Date Seed-Derived Activated Carbon: A Comparative Study on Heavy Metal Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursor Materials and Preparation of Activated Carbon
2.2. Chemicals Used
2.3. Adsorbate
2.4. Chemical Preparation
2.5. Adsorption Experiment
2.6. Statistical Analysis
3. Results
3.1. Sample Characterization
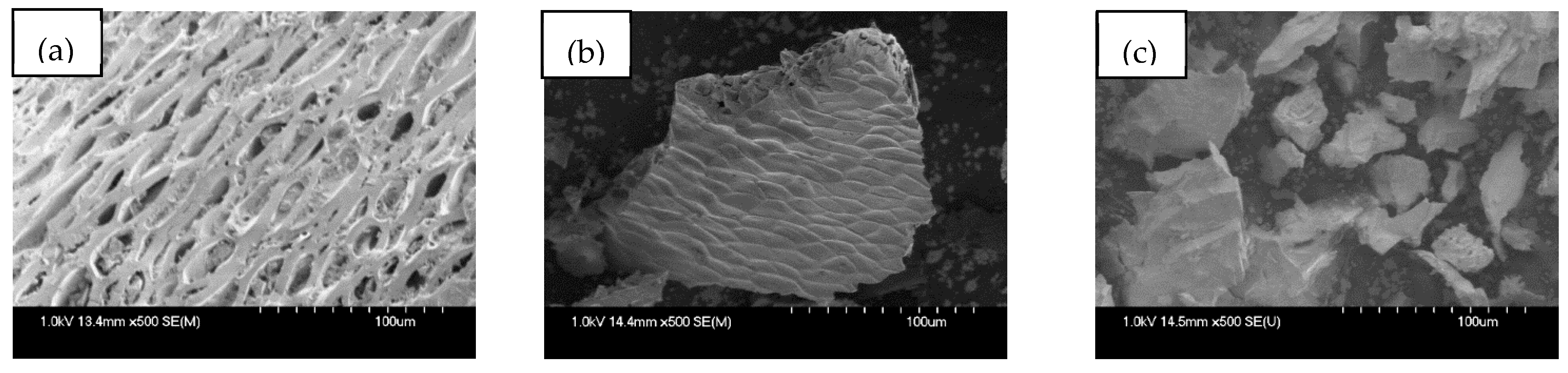
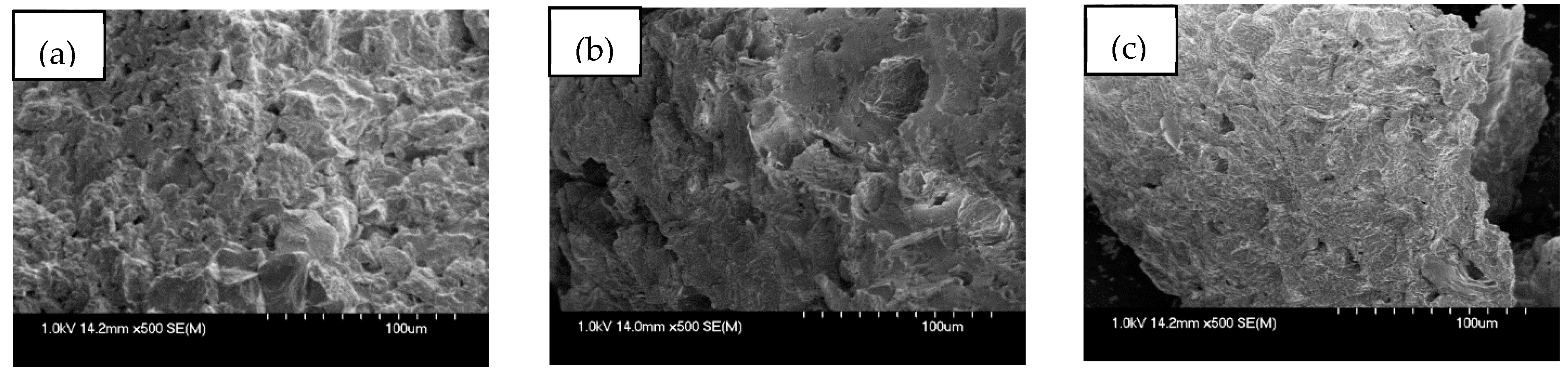
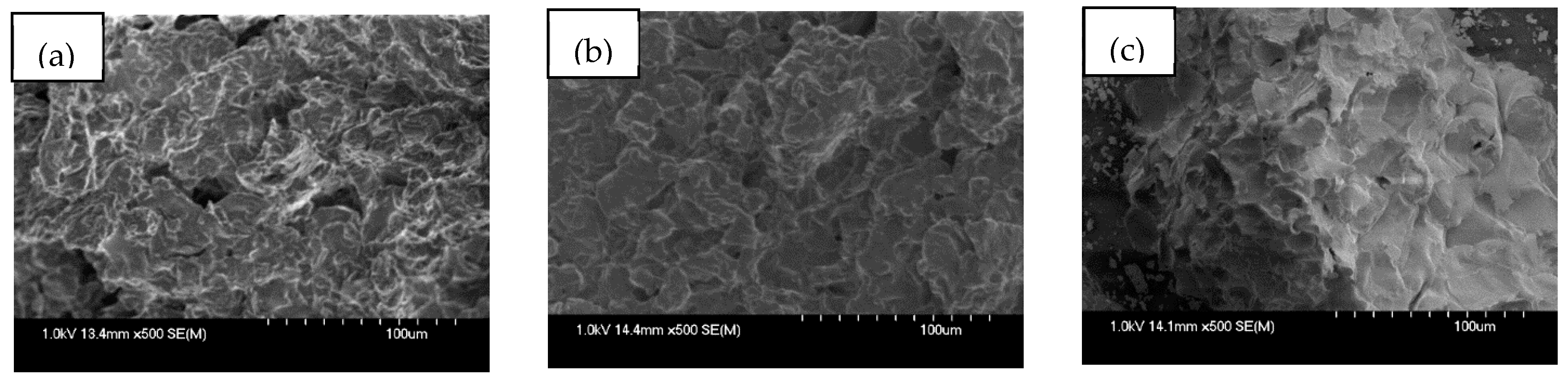
3.1.1. SEM Analysis
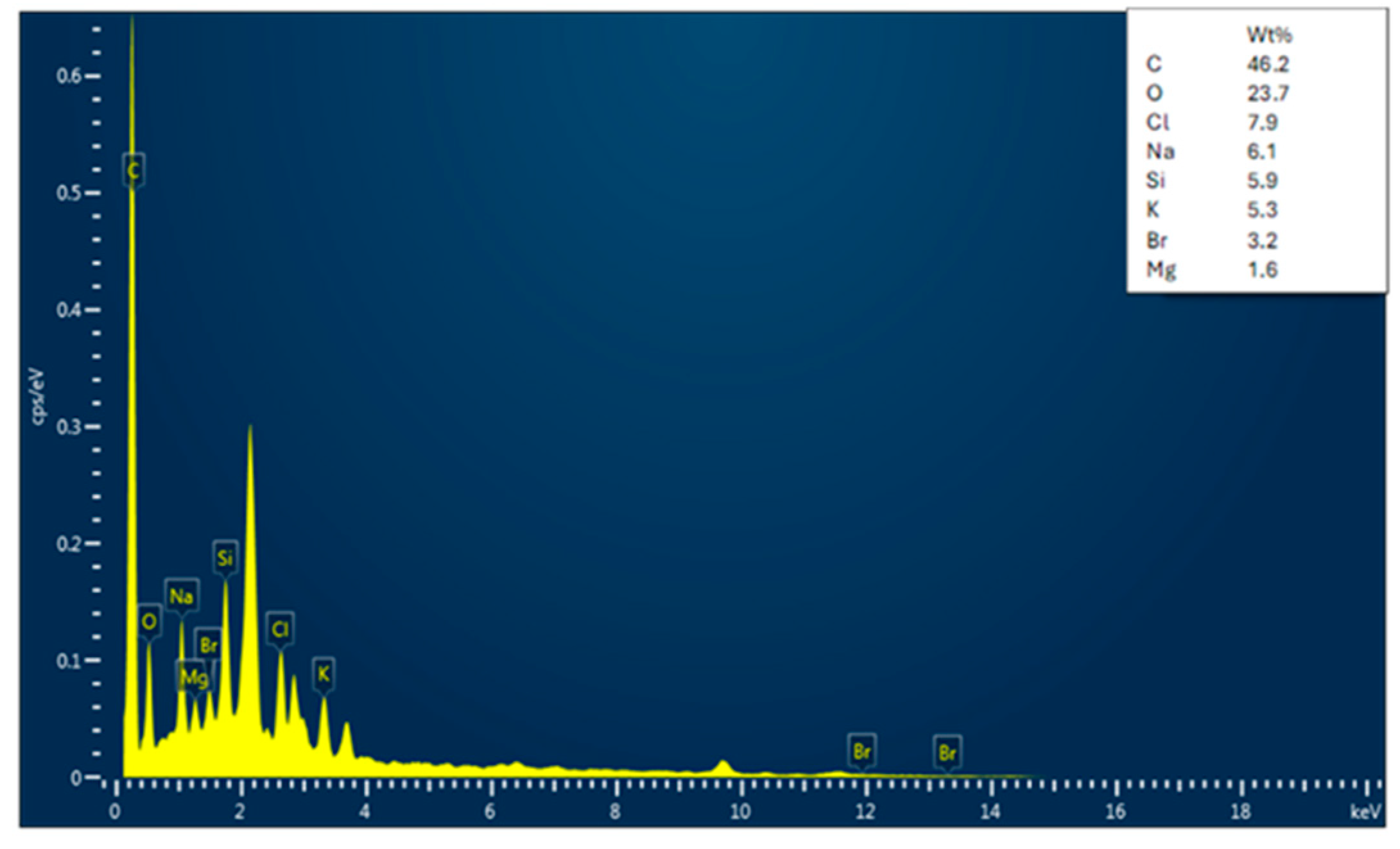
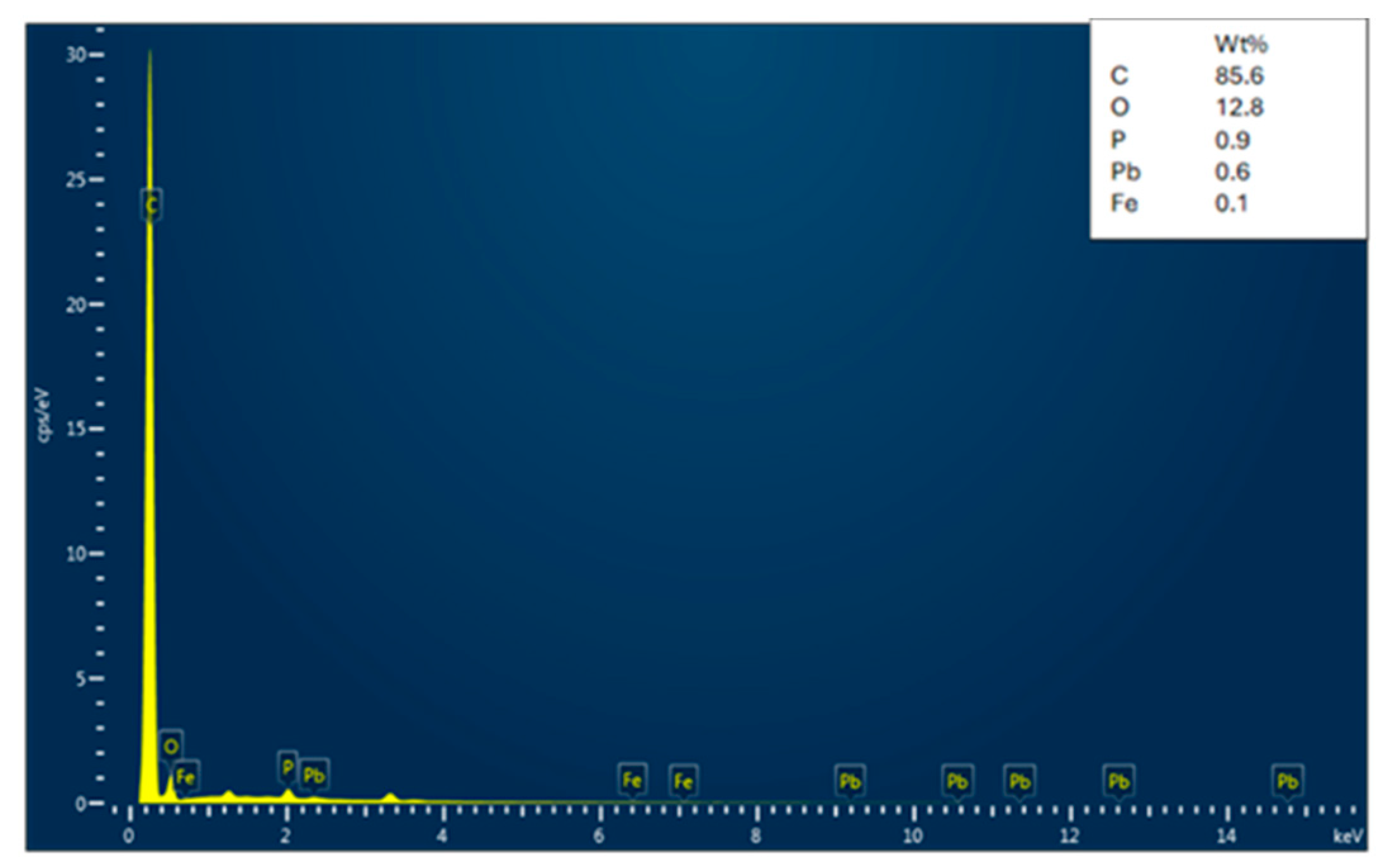
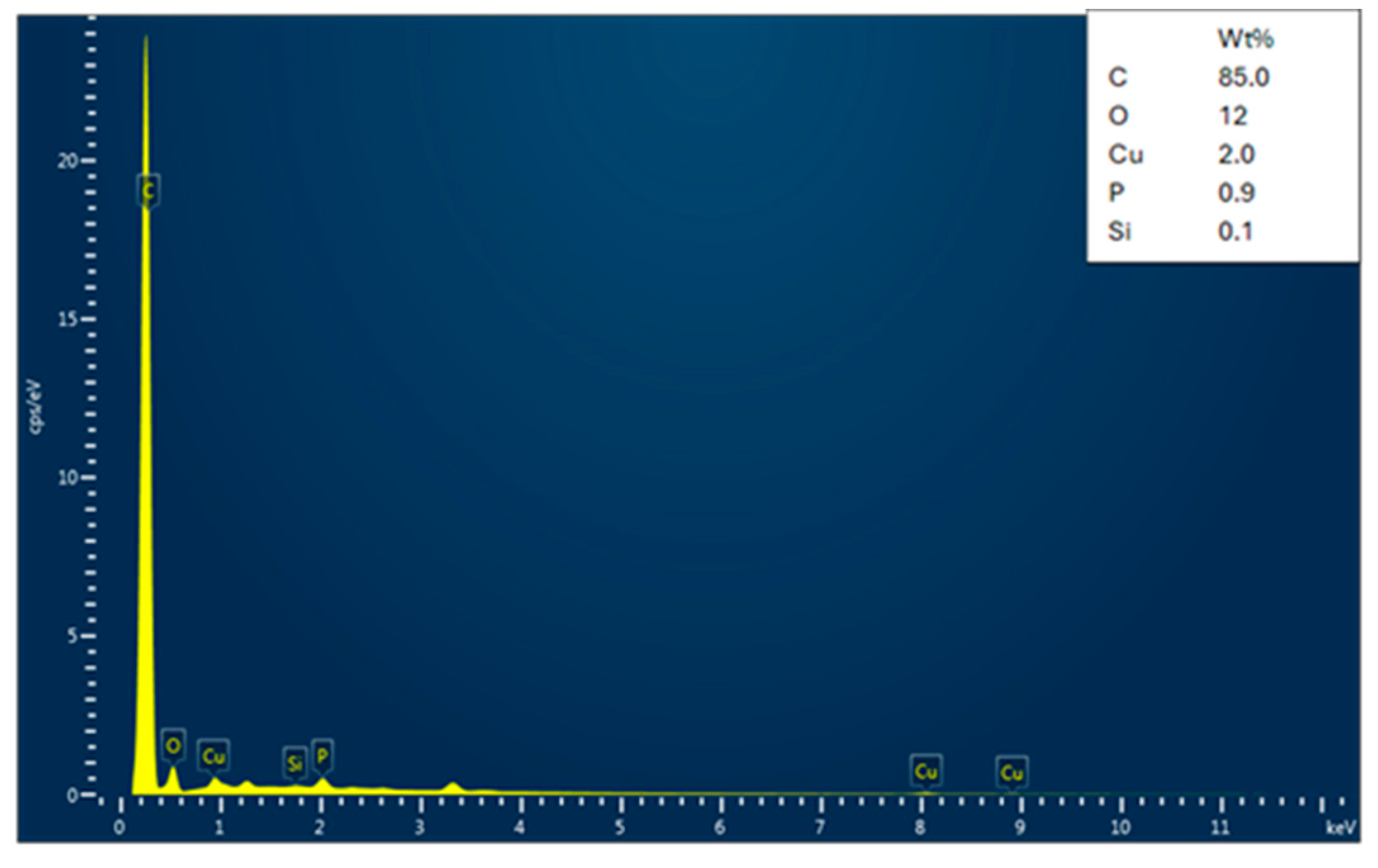
3.1.2. EDX Analysis
3.1.3. XRD Analysis
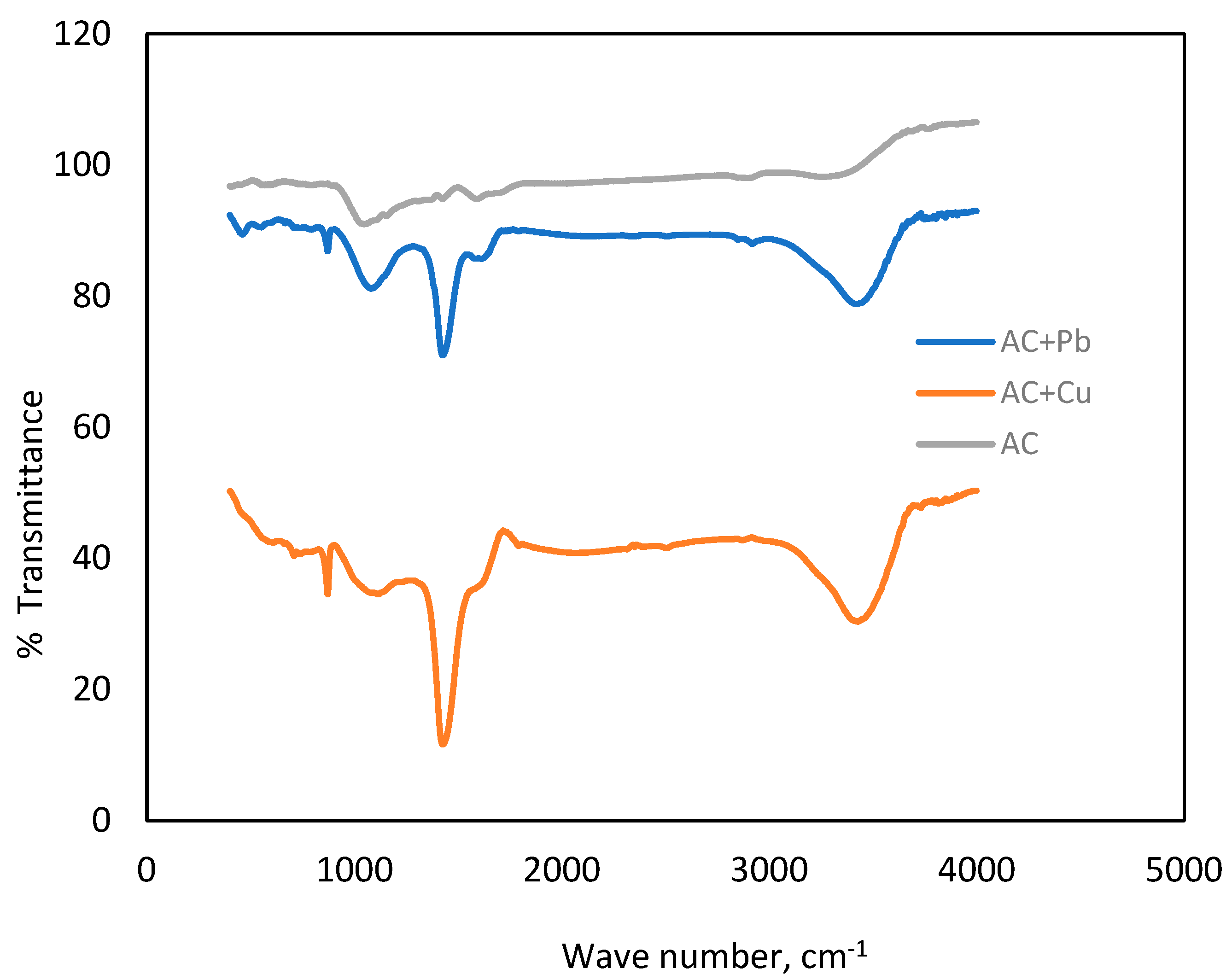
3.1.4. FTIR Analysis
3.2. Selection of Adsorbent
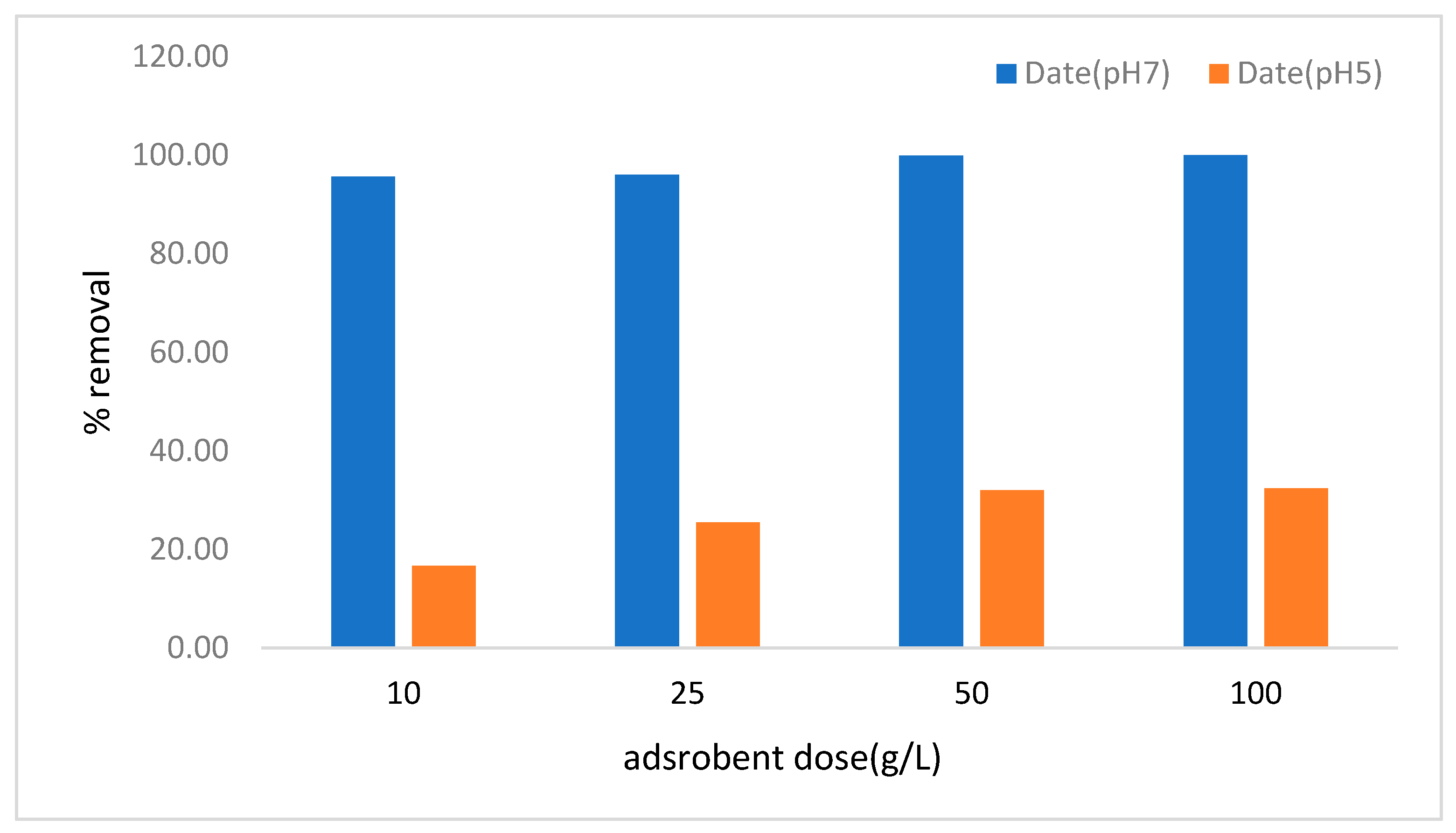
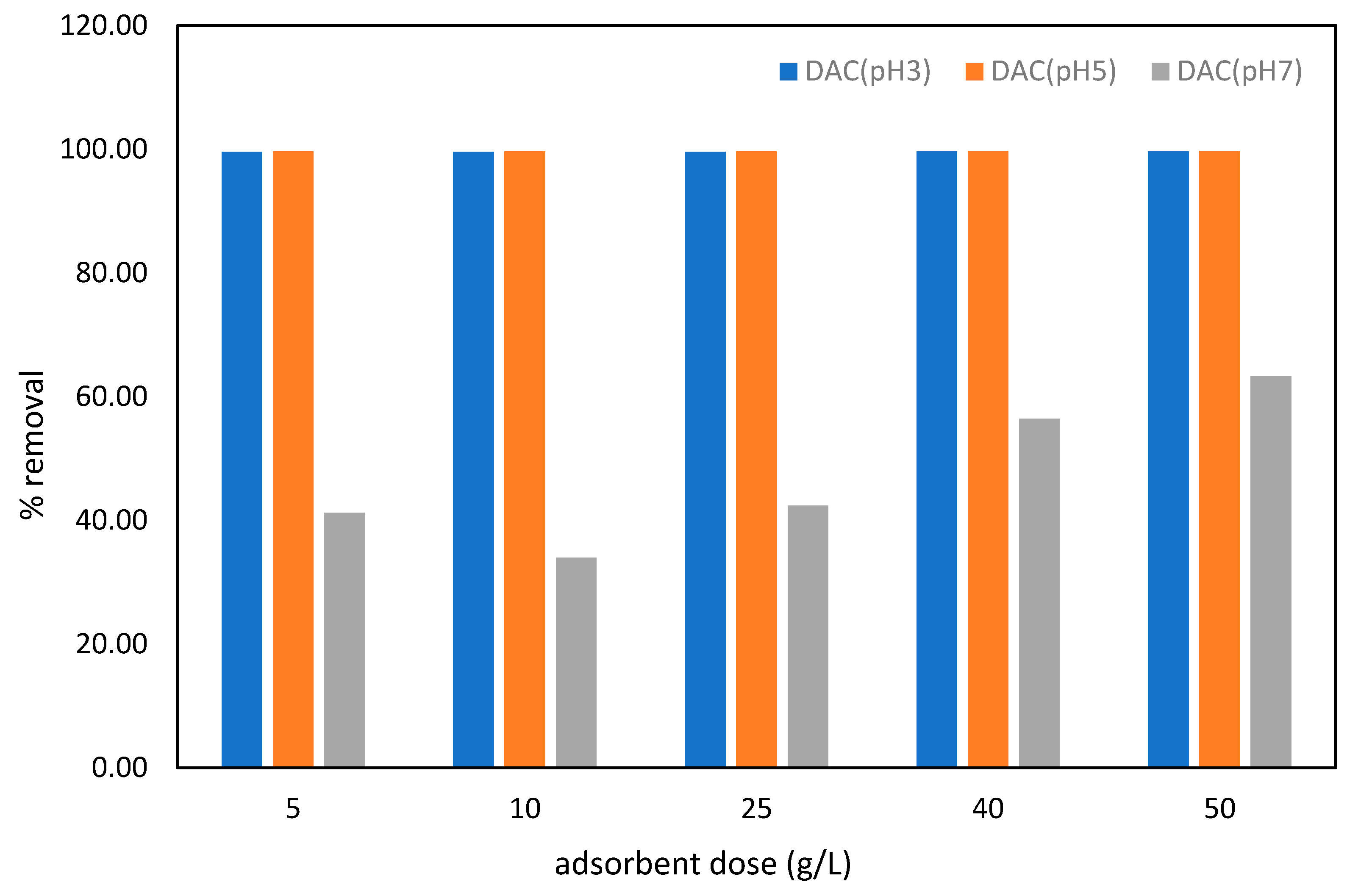
3.3. Effect of pH and Adsorbent Dose
3.4. Effect of Disinfectants
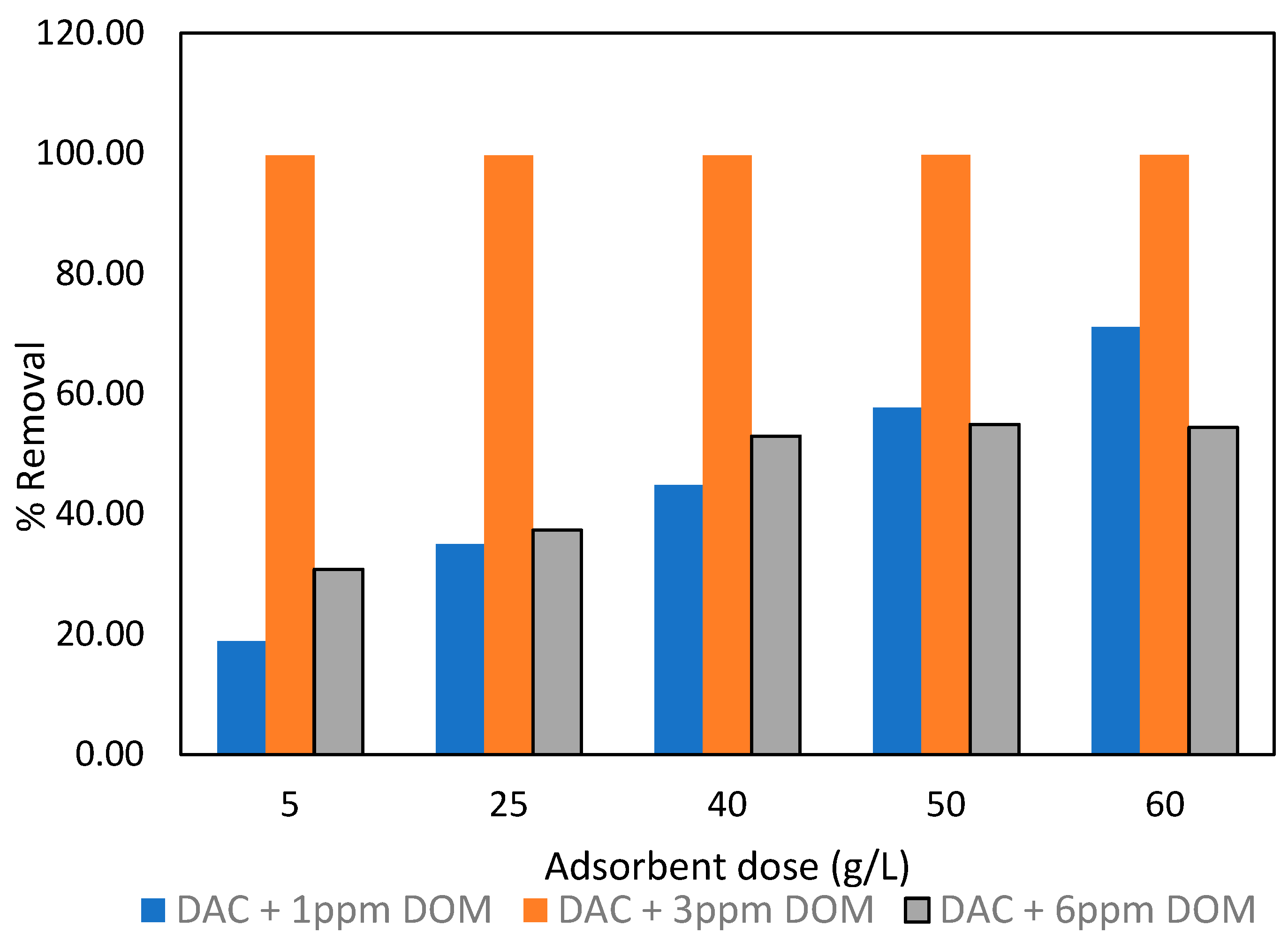
3.5. Effect of DOM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zada, A.; Qu, Y.; Ali, S.; Sun, N.; Lu, H.; Yan, R.; Zhang, X.; Jing, L. Improved Visible-Light Activities for Degrading Pollutants on TiO2/g-C3N4 Nanocomposites by Decorating SPR Au Nanoparticles and 2,4-Dichlorophenol Decomposition Path. J. Hazard. Mater. 2018, 342, 715–723. [Google Scholar] [CrossRef]
- World Health Organization. Lead in Drinking-Water: Health Risks, Monitoring and Corrective Actions: Technical Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- González-Fernández, L.A.; Medellín-Castillo, N.A.; Navarro-Frómeta, A.E.; Carranza-Álvarez, C.; Castillo-Ramos, V.; Sánchez-Polo, M.; Vilasó-Cadre, J.E.; Díaz-Flores, P.E.; Morales-Oyervides, L.; Pérez-Aguilar, N.V.; et al. Heavy Metal Pollution in Water: Cause and Remediation Strategies. In Current Status of Marine Water Microbiology; Springer: Singapore, 2023; pp. 221–262. [Google Scholar]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Jarvis, P.; Fawell, J. Lead in Drinking Water—An Ongoing Public Health Concern? Curr. Opin. Environ. Sci. Health 2021, 20, 100239. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Huq, A.O.; binti Yahya, R. The Removal of Heavy Metal Ions from Wastewater/Aqueous Solution Using Polypyrrole-Based Adsorbents: A Review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- El-Fahla, N.A.; Mohallal, M.E.; Gad El-Hak, H.N.; Dessouki, A.A.; Abdelrazek, H.M.A. Effect of Lead Toxicity on The Kidney of Nile Tilapia: Amelioration by Beta-MOS®. Egypt. Acad. J. Biol. Sci. Histol. Histochem. 2022, 14, 31–45. [Google Scholar] [CrossRef]
- Fabolude, G.; Knoble, C.; Vu, A.; Yu, D. Comprehensive Lead Exposure Vulnerability for New Jersey: Insights from a Multi-Criteria Risk Assessment and Community Impact Analysis Framework. Ecol. Indic. 2024, 167, 112585. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global Health Burden and Cost of Lead Exposure in Children and Adults: A Health Impact and Economic Modelling Analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef]
- Hiwatari, M.; Yamada, D.; Narita, D.; Hangoma, P.; Chitah, B. Toxic Pollution and Poverty: Economic Impacts of Lead (Pb) Exposure on Household Welfare in Zambia. Ecol. Econ. 2024, 221, 108209. [Google Scholar] [CrossRef]
- Li, B.; Trueman, B.F.; Rahman, M.S.; Gagnon, G.A. Controlling Lead Release Due to Uniform and Galvanic Corrosion—An Evaluation of Silicate-Based Inhibitors. J. Hazard. Mater. 2021, 407, 124707. [Google Scholar] [CrossRef]
- Health Canada. Water Talk. Minimizing Exposure to Lead from Drinking Water Distribution Systems; Health Canada: Ottawa, ON, Canada, 2016.
- US Environmental Protection Agency. Use of Lead Free Pipes, Fittings, Fixtures, Solder and Flux for Drinking Water; US Environmental Protection Agency: Washington, DC, USA, 2024.
- Santucci, R.J.; Scully, J.R. The Pervasive Threat of Lead (Pb) in Drinking Water: Unmasking and Pursuing Scientific Factors That Govern Lead Release. Proc. Natl. Acad. Sci. USA 2020, 117, 23211–23218. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological Relevance and Mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Ghosh, S.; Sadhu, A.; Mandal, A.H.; Biswas, J.K.; Sarkar, D.; Saha, S. Copper Oxide Nanoparticles as an Emergent Threat to Aquatic Invertebrates and Photosynthetic Organisms: A Synthesis of the Known and Exploration of the Unknown. Curr. Pollut. Rep. 2025, 11, 6. [Google Scholar] [CrossRef]
- El-Araby, H.A.; Ibrahim, A.M.M.A.; Mangood, A.H.; Abdel-Rahman, A.A.-H.; El-Araby, H.A.; Ibrahim, A.M.M.A.; Mangood, A.H.; Abdel-Rahman, A.A.-H. Sesame Husk as Adsorbent for Copper(II) Ions Removal from Aqueous Solution. J. Geosci. Environ. Prot. 2017, 5, 109–152. [Google Scholar] [CrossRef]
- Rahman, M.S. Experimental Investigation on the Effect of Physio-Chemical Properties of Water on Galvanic Corrosion and Turbidity Due to Iron Oxidation. Environ. Process. 2023, 10, 37. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hachisuga, N.; Serpone, N. Recycling of E-Waste Power Cables Using Microwave-Induced Pyrolysis—Process Characteristics and Facile Recovery of Copper Metal. RSC Adv. 2024, 14, 29955–29964. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef]
- Manne, R.; Kumaradoss, M.M.R.M.; Iska, R.S.R.; Devarajan, A.; Mekala, N. Water Quality and Risk Assessment of Copper Content in Drinking Water Stored in Copper Container. Appl. Water Sci. 2022, 12, 27. [Google Scholar] [CrossRef]
- Schwartz, H.; Marushka, L.; Chan, H.M.; Batal, M.; Sadik, T.; Ing, A.; Fediuk, K.; Tikhonov, C. Metals in the Drinking Water of First Nations across Canada. Can. J. Public Health 2021, 112, 113–132. [Google Scholar] [CrossRef]
- Althomali, R.H.; Abbood, M.A.; Saleh, E.A.M.; Djuraeva, L.; Abdullaeva, B.S.; Habash, R.T.; Alhassan, M.S.; Alawady, A.H.R.; Alsaalamy, A.H.; Najafi, M.L. Exposure to Heavy Metals and Neurocognitive Function in Adults: A Systematic Review. Environ. Sci. Eur. 2024, 36, 18. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Fadila, C.R.; Othman, M.H.D.; Adam, M.R.; Takagi, R.; Yoshioka, T.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Adsorptive Membrane for Heavy Metal Removal: Material, Fabrication, and Performance. Mater. Today Proc. 2022, 65, 3037–3045. [Google Scholar] [CrossRef]
- Ren, D.; Zhang, M.; Han, G.; Li, Z.; Yang, L.; Li, X.; Gong, Y.; Shang, Z.; Yang, F. Preparation of CO2/N2-Switchable Hydrogel Beads and Adsorption, Desorption, Regeneration for Cu(II). Colloids Surf. Physicochem. Eng. Asp. 2023, 674, 131882. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Wu, Y.; Geng, L.; Zhang, X.; Zhang, D.; Hu, H.; Zhang, Y.; Li, X.; Liu, W.; et al. Synthesis of Uniform-Sized and Microporous MIL-125(Ti) to Boost Arsenic Removal by Chemical Adsorption. Polyhedron 2021, 196, 114980. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent Advances on Sustainable Adsorbents for the Remediation of Noxious Pollutants from Water and Wastewater: A Critical Review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Rahman, M.H.; Wasiuddin, N.M.; Islam, M.R. Experimental and Numerical Modeling Studies of Arsenic Removal with Wood Ash from Aqueous Streams. Can. J. Chem. Eng. 2004, 82, 968–977. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Mtei, K.M.; Choi, J.W. Adsorptive Removal of Heavy Metals from Wastewater Using Low-Cost Adsorbents Derived from Agro-Based Materials. In Heavy Metal Remediation: Sustainable Nexus Approach; Springer: Cham, Switzerland, 2024; pp. 237–271. [Google Scholar]
- You, Z.; Yu, Z.; Hu, Y.; Dong, J.; Yue, W.; Li, J.; Ma, X. Effect of Modified Ca-Al Adsorbents on Heavy Metal Fixation during Co-Combustion of Coal and Coconut Shells: Experiments and Simulations. Environ. Pollut. 2024, 361, 124856. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Mohan, B.C.; Tai, M.H.; Yao, Z.; Su, W.; Lay-Ming Teo, S.; Wang, C.-H. Green, Non-Toxic and Efficient Adsorbent from Hazardous Ash Waste for the Recovery of Valuable Metals and Heavy Metal Removal from Waste Streams. Chemosphere 2023, 329, 138524. [Google Scholar] [CrossRef]
- Singh, J.; Jadeja, R. Recent Advances in Agricultural Waste Derived Magnetic Biochar for Removal of Heavy Metal Ions: Mechanistic Insights and Technological Innovation. J. Mol. Struct. 2025, 1325, 141005. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Wu, J.; Yu, X. Eggshell-Enhanced Biochar with in-Situ Formed CaO/Ca(OH)2 for Efficient Removal of Pb2+ and Cd2+ from Wastewater: Performance and Mechanistic Insights. Sep. Purif. Technol. 2025, 354, 129352. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, K.; Wang, L.; Wang, W.; Du, M.; Li, R.; Fang, J.; Jiang, Z. Utilization of Rice Husk Ash as a Potential Catalyst for Diatom Growth and Adsorbent for Heavy Metals in Aquaculture Systems. Aquaculture 2025, 595, 741533. [Google Scholar] [CrossRef]
- Gama de Almeida, M.B.; Dantas de Jesus, A.M.; Pereira, A.S.; Fiore, F.A. Evaluating Centrifuged Water Treatment Plant Sludge as an Adsorbent for Nutrients, Microorganisms, and Heavy Metals Removal from Wastewater. J. Clean. Prod. 2024, 468, 142975. [Google Scholar] [CrossRef]
- Yazid, H.; Amour, L.; Terkmani, A.; Maachi, R. Biosorption of Lead from Aqueous Solution by Biologically Activated Date Pedicels: Batch and Column Study. Desalin. Water Treat. 2013, 51, 1690–1699. [Google Scholar] [CrossRef]
- Tariq, A.; Yahaya, N.; Sajid, M. Low Cost Adsorbents Derived from Vegetables and Fruits: Synthesis, Properties, and Applications in Removal of Heavy Metals from Water. Desalin. Water Treat. 2024, 320, 100626. [Google Scholar] [CrossRef]
- Naseer, A. Role of Nanocomposites and Nano Adsorbents for Heavy Metals Removal and Dyes. An Overview. Desalina. Water Treat. 2024, 320, 100662. [Google Scholar] [CrossRef]
- Boughrara, L.; Sebba, F.Z.; Sebti, H.; Choukchou-Braham, E.; Bounaceur, B.; Kada, S.O.; Zaoui, F. Removal of Zn(II) and Ni(II) Heavy Metal Ions by New Alginic Acid-Ester Derivatives Materials. Carbohydr. Polym. 2021, 272, 118439. [Google Scholar] [CrossRef] [PubMed]
- Gomravi, Y.; Karimi, A.; Azimi, H. Adsorption of Heavy Metal Ions via Apple Waste Low-Cost Adsorbent: Characterization and Performance. Korean J. Chem. Eng. 2021, 38, 1843–1858. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Palm Kernel Shell as an Effective Adsorbent for the Treatment of Heavy Metal Contaminated Water. Sci. Rep. 2019, 9, 18955. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R. Oak (Quercus Robur) Acorn Peel as a Low-Cost Adsorbent for Hexavalent Chromium Removal from Aquatic Ecosystems and Industrial Effluents. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Madzin, Z.; Zahidi, I.; Talei, A.; Raghunandan, M.E.; Hermawan, A.A.; Karam, D.S. Optimising Spent Mushroom Compost Biochar for Heavy Metal Removal: Mechanisms and Kinetics in Mine Water Treatment. J. Water Process Eng. 2025, 69, 106829. [Google Scholar] [CrossRef]
- Rahman, M.S. Adsorption of Methyl Blue onto Activated Carbon Derived from Red Oak (Quercus rubra) Acorns: A 26 Factorial Design and Analysis. Water. Air. Soil Pollut. 2021, 232, 1. [Google Scholar] [CrossRef]
- Lebel Desrosiers, S.; Collin, A.; Bélanger, N. Factors Affecting Early Red Oak (Quercus rubra L.) Regeneration near Its Northern Distribution Limit in Quebec. Front. For. Glob. Change 2024, 7, 1451161. [Google Scholar] [CrossRef]
- FAO Statistics. World Food and Agriculture—Statistical Yearbook 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Nasser, R.A.; Salem, M.Z.M.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical Analysis of Different Parts of Date Palm (Phoenix dactylifera L.) Using Ultimate, Proximate and Thermo-Gravimetric Techniques for Energy Production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- Alsulaili, A.D.; Refaie, A.A.; Garcia, H.A. Adsorption Capacity of Activated Carbon Derived from Date Seeds: Characterization, Optimization, Kinetic and Equilibrium Studies. Chemosphere 2023, 313, 137554. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of Lead(II) from Aqueous Solution Using Date Seed-Derived Biochar: Batch and Column Studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Peaches and Nectarines: Global Growth in Production Slowing While Exports Plateau. 2023. Available online: https://fas.usda.gov/data/peaches-and-nectarines-global-growth-production-slowing-while-exports-plateau (accessed on 5 February 2025).
- Eisnor, J.D.; Gagnon, G.A. Impact of Secondary Disinfection on Corrosion in a Model Water Distribution System. J. Water Supply Res. Technol. Aqua 2004, 53, 441–452. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G. A Comparative Study on the Removal Efficiency of Metal Ions (Cu2+, Ni2+, and Pb2+) Using Sugarcane Bagasse-Derived ZnCl2-Activated Carbon by the Response Surface Methodology. Adsorpt. Sci. Technol. 2016, 35, 72–85. [Google Scholar] [CrossRef]
- Vunain, E.; Kenneth, D.; Biswick, T. Synthesis and Characterization of Low-Cost Activated Carbon Prepared from Malawian Baobab Fruit Shells by H3PO4 Activation for Removal of Cu(II) Ions: Equilibrium and Kinetics Studies. Appl. Water Sci. 2017, 7, 4301–4319. [Google Scholar] [CrossRef]
- Nasirian, A. Synthesis and Characterization of Cu Nanoparticles and Studying of Their Catalytic Properties. Int. J. Nano Dim. 2012, 2, 159–164. [Google Scholar]
- Theivasanthi, T.; Alagar, M. X-Ray Diffraction Studies of Copper Nanopowder. arXiv 2010, arXiv:1003.6068. [Google Scholar]
- Roushdy, N.; Elnouby, M.S.; Farag, A.A.M.; Ramadan, M.; El-Shazly, O.; El-Wahidy, E.F. Structural and Electrical Characterization of Nickel Sulfide Nanoparticles. Opt. Quantum Electron. 2024, 56, 1794. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The Comparative Analyses of Reduced Graphene Oxide (RGO) Prepared via Green, Mild and Chemical Approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Allahkarami, E.; Dehghan Monfared, A.; Silva, L.F.O.; Dotto, G.L. Toward a Mechanistic Understanding of Adsorption Behavior of Phenol onto a Novel Activated Carbon Composite. Sci. Rep. 2023, 13, 167. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Sreematha, B.; Arundhathi, N.; Ravinder, D. Influence of Cerium Substitution on Structural, Optical, and Electrical Transport Properties of Ni-Nano Ferrites Prepared by Citrate Gel Auto Combustion Method. Results Chem. 2023, 5, 100929. [Google Scholar] [CrossRef]
- Benjamine, M.; Rahman, A. Structural, Morphological and Optical Properties of CuO Nanoparticles Prepared through Chemical Method. J. Emerg. Technol. Innov. Res. 2018, 5, 273–278. [Google Scholar]
- Kamalabadi, M.; Madrakian, T.; Afkhami, A.; Ghoorchian, A. Crystal Violet-Modified HKUST-1 Framework with Improved Hydrostability as an Efficient Adsorbent for Direct Solid-Phase Microextraction. Microchim. Acta 2021, 188, 305. [Google Scholar] [CrossRef]
- Nasir, H.; Rahman, N.; Zulfiqar; Khan, T.; Ali, S.; Khan, R.; Hayat, K. Variations in Structural, Optical, and Dielectric Properties of CuO Nanostructures with Thermal Decomposition. J. Mater. Sci. Mater. Electron. 2020, 31, 10649–10656. [Google Scholar] [CrossRef]
- Koseoglu, E.; Akmil-Başar, C. Preparation, Structural Evaluation and Adsorptive Properties of Activated Carbon from Agricultural Waste Biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Devi, B.; Goswami, M.; Rabha, S.; Kalita, S.; Sarma, H.P.; Devi, A. Efficacious Sorption Capacities for Pb(II) from Contaminated Water: A Comparative Study Using Biowaste and Its Activated Carbon as Potential Adsorbents. ACS Omega 2023, 8, 15141–15151. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Farag, S.; Fikry, M.; Aly, A.A.; Amr, A. Evaluation of the Adsorption Capacity of Surfactant-Modified Biomass in an Aqueous Acid Blue 193 System. Waste Manag. Bull. 2024, 2, 172–183. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Sellaoui, L.; Dhaouadi, F.; Taamalli, S.; Louis, F.; Bakali, A.E.; Badawi, M.; Bonilla-Petriciolet, A.; Silva, L.; da Boit Martinello, K.; Dotto, G.L. Understanding the Cu2+ Adsorption Mechanism on Activated Carbon Using Advanced Statistical Physics Modelling. Environ. Sci. Pollut. Res. 2022, 29, 54882–54889. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Cheng, S.; Xia, H.; Zhang, L.; Peng, J.; Li, C.; Zhang, S. Copper Loaded on Activated Carbon as an Efficient Adsorbent for Removal of Methylene Blue. RSC Adv. 2017, 7, 14395–14405. [Google Scholar] [CrossRef]
- Djezzar, Z.; Aidi, A.; Rehali, H.; Ziad, S.; Othmane, T. Characterization of Activated Carbon Produced from the Green Algae Spirogyra Used as a Cost-Effective Adsorbent for Enhanced Removal of Copper(Ii): Application in Industrial Wastewater Treatment. RSC Adv. 2024, 14, 5276–5289. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.; García-Díaz, I.; Alguacil, F.J.; López Gómez, F.A. Removal of Copper Ions from Wastewater by Adsorption onto a Green Adsorbent from Winemaking Wastes. BioResources 2020, 15, 1112–1133. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Removal of Lead (II) Ions from Aqueous Solutions Using Manganese Oxide-Coated Adsorbents: Characterization and Kinetic Study. Adsorpt. Sci. Technol. 2009, 27, 177–191. [Google Scholar] [CrossRef]
- Amrutha; Jeppu, G.; Girish, C.R.; Prabhu, B.; Mayer, K. Multi-Component Adsorption Isotherms: Review and Modeling Studies. Environ. Process. 2023, 10, 38. [Google Scholar] [CrossRef]
- Aguilar, K.M.M.; Kose, Y.; Amano, Y.; Machida, M.; Imazeki, F. Influence of Oxidation Conditions of Activated Carbon on Adsorption of Pb (II) from Aqueous Solution. J. Environ. Chem. 2016, 26, 109–114. [Google Scholar] [CrossRef]
- Evans, M.J.B.; Halliop, E.; Liang, S.; MacDonald, J.A.F. The Effect of Chlorination on Surface Properties of Activated Carbon. Carbon 1998, 36, 1677–1682. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans World Health Organization International Agency for Research on Cancer. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans World Health Organization International Agency for Research on Cancer: Lyon, France, 2004; Volume 84. [Google Scholar]
- Minkina, T.M.; Pinskii, D.L.; Zamulina, I.V.; Nevidomskaya, D.G.; Gülser, C.; Mandzhieva, S.S.; Bauer, T.V.; Morozov, I.V.; Sushkova, S.N.; Kizilkaya, R. Chemical Contamination in Upper Horizon of Haplic Chernozem as a Transformation Factor of Its Physicochemical Properties. J. Soils Sediments 2018, 18, 2418–2430. [Google Scholar] [CrossRef]
- Zamulina, I.V.; Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Bauer, T.V.; Burachevskaya, M.V. The Influence of Long-Term Zn and Cu Contamination in Spolic Technosols on Water-Soluble Organic Matter and Soil Biological Activity. Ecotoxicol. Environ. Saf. 2021, 208, 111471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H. Co-Transport of Dissolved Organic Matter and Heavy Metals in Soils Induced by Excessive Phosphorus Applications. J. Environ. Sci. 2010, 22, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Katoh, M. Feature of Lead Complexed with Dissolved Organic Matter on Lead Immobilization by Hydroxyapatite in Aqueous Solutions and Soils. Chemosphere 2020, 249, 126122. [Google Scholar] [CrossRef]
- Qiu, L.; Suo, C.; Zhang, N.; Yuan, R.; Chen, H.; Zhou, B. Adsorption of Heavy Metals by Activated Carbon: Effect of Natural Organic Matter and Regeneration Methods of the Adsorbent. Desalin. Water Treat. 2022, 252, 148–166. [Google Scholar] [CrossRef]
- Da’na, E.; Awad, A. Regeneration of Spent Activated Carbon Obtained from Home Filtration System and Applying It for Heavy Metals Adsorption. J. Environ. Chem. Eng. 2017, 5, 3091–3099. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.S.; Bansal, N.; Rahman, M.H.; Mortula, M. Date Seed-Derived Activated Carbon: A Comparative Study on Heavy Metal Removal from Aqueous Solutions. Appl. Sci. 2025, 15, 3257. https://doi.org/10.3390/app15063257
Rahman MS, Bansal N, Rahman MH, Mortula M. Date Seed-Derived Activated Carbon: A Comparative Study on Heavy Metal Removal from Aqueous Solutions. Applied Sciences. 2025; 15(6):3257. https://doi.org/10.3390/app15063257
Chicago/Turabian StyleRahman, Mohammad Shahedur, Neetu Bansal, Mohammod Hafizur Rahman, and Maruf Mortula. 2025. "Date Seed-Derived Activated Carbon: A Comparative Study on Heavy Metal Removal from Aqueous Solutions" Applied Sciences 15, no. 6: 3257. https://doi.org/10.3390/app15063257
APA StyleRahman, M. S., Bansal, N., Rahman, M. H., & Mortula, M. (2025). Date Seed-Derived Activated Carbon: A Comparative Study on Heavy Metal Removal from Aqueous Solutions. Applied Sciences, 15(6), 3257. https://doi.org/10.3390/app15063257







