Abstract
Early identification of Autism Spectrum Disorders (ASDs) can significantly improve outcomes. Deficits in joint attention (JA) abilities, considered a milestone in socio-communicative development, are among the earliest indicators of ASD. The purpose of this study is to examine if the ability to disengage visual attention (DA) at 12 months could predict joint attention abilities and socio-communicative development at 24 months in a population of infants at increased likelihood and reduced likelihood to develop ASD. Latency of DA at 12 months was analyzed through an eye-tracking paradigm in a group of 56 infants at increased (IL = 29) or reduced (RL = 27) likelihood to develop ASD. JA at 12 months was assessed through items from the Early Social Communication Scales. Diagnostic status was established at 24 months, with 10 children receiving a diagnosis of ASD. A higher DA latency at 12 months is correlated with a lower frequency of JA behaviors at 12 months and with poorer JA abilities at 24 months. Altered visual attention at 12 months was also correlated with socio-communicative development at 24 months and, together with lower JA abilities at 12 months, correlated with diagnostic status. Our findings point to the potential relevance of DA and JA skills as prognostic markers and intervention targets.
1. Introduction
A diagnosis of Autism Spectrum Disorder (ASD) requires the behavioral detection of impairments in social interaction and social communication, along with the presence of restricted and repetitive patterns of behaviors, interests and activities [1]. Although early access to evidence-based intervention has been linked to improved outcomes [2,3], establishing an unequivocal ASD diagnosis can be challenging before the second year of life [4,5], as no reliable behavioral markers of ASD have been identified during infancy and promising neuroimaging biomarkers are still far from being applied in clinical practice [6]. However, a body of literature points to the importance of early emerging differences in joint attention (JA) as a reliable early sign of ASD [7,8,9]. JA is manifested from infancy onward as the ability to coordinate and share a common point of view with other people, including following another person’s gaze or pointing towards an object or event of interest (response to joint attention, RJA) and directing another person’s attention towards a point of interest through gaze and/or gestures (initiating joint attention, IJA) [10]. JA difficulties are considered to be a core manifestation of ASD [1] and are a prominent intervention target across numerous early intervention programs [11,12,13,14,15]. Therefore, a better understanding of how JA emerges and develops is crucial for both diagnostic and intervention purposes.
Different theories have been proposed to explain how JA abilities emerge and develop: for example, the Social Motivation Model [16] argues that ASD children show JA deficits as a consequence of disfunctions in the dopaminergic reward circuits. According to this view, ASD infants, compared to neurotypical peers, pay less attention to social stimuli (i.e., faces) because they are less pleasant and rewarding to them. In this model, reduced exposure to social stimuli reduces the specialization of the social brain areas, with cascading effects on the development of social behaviors such as joint attention [17]. This theory has been recently discussed in a study of Palomo et al. [18], who found that ASD infants at 9–12 months showed reduced alternating gaze to initiate JA, but they did not show a reduced orienting to social faces (social gaze). The authors concluded that social motivation is not a primary deficit in ASD [18,19].
In contrast to the “Social Motivation Model”, other authors argued that JA alterations in ASD may be linked to atypicalities in the executive attention system that underlies the orienting response, which implies the ability to disengage the attention from a central stimulus and to subsequently orient it to a new target [19,20,21]. During a typical JA episode, one has to rapidly shift the attentional focus from an object or an event to the social partner’s face and back again toward the object or event he/she wants to share with the social partner. While JA skills begin to emerge from 6 months of life, with a high rate of variability among individuals [10], the ability to flexibly switch visual attention matures rapidly during the first months of life and allows infants to quickly and selectively orient towards salient stimuli and thus to learn from the environment [22]. Atypical reorienting efficiency during the first year of life has been associated with the later emergence of ASD [8,23]. Reorienting visual attention can be conceptualized as the time taken by the infant to make a saccade from a central stimulus to a peripheral one. Saccadic movements are driven by the visual attention system that selects the direction of each saccade by disengaging attention from a fixation point and shifting it to another point. The neural mechanisms underlying impaired attentional disengagement in ASD remain poorly understood. However, elevated levels of tonic locus coeruleus–norepinephrine activity may play a role [24], as well as atypical activation and connectivity of the ventral and dorsal subdivision of the anterior insula, which is supposed to regulate the salience network involved in the regulation of attention [25].
In summary, a reduction in the efficiency of the attention disengagement and in the frequency of joint attention during the first year of life can be considered as supporting evidence for both of the explanatory theories described above.
Recent research has focused on attentional processes in ASD using eye-tracking (ET) a non-invasive methodology designed to provide an objective quantification of eye movements [26,27,28,29,30]. The precision and cost effectiveness of ET render it a promising tool for the identification of biomarkers useful for clinical trials [31] and the examination of processes associated with autism symptoms [32,33]. A substantial body of scientific literature has demonstrated that eye tracking can be considered a valid and reliable methodology, especially in very young subjects or those with severe clinical impairments who exhibit lower levels of cooperation [28,33,34,35].
Several studies analyzed the reorienting efficiency, more specifically the disengagement of attention, through ET in infants at risk for ASD and documented that attentional patterns differ between infants subsequently diagnosed with ASD and neurotypical peers, with the former displaying increased saccadic latencies as early as 7 months of life [36] and continuing to show this pattern during the second year of life [37]. Additional studies have generated mixed findings, perhaps reflecting variability in methodological paradigms and tasks, including whether the stimuli used to measure disengagement latencies are social or non-social in nature. Accordingly, the nature and the complexity of the stimuli (e.g., faces and objects) could affect the disengagement of attention, making it difficult to ascertain whether altered disengagement reflects altered social processing (i.e., longer latency specifically in response to social stimuli such as faces) versus a basic impairment of attentional disengagement (regardless of the nature of the stimuli being attended to) [38].
The aims of this study are to explore the association between the ability to disengage attention and the development of joint attention abilities: in particular, we tested the hypothesis that the ability to disengage attention is correlated with JA abilities. The second aim of the study is to verify the hypothesis that a slower disengagement of attention and a lower frequency of JA behaviors at 12 months are early precursors of later socio-communicative abilities.
2. Methods
2.1. Participants
The sample is composed of 29 infants (M/F:21/8) at increased likelihood of being diagnosed with ASD (IL) [39,40] by virtue of having older siblings with ASD, which were compared to 27 infants (M/F:12/15) at reduced likelihood (RL) to be diagnosed with ASD. The sample was 96% Caucasian and 4% Hispanic. The maternal education was as follows: 12% secondary school, 42% high school and 46% university degree.
This study had a longitudinal design, with disengagement of attention and JA assessed at 12 months (T0). T0 examiners, who administered the eye-tracking tasks and joint attention tasks, were different from T1 examiners, who administered the diagnostic assessment.
At 24 months (T1), a diagnostic assessment was conducted by two different clinicians with extensive experience in the evaluation of toddlers with NDDs (a psychologist and a neuro-psychiatrist) within a tertiary care university hospital. The diagnostic assessment included the ADOS-2 Toddler Module administered by research-reliable raters [41], the Griffiths Mental Developmental Scales—ER (GMDS-ER, [42]) to obtain the developmental level, parental questionnaires and interviews (such as Vineland Adaptive Behavior Scale -II [43] and clinical observation. Neurological or sensory deficits were excluded by the neuro-psychiatrist through a clinical visit or through specific instrumental analysis, if needed. Anamnestic information (infant’s history and family history) was collected by the neuro-psychiatrist as well. Clinical diagnosis was finally made according to the DSM-5 criteria. Blindness of group membership was possible for those who administered the eye-tracking measures and assessment measures (joint attention task and ADOS-2) but not for the neuropsychiatrist, who was responsible for the collection of the anamnestic data.
A total of 13 infants belonging to the RL group and 3 to the IL group did not complete the T1 assessment due to the March 2020 lockdown or dropped out from the study; for this reason, they were included only in the T0 analysis. At T1, IL and RL infants were classified into three groups according to their diagnostic status, including ASD (n = 10), NO-ASD (n = 25) or other neurodevelopmental disorders (NDDs) (n = 5, 3 with a language disorder and 2 with a neurodevelopmental disorder not otherwise specified). These latter five infants with an NDD diagnosis were then excluded from the T1 analyses (see Figure 1 for a participant flowchart).
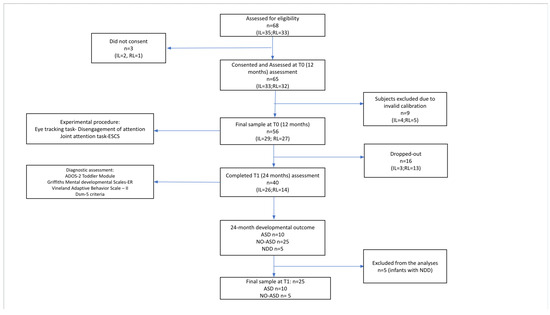
Figure 1.
Participant flowchart.
IL infants were recruited from two distinct ongoing research projects at the IRCCS Fondazione Stella Maris (project 1: “Early Bird Diagnostic Protocol for ASD”, code: NET -2013-02355263, and project 2: NIDA Network—Network Italiano per i Disturbi dello Spettro Autistico). Both projects focused on the developmental surveillance of infants at IL to develop ASD. Those projects involved experimental protocols at different developmental time points. Despite these differences, there were some overlaps in the procedures and in the measures that facilitated data sharing between the two projects.
RL infants were recruited through local pediatricians, who enrolled infants without a family history of ASD and/or developmental concerns.
The subjects were reimbursed for travel expenses incurred in reaching the center where the evaluation was carried out.
The sample size was verified according to the existing literature [44,45] and a power analysis using G*Power 3 [46], with a power level of 0.80, an effect size (f2) of 0.20, α = 0.025 and an estimated sample size of 42 [47]. The initial sample consisted of 57 children; however, as reported in the manuscript, 13 children in the RL group and 3 in the IL group did not complete the T1 assessment due to the March 2020 lockdown or dropped out of the study. To assess the potential impact of this reduction, a post hoc power analysis was conducted, which indicated a reduction in power. However, the power remains acceptable for detecting the expected effects, given the specificity of the sample (G*Power: power level = 0.73, effect size (f2) = 0.20, α = 0.025, estimated sample size = 35).
An informed consent form was obtained for all the participants.
2.2. Procedure
Participants were tested in a quiet room in our clinical setting. Infant’s gaze during the task was recorded with an SMI Eye-Tracking device provided by SensoMotoric Instruments (Teltow, Germany), with a sample rate of 500 Hz and accuracy better than 31 degrees of visual angle. No explicit instruction was given to the infant. The eye-tracking device was positioned in front of each participant and below a 22-inch flat-screen monitor where the stimuli were presented. The distance of the infants from the screen was approximately 50 cm, and the inclination angle of the eye-tracking device was adjusted for each infant. Infants were seated on their parents’ lap: Parents were instructed to wear dark sunglasses to prevent the eye-tracking system from capturing their gaze. Additionally, they were asked not to intervene during the eye-tracking procedure and to hold the child to enhance his/her comfort. Before the stimuli were presented, a 5-point calibration procedure was conducted. According to the literature on using eye-tracking procedures with very young subjects [48], this calibration balances a good result with minimal perceived intrusiveness for the user.
Following this, each child passively viewed the experimental stimuli. The calibration was repeated until the deviation from the known calibration target for both the x and y components was below 2°. The accuracy of calibration between the two groups was calculated as the root mean square value of the deviation of the x and y components. The root mean square was not significantly different between the two groups. A total of 9 subjects (IL = 4, RL = 5) were excluded due to invalid calibration.
The experimental paradigm included 4 blocks of 6 trials each for a total of 24 trials (7 min in total), presented in random order. Experimental stimuli were interspersed with filler stimuli (animations or cartoon scenes) to reorient infant attention towards the screen and to avoid fuzziness.
2.3. Attention Disengagement
Attention disengagement was examined through an eye-tracking Fixation Shift paradigm (see [22,38] for a definition of the Fixation Shift paradigm), created ad hoc for this project. Specifically, each trial included a central stimulus that remained visible for 2 s on the screen followed by the appearance of a peripheral stimulus, with the two stimuli remaining simultaneously on the screen for the 3 s. Each stimulus was a simple geometrical shape without social or object connotation (Figure 2), to avoid the potential confounding factor of altered social processing (i.e., an increased time to process human faces that could affect the disengagement latency). Disengagement latency was measured as the time participants remained fixated on the central stimulus once the peripheral one had appeared before making a saccade towards the peripheral stimulus. The rationale was that longer fixations on the central stimulus after the appearance of the peripheral stimulus reflected difficulties in disengaging visual attention from the central stimulus. This approach was used in previous literature to measure disengagement latency [38] and is consistent with other studies measuring disengagement at an early age with the eye-tracking method [36,37]. Disengagement latency was measured by analyzing raw data obtained by the SMI eye-tracker using a Matlab script.
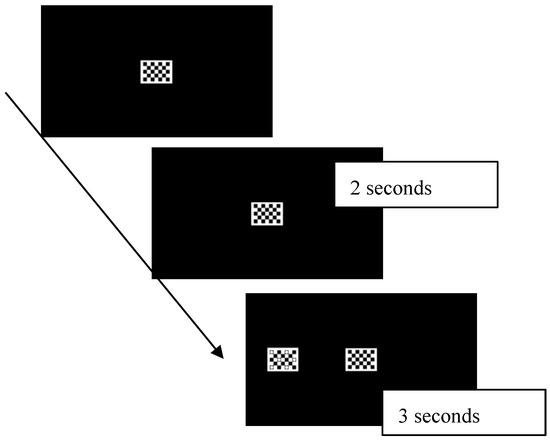
Figure 2.
Each trial began with a central stimulus that remained visible for 2 s on the screen; then a peripheral one appeared and the two stimuli remained on the screen for 3 s. Disengagement latency was measured as the time participants remained fixated on the central stimulus once the peripheral one had appeared before making a saccade towards the peripheral stimulus.
In the current study, trials were considered invalid based on the following criteria: (1) once the peripheral stimulus appeared, the time spent in looking at the central stimulus (in terms of net dwell time [49]) was lower than 200 ms (the minimum duration of a fixation, according to [50]), indicating that the subject was not looking at the central stimulus when the peripheral one appeared; (2) during the presentation of the peripheral stimulus, the infant remained fixated on the central stimulus and did not orient towards the peripheral stimulus at all (sticky fixation). Those trials were excluded because they were considered a failure to disengage attention. No significant differences were found between the number of sticky fixations between groups (IL = 1, RL = 1). Despite high rates of sticky fixations reflecting difficulties in switching visual attention, they represent the likelihood of disengaging to orient to peripheral targets rather than the speed of orienting [37]. (3) After the presentation of the central stimulus, the infant lost his/her attention for the task and looked away from the screen.
2.4. Joint Attention
Joint attention was assessed through specific tasks from the Early Social Communication Scales (ESCS, [51]), a semi-structured observation of social–communicative behaviors. For our purposes, only the tasks eliciting joint attention behaviors were administered, in particular, the following:
- Object Spectacle Task: In this task, the examiner activates mechanical toys, and the child’s response to the activation of a toy is assessed (a total of 4 trials).
- Book Presentation Task: In this task, the examiner presents images to the child (a total of 6 images), evaluating the child’s ability to follow proximal pointing and any initiation of joint attention or requesting behaviors.
- Gaze-Following Task: This task assesses the child’s ability to follow distal pointing and shift their gaze toward a distant object (a total of 5 trials).
Overall, the examiner employs in these tasks strategies that elicit target behaviors in the child, both in terms of initiating and responding to joint attention.
The total duration of the observation was approximately 10 min. Target behaviors were coded with the software “The Observer 10.0” [52]. An ad hoc observational analysis was created, including the specific behaviors used to initiate or respond to joint attention: alternating gaze (AG-IJA), declarative pointing (DP-IJA), following proximal pointing (FPP-RJA) and following distal pointing (FDP-RJA).
Video recordings of JA behaviors during the ESCS were coded offline by a research assistant, blind to group membership, who achieved a satisfactory agreement (Cohen’s K = 0.80) with the principal investigator.
3. Statistical Analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS, version 23.0.0, IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk test was performed to evaluate the normality of the data. Given the non-normal distributions, Spearman’s rank correlation analysis was employed to assess the relationship between the disengagement of attention and joint attention variables at T0. A Bonferroni correction was applied to adjust for multiple comparisons. Linear regression was used to examine whether disengagement latency at 12 months could predict joint attention behaviors at 24 months, as assessed through item b13 (entitled “Initiation of Joint Attention”) of the ADOS-2 Toddler Module. Additionally, linear regression was used to test whether disengagement latency and JA behaviors at 12 months could predict socio-communicative abilities at 24 months (measured with the ADOS-2 Toddler Module) and developmental level at 24 months (assessed using the GMDS-ER). The developmental quotient, measured with the GMDS-ER, was included as a covariate in all analyses. The underlying assumptions were verified before applying the linear regression (linearity, independence, homoscedasticity and normality of residuals).
At T1, outcome groups were categorized into ASD (n = 10) and NO-ASD (n = 25), excluding infants with neurodevelopmental disorders (NDD, n = 5). Despite the small number of infants diagnosed with ASD at 24 months (n = 10), we also applied binary logistic regression to determine whether disengagement of attention and JA behaviors at T0 could predict the diagnostic outcome at T1. The underlying assumptions were verified before applying the logistic regression (linearity, independence, multicollinearity and absence of outliers).
4. Results
4.1. Correlation Between Disengagement Latency and Joint Attention Behaviors at 12 Months
A negative correlation was found between disengagement latency and two joint attention behaviors (Bonferroni correction was applied and significance was set at p < 0.0125), namely declarative pointing (DP-IJA) (r = −0.395, p = 0.004, Figure 3) and following distal pointing (FDP-RJA) (r = −0.435, p = 0.001, Figure 4). See Appendix A, Table A1 for descriptive statistics of T0 variables.
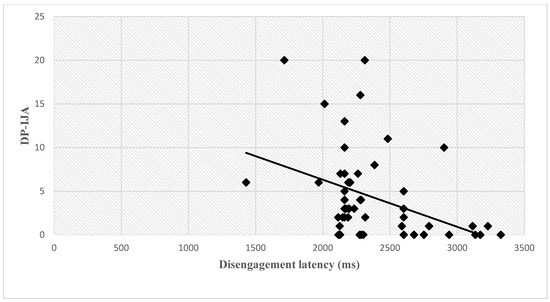
Figure 3.
Correlation between disengagement latency and DP-IJA at 12 months. Abbreviations: (ms), milliseconds; DP-IJA, declarative pointing-initiation of joint attention.
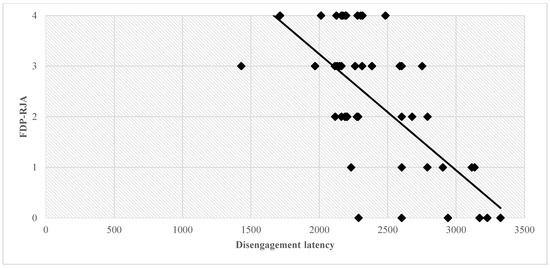
Figure 4.
Correlation between disengagement latency and FDP-IJA. Abbreviations: ms, milliseconds; FDP-RJA, following distal pointing-responding to joint attention.
4.2. T0 Predictors of Outcome at T1 (Table 1)
DA at 12 months as a predictor of developmental level (GMDS score) at 24 months: The model demonstrated a significant overall fit, F(1,32) = 5.87, p = 0.021. The results indicated that disengagement at 12 months significantly predicted developmental level at 24 months (β = −0.399, p = 0.021).
DA at 12 months as a predictor of joint attention (item b13-ADOS-2 of the Toddler Module) at 24 months: The model demonstrated a significant overall fit, F(1,28) = 5.037, p = 0.033. It was found that disengagement at 12 months significantly predicted JA abilities at 24 months (β = 0.397, p = 0.033).
DA at 12 months as a predictor of social affect (SA) score (Ados-2 Toddler Module) at 24 months: The model demonstrated a significant overall fit, F(1,32) = 4.187, p = 0.049. It was found that disengagement at 12 months significantly predicted the SA score at 24 months (β = 0.345, p = 0.049).
DA at 12 months as a predictor of ADOS-2 total score at 24 months: The model demonstrated a significant overall fit, F(1,32) = 4.237, p = 0.048. The results revealed that disengagement at 12 months significantly predicted the ADOS-2 total score at 24 months (β = 0.347, p = 0.048).
Alternating gaze of initiation of joint attention (AG-IJA) at 12 months as a predictor of developmental level (GMDS score) at 24 months: The model demonstrated a significant overall fit, F(1,31) = 5.525, p = 0.026. AG-IJA at 12 months significantly predicted the GMDS score at 24 months (β = 0.394, p = 0.026).
Alternating gaze of initiation of joint attention (AG-IJA) at 12 months as a predictor of outcome (ASD/NO-ASD) at 24 months: The model was statistically significant (χ2 [1] = 10.98, p = 0.001), explaining between 27.6% (Cox and Snell R Square) and 40.3% (Nagelkerke R Square) of the variance in diagnostic outcome and correctly classifying 73.5% of cases.
In the model, AG-IJA at 12 months was significant (B = −0.746, Wald = 5.532, p = 0.019, 95% CI [0.37, 0.61]), indicating a trend where increasing AG-IJA is associated with lower odds of the ASD diagnostic outcome.
Disengagement latency (DA) at 12 months as a predictor of outcome (ASD/NO-ASD) at 24 months: The model was statistically significant (χ2 [1] = 7.602, p = 0.006), explaining between 19.5% (Cox and Snell R Square) and 28% (Nagelkerke R Square) of the variance in diagnostic outcome and correctly classifying 74.3% of cases.
In the model, DA at 12 months was significant (B = 1.003, Wald = 5.878, p = 0.015, 95% CI [2.72, 2.73]), indicating a trend where increasing DA latency is associated with higher odds of the ASD diagnostic outcome.
These findings indicate that both disengagement latency and alternating gaze are important factors in determining the likelihood of determining the diagnostic outcome for ASD, with disengagement latency being a more influential predictor than alternating gaze.
See Appendix A, Table A2 for descriptive statistics.

Table 1.
The 12-month predictors of clinical outcome at 24 months. A binary logistic regression was used to test if disengagement latency and alternating gaze-initiation of joint attention (AG-IJA) at 12 months could correlate with clinical outcome at 24 months (ASD/NO-ASD). A linear regression was used to test if disengagement latency and AG-IJA could correlate with the ADOS-2 scores of joint attention (JA), social affect (SA), repetitive restricted behavior (RRB), total score and developmental quotient (DQ) on the Griffiths Mental Developmental Scale (GMDS-ER) at 24 months.
Table 1.
The 12-month predictors of clinical outcome at 24 months. A binary logistic regression was used to test if disengagement latency and alternating gaze-initiation of joint attention (AG-IJA) at 12 months could correlate with clinical outcome at 24 months (ASD/NO-ASD). A linear regression was used to test if disengagement latency and AG-IJA could correlate with the ADOS-2 scores of joint attention (JA), social affect (SA), repetitive restricted behavior (RRB), total score and developmental quotient (DQ) on the Griffiths Mental Developmental Scale (GMDS-ER) at 24 months.
| 12-Month Predictors | ASD and NO-ASD | ADOS-2 JA (Item b13) | ADOS-2 SA | ADOS-2 RRB | ADOS-2 Total | GMDS-ER DQ |
|---|---|---|---|---|---|---|
| Disengagement latency (ms) | p = 0.015 * B = 1.003 | F = 5.037 β = 0.397 p = 0.033 * | F = 4.187 β = 0.345 p = 0.049 * | ns | F = 4.237, β = 0.347 p = 0.048 * | F = 5.870 β = −0.399 p = 0.021 * |
| AG-IJA | p = 0.019 * B = −0.746 | ns | ns | ns | ns | F = 5.525, β = 0.394, p = 0.026 |
Abbreviations: ms, milliseconds; ns, not significant. * p < 0.05.
5. Discussion
In this study, we wanted to test the hypothesis whether the basic ability to disengage visual attention could be associated with the ability to initiate and respond to JA, a more complex dimension of social functioning. Our hypothesis was supported by our findings, as a slower disengagement of attention was correlated with a lower frequency of JA behaviors at 12 months, both in IJA (declarative pointing) and in RJA (responding to distal pointing). Moreover, we found that a slower disengagement of attention at 12 months correlates with a higher impairment of IJA abilities at 24, measured with item B13 of the ADOS-2 Toddler Module, and higher ADOS-2 total scores and ADOS-2 social affect scores. These findings suggested that the association between disengagement of attention and JA, as well as the correlation between disengagement of attention and socio-communicative development, are both concurrent and predictive. Finally, we also detected that a reduced frequency of alternating gaze at 12 months was associated with lower cognitive development at 24 months.
These findings are consistent with other studies showing that lower-level attentional processes might interfere with the development of a higher-level cognitive and behavioral domain [23,53,54].
Assuming a domain-relevant theoretical model approach, the infant brain is equipped with some basic-level orientations, non-specific but more relevant to the processing of certain class of stimuli, with domain specificity emerging over time across interactions with the environment [55]. According to this view, firstly applied to this topic by Landry and Bryson [56], the ability to disengage visual attention might play a key role in the orienting network, which is implied in the goal-directed selection of information from the environment. Of note, the orienting network is not strictly specific for the development of JA but is needed to self-regulate arousal and affect, and it is supposed to be important for appropriate social interactions, like those occurring during a JA act [57]. Consistently with domain-general theories of autism [19,58,59,60], an early impairment in the disengagement of visual attention might interfere with the efficiency of the orienting system, which in turn might reduce the exposure to a certain class of stimuli (like the social ones) that are necessary for the specialization of the social brain areas and for the development of social abilities, such as joint attention. Adopting a dimensional rather than a categorical perspective in the study of ASD, where various dimensions of development are examined in their phenotypic expression rather than searching for singular markers, could lead to a more comprehensive understanding of clinical heterogeneity and the development of personalized intervention programs. The investigation of joint attention aligns with this approach, as it serves as a critical milestone for the subsequent development of language and various other social skills in both neurotypical and neurodivergent populations [10,13,61]. Understanding the early precursors of joint attention is essential for identifying developmental risk factors and for implementing timely interventions through the development of tailored treatments. Our findings, which highlighted the association between disengagement and joint attention in both low- and high-risk groups of children, if replicated in larger samples, may enhance our understanding of joint attention development, thereby improving early treatment targets. For instance, Wass and colleagues [62] provide evidence that attentional control can be enhanced through structured interventions, emphasizing techniques that engage infants in tasks requiring focus and sustained attention. These findings suggest that early training can lead to improvements in attentional abilities, such as reduced disengagement latencies, which may have cascading effects on later cognitive and social development.
Despite the low number of infants in our study who received a diagnosis of ASD (n = 10), and thus our results should be interpreted with extreme caution, our study also suggested that a slower disengagement of visual attention and reduced frequency of JA abilities at 12 months is associated with an ASD diagnosis at 24 months.
The importance of assessing JA and DA for an early identification of ASD is in line with previous studies that prospectively followed up IL infants from the first year of life until the age of their diagnosis [18,36,37,63,64,65,66,67,68]. Different associations between disengagement latency and alternating gaze frequencies might help to better predict different clinical phenotypes. This possibility has already been explored by Bedford et al. [69], who found that the coexistence of a slower disengagement of attention together with a difficulty in responding to JA might have additive and cumulative effects on the risk of developing ASD. Further studies, with larger samples and with different ASD phenotypic expressions, need to be implemented to examine this hypothesis.
Additionally, we found a correlation between slower disengagement latency and lower cognitive level, which is also consistent with previous literature [70,71], including studies documenting a link between attentional disengagement and neurocognitive outcome in various populations such as children with perinatal brain injury and preterm birth [72,73]. Such association between disengagement and cognitive development across different populations poses the question about the specificity of this marker in autism. Even though in this study slower disengagement correlates with ASD symptom severity as well as cognitive functioning, it remains unclear whether this early marker is specific to ASD or if it is more predictive of a lower cognitive level in general, which, in turn, might affect the results of tests measuring ASD symptoms [74]. Further research is needed to address this issue.
Taking together, our findings, if replicated, might point to the potential utility of assessing the disengagement of attention and JA skills during infancy in order to detect infants at risk for neurodevelopmental conditions and to develop tailored interventions [71,75]. In addition, multimodal assessments, such as the integration of electroencephalogram (EEG) and eye-tracking data [76,77], may help elucidate neural mechanisms underlying early neurocognitive markers in ASD.
6. Limitations
An important limitation of the current study is the small sample size (IL n = 29, RL n = 27). Prospective studies with infants at increased likelihood to receive a diagnosis of ASD, in which different developmental outcomes are expected [78,79], benefit from larger samples in order to clarify the link between putative predictors and different outcomes. Therefore, our findings need to be replicated with a larger sample to improve the reliability of the findings.
Despite that different evidence suggests a moderate diagnostic stability at 24 months and even earlier [80,81,82,83], another limitation is the absence of diagnostic outcome at 36 months or later: indeed, a recent study that followed up IL infants from the first year of life (mean age 15.4 months) to preschool age (mean age 5.7 years) indicated modest diagnostic stability when a diagnosis is made during the second year of life (63%) but higher (68%) at 36 months [80]. Although not all children on the autism spectrum are fully symptomatic at 24 months [83], detecting early alterations associated with diagnostic status for at least some children can accelerate the provision of well-timed interventions.
7. Conclusions
In the current study, a delayed disengagement of visual attention at 12 months has been correlated with poorer joint attention abilities (both at 12 months and at 24 months) and with difficulties in socio-communicative and cognitive development. Altered DA and reduced JA at 12 months correlates with diagnostic status in infants who went on to develop ASD at 24 months.
Additional studies with larger samples are needed to replicate these findings and to better define the contribution of non-social dimensions (such as the visual attention abilities) as well as social dimensions in the development of JA. If replicated, our results might provide actionable insight on early intervention targets, including early training of the orienting system for infants at higher likelihood to develop ASD during the first year. This has the potential to have a positive cascading effect on social abilities, including the ability to share attention, potentially improving outcomes during toddlerhood.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by V.C. Data analysis was performed by L.B. Offline video coding of the experimental session was performed by C.B., F.A., A.M. and R.T. confirmed the clinical diagnosis of the participants. F.M., F.A. and S.C. supervised the experimental procedure and the writing of the manuscript. G.V. was the external consultant for the experimental procedure, data analysis and writing of the manuscript. The first draft of the manuscript was written by V.C., and all authors commented on a previous version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work leading to these results was supported by the Italian Ministry of Health Grant—NET-2013-02355263-3, by the 5 × 1000 voluntary contributions and by the FIA-Fondazione Italiana Autismo.
Institutional Review Board Statement
All research activities described in the article meet ethical guidelines; these were performed in line with the principles of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was conducted within the research activities of the project NET-2013-02355263-3, which was reviewed and approved by the Pediatric Ethical Committee of Tuscany region at Meyer Children’s Hospital on 28 February 2017 (n.26/2017).
Informed Consent Statement
All participants and their parents or legal guardians, where appropriate, gave written informed consent to the conduct of clinical care and data collection for research purposes.
Data Availability Statement
The dataset presented in this study can be requested to the authors.
Acknowledgments
Participants were enrolled thanks to the research activities of the NIDA network (Network Italiano per i disturbi dello spettro autistico), coordinated by Maria Luisa Scattoni, and thanks to the clinical activities of three local pediatricians: Neri, Tarabella and Baracchini.
Conflicts of Interest
The authors declare they have no financial interests.
Appendix A

Table A1.
Descriptive statistics of T0 variables in IL and RL groups.
Table A1.
Descriptive statistics of T0 variables in IL and RL groups.
| Variables | IL (n = 29) | RL (n = 27) | ||
|---|---|---|---|---|
| Mean | Sd | Mean | Sd | |
| Disengagement latency (ms) | 2554.64 | 302.7 | 2157.45 | 147.8 |
| AG-IJA | 10.12 | 4.81 | 19 | 7.86 |
| DP-IJA | 3.25 | 5.15 | 6.71 | 6.03 |
| FPP-RJA | 3.96 | 2.56 | 6.21 | 1.31 |
| FDP-RJA | 1.83 | 1.4 | 3.57 | 0.75 |
Abbreviations: ms, milliseconds; AG-IJA, alternating gaze-initiation of joint attention; DP-IJA, declarative pointing-initiation of joint attention; FPP-RJA, following proximal pointing-responding to joint attention; FDP-RJA, following distal pointing-responding to joint attention; IL, increased likelihood to develop ASD; RL, reduced likelihood to develop ASD; sd, standard deviation.

Table A2.
Descriptive statistics of T1 variables in ASD and NO-ASD groups.
Table A2.
Descriptive statistics of T1 variables in ASD and NO-ASD groups.
| Variables | ASD (n = 10) | NO-ASD (n = 25) | ||
|---|---|---|---|---|
| Mean | Sd | Mean | Sd | |
| SA—Ados-2 Toddler Module | 11.70 | 3.77 | 3.65 | 2.98 |
| RRB—Ados-2 Toddler Module | 4.20 | 1.93 | 1.91 | 1.81 |
| Total ADOS-2 score | 15.90 | 4.77 | 5.57 | 4.05 |
| DQ-GMDS-ER | 89.17 | 17.22 | 105.95 | 11.86 |
Abbreviations: SA, social affect; RRB, restricted and repetitive behaviors; DQ, developmental quotient; GMDS-ER, Griffiths Mental Developmental Scale—ER.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR®; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Frazier, T.W.; Klingemier, E.W.; Anderson, C.J.; Gengoux, G.W.; Youngstrom, E.A.; Hardan, A.Y. A longitudinal study of language trajectories and treatment outcomes of early intensive behavioral intervention for autism. J. Autism Dev. Disord. 2021, 51, 4534–4550. [Google Scholar] [CrossRef] [PubMed]
- Sandbank, M.; Bottema-Beutel, K.; Crowley, S.; Cassidy, M.; Feldman, J.I.; Canihuante, M.; Woynaroski, T. Intervention effects on language in children with autism: A Project AIM meta-analysis. J. Speech Lang. Hear. Res. 2020, 63, 1537–1560. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.; Frye, R.E. Early detection and diagnosis of autism spectrum disorder: Why is it so difficult? Semin. Pediatr. Neurol. 2020, 35, 100831. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bauman, M.L.; Stone, W.L.; Yirmiya, N.; Estes, A.; Hansen, R.L.; McPartland, J.C.; Natowicz, M.R.; Choueiri, R.; Fein, D. Early Identification of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics 2015, 136 (Suppl. S1), S10–S14. [Google Scholar] [CrossRef]
- Hiremath, C.S.; Sagar, K.J.V.; Yamini, B.K.; Girimaji, A.S.; Kumar, R.; Sravanti, S.L.; Padmanabha, H.; Vykunta Raju, K.N.; Kishore, M.T.; Jacob, P.; et al. Emerging behavioral and neuroimaging biomarkers for early and accurate characterization of autism spectrum disorders: A systematic review. Transl. Psychiatry 2021, 11, 42. [Google Scholar] [CrossRef]
- Franchini, M.; Armstrong, V.L.; Schaer, M.; Smith, I.M. Initiation of joint attention and related visual attention processes in infants with autism spectrum disorder: Literature review. Child Neuropsychol. 2019, 25, 287–317. [Google Scholar] [CrossRef] [PubMed]
- Gliga, T.; Jones, E.J.; Bedford, R.; Charman, T.; Johnson, M.H. From early markers to neuro-developmental mechanisms of autism. Dev. Rev. 2014, 34, 189–207. [Google Scholar] [CrossRef]
- Shen, M.D.; Piven, J. Brain and behavior development in autism from birth through infancy. Dialogues Clin. Neurosci. 2017, 19, 325–333. [Google Scholar] [CrossRef]
- Mundy, P.; Newell, L. Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 2007, 16, 269–274. [Google Scholar] [CrossRef]
- Kaale, A.; Smith, L.; Sponheim, E. A randomized controlled trial of preschool-based joint attention intervention for children with autism. J. Child Psychol. Psychiatry 2012, 53, 97–105. [Google Scholar] [CrossRef]
- Rogers, S.J.; Estes, A.; Lord, C.; Vismara, L.; Winter, J.; Fitzpatrick, A.; Guo, M.; Dawson, G. Effects of a brief Early Start Denver Model (ESDM)–based parent intervention on toddlers at risk for autism spectrum disorders: A randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.; Shire, S.; Chang, Y.C.; Kasari, C. Joint engagement is a potential mechanism leading to increased initiations of joint attention and downstream effects on language: JASPER early intervention for children with ASD. J. Child Psychol. Psychiatry 2021, 62, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- So, W.C.; Law, W.W.; Cheng, C.H.; Lee, C.; Ng, K.C.; Kwok, F.Y.; Lam, H.W.; Lam, K.Y. Comparing the effectiveness of robot-based to human-based intervention in improving joint attention in autistic children. Front. Psychiatry 2023, 14, 1114907. [Google Scholar] [CrossRef]
- Waddington, H.; Reynolds, J.E.; Macaskill, E.; Curtis, S.; Taylor, L.J.; Whitehouse, A.J. The effects of JASPER intervention for children with autism spectrum disorder: A systematic review. Autism 2021, 25, 2370–2385. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Webb, S.J.; Wijsman, E.; Schellenberg, G.; Estes, A.; Munson, J.; Faja, S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Dev. Psychopathol. 2005, 17, 679–697. [Google Scholar] [CrossRef]
- Falck-Ytter, T.; Kleberg, J.L.; Portugal, A.M.; Thorup, E. Social Attention: Developmental Foundations and Relevance for Autism Spectrum Disorder. Biol. Psychiatry 2023, 94, 8–17. [Google Scholar] [CrossRef]
- Palomo, R.; Ozonoff, S.; Young, G.S.; Belinchon Carmona, M. Social orienting and initiated joint attention behaviors in 9 to 12 month old children with autism spectrum disorder: A family home movies study. Autism Res. 2022, 15, 1109–1119. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Johnson, M.H. Autism and the social brain: The first-year puzzle. Biol. Psychiatry 2016, 80, 94–99. [Google Scholar] [CrossRef]
- Clohessy, A.B.; Posner, M.I.; Rothbart, M.K. Development of the functional visual field. Acta Psychol. 2001, 106, 51–68. [Google Scholar] [CrossRef]
- Mundy, P.; Card, J.; Fox, N. EEG correlates of the development of infant joint attention skills. Dev. Psychobiol. 2000, 36, 325–338. [Google Scholar] [CrossRef]
- Kulke, L.; Atkinson, J.; Braddick, O. Automatic detection of attention shifts in infancy: Eye tracking in the fixation shift paradigm. PLoS ONE 2015, 10, e0142505. [Google Scholar] [CrossRef]
- Sacrey, L.A.R.; Armstrong, V.L.; Bryson, S.E.; Zwaigenbaum, L. Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neurosci. Biobehav. Rev. 2014, 47, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Keehn, B.; Kadlaskar, G.; Bergmann, S.; McNally Keehn, R.; Francis, A. Attentional disengagement and the locus coeruleus–norepinephrine system in children with autism spectrum disorder. Front. Integr. Neurosci. 2021, 15, 716447. [Google Scholar] [CrossRef]
- Odriozola, P.; Uddin, L.Q.; Lynch, C.J.; Kochalka, J.; Chen, T.; Menon, V. Insula response and connectivity during social and non-social attention in children with autism. Soc. Cogn. Affect. Neurosci. 2016, 11, 433–444. [Google Scholar] [CrossRef]
- Billeci, L.; Narzisi, A.; Campatelli, G.; Crifaci, G.; Calderoni, S.; Gagliano, A.; Calzone, C.; Colombi, C.; Pioggia, G.; Muratori, F.; et al. Disentangling the initiation from the response in joint attention: An eye-tracking study in toddlers with autism spectrum disorders. Transl. Psychiatry 2016, 6, e808. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Billeci, L.; Calderoni, S.; Boncoddo, M.; Lattarulo, C.; Costanzo, V.; Turi, M.; Colombi, C.; Narzisi, A. How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study. Brain Sci. 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Klaiman, C.; Richardson, S.; Aoki, C.; Smith, C.; Minjarez, M.; Bernier, R.; Pedapati, E.; Bishop, S.; Ence, W.; et al. Eye-Tracking-Based measurement of Social Visual Engagement compared with Expert Clinical Diagnosis of Autism. J. Am. Med. Assoc. 2023, 330, 854–865. [Google Scholar] [CrossRef]
- Ozdemir, S.; Akin-Bulbul, I.; Yildiz, E. Visual Attention in Joint Attention Bids: A Comparison Between Toddlers with Autism Spectrum Disorder and Typically Developing Toddlers. J. Autism Develompental Disord. 2024, 55, 408–427. [Google Scholar] [CrossRef]
- Vernetti, A.; Butler, M.; Banarjee, C.; Boxberger, A.; All, K.; Macari, S.; Chawarska, K. Face-to-face live eye-tracking in toddlers with autism: Feasibility and impact of familiarity and face covering. Autism Res. 2023, 17, 1381–1390. [Google Scholar] [CrossRef]
- Shic, F.; Naples, A.J.; Barney, E.C.; Chang, S.A.; Li, B.; McAllister, T.; Kim, M.; Dommer, K.J.; Hasselmo, S.; Atyabi, A.; et al. The autism biomarkers consortium for clinical trials: Evaluation of a battery of candidate eye-tracking biomarkers for use in autism clinical trials. Mol. Autism 2022, 13, 15. [Google Scholar] [CrossRef]
- Frazier, T.W.; Strauss, M.; Klingemier, E.W.; Zetzer, E.E.; Hardan, A.Y.; Eng, C.; Youngstrom, E.A. A meta-analysis of gaze differences to social and nonsocial information between individuals with and without autism. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.H.; Cheng, A.; Andreason, C.; Zahiri, J.; Xiao, Y.; Xu, R.; Bao, B.; Courchesne, E.; Barnes, C.C.; Arias, S.J.; et al. Large scale validation of an early-age eye-tracking biomarker of an autism spectrum disorder subtype. Sci. Rep. 2022, 12, 4253. [Google Scholar] [CrossRef]
- Hamner, T.; Vivanti, G. Eye-Tracking Research in Autism Spectrum Disorder: What Are We Measuring and for What Purposes? J. Autism Dev. Disord. 2022, 52, 1360–1374. [Google Scholar] [CrossRef]
- Murias, M.; Major, S.; Davlantis, K.; Franz, L.; Harris, A.; Rardin, B.; Sabatos-DeVito, M.; Dawson, G. Validation of eye-tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Res. 2021, 14, 2620–2633. [Google Scholar] [CrossRef] [PubMed]
- Elison, J.T.; Paterson, S.J.; Wolff, J.J.; Reznick, J.S.; Sasson, N.J.; Gu, H.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; Evans, A.C.; et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 2013, 170, 899–908. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Fernandes, J.; Webb, S.J.; Dawson, G.; Charman, T.; Johnson, M.H.; British Autism Study of Infant Siblings Team. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry 2013, 74, 189–194. [Google Scholar] [CrossRef]
- Chawarska, K.; Volkmar, F.; Klin, A. Limited attentional bias for faces in toddlers with autism spectrum disorders. Arch. Gen. Psychiatry 2010, 67, 178–185. [Google Scholar]
- Hansen, S.N.; Schendel, D.E.; Francis, R.W.; Windham, G.C.; Bresnahan, M.; Levine, S.Z.; Reichenberg, A.; Gissler, M.; Kodesh, A.; Bai, D.; et al. Recurrence Risk of Autism in Siblings and Cousins: A Multinational, Population-Based Study. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 866–875. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef]
- Luyster, R.; Gotham, K.; Guthrie, W.; Coffing, M.; Petrak, R.; Pierce, K.; Bishop, S.; Esler, A.; Hus, V.; Oti, R.; et al. The Autism Diagnostic Observation Schedule—Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1305–1320. [Google Scholar] [CrossRef]
- Griffiths, R. Griffiths Mental Developmental Scales-Extended Revised; Hogrefe Limited: Oxford, UK, 2006. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; American Guidance Service: Circle Pines, MN, USA, 2005. [Google Scholar]
- Chita-Tegmark, M. Social attention in ASD: A review and meta-analysis of eye-tracking studies. Res. Dev. Disabil. 2016, 48, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.; Shic, F.; Holden, A.N.; Horowitz, E.J.; Barrett, A.C.; German, T.C.; Vernon, T.W. The Use of Eye Tracking as a Biomarker of Treatment Outcome in a Pilot Randomized Clinical Trial for Young Children with Autism. Autism Res. Off. J. Int. Soc. Autism Res. 2019, 12, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. The effect size. In Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 1988; pp. 77–83. [Google Scholar]
- Costanzo, V.; Narzisi, A.; Cerullo, S.; Crifaci, G.; Boncoddo, M.; Turi, M.; Apicella, F.; Tancredi, R.; Muratori, F.; Calderoni, S.; et al. High-Risk Siblings without Autism: Insights from a Clinical and Eye-Tracking Study. J. Pers. Med. 2022, 12, 1789. [Google Scholar] [CrossRef]
- Souter, N.E.; Arunachalam, S.; Luyster, R.J. The robustness of eye–mouth index as an eye-tracking metric of social attention in toddlers. Int. J. Behav. Dev. 2020, 44, 469–478. [Google Scholar] [CrossRef]
- Frick, J.E.; Colombo, J.; Saxon, T.F. Individual and developmental differences in disengagement of fixation in early infancy. Child Dev. 1999, 70, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.; Delgado, C.; Block, J.; Venezia, M.; Hogan, A.; Seibert, J. Early Social Communication Scales (ESCS); University of Miami: Coral Gables, FL, USA, 2003. [Google Scholar]
- Noldus. The Observer Reference Manual, Version 10.0; Noldus Information Technology bv Sede Internazionale: Wageningen, The Netherlands, 2010.
- Ronconi, L.; Cantiani, C.; Riva, V.; Franchin, L.; Bettoni, R.; Gori, S.; Bulf, H.; Valenza, E.; Facoetti, A. Infants’ reorienting efficiency depends on parental autistic traits and predicts future socio-communicative behaviors. Cereb. Cortex 2024, 34, 40–49. [Google Scholar] [CrossRef]
- Schietecatte, I.; Roeyers, H.; Warreyn, P. Exploring the nature of joint attention impairments in young children with autism spectrum disorder: Associated social and cognitive skills. J. Autism Dev. Disord. 2012, 42, 1–12. [Google Scholar] [CrossRef]
- Karmiloff-Smith, A. An Alternative to Domain-general or Domain-specific Frameworks for Theorizing about Human Evolution and Ontogenesis. AIMS Neurosci. 2015, 2, 91–104. [Google Scholar] [CrossRef]
- Landry, R.; Bryson, S.E. Impaired disengagement of attention in young children with autism. J. Child Psychol. Psychiatry 2004, 45, 1115–1122. [Google Scholar] [CrossRef]
- Bryson, S.E.; Zwaigenbaum, L.; Brian, J.; Roberts, W.; Szatmari, P.; Rombough, V.; McDermott, C. A prospective case series of high-risk infants who developed autism. J. Autism Dev. Disord. 2007, 37, 12–24. [Google Scholar] [CrossRef]
- Canu, D.; Van der Paelt, S.; Canal-Bedia, R.; Posada, M.; Vanvuchelen, M.; Roeyers, H. Early non social behavioural indicators of autism spectrum disorder (ASD) in siblings at elevated likelihood for ASD: A systematic review. Eur. Child Adolesc. Psychiatry 2021, 30, 497–538. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Kumagaya, S.; Myowa-Yamakoshi, M. Neurodevelopmental hypothesis about the etiology of autism spectrum disorders. Front. Hum. Neurosci. 2017, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.; Bullen, J. The bidirectional social-cognitive mechanisms of the social-attention symptoms of autism. Front. Psychiatry 2022, 12, 2570. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Duku, E.; Armstrong, V.; Brian, J.; Bryson, S.E.; Garon, N.; Roberts, W.; Roncadin, C.; Zwaigenbaum, L.; Smith, I.M. Variability in verbal and nonverbal communication in infants at risk for autism spectrum disorder: Predictors and outcomes. J. Autism Dev. Disord. 2018, 48, 3417–3431. [Google Scholar] [CrossRef]
- Wass, S.; Porayska-Pomsta, K.; Johnson, M.H. Training attentional control in infancy. Curr. Biol. 2011, 21, 1543–1547. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bryson, S.; Rogers, T.; Roberts, W.; Brian, J.; Szatmari, P. Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 2005, 23, 143–152. [Google Scholar] [CrossRef]
- Gangi, D.N.; Ibañez, L.V.; Messinger, D.S. Joint attention initiation with and without positive affect: Risk group differences and associations with ASD symptoms. J. Autism Dev. Disord. 2014, 44, 1414–1424. [Google Scholar] [CrossRef]
- Ibanez, L.V.; Grantz, C.J.; Messinger, D.S. The development of referential communication and autism symptomatology in high-risk infants. Infancy 2013, 18, 687–707. [Google Scholar] [CrossRef]
- Landa, R.J.; Gross, A.L.; Stuart, E.A.; Faherty, A. Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Dev. 2013, 84, 429–442. [Google Scholar] [CrossRef]
- Parlade, M.V.; Iverson, J.M. The development of coordinated communication in infants at heightened risk for autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 2218–2234. [Google Scholar] [CrossRef]
- Thorup, E.; Nyström, P.; Gredebäck, G.; Bölte, S.; Falck-Ytter, T.; EASE Team. Altered gaze following during live interaction in infants at risk for autism: An eye tracking study. Mol. Autism 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.; Pickles, A.; Gliga, T.; Elsabbagh, M.; Charman, T.; Johnson, M.H.; BASIS Team. Additive effects of social and non-social attention during infancy relate to later autism spectrum disorder. Dev. Sci. 2014, 17, 612–620. [Google Scholar] [CrossRef]
- Atkinson, J.; Braddick, O. Visual attention in the first years: Typical development and developmental disorders. Dev. Med. Child Neurol. 2012, 54, 589–595. [Google Scholar] [CrossRef]
- Hitzert, M.M.; Van Braeckel, K.N.; Bos, A.F.; Hunnius, S.; Geuze, R.H. Early visual attention in preterm and fullterm infants in relation to cognitive and motor outcomes at school age: An exploratory study. Front. Pediatr. 2014, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O.; Anker, S.; Nardini, M.; Birtles, D.; Rutherford, M.A.; Mercuri, E.; Dyet, L.E.; Edwards, A.D.; Cowan, F.M. Cortical vision, MRI and developmental outcome in preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2008, 93, F292–F297. [Google Scholar] [CrossRef]
- Mercuri, E.; Rutherford, M.; Cowan, F.; Pennock, J.; Counsell, S.; Papadimitriou, M.; Azzopardi, D.; Bydder, G.; Dubowitz, L. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: A clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics 1999, 103, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hus, V.; Pickles, A.; Cook, E.H., Jr.; Risi, S.; Lord, C. Using the autism diagnostic interview—Revised to increase phenotypic homogeneity in genetic studies of autism. Biol. Psychiatry 2007, 61, 438–448. [Google Scholar] [CrossRef]
- Ozdemir, S.; Akin-Bulbul, I.; Kok, I.; Ozdemir, S. Development of a visual attention based decision support system for autism spectrum disorder screening. Int. J. Psychophysiol. 2022, 173, 69–81. [Google Scholar] [CrossRef]
- Billeci, L.; Narzisi, A.; Tonacci, A.; Sbriscia-Fioretti, B.; Serasini, L.; Fulceri, F.; Apicella, F.; Sicca, F.; Calderoni, S.; Muratori, F. An integrated EEG and eye-tracking approach for the study of responding and initiating joint attention in Autism Spectrum Disorders. Sci. Rep. 2017, 7, 13560. [Google Scholar] [CrossRef]
- Han, J.; Jiang, G.; Ouyang, G.; Li, X. A multimodal approach for identifying autism spectrum disorders in children. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Charman, T.; Young, G.S.; Brian, J.; Carter, A.; Carver, L.J.; Chawarska, K.; Hertz-Picciotto, I. Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A baby siblings research consortium (BSRC) study. Autism Res. 2017, 10, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Milosavljevic, B.; Pasco, G.; Jones, E.J.; Gliga, T.; Happé, F.; Johnson, M.H.; Charman, T. the BASIS team Mid-childhood outcomes of infant siblings at familial high-risk of autism spectrum disorder. Autism Res. 2017, 10, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Landa, R.J.; Reetzke, R.; Holingue, C.B.; Herman, D.; Hess, C.R. Diagnostic stability and phenotypic differences among school-age children diagnosed with ASD before age 2. Front. Psychiatry 2022, 13, 805686. [Google Scholar] [CrossRef]
- Pierce, K.; Gazestani, V.H.; Bacon, E.; Barnes, C.C.; Cha, D.; Nalabolu, S.; Lopez, L.; Moore, A.; Pence-Stophaeros, S.; Courchesne, E. Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatr. 2019, 173, 578–587. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bryson, S.E.; Brian, J.; Smith, I.M.; Roberts, W.; Szatmari, P.; Roncadin, C.; Garon, N.; Vaillancourt, T. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016, 9, 790–800. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Landa, R.J.; Brian, J.; Bryson, S.; Charman, T.; Chawarska, K.; Macari, S.L.; Messinger, D.; Stone, W.L.; et al. Diagnostic stability in young children at risk for autism spectrum disorder: A baby siblings research consortium study. J. Child Psychol. Psychiatry 2015, 56, 988–998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).