Effect of the α2-Receptor Agonists Medetomidine, Detomidine, Xylazine, and Romifidine on the Ketamine Metabolism in Equines Assessed with Enantioselective Capillary Electrophoresis. Electrophoresis 2017, 38, 1895–1904. [Google Scholar] [CrossRef]
- Skelding, A.; Queiroz-Williams, P.; Bettschart-Wolfensberger, R.; Ringer, S.K. Pharmacology of Drugs Used in Equine Anesthesia Phenothiazines. In Manual of Equine Anesthesia and Analgesia, 2nd ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 184–222. [Google Scholar] [CrossRef]
- Grubb, T.L.; Riebold, T.W.; Huber, M.J. Evaluation of Lidocaine, Xylazine, and a Combination of Lidocaine and Xylazine for Epidural Analgesia in Llamas. J. Am. Vet. Med. Assoc. 1993, 203, 1441–1444. [Google Scholar] [CrossRef]
- DeRossi, R.; Junqueira, A.L.; Beretta, M.P. Analgesic and Systemic Effects of Xylazine, Lidocaine and their Combination after Subarachnoid Administration in Goats. J. S. Afr. Vet. Assoc. 2005, 76, 79–84. [Google Scholar] [CrossRef]
- Lee, I.; Ayukawa, Y.; Sasaki, N.; Yamada, H.; Yamagishi, N.; Oboshi, K. Comparison of Xylazine, Lidocaine and the Two Drugs Combined for Modified Dorsolumbar Epidural Anaesthesia in Cattle. Vet. Rec. 2004, 155, 797–799. [Google Scholar]
- Klein, S.E.; Dodam, J.R.; Ge, B.; Strawn, M.; Varner, K.M. Comparison of Lidocaine and Lidocaine-xylazine for Distal Paravertebral Anesthesia in Dairy Cattle. J. Am. Vet. Med. Assoc. 2023, 262, 241–245. [Google Scholar] [CrossRef]
- Khalil, K.A.; Mousa, Y.J.; Alzubaidy, M.H. Pharmacokinetic Criteria of Ketoprofen and its Cyclooxygenase-2 Inhibition in Mice: Influence of Xylazine Administration. Maced. Vet. Rev. 2022, 46, 27–33. [Google Scholar] [CrossRef]
- Uney, K.; Yuksel, M.; Durna Corum, D.; Coskun, D.; Turk, E.; Dingil, H.B.; Corum, O. Effect of Xylazine on Pharmacokinetics and Physiological Efficacy of Intravenous Carprofen in Castrated Goats Kids. Animals 2023, 13, 2700. [Google Scholar] [CrossRef] [PubMed]
- Ukwueze, O.C.; Eze, C.A.; Anaga, A.O. Evaluation of Analgesic Effect of Xylazine, Tramadol and Lignocaine on Propofol Anaesthesia in West African Dwarf (WAD) Goat. Veterinaria 2024, 73, 150–162. [Google Scholar] [CrossRef]
- Nash, P.B.; Hemphill, M.A.; Barron, J.N. Administration of Ketamine/Xylazine Increases Severity of Influenza (A/Puerto Rico/8/34) in Mice. Heliyon 2023, 9, e14368. [Google Scholar] [CrossRef]
- Fitzgerald, N.D.; Palamar, J.J.; Cottler, L.B. Use of Illegally Manufactured Fentanyl in the United States: Current Trends. Curr. Addict. Rep. 2025, 12, 6. [Google Scholar] [CrossRef]
- Eger, W.H.; Plesons, M.; Bartholomew, T.S.; Bazzi, A.R.; Hauschild, M.H.; McElrath, C.C.; Owens, C.; Forrest, D.W.; Tookes, H.E.; Crable, E.L. Protective or Potentially Harmful? Altering Drug Consumption Behaviors in Response to Xylazine Adulteration. Res. Squ. 2024, in press. [Google Scholar] [CrossRef]
- Quijano, T.; Crowell, J.; Eggert, K.; Clark, K.; Alexander, M.; Grau, L.; Heimer, R. Xylazine in the Drug Supply: Emerging Threats and Lessons Learned in Areas with High Levels of Adulteration. Int. J. Drug Policy 2023, 120, 104154. [Google Scholar] [CrossRef]
- Reed, M.K.; Imperato, N.S.; Bowles, J.M.; Salcedo, V.J.; Guth, A.; Rising, K.L. Perspectives of People in Philadelphia Who Use Fentanyl/Heroin Adulterated with the Animal Tranquilizer Xylazine; Making a Case for Xylazine Test Strips. Drug Alc. Depend. Rep. 2022, 4, 100074. [Google Scholar] [CrossRef]
- Chavez, C.; Sanabria, D.; Ruiz, K. Nine Xylazine Related Deaths in Puerto Rico, Oral Presentation (K44). In Forensic Toxicology Proceedings: Forensic Sci 2002–2011; American Academy of Forensic Sciences: Colorado Springs, CO, USA, 2009; p. 77. [Google Scholar]
- Rai, N.S.; Friend, C.A. Veterinary Drug Causes Heart Failure? A Rare Case of Xylazine Induced Cardiomyopathy in a 22 Year Old. J. Am. Coll. Cardiol. 2022, 79 (Suppl. S9), 2752. [Google Scholar]
- Malayala, S.V.; Papudesi, B.N.; Bobb, R.; Wimbush, A. Xylazine-Induced Skin Ulcers in a Person Who Injects Drugs in Philadelphia, Pennsylvania, USA. Cureus 2022, 14, e28160. [Google Scholar] [CrossRef]
- Bishnoi, A.; Singh, V.; Khanna, U.; Vinay, K. Skin Ulcerations Caused by Xylazine: A Lesser-Known Entity. J. Am. Acad. Dermatol. 2023, 89, e99–e102. [Google Scholar] [CrossRef] [PubMed]
- Zagorski, C.M.; Hosey, R.A.; Moraff, C.; Ferguson, A.; Figgatt, M.; Aronowitz, S.; Stahl, L.E.; Hill, L.G.; McElligott, Z.; Dasgupta, N. Reducing the Harms of Xylazine: Clinical Approaches, Research Deficits, and Public Health Context. Harm Reduct. J. 2023, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Levy, J.; Rubenstein, R.; Ilyas, E.N.; Ramtin, S.; Jones, C. Xylazine-induced Skin Necrosis, Emerging Public Health Crisis and the Ethical Considerations in Surgical Reconstruction: A Case Report. SurgiColl 2024, 2, 1–8. [Google Scholar] [CrossRef]
- Eid, S.M.; Attia, K.A.; El-Olemy, A.; Abbas, A.E.F.; Abdelshafi, N.A. An Innovative Nanoparticle-modified Carbon Paste Sensor for Ultrasensitive Detection of Lignocaine and Its Extremely Carcinogenic Metabolite Residues in Bovine Food Samples: Application of NEMI, ESA, AGREE, ComplexGAPI, and RGB12 algorithms. Food Chem. 2023, 426, 136579. [Google Scholar] [CrossRef]
- Kirkland, D.J.; Sheil, M.L.; Streicker, M.A.; Johnson, G.E. A Weight of Evidence Assessment of the Genotoxicity of 2,6-Xylidine Based on Existing and New Data, with Relevance to Safety of Lidocaine Exposure. Reg. Toxicol. Pharm. 2021, 119, 104838. [Google Scholar] [CrossRef]
- Acosta-Mares, P.; Violante-Soria, V.; Browne, T., Jr.; Cruz, S.L. Xylazine Potentiates the Lethal but not the Rewarding Effects of Fentanyl in Mice. Drug Alcohol Depend. 2023, 253, 110993. [Google Scholar] [CrossRef]
- Choi, S.; Irwin, M.R.; Kiyatkin, E.A. Xylazine Effects on Opioid-induced Brain Hypoxia. Psychopharmacology 2023, 240, 1561–1571. [Google Scholar] [CrossRef]
- Choi, S.; Irwin, M.R.; Noya, M.R.; Shaham, Y.; Kiyatkin, E.A. Combined Treatment with Naloxone and the Alpha2 Adrenoceptor Antagonist Atipamezole Reversed Brain Hypoxia Induced by a Fentanyl-Xylazine Mixture in a Rat Model. Neuropsychopharmacology 2024, 49, 1104–1112. [Google Scholar] [CrossRef]
- Mittleman, R.E.; Hearn, W.L.; Hime, G.W. Xylazine Toxicity—Literature Review and Report of Two Cases. J. Forensic Sci. 1998, 43, 400–402. [Google Scholar] [PubMed]
- Ayub, S.; Parnia, S.; Poddar, K.; Bachu, A.K.; Sullivan, A.; Khan, A.M.; Ahmed, S.; Jain, L. Xylazine in the Opioid Epidemic: A Systematic Review of Case Reports and Clinical Implications. Cureus 2023, 15, e36864. [Google Scholar] [CrossRef]
- Capraro, A.J.; Wiley, J.F., 2nd; Tucker, J.R. Severe Intoxication from Xylazine Inhalation. Pediatr. Emerg. Care 2001, 17, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Meister, C.M.; Golle, K.; Zschiesche, M. Severe Intoxication with the Veterinary Tranquilizer Xylazine in Humans. J. Anal. Toxicol. 2001, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, P. ‘Zombie Drug’ Tranq: Flesh-Rotting Drug Surges in US, Leaving a Trail of Deaths and Destruction: Hindustan Times 2023. Available online: https://www.hindustantimes.com/world-news/zombie-drug-tranq-flesh-rotting-drug-surges-in-us-leaving-a-trail-of-deaths-and-destruction-101688489718164.html (accessed on 20 August 2024).
- Warp, P.V.; Hauschild, M.; Serota, D.P.; Ciraldo, K.; Cruz, I.; Bartholomew, T.S.; Tookes, H.E. A Confirmed Case of Xylazine-induced Skin Ulcers in a Person Who Injects Drugs in Miami, Florida, USA. Harm Reduct. J. 2024, 21, 64. [Google Scholar] [CrossRef]
- Sisco, E.; Appley, M.G.; Pyfrom, E.M.; Banta-Green, C.J.; Shover, C.L.; Molina, C.A.; Biamont, B.; Robinson, E.L. Beyond Fentanyl Test Strips: Investigating Other Urine Drug Test Strips for Drug Checking Applications. Forensic Chem. 2024, 40, 100594. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.; Tardif, J.; Ujeneza, M.; Badea, A.; Green, T.C.; McKee, H.; McKenzie, M.; Park, J.N. Pilot Findings on the Real-world Performance of Xylazine Test Strips for Drug Residue Testing and the Importance of Secondary Testing Methods. Drug Alcohol Depend. Rep. 2024, 11, 100241. [Google Scholar] [CrossRef]
- Shrestha, S.; Cyr, K.; Hajinazarian, G.; Dillon, J.; Oh, T.; Pustz, J.; Stopka, T.J. Exploring Xylazine Awareness, Health Impacts, and Harm Reduction Strategies: Findings from a Multimethods Study in Lowell, Massachusetts. Subst. Use Addict. J. 2024, in press. [Google Scholar] [CrossRef]
- Friedman, J.R.; Montoya, A.G.; Ruiz, C.; Tejeda, M.A.G.; Segovia, L.A.; Godvin, M.E.; Sisco, E.; Pyfrom, E.M.; Appley, M.G.; Shover, C.L.; et al. The Detection of Xylazine in Tijuana, Mexico: Triangulating Drug Checking and Clinical Urine Testing Data. medRxiv 2024. [Google Scholar] [CrossRef]
- Sugarman, O.K.; Shah, H.; Whaley, S.; McCourt, A.; Saloner, B.; Bandara, S. A Content Analysis of Legal Policy Responses to Xylazine in the Illicit Drug Supply in the United States. Int. J. Drug Policy 2024, 129, 104472. [Google Scholar] [CrossRef]
- Dai, P.; Chen, Y.; Luo, X.; Zhou, Z.; Shi, M.; Genjiafu, A.; Jian, X. Fatal Hyperpyrexia Caused by Xylazine: A Case Report. Front. Pharmacol. 2024, 15, 1437960. [Google Scholar] [CrossRef]
- Rock, K.L.; Lawson, A.J.; Duffy, J.; Mellor, A.; Treble, R.; Copeland, C.S. The First Drug-Related Death Associated with Xylazine Use in the UK and Europe. J. Forensic Leg. Med. 2023, 97, 102542. [Google Scholar] [CrossRef]
- Di Trana, A.; Di Giorgi, A.; Carlier, J.; Serra, F.; Busardò, F.P.; Pichini, S. “Tranq-dope”: The First Fatal Intoxication Due to Xylazine-adulterated Heroin in Italy. Clin. Chim. Acta 2024, 561, 119826. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.A.; De Jong, A.R. Xylazine Complicating Opioid Ingestions in Young Children. Pediatrics 2023, 151, e2022058684. [Google Scholar] [CrossRef] [PubMed]
- Hull, I.; Jawa, R.; Shang, M.; Davis, C.; King, C.; McMurtrie, G.; Krans, E. Implications of Xylazine Exposure in Pregnancy: A Narrative Review. J. Addict. Dis. 2024, in press. [Google Scholar] [CrossRef]
- Administration, D.E. The Growing Threat of Xylazine and Its Mixture with Illicit Drugs. 2024. Available online: https://www.dea.gov/documents/2022/2022-12/2022-12-21/growing-threat-xylazine-and-its-mixture-illicit-drugs (accessed on 16 October 2024).
- Johnson, J.; Pizzicato, L.; Johnson, C.; Viner, K. Increasing Presence of Xylazine in Heroin and/or Fentanyl Deaths, Philadelphia, Pennsylvania, 2010–2019. Inj. Prev. 2021, 27, 395–398. [Google Scholar] [CrossRef]
- Thuillier, A.V.; Qiao, Y.; Wu, Z.H. Modeling Changes of Fatal Xylazine-Involved Drug Overdoses in Connecticut Across Time. Subst. Use Misuse 2024, 59, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Delcher, C.; Anthony, N.; Mir, M. Xylazine-involved Fatal Overdoses and Localized Geographic Clustering: Cook County, IL, 2019–2022. Drug Alcohol Depend. 2023, 249, 110833. [Google Scholar] [CrossRef]
- Michaels, N.L.; Bista, S.; Short Mejia, A.; Hays, H.; Smith, G.A. Xylazine Awareness and Attitudes among People Who Use Drugs in Ohio, 2023–2024. Harm Reduct. J. 2024, 21, 182. [Google Scholar] [CrossRef]
- Rubin, R. Here’s What to Know about Xylazine, Aka Tranq, the Animal Tranquilizer Increasingly Found in Illicit Fentanyl Samples. JAMA 2023, 329, 1904–1906. [Google Scholar] [CrossRef]
- United States Drug Enforcement Administration. DEA Reports Widespread Threat of Fentanyl Mixed with Xylazine. 2023. Available online: https://www.dea.gov/alert/dea-reports-widespread-threat-fentanyl-mixed-xylazine (accessed on 16 October 2024).
- Cano, M.; Daniulaityte, R.; Marsiglia, F. Xylazine in Overdose Deaths and Forensic Drug Reports in US States, 2019-2022. JAMA Netw. Open 2024, 7, e2350630. [Google Scholar] [CrossRef]
- Marra, M.; Catalano, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Salpini, R.; Svicher, V.; Marsico, S.; Aquaro, S.; Pellegrino, M. New Therapies and Strategies to Curb HIV Infections with a Focus on Macrophages and Reservoirs. Viruses 2024, 16, 1484. [Google Scholar] [CrossRef]
- SAMHSA. Harm Reduction. Available online: https://www.samhsa.gov/find-help/harm-reduction (accessed on 8 January 2025).

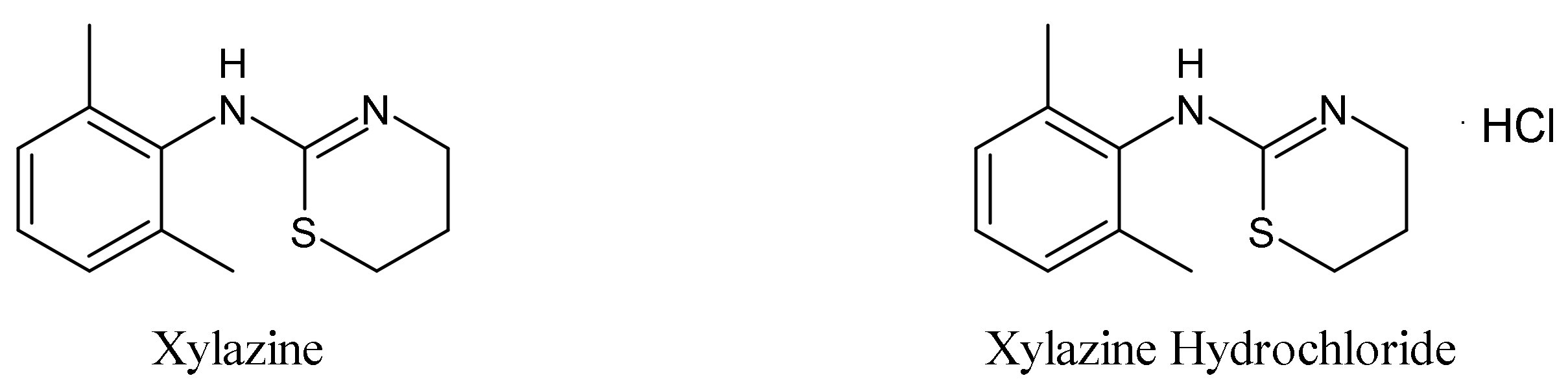

| t1/2 | Tmax | Fatal Dose Range | Approximate Effect Duration |
|---|---|---|---|
| 23–50 min | 12–14 min | 40–2400 mg | 4–6 h |
| Method of Detection | LOD | Sample | Ref. |
|---|---|---|---|
| Electroanalytical method using glassy carbon electrode | 120 nmol/L | Pharmaceutical and Urine samples | [126] |
| Portable electrochemical devices integrated with a smartphone | 0.06 μg/mL | Beverage samples | [75] |
| Laser-scribed graphene on polyetherimide substrate | 1.39 × 10−7 mol/L | Urine and Beverage samples | [127] |
| Monoclonal-antibody-based lateral flow immunoassay | 0.1 ng/mL | Milk | [128] |
| Colorimetric assay using molecularly imprinted polymers | 1.36 mM * | Gin and tonic drinks | [129] |
| Enzyme-linked immunosorbent assay | 0.1 ng/mL ** | Oral fluid | [130] |
| Portable electrochemical test strips based on Au/graphene | 0.02 µg/L | Raw milk | [131] |
| Portable amperometric sensor with anti-fouling properties | ~1 ppm * | Alcoholic and Non-alcoholic beverages | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Catalano, A.; Aiello, F.; Andreu, I.; Sinicropi, M.S.; Lentini, G. Xylazine, a Drug Adulterant Whose Use Is Spreading in the Human Population from the U.S. to the U.K. and All Europe: An Updated Review. Appl. Sci. 2025, 15, 3410. https://doi.org/10.3390/app15063410
Iacopetta D, Catalano A, Aiello F, Andreu I, Sinicropi MS, Lentini G. Xylazine, a Drug Adulterant Whose Use Is Spreading in the Human Population from the U.S. to the U.K. and All Europe: An Updated Review. Applied Sciences. 2025; 15(6):3410. https://doi.org/10.3390/app15063410
Chicago/Turabian StyleIacopetta, Domenico, Alessia Catalano, Francesca Aiello, Inmaculada Andreu, Maria Stefania Sinicropi, and Giovanni Lentini. 2025. "Xylazine, a Drug Adulterant Whose Use Is Spreading in the Human Population from the U.S. to the U.K. and All Europe: An Updated Review" Applied Sciences 15, no. 6: 3410. https://doi.org/10.3390/app15063410
APA StyleIacopetta, D., Catalano, A., Aiello, F., Andreu, I., Sinicropi, M. S., & Lentini, G. (2025). Xylazine, a Drug Adulterant Whose Use Is Spreading in the Human Population from the U.S. to the U.K. and All Europe: An Updated Review. Applied Sciences, 15(6), 3410. https://doi.org/10.3390/app15063410










