Efficiency of H2O2-Modified Ferrite Process for High-Concentration PVA Removal and Magnetic Nanoparticle Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.3. Preparation and Recovery of Magnetic Precipitates
2.4. Additional Information Regarding the Sample Preparation
3. Results and Discussion
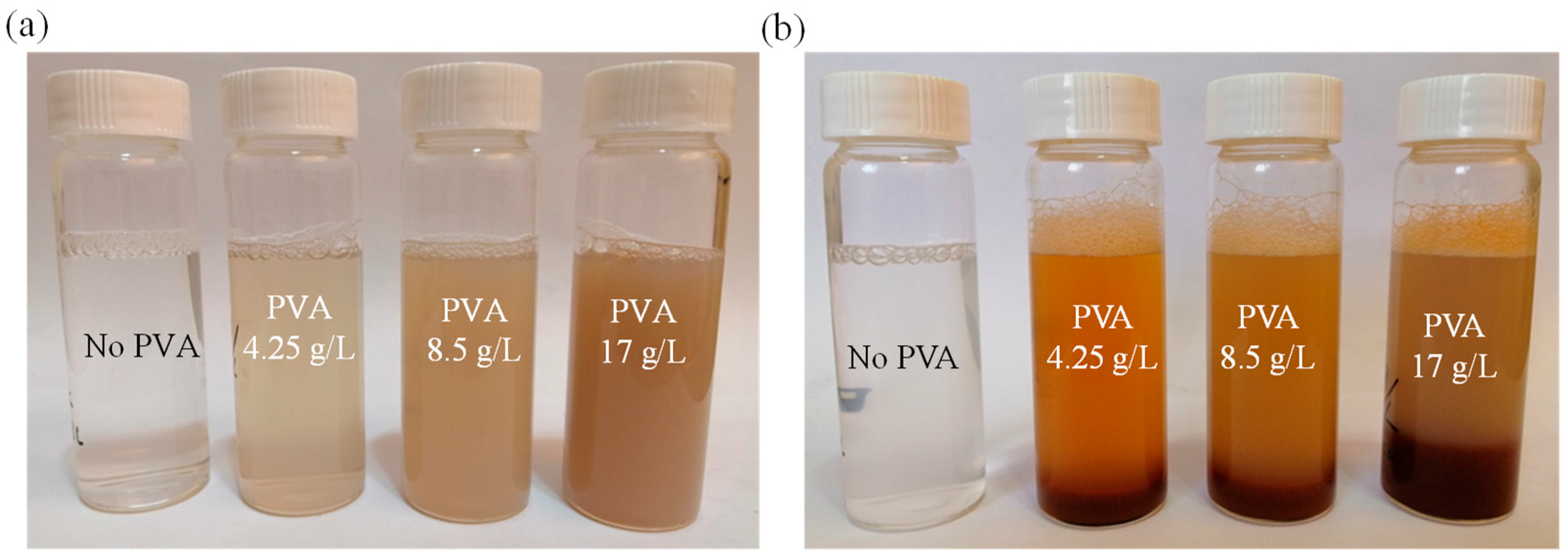
3.1. Visual Observation of PVA Treatment Effects by Ferrite Process
3.2. Effects of Modified Ferrite Treatment on PVA Molecular Structure
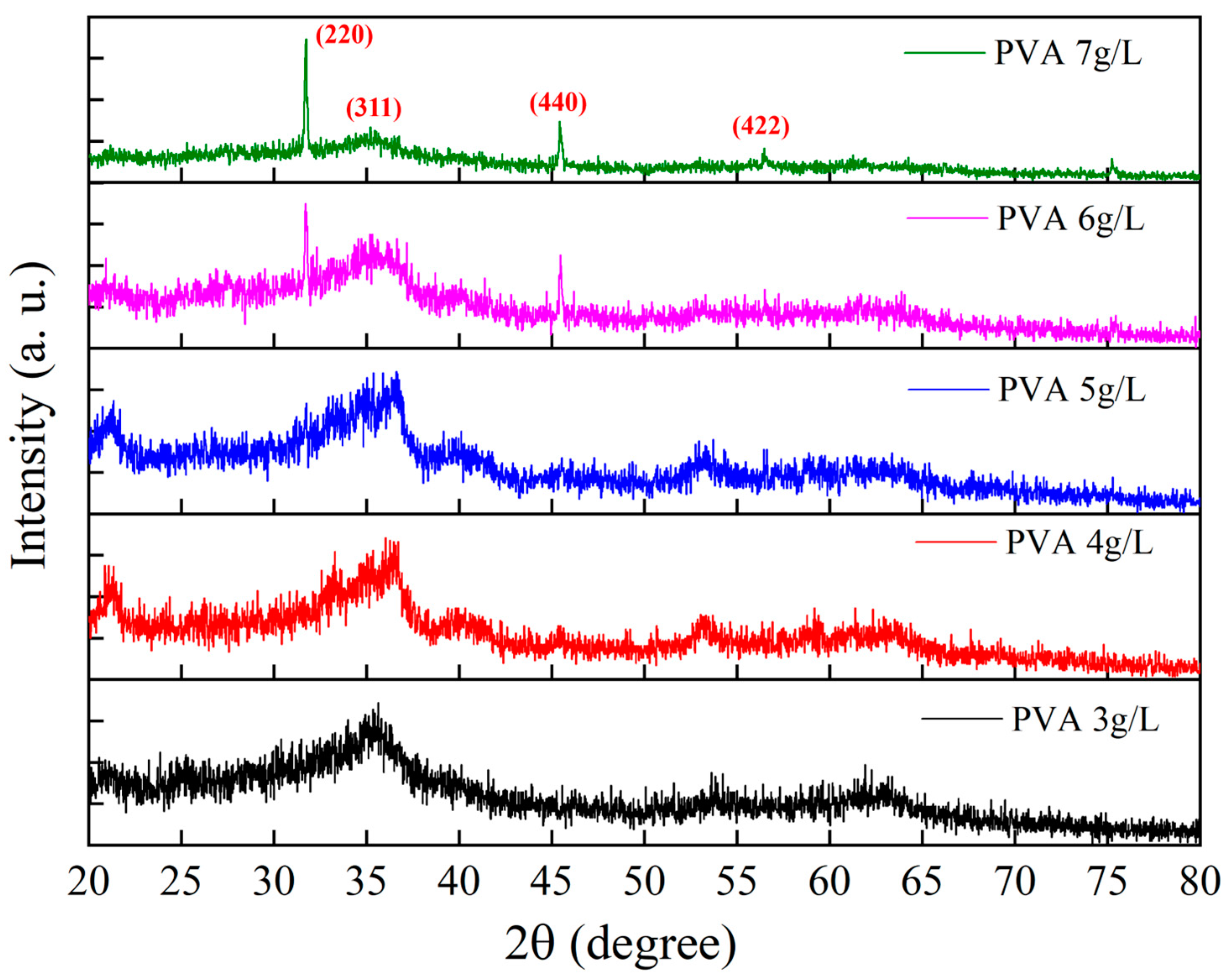
3.3. Crystal Structure Analysis of Filter Residues at Different PVA Concentrations
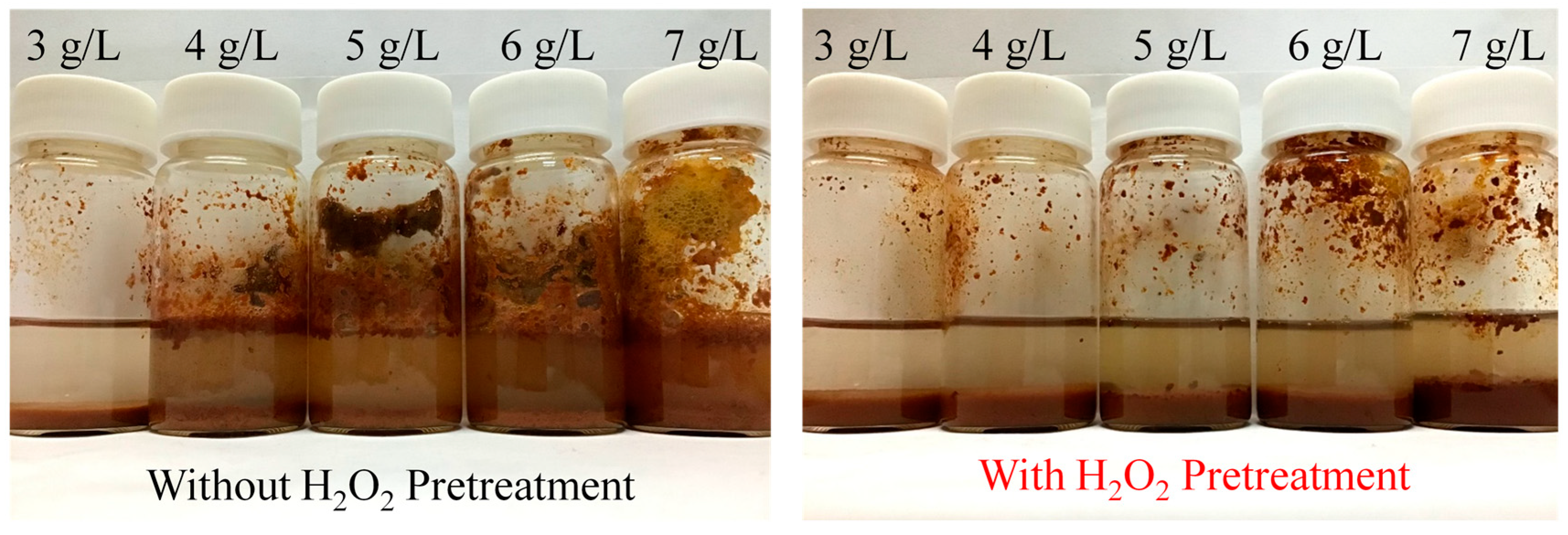
3.4. Visual Observation of H2O2 Pre-Oxidation Effects on PVA Removal
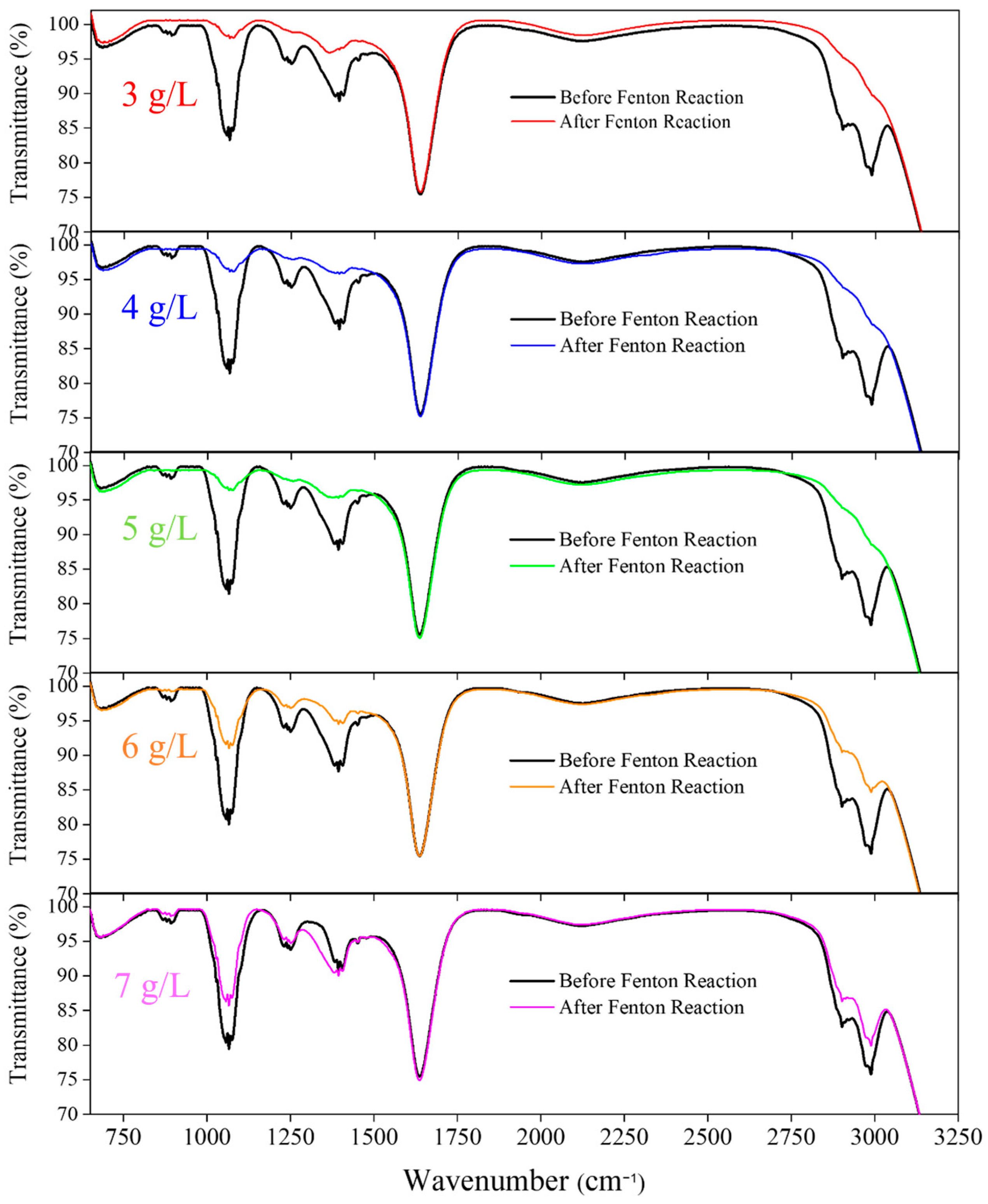
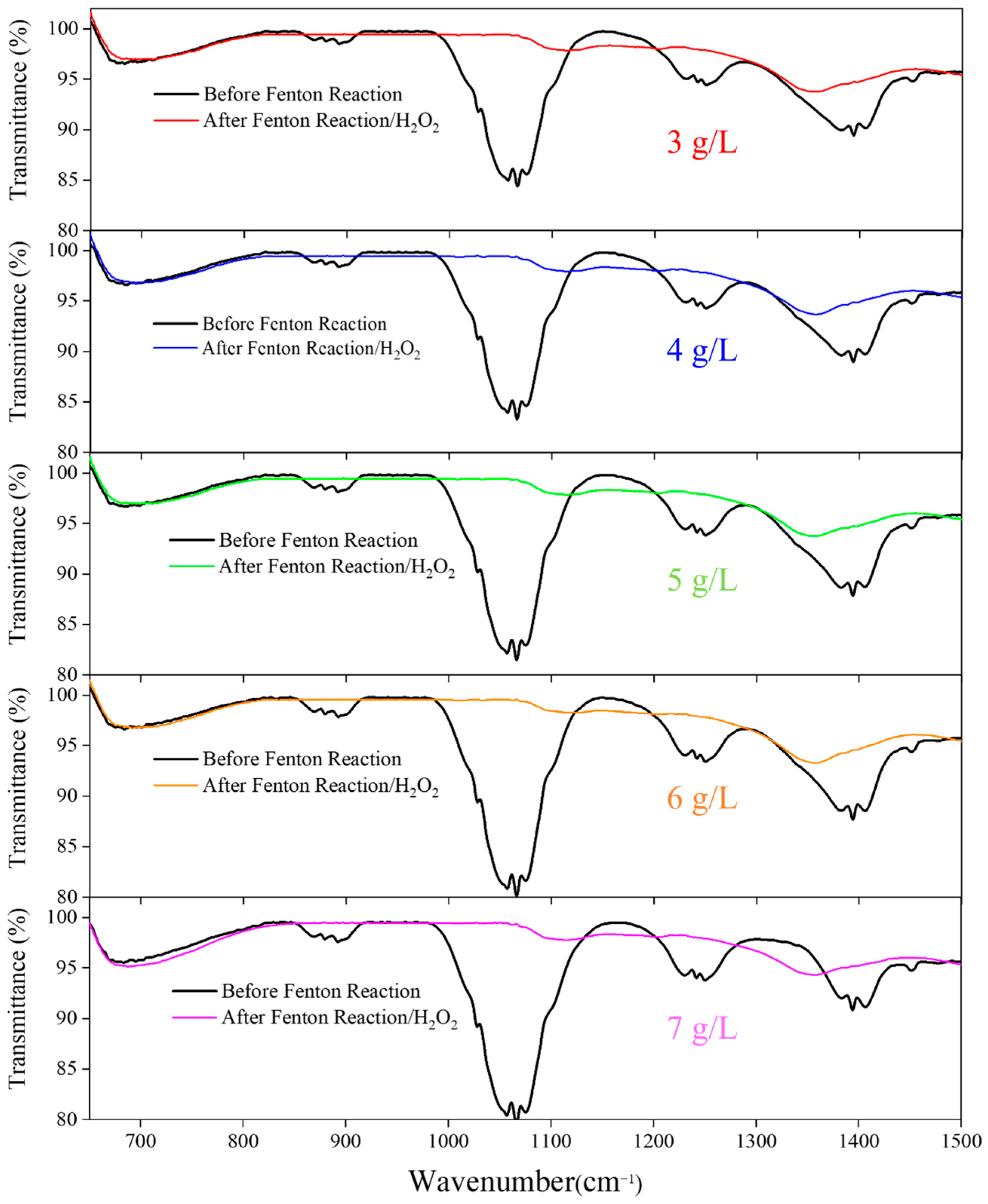
3.5. FTIR Analysis of H2O2 Pre-Oxidation Effects and Treatment Efficiency
3.6. Characteristics of Filter Residues
3.6.1. Magnetic Response Analysis of Filter Residues in Solution and After Heat Treatment
3.6.2. Visual Appearance Analysis
3.6.3. Magnetic Properties Analysis of Filter Residues
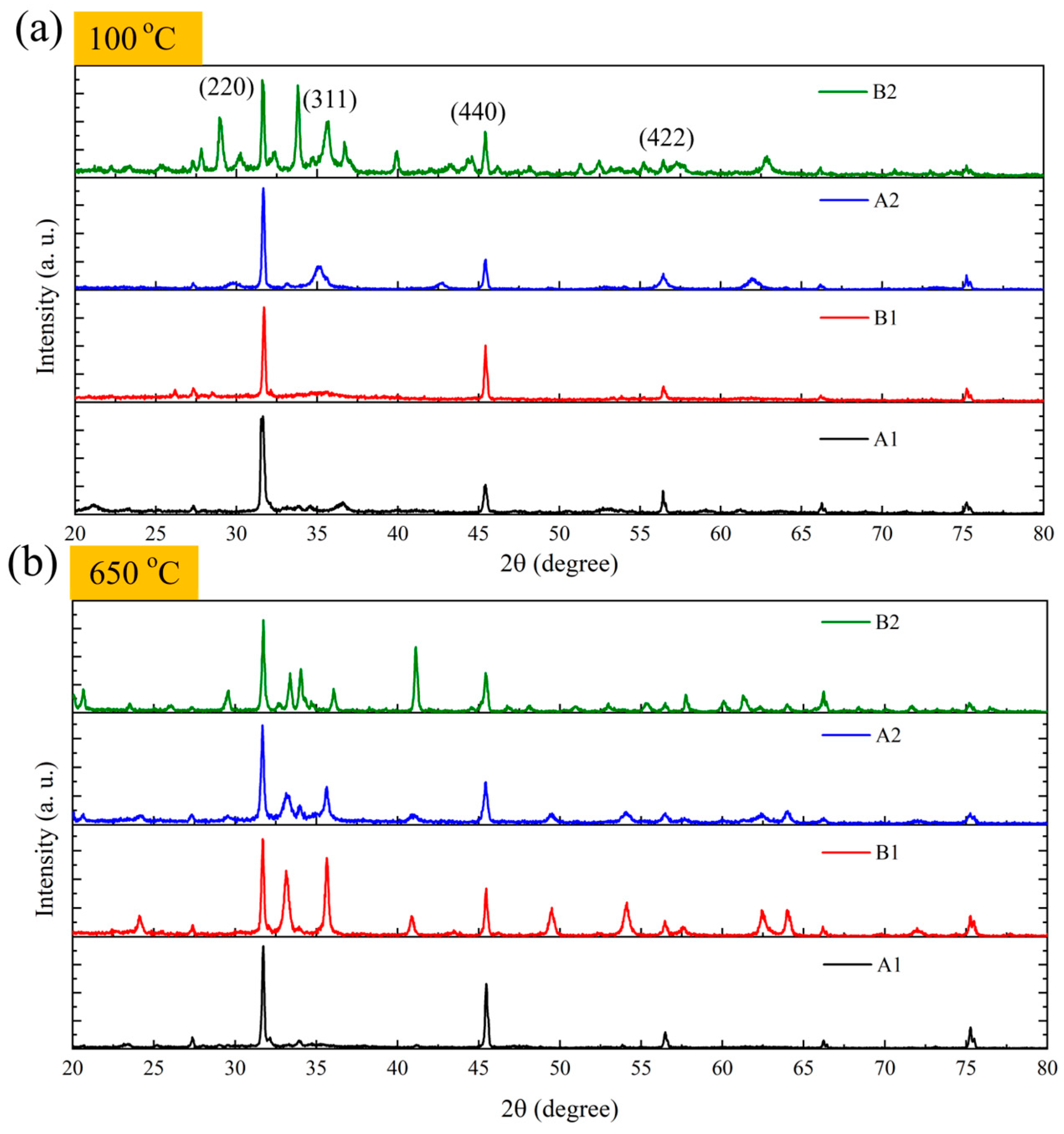
3.6.4. Correlation Between XRD Analysis and Magnetic Properties of Filter Residues
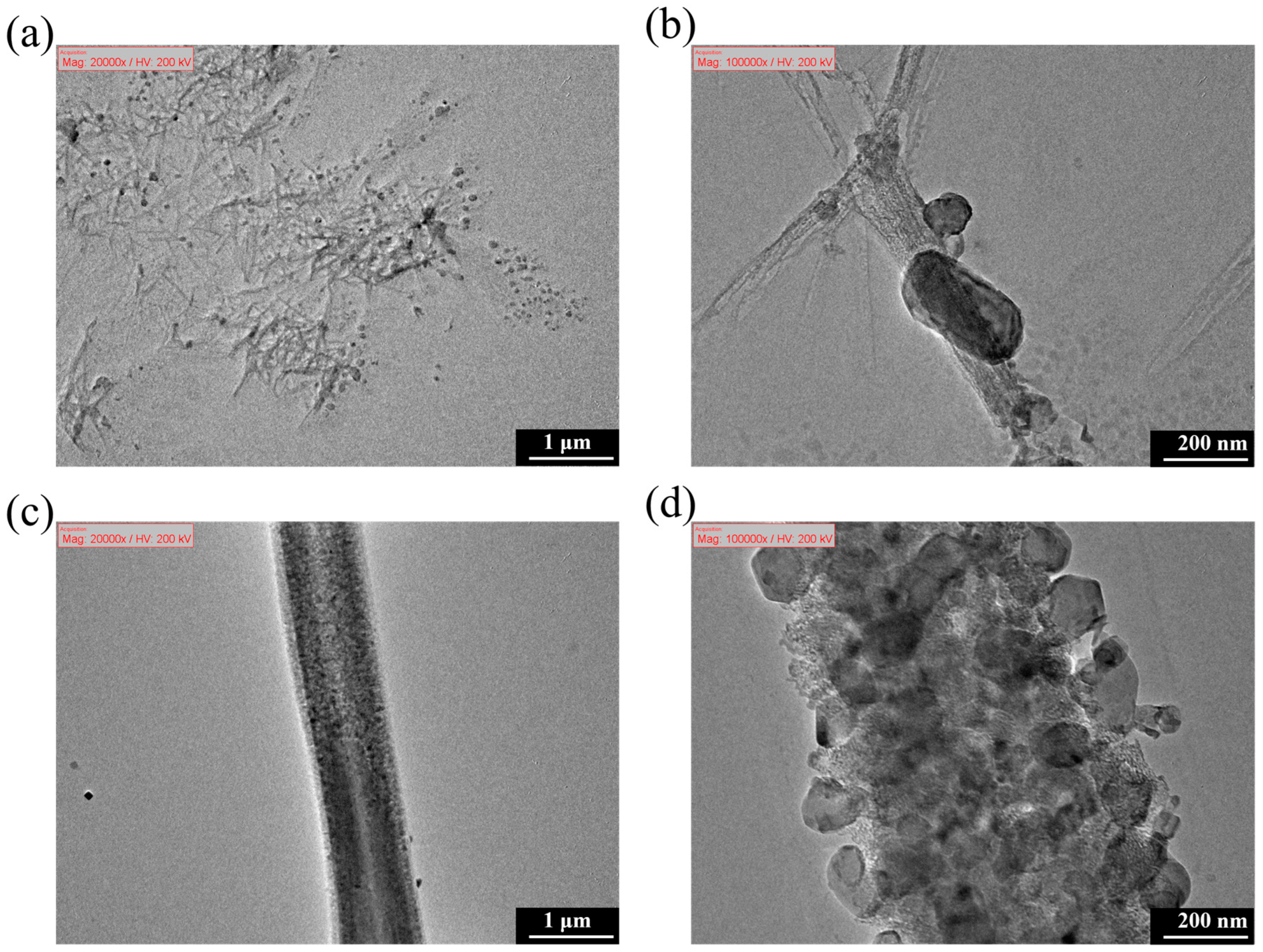
3.6.5. Microscopic Morphology Analysis of Filter Residues
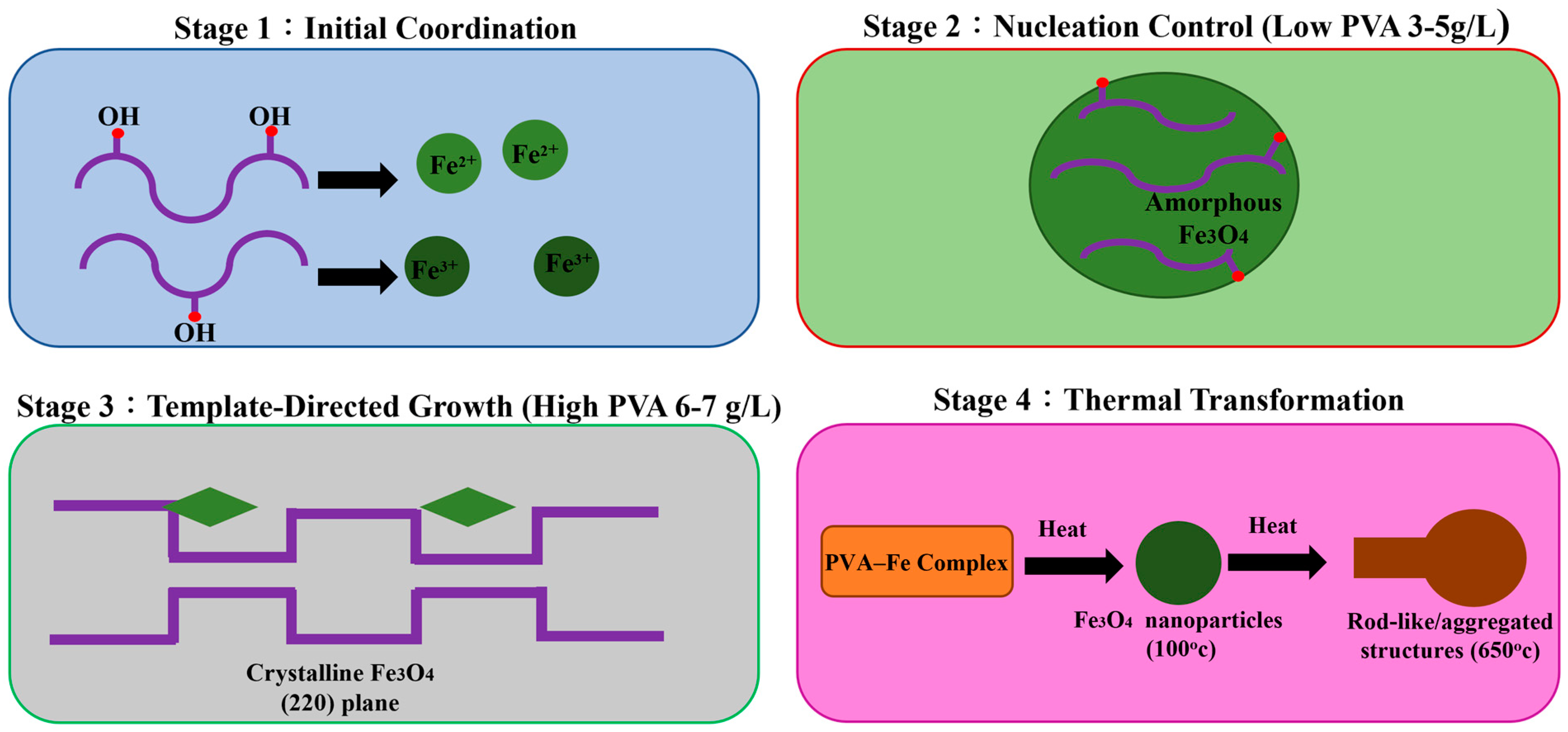
3.6.6. Proposed Interaction Mechanism Between PVA and Iron Oxides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Couți, N.; Porfire, A.; Iovanov, R.; Crișan, A.G.; Iurian, S.; Casian, T.; Tomuță, I. Polyvinyl Alcohol, a Versatile Excipient for Pharmaceutical 3D Printing. Polymers 2024, 16, 517. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; He, J. Polyvinyl alcohol (PVA)-based hydrogels: Recent progress in fabrication, properties, and multifunctional applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.H.; Araújo, E.S. An approach to 3D printing techniques, polymer materials, and their applications in the production of drug delivery systems. Compounds 2024, 4, 71–105. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Li, D.; Wang, Y.; Geng, S.; Qian, K. Study on the fracture behavior and anisotropy of 3D-printing PVA fiber-reinforced concrete. Constr. Build. Mater. 2024, 447, 138051. [Google Scholar] [CrossRef]
- Luo, S.; Li, W.; Wang, D. Study on bending performance of 3D printed PVA fiber reinforced cement-based material. Constr. Build. Mater. 2024, 433, 136637. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V. Degradation of polyvinyl alcohol in US wastewater treatment plants and subsequent nationwide emission estimate. Int. J. Environ. Res. Public Health 2021, 18, 6027. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Wang, J. Degradation of PVA (polyvinyl alcohol) in wastewater by advanced oxidation processes. J. Adv. Oxid. Technol. 2017, 20, 20170018. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Yadav, L.; Agarwal, M.; Gupta, R. Sustainable solution for wastewater management: Fabrication of cost-effective β-cyclodextrin incorporated chitosan polyvinyl alcohol composite hydrogel film for the efficient adsorption of anionic congo red and tartrazine dyes. Sep. Purif. Technol. 2025, 355, 129750. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Ruman, U.E.; He, G.; Sabir, A.; Shafiq, M.; Zubair, M. Environmental Issues Concerned with Poly (Vinyl Alcohol)(PVA) in Textile Wastewater. In Polymer Technology in Dye-containing Wastewater: Volume 1; Springer Nature: Singapore, 2022; pp. 225–236. [Google Scholar]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- Yonar, T.; Kestioglu, K.; Azbar, N. Treatability studies on domestic wastewater using UV/H2O2 process. Appl. Catal. B Environ. 2006, 67, 223–228. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; Ismail, A.F. A review on the use of membrane technology systems in developing countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Dhib, R.; Mehrvar, M. Recent advances in dynamic modeling and process control of PVA degradation by biological and advanced oxidation processes: A review on trends and advances. Environments 2021, 8, 116. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Xiao, B.; Zhou, C.; Jiang, Z.; Liang, Y.; Wang, S. Visible-light photocatalytic degradation of water-soluble polyvinyl alcohol in aqueous solution by Cu2O@ TiO2: Optimization of conditions, mechanisms and toxicity analysis. J. Environ. Manag. 2023, 341, 118054. [Google Scholar] [CrossRef]
- Parsa, Z.; Dhib, R.; Mehrvar, M. Continuous UV/H2O2 Process: A Sustainable Wastewater Treatment Approach for Enhancing the Biodegradability of Aqueous PVA. Sustainability 2024, 16, 7060. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Prozorovich, V.G.; Krivoshapkina, E.F.; Kuznetsova, T.F.; Krivoshapkin, P.V.; Katsoshvili, L.L. Physicochemical properties of manganese oxides obtained via the sol–gel method: The reduction of potassium permanganate by polyvinyl alcohol. Russ. J. Phys. Chem. A 2017, 91, 1486–1492. [Google Scholar] [CrossRef]
- Ye, B.; Li, Y.; Chen, Z.; Wu, Q.Y.; Wang, W.L.; Wang, T.; Hu, H.Y. Degradation of polyvinyl alcohol (PVA) by UV/chlorine oxidation: Radical roles, influencing factors, and degradation pathway. Water Res. 2017, 124, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Huang, K.Y.; Wang, C.T.; Chou, W.L.; Shu, C.M. Removal of polyvinyl alcohol using photoelectrochemical oxidation processes based on hydrogen peroxide electrogeneration. Int. J. Photoenergy 2013, 2013, 841762. [Google Scholar] [CrossRef]
- Chou, W.L. Removal and adsorption characteristics of polyvinyl alcohol from aqueous solutions using electrocoagulation. J. Hazard. Mater. 2010, 177, 842–850. [Google Scholar] [CrossRef]
- Lin, D.; Fu, Y.; Li, X.; Wang, L.; Hou, M.; Hu, D.; Boczkaj, G. Application of persulfate-based oxidation processes to address diverse sustainability challenges: A critical review. J. Hazard. Mater. 2022, 440, 129722. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, H.W.; Park, J.M.; Park, H.S.; Yoon, C. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Dong, Y.; Fu, Y.; Wang, L.; Li, Q.; Liu, Y.; Wang, Z. Non-radical reactions in persulfate-based homogeneous degradation processes: A review. Chem. Eng. J. 2021, 421, 127818. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, C.; Shao, P.; Luo, X.; Zhang, H.; Wang, S.; Duan, X. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes. Environ. Sci. Technol. 2021, 56, 78–97. [Google Scholar] [CrossRef]
- Zohrabi, Y. Synthesis and application of magnetic ferrites (MFe2O4) in the removal of heavy metals from aqueous solutions: An updated review. Mater. Sci. Eng. B 2024, 299, 117024. [Google Scholar] [CrossRef]
- Abidin, M.Z.U.; Ikram, M.; Moeen, S.; Nazir, G.; Kanoun, M.B.; Goumri-Said, S. A comprehensive review on the synthesis of ferrite nanomaterials via bottom-up and top-down approaches advantages, disadvantages, characterizations and computational insights. Coord. Chem. Rev. 2024, 520, 216158. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Boumya, W.; Elmouwahidi, A.; Baillón-García, E.; Abdennouri, M.; Barka, N. Recent trends in magnetic spinel ferrites and their composites as heterogeneous Fenton-like catalysts: A review. J. Environ. Manag. 2024, 367, 121971. [Google Scholar] [CrossRef]
- Saleem, M.U.; Hussain, H.; Shukrullah, S.; Yasin Naz, M.; Irfan, M.; Rahman, S.; Ghanim, A.A.J. Study of Kinetics and the Working Mechanism of Silica-Coated Amino-Functionalized CoFe2O4 Ferrite Nanoparticles to Treat Wastewater for Heavy Metals. ACS Omega 2024, 9, 3507–3524. [Google Scholar] [CrossRef]
- Olivito, F.; Algieri, V.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Costanzo, P.; De Nino, A. Cellulose citrate: A convenient and reusable bio-adsorbent for effective removal of methylene blue dye from artificially contaminated water. RSC Adv. 2021, 11, 34309–34318. [Google Scholar] [CrossRef]
- Jasrotia, R.; Jaswal, N.; Prakash, J.; Kit, C.C.; Singh, J.; Kandwal, A. Photocatalytic application of magnesium spinel ferrite in wastewater remediation: A review. J. Magnes. Alloys 2024, 12, 2433–2446. [Google Scholar] [CrossRef]
- Mahesh, B. A comprehensive review on current trends in greener and sustainable synthesis of ferrite nanoparticles and their promising applications. Results Eng. 2024, 21, 101702. [Google Scholar]
- Antony Arockiaraj, M.; Mani, G.; ALOthman, Z.A. Effect of chitosan-incorporated Fe3O4 nanocomposites on the photocatalytic removal of organic molecules. J. Mater. Sci. Mater. Electron. 2022, 33, 9570–9579. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Hasan, G.G.; Meneceur, S.; Salmi, C.; Abdullah, J.A.A.; Menaa, F. Efficient Removal of Heavy Metals, Dyes, and Contaminants from Industrial Wastewater Using Chitosan-Coated Fe3O4 Nanocomposites: Biosynthesis, Characterizations, and Performance Evaluation. Biomass Convers. Biorefin. 2024, 14, 30719–30734. [Google Scholar] [CrossRef]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene oxide/Fe3O4/chitosan nanocomposite: A recoverable and recyclable adsorbent for organic dyes removal. Application to methylene blue. Mater. Res. Express 2017, 4, 035701. [Google Scholar] [CrossRef]
- Khodadadi, A.; Talebtash, M.R.; Farahmandjou, M. Effect of PVA/PEG-coated Fe3O4 nanoparticles on the structure, morphology and magnetic properties. Phys. Chem. Res. 2022, 10, 537–547. [Google Scholar]
- Ghanbari, D.; Salavati-Niasari, M.; Ghasemi-Kooch, M. A sonochemical method for synthesis of Fe3O4 nanoparticles and thermal stable PVA-based magnetic nanocomposite. J. Ind. Eng. Chem. 2014, 20, 3970–3974. [Google Scholar] [CrossRef]
- Gupta, P.K.; Palanisamy, S.; Gopal, T.; Rajamani, R.; Pandit, S.; Sinha, S.; Thakur, V.K. Synthesis and characterization of novel Fe3O4/PVA/eggshell hybrid nanocomposite for photodegradation and antibacterial activity. J. Compos. Sci. 2021, 5, 267. [Google Scholar] [CrossRef]
- Gayed, H.M.; Masry, B.A.; Sayed, M.A.; Awadallah-F, A. Development of Fe3O4/polyvinylalcohol-nanocomposite hydrogel by chemical and irradiation approaches for Sb (III) sorption from acidic medium. Nanotechnol. Environ. Eng. 2023, 8, 691–705. [Google Scholar] [CrossRef]
- Riva’i, I.; Wulandari, I.O.; Sulistyarti, H.; Sabarudin, A. Ex-situ synthesis of polyvinyl alcohol (PVA)-coated Fe3O4 nanoparticles by coprecipitation-ultrasonication method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012065. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Abdul Hamid, M.A.; Kamil Othman, N. Radiolytic formation of Fe3O4 nanoparticles: Influence of radiation dose on structure and magnetic properties. PLoS ONE 2014, 9, e90055. [Google Scholar] [CrossRef]
- Rahman, M.M.; Elaissari, A. Organic–inorganic hybrid magnetic latex. In Hybrid Latex Particles: Preparation with (Mini) Emulsion Polymerization; Springer: Berlin/Heidelberg, Germany, 2010; pp. 237–281. [Google Scholar] [CrossRef]
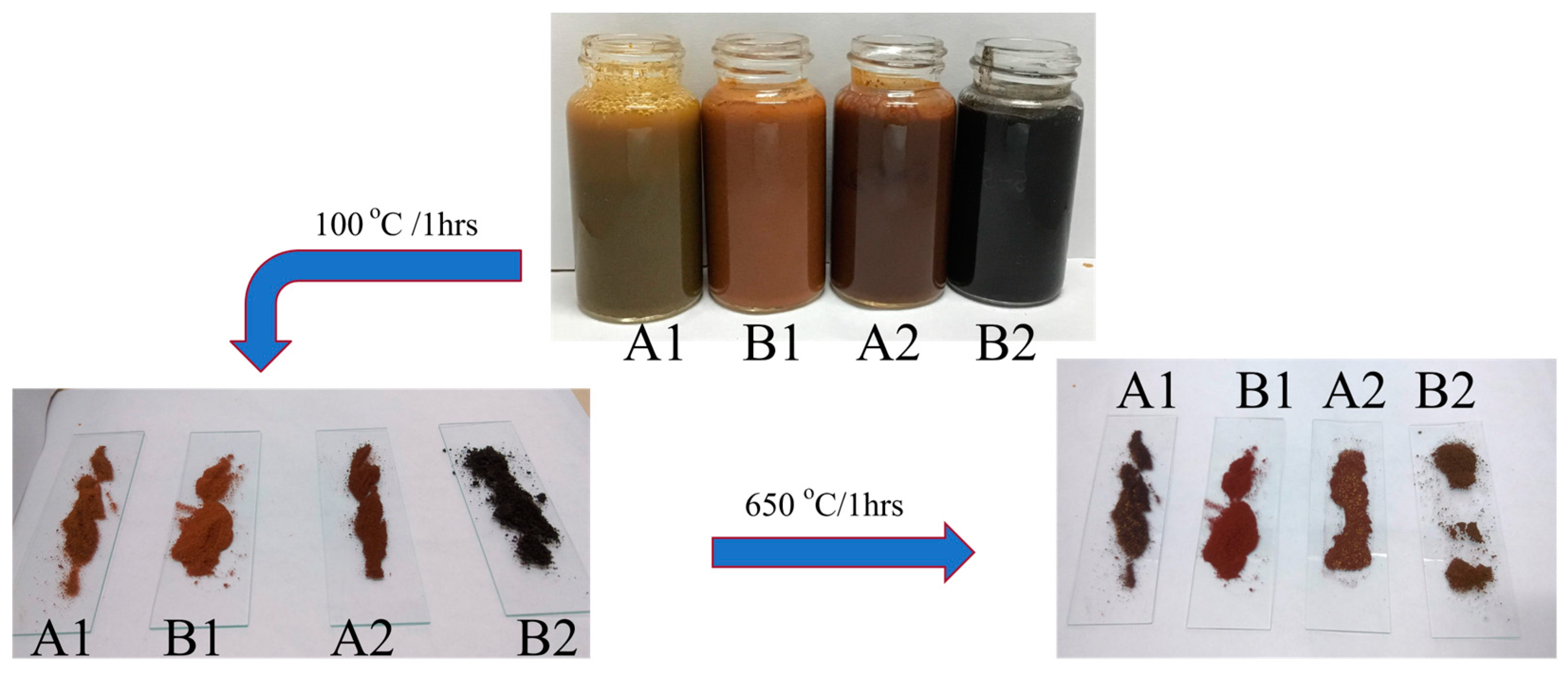

| Sample Code | Treatment Method | Magnetic Properties in Solution | 100 °C Thermal Treatment | 650 °C Thermal Treatment |
|---|---|---|---|---|
| A1 | Single Ferrite Addition | No magnetic response | No magnetic response | Complete magnetic attraction |
| B1 | H2O2 Pretreatment/ Ferrite Addition | Slight magnetic response | No magnetic response | Nearly complete magnetic attraction |
| A2 | H2O2 Pretreatment/ Sequential Ferrite Addition | Partial precipitate magnetic separation | Nearly complete magnetic attraction | Complete magnetic attraction |
| B2 | Sequential Ferrite Addition | Complete magnetic control of precipitates | Complete magnetic attraction | No magnetic response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.-C.; Hsiao, V.K.S. Efficiency of H2O2-Modified Ferrite Process for High-Concentration PVA Removal and Magnetic Nanoparticle Formation. Appl. Sci. 2025, 15, 3367. https://doi.org/10.3390/app15063367
Fu Y-C, Hsiao VKS. Efficiency of H2O2-Modified Ferrite Process for High-Concentration PVA Removal and Magnetic Nanoparticle Formation. Applied Sciences. 2025; 15(6):3367. https://doi.org/10.3390/app15063367
Chicago/Turabian StyleFu, Yu-Chih, and Vincent K. S. Hsiao. 2025. "Efficiency of H2O2-Modified Ferrite Process for High-Concentration PVA Removal and Magnetic Nanoparticle Formation" Applied Sciences 15, no. 6: 3367. https://doi.org/10.3390/app15063367
APA StyleFu, Y.-C., & Hsiao, V. K. S. (2025). Efficiency of H2O2-Modified Ferrite Process for High-Concentration PVA Removal and Magnetic Nanoparticle Formation. Applied Sciences, 15(6), 3367. https://doi.org/10.3390/app15063367








