Abstract
The presence of heavy metals in groundwater and wastewater has been a concern for health organizations. This study investigated the effectiveness of activated carbon derived from various natural precursors, including acorns from red oak trees (Quercus rubra), date seeds, and peach seeds, employing the thermal activation method for the removal of heavy metals from aqueous solutions. Batch adsorption tests investigated the effects of sorbent quantity, pH levels, disinfectant presence, and dissolved organic matter (DOM) on the removal efficiency of Pb and Cu. Characterization of the prepared activated carbon was conducted using scanning electron microscopy (SEM). Lead removal efficiency diminished at pH 7 relative to pH 3 and 5, but copper exhibited superior removal efficiencies at pH 7 compared to pH 5. The addition of monochloramine at 4 parts per million (ppm) effectively eliminated lead from the solution. A rise in free chlorine concentration from 2 to 4 mg/L led to a reduction in metal removal from water by 20 to 60%. DOM at concentrations of 1 and 6 mg/L reduced metal removal efficacy relative to DOM at 3 mg/L. Date seed-activated carbons underscore their distinctive potential, offering useful insights for the enhancement of water and wastewater treatment systems.
1. Introduction
The advancement and swift progress of civilization have led to the emission of several undesirable contaminants into the environment [1]. Heavy metal pollutants contamination is a significant issue, according to current data, particularly in water systems. For instance, about 10% of the water sources in underdeveloped rural areas are said to have lead levels greater than 50 µg/L, which is the maximum amount allowed by the WHO guidelines [2]. In industrial areas, heavy metal contamination in water sources frequently surpasses safety thresholds; for example, in specific regions, copper levels have been documented at concentrations markedly above the World Health Organization’s advised limit of 2 mg/L [3]. The introduction of heavy metals, originating from both natural and human activities, has concerned the scientific community in recent decades. These metals have the ability to be adsorbed onto cell membranes, leading to the development of numerous incurable diseases in both humans and aquatic plants and animals. Metals such as lead (Pb) and copper (Cu) are widely present in the environment and pose significant risks [4].
Lead (Pb), listed as a priority pollutant by the Environmental Protection Agency, surpasses other heavy metals in contamination levels in water bodies and poses severe cumulative health risks globally. In Addis Ababa, lead levels in water have been found to average 62.6 μg/L [5], which exceeds the permissible lead concentration of 50 μg/L set by the World Health Organization for drinking water [6]. The exposure to lead (Pb) disrupts physiological functions, damages vital organs, and causes kidney failure. Furthermore, Pb can accumulate in many tissues of fish exposed to it, leading to harm in the liver and kidneys, as well as stunted growth. This accumulation also causes stress, resulting in disrupted cortisol levels and metabolic enzymes [7].
Studies have shown that elevated blood lead levels are associated with cognitive deficits in children [8] and an increased risk of cardiovascular disease mortality [9], hypertension, and renal dysfunction [10]. Lead has been extensively utilized in drinking water distribution systems throughout history due to its corrosion resistance and malleability [11]. Although there have been extensive prohibitions on lead pipes and lead-tin solders, there still exists a residue of plumbing that contains lead [12,13]. Despite the well-known lead-in-water emergencies in Flint, MI, and Washington, D.C., the risk of lead exposure through drinking water remains a persistent danger to public health [14].
Copper is a vital metal in several enzymes for all living organisms; complications arise when there is a deficiency or excess of it [15]. Copper is one of the most widely used heavy metals, with the cupric ion [Cu(II)] being the predominant species in the environment. In this form, copper exhibits toxicity towards numerous living creatures [16]. Individuals are subjected to excessively elevated concentrations of harmful metals in their beverages and meals as a result of some habits, such as preparing food in pots lined or glazed with copper and utilizing copper pipes for water delivery [17,18]. Copper waste originates from several sources, such as drainage discharge, fertilizer companies, mining wastes, electroplating baths, dyes and pigments, as well as electronic waste (e-waste) and discarded electrical equipment, among others [19]. While a small quantity of copper is necessary for living organisms, an overabundance of copper in water can lead to detrimental effects, such as neurotoxicity, depression, brain damage, jaundice, respiratory problems, liver and kidney failure, cardiovascular disease, gastrointestinal problems, and potentially death [20]. The permissible concentration of copper in drinking water is 1.3 mg/L as per the U.S. Environmental Protection Agency (EPA) [21], less than 2 mg/L in Canada [22], and 2.0 mg/L according to the World Health Organization (WHO) [21].
These metals gradually build up in the human nervous system when exposed to polluted water and air sources, leading to lethal disorders even at extremely low levels. Consequently, it is crucial to dispose of them promptly to prevent their concentration from exceeding acceptable thresholds [23].
Numerous physical and chemical methods have been developed to reduce heavy metal concentrations, including membrane separation, accelerated oxidation, and adsorption [24,25,26,27]. However, most of these methods have inherent limitations, such as operational complexity and high costs. In comparison to other technologies, adsorption is a simpler and more convenient approach for successfully removing pollutants [28,29]. However, the cost and regeneration of activated carbon are a major hindrance for its use. Therefore, researchers have focused their attention on waste-based adsorbents for heavy metal removal due to their low cost, renewable nature, and effectiveness. Additionally, by utilizing waste materials such as agricultural by-products and forestry wastes, we can reduce environmental impact and boost the added value of waste materials [30]. This is why, in recent years, adsorbent materials have been prepared from a variety of natural low-cost materials such as coconut shells [31], wood ash [32], sugarcane [33], eggshell [34], rice husk [35], sludge [36], the pedicels of dates [37], sugar beet pulp [38], waste from the pulp and paper industry [39], gulfweed [40], apple pulp [41], palm tree derivatives [42], red oak acorn [43], mushrooms [44] etc. A single low-cost adsorbent will not be able to remove all pollutants adequately [45]. Additionally, a larger quantity of adsorbents is required throughout the removal process, as the majority of low-cost adsorbents have limited adsorption capabilities. As a result, novel, readily available, low-cost, environmentally friendly, and highly effective adsorbents are required.
In addition, most past research has concentrated on heavy metal removal from wastewater, soil, or natural water. In the realm of water purification, the utilization of novel activated carbon for the removal of heavy metals represents a distinctive and innovative approach, particularly in the context of enhancing drinking water quality. While activated carbon has been widely explored for water treatment, the novelty of this study lies in its exclusive focus on addressing heavy metal contamination, specifically in drinking water, diverging from conventional research that often targets wastewater or natural sources. Plant and agricultural waste such as red oak acorns, date seeds, and peach seeds were used in this project for removing heavy metals from drinking water.
Red oak, native to Canada and the US, is cost-effective and abundant. Date palm tree, a cost-effective and abundant plant, is cultivated in arid and semi-arid environments [46]. The global date production is around 9.7 million tons [47], with palm waste generated at a rate of 35 kg per tree [48]. The date palm, a staple crop, generates 750,000 tons of date seeds annually, offering potential for sustainable applications such as water treatment and environmental remediation. Using this agricultural waste for value-added products and responsible waste management is beneficial for date farmers [49,50]. Peaches, renowned for their sweet, vibrant flavor, are celebrated globally, with global production estimated at 24.2 million in 2022–2023 [51].
There are several factors affecting the adsorption capacity of any adsorbents. The most common factors influencing the adsorption process include pH, adsorbent dosage, and the presence of other competing ions in the solution. Disinfection is common in water and wastewater treatment plants. The presence of chlorine may have consequences on the adsorption process. In addition, dissolved organic material is a known adsorbate competing against heavy metal ions.
This research highlights the potential of plant and agricultural waste-derived activated carbon as a sustainable solution for heavy metal adsorption in drinking water purification. By offering an environmentally friendly, cost-effective, and readily available adsorbent, it addresses critical public health concerns while enhancing water safety. The comparative study demonstrates the effectiveness of these novel activated carbons, providing valuable insights that contribute to the advancement of water treatment technologies.
From our initial research, some agricultural tree residues, such as oak and peach tree wastes, were studied with the intent to use them for activated carbon production and to eliminate lead (Pb) and copper (Cu) from water solutions. However, the decision for this study was influenced by the performance and availability of date seed waste and its ability to solve ecological problems. Activated carbon from date seeds has a high adsorption capacity, as well as the desired combination of heavy metal removal and waste utilization. Nowadays, such a strategic move not only utilizes the huge amount of biomass produced annually by the date industry, but it also helps solve important environmental problems. It is therefore safe to assume that the present study expands on this base by dealing with the preparation, characterization, and application of date seed-based activated carbon for Pb and Cu removal from water systems, which is a novel approach to tackling water treatment.
2. Materials and Methods
2.1. Precursor Materials and Preparation of Activated Carbon
The acorns were gathered from the ground beneath the red oak trees located in the Dartmouth district of Atlantic Canada, in Nova Scotia. The fruits underwent a drying process in ambient air conditions for a minimum duration of 24 h, during which they were meticulously cleaned of any foliage or small remnants of branches and trees. Locally grown peaches were purchased from the local market in Halifax, NS, Canada. Dates imported from the Middle East were also purchased from local stores in Halifax, NS. The seeds from the dates and the peaches were extracted, washed, and air-dried (Figure 1). Prior to commencing the studies, the samples underwent a process of drying and grinding using a high-speed rotary cutting mill. Grinded seeds were thermally activated in a muffle furnace under limited oxygen conditions. The temperature increased at a rate of 10 °C per minute and was maintained at 500 °C for one hour during carbonation. After cooling in the oven, the carbonized sample was pulverized into tiny particles using a mortar and pestle. A fraction of 0.5–1.00 mm diameter red oak acorn activated carbon (RAC), date seed activated carbon (DAC), and peach seed activated carbon (PAC) was used in this investigation.

Figure 1.
(a) Acorn tree, (b) date seeds, (c) peach seeds, (d) milled acorn, and (e) active carbon from dates.
2.2. Chemicals Used
All compounds utilized in this study were analytical or trace metal grade reagents. Analytical grade cupric sulfate anhydrous (CuSO4) and trace metal grade lead (II) nitrate [Pb(NO3)2] were purchased from Fisher Scientific, Waltham, MA, USA. Humic acid as a model DOM was obtained from Sigma Corp., Cream Ridge, NJ, USA. In addition, sodium hydroxide (NaOH), hydrochloric acid (HCl), and sodium hypochlorite (NaOCl) were procured from Fisher Scientific, USA. Sodium hypochlorite (NaOCl) stock solution of about 60,000 mg/L was used to make the free chlorine solution used in this project.
2.3. Adsorbate
Stock solutions of heavy metals were prepared by dissolving the accurate amount of CuSO4 and Pb(NO3)2 in distilled water; the other solutions were prepared by dilution. The pH was adjusted with 0.1 M HCl or 0.1 M NaOH.
2.4. Chemical Preparation
The NaOCl stock solution, which had a high concentration, was diluted to achieve a solution with a concentration of 100 mg/L. Throughout this project, only fresh solutions were utilized. The preparation of monochloramine was carried out according to the methodology described by Eisnor [52]. This technique combined sodium hypochlorite and ammonium chloride with phosphate buffer saline (PBS) at a pH of 9.5. PBS consisted of 8.0 g of sodium chloride (NaCl), 0.2 g of potassium dihydrogen phosphate (KH2PO4), 2.9 g of disodium hydrogen phosphate dodecahydrate (Na2HPO4 12H2O), and 0.2 g of potassium chloride (KCl) dissolved in 1 L of deionized water. For the preparation of this solution, 1.85 mL of sodium hypochlorite (at a concentration of 60,000 mg/L) was added to 500 mL of a phosphate-buffered saline (PBS) solution. An amount of 400 mg (0.4 g) of NH4Cl was introduced into a 2 L glass Erlenmeyer flask, which was separate from the one being used. This was achieved by combining it with 500 mL of PBS solution.
The sodium hypochlorite solution was introduced into the NH4Cl solution using a gradual drip process. Throughout this procedure, the mixture was agitated incessantly until the entire sodium hypochlorite solution was included. The pH of the monochloramine solution was adjusted to 9.5 using 0.1 N NaOH and then stored in the refrigerator at 4 °C. Monochloramine was synthesized and kept for a maximum duration of 5 days. A DOM stock solution of 350 mg/L was prepared by dissolving the required amount of humic acid in distilled water and was kept in the refrigerator. The DOM stock solution was diluted to achieve a solution with the desired concentration.
2.5. Adsorption Experiment
Batch adsorption tests for heavy metals (i.e., Cu, Pb) were conducted utilizing VWR® polypropylene tubes with lids. A stock solution of heavy metal was created and subsequently diluted to various quantities, with the pH of the solution adjusted using 0.1 M NaOH or 0.1 M HCl. Following pH correction, the necessary quantity of the prepared activated carbon (RAC, DAC, PAC) was incorporated. The sample was subsequently combined in an orbital shaker (Barnstead/Lab-Line MaxQ™ 2000) at 175, rpm for a duration of 2 h. Following the conclusion of the mixing period, the samples (suspensions) were centrifuged using a centrifuge machine (IEC Centra GP8R, Thermo Electronic Corporation, Waltham, MA, USA) for ten minutes at 25,000 revolutions per minute. Aliquots of the supernatant were taken from the mixture by decantation, acidified using concentrated HNO3 (to a pH less than 2.0), and then stored in the refrigerator. Inductively coupled plasma mass spectrometry (ICP-MS, X Series II, Thermo Scientific, reporting limits: Pb = 0.4 µg/L and Cu = 0.7 µg/L) was utilized to determine the amounts of metals in the samples. The efficiency of metal removal (expressed as a percentage, R%) was estimated using the following equation:
The symbols Ci and Cf represent the starting and final concentrations of the metal, respectively, measured in milligrams per liter (mg/L).
2.6. Statistical Analysis
Paired t-test analysis of the data was performed using Microsoft Excel 2019 to see if there were significant differences between samples. Unless otherwise mentioned, all statistical analyses were conducted at a 95% confidence level.
3. Results
The outcomes of the sample characterization study are presented at the beginning of this section, followed by the findings of several adsorption tests pertaining to all the adsorbents that were chosen for this investigation.
3.1. Sample Characterization
Samples were characterized by (i) SEM, (ii) EDX, (iii) XRD, and (iv) FTIR.
3.1.1. SEM Analysis
SEM images are shown in Figure 2, Figure 3 and Figure 4. The SEM micrographs show a significant difference in the surface between before and after adsorption. The scanning electron microscopy (SEM) images of all activated samples exhibited an uneven and well-defined porous structure, suggesting a comparatively large surface area. Upon activation, the outer surface of activated carbon exhibits fissures, crevices, and a range of grain sizes within its macroscopic pores. The porous and defective structure of carbon is attributed to the liberation of non-carbon components, such as hydrogen, oxygen, and nitrogen, from the char’s surface during the pyrolysis process [53]. This liberation leads to the creation of a stiff carbon framework with a rudimentary pore structure. An efficient adsorbent requires the presence of pores and an internal surface area. The formation of pores in activated carbon is crucial, as pores serve as active sites, playing a major part in the process of adsorption [54].

Figure 2.
Scanning electron micrographs (SEM) of (a) RAC before adsorption, (b) RAC after Cu adsorption, and (c) RAC after Pb adsorption.

Figure 3.
Scanning electron micrographs (SEM) of (a) DAC before adsorption, (b) DAC after Cu adsorption, and (c) DAC after Pb adsorption.

Figure 4.
Scanning electron micrographs (SEM) of (a) PAC before adsorption, (b) PAC after Cu adsorption, and (c) PAC after Pb adsorption.
After the adsorption of Cu and Pb, visible modifications in the surface morphology confirm the adsorption of metal ions onto the activated carbon. In the Cu-adsorbed samples, some blocked pores and increased surface roughness are observed, indicating that the copper ions penetrated the more compressed portions of the activated carbon. Copper ions are retained as coordination complexes by the oxygenated functional groups (-OH and -COOH). By contrast, the Pb-adsorbed samples exhibit greater surface aggregation and structural compaction. This behavior can be attributed to the precipitation of lead in the pores or the formation of surface complexes with functional groups. The surface morphology of bioadsorbents RAC, DAC, and PAC significantly differs due to their precursor materials. All three adsorbents exhibit substantial adsorption potential, making them viable candidates for metal removal.
3.1.2. EDX Analysis
The EDX analysis of the adsorbent (date seed activated carbon) before and after Cu and Pb adsorption is shown in Figure 5, Figure 6 and Figure 7. Before adsorption, the activated carbon contains various elements, such as O, Cl, Na, Si, K, Br, and Mg, along with a major peak for C. The presence of trace elements indicates residual components originating from the seed’s inherent mineral content. The EDX analyses of lead- and copper-adsorbed activated carbon confirm a high carbon content, characteristic of activated carbon. After adsorption, distinct Cu and Pb peaks appear, which directly signify metal uptake by activated carbon. At the same time, the oxygen content is decreased, which is indicative of the fact that metal ions interacted with surface functional groups by ion exchange or complexation. The removal of elements such as sodium, silicon, and potassium also provides support to the ion replacement hypothesis for heavy metals. Additionally, the significant loss of oxygen content is indicative of the participation of oxygenated functional groups such as hydroxyl (-OH) and carboxyl (-COOH) in chelating Cu2⁺ and Pb2⁺ ions. In the copper-adsorbed activated carbon samples, copper, oxygen, phosphorus, and silicon were detected, while the lead-adsorbed activated carbon exhibited the presence of lead, oxygen, phosphorus, and iron. These results confirm the potency of activated carbon in adsorbing both copper and lead while maintaining its carbon-rich structure.
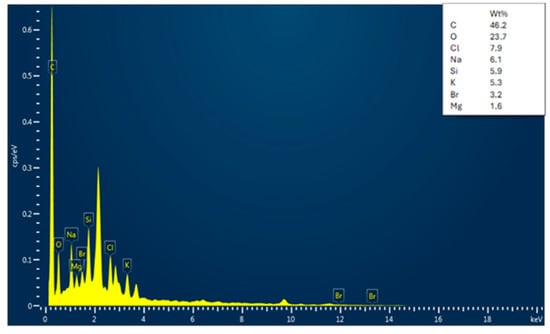
Figure 5.
Energy Dispersive X-ray (EDX) spectrum of date seed activated carbon before adsorption.
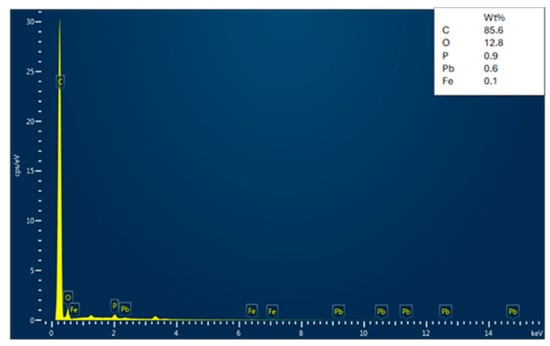
Figure 6.
Energy Dispersive X-ray (EDX) spectrum of date seed activated carbon after lead adsorption.
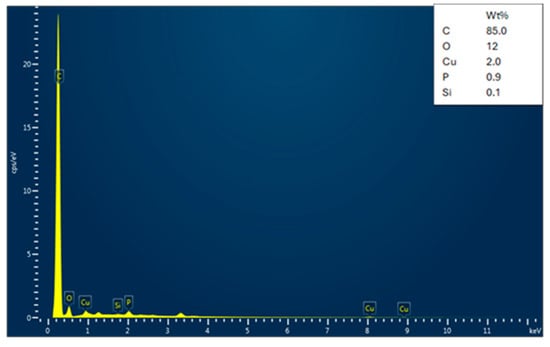
Figure 7.
Energy Dispersive X-ray (EDX) spectrum of date seed activated carbon after copper adsorption.
In the Pb- and Cu-adsorbed samples, metal ion binding may reduce or displace the various functional groups present in activated carbon, leading to a relative increase in the carbon signal due to the decrease in oxygen and other surface elements.
3.1.3. XRD Analysis
The structural analysis of activated carbon samples after heavy metal adsorption (Figure 8 and Figure 9) provides crucial insights into their effectiveness and interaction mechanisms. X-ray diffraction (XRD) analysis is a commonly employed technique to identify the crystalline phases and structural changes in adsorbents. By analyzing the XRD patterns, it is possible to determine the presence (and crystalline nature) of adsorbed metals. This offers valuable information on the adsorption behavior and material properties.
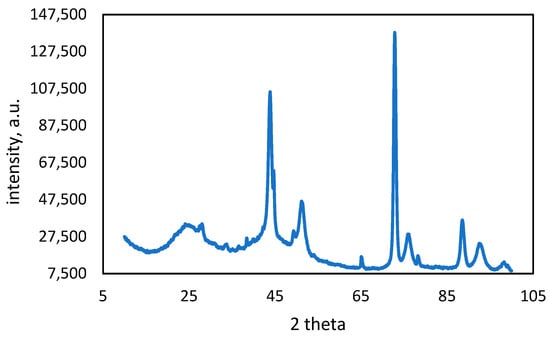
Figure 8.
XRD pattern of copper-adsorbed activated carbon.
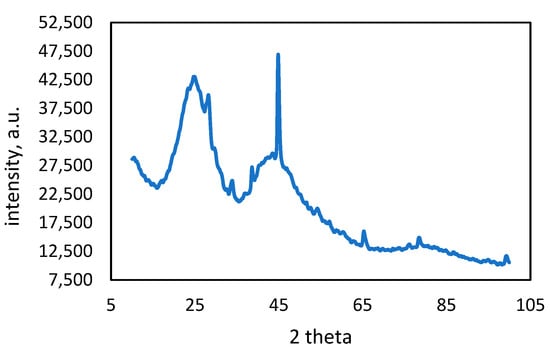
Figure 9.
XRD pattern of lead-adsorbed activated carbon.
The XRD pattern of activated carbon with adsorbed copper (Figure 8) exhibits peaks located around 43.8°, 51.31°, and 74°. These peaks closely match the characteristic peaks of copper reported in the literature, corresponding to the (111), (200), and (220) planes of FCC copper [55]. The literature confirmed the use of the standard JCPDS card (No. 04-0836) for copper, indicating that the peaks in the activated carbon sample can also be indexed to these crystal planes of copper [56]. This alignment with the literature confirms the presence of copper in the activated carbon and supports its structure as FCC copper [55,56].
The broad peak between 20° and 30° shows the amorphous nature of the activated carbon. This peak, which can be observed in both activated carbon samples, is due to amorphous carbon. There is no characteristic peak of lead observed in the XRD pattern of Pb-adsorbed activated carbon.
3.1.4. FTIR Analysis
Functional groups significantly influence the surface adsorption capabilities of activated carbon. The FTIR diagram (Figure 10) shows the spectra of activated carbon (AC) and AC after adsorption of Pb and Cu ions. In the pure AC, the broad peak is observed around 3400 cm−1, which is assigned to -OH stretching vibrations, which proves the presence of hydroxyl groups on the surface [57]. The peak around 1600 cm−1 is the C=C stretching peak, which is characteristic of graphitic carbon [58], while the other smaller peak around 1000 cm−1 is due to C-O stretching, which may include carboxyl or hydroxyl groups [59,60].
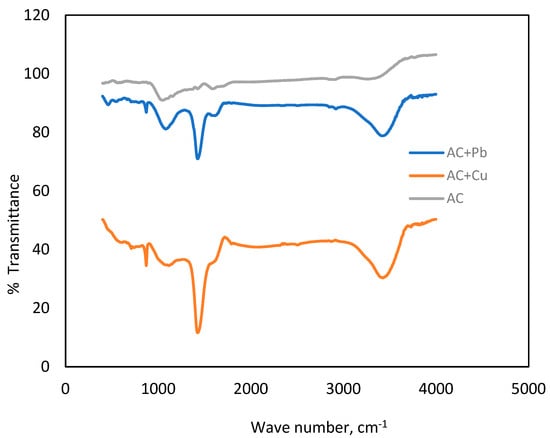
Figure 10.
FTIR spectra of activated carbon (AC) before and after adsorption of Pb and Cu ions.
In the case of Pb-adsorbed AC, the broad peak observed around 3400 cm−1 wavenumber shifts, which may suggest that the hydroxyl groups on the carbon surface are engaged in a bond with Pb ions, which may lead to the formation of surface complexes. Additionally, new absorption bands in the 500–600 cm−1 region are attributed to Pb-O stretching absorptions, suggesting the formation of lead oxides or other lead-containing species on the surface [59,61]. Likewise, in the Cu-adsorbed AC spectrum, the shift of the 3400 cm−1 peak implies the bonding between Cu ions and the surface hydroxyl groups. The new absorption bands appearing at 600–700 cm−1 are attributed to Cu-O stretching, and this may be due to the formation of copper oxides or complexes on the surface [62,63,64].
These shifts and new peaks in the spectra show that both Pb and Cu ions interact with functional groups on the activated carbon, mostly hydroxyl (-OH) and carbonyl (C=O). This leads to the formation of metal–oxygen bonds, hence suggesting the formation of metal complexes or oxides on the carbon surface after ion adsorption.
The adsorbent’s surface contains pores that serve as sites for the adsorption of metals. Due to the presence of substantial pores, there is a high likelihood that the heavy metal ions will become trapped and adsorbed within them. Koseoglu and Akmil-Başar [65] observed a similar phenomenon regarding the adsorptive characteristics of activated carbon generated from orange peel. Upon the adsorption of heavy metals, it is seen that the surfaces of activated carbon undergo a smoothing effect as a result of the filling of pores by ions.
3.2. Selection of Adsorbent
Based on superior experimental performance and existing research data, date seed activated carbon (DAC) was chosen over the peach- and acorn-based activated carbons The preliminary adsorption experiments revealed significantly higher removal efficiencies of DAC for both lead and copper in the same experimental conditions. Hence, it was concluded that DAC possesses an enhanced capacity for binding heavy metals, which is likely due to a greater surface area, higher pore volume, and more favorable surface chemistry compared to the materials tested. Studies reveal that the carbon produced from date seeds is characterized by high thermal stability, possesses abundant micropores, and has a distribution of hydroxyl and carboxyl groups, which are functional groups that improve the adsorption of metal ions. Furthermore, given that date seeds have vast availability as agricultural byproducts, DAC is considered more sustainable and cost-effective in comparison with other methods for waste valorization and environmental remediation.
3.3. Effect of pH and Adsorbent Dose
The availability and accessibility of adsorption sites were significantly influenced by the adsorbent dose [66,67,68]. The concentration of hydrogen ions is considered to be an important factor in adsorption operations, which can have a significant effect on how heavy metals behave in aqueous solutions. One of the deciding elements is the concentration of hydrogen ions, which affects the solubility of heavy metal ions in a solution [17]. Throughout the process, it also influences the extent of ionization of the adsorbate and replaces certain positive ions that may exist in the active sites of the adsorbent [54].
An investigation was conducted to examine the effect of different dosages of DAC (10–100 g/L for the Cu solution and 5–50 g/L for the Pb solution) on two metals (Cu and Pb), with an initial concentration of 50 mg/L. The impact of varying amounts of adsorbent on the removal of Cu and Pb ions is illustrated in Figure 11 and Figure 12 at various pH levels.
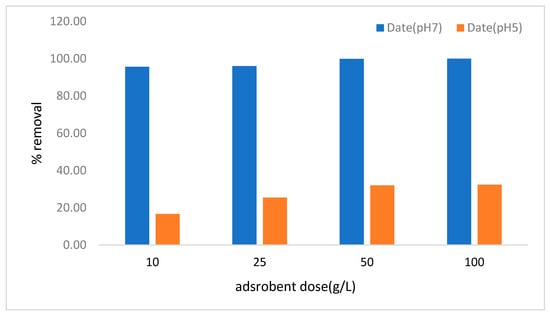
Figure 11.
Copper removal efficiency (%) at different pH (5 and 7) levels by DAC at four different doses: 10, 25, 50, and 100 g/L.
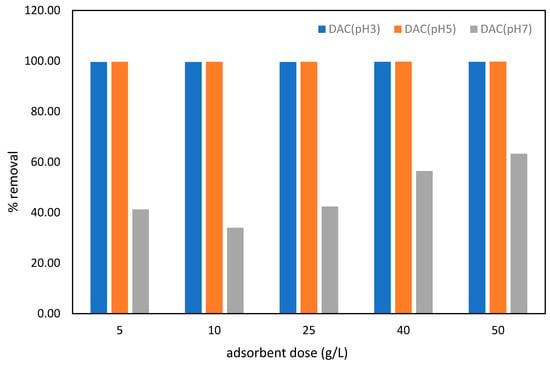
Figure 12.
Efficiency (%) of lead removal by DAC at different pH levels (3, 5 and 7). Adsorbents are introduced at 5, 10, 25, and 50 g/L.
The removal efficiencies of Cu2⁺ and Pb2⁺ by date seed activated carbon (DAC) are highly affected by adsorbent dose and pH, as illustrated in Figure 11 and Figure 12. A strong adsorption potential of DAC was reported, which is driven by its surface chemistry and active functional groups. The results indicate a positive correlation between the dosage of adsorbent and the percentage of lead and copper removal efficiency. It is anticipated that the adsorption will rise as the dose of adsorbent increases, as a result of the expansion in the surface area of the adsorbent and the greater number of available adsorption sites [54,66]. The Cu removal efficiency for DAC was investigated at pH 5 and pH 7, with varying adsorbent doses in g/L. At pH 7 (Figure 11) pH 7, DAC demonstrated consistent performance, achieving high Cu removal percentages, between 95% and 99%. This implies that a neutral pH is the key parameter for the adsorption of Cu ions. Paired t-test analysis showed that the Cu removal rates at pH 5 and 7 are significantly different (p = 1.67 × 10−6 < 0.05).
The copper removal efficiency is consistently high at pH 7, surpassing 95% for all adsorbent concentrations. This suggests that the neutral pH promotes the optimal adsorption of copper ions, presumably as a result of the reduced competition between H⁺ ions and Cu2⁺ ions for active adsorption sites, as observed in previous studies [69,70]. At pH 5, however, the removal rate is comparatively lower, around 32% at the highest adsorbent concentration of 100 g/L. The inferior performance at lower pH is due to the protonation of the DAC active sites, which in turn results in the reduction of the active sites and is responsible for lower Cu removal [71].
The trend, on the other hand, shows that the increase in the adsorbent dose from 10 g/L to 100 g/L results in a better removal efficiency at both pH levels. This is due to the increased availability of surface area and active adsorption sites with the higher adsorbent doses, which aligns with findings in other previous adsorption studies [72].
The efficacy of Pb removal by DAC was evaluated at several pH values (3, 5, and 7), with varied adsorbent dosages in g/L (Figure 12). The results demonstrate that the efficacy of lead adsorbent is superior at low pH levels (pH 3 and 5), achieving around 99% to 100% effectiveness. This phenomenon may be attributed to the prevalence of Pb2⁺ ions available for adsorption and to reduced competition for adsorption in an acidic pH environment. At pH 3 and 5, the surface charge of DAC may be more conducive to electrostatic attraction to Pb2⁺ ions. Similar findings were observed in a prior study, indicating that pH 5 settings facilitate lead adsorption due to increased electrostatic interactions [73]. The decrease in lead adsorption efficiency at higher pH levels can be attributed to the formation of soluble hydroxylated species, such as Pb(OH)⁺ and Pb(OH)2, which remain in the solution rather than binding to the adsorbent surface [74]. The paired t-test analysis indicated a significant difference in Pb removal rates at pH 3, 5, and 7 (p = 1.04 × 10−5 to 0.02 < 0.05).
The extent of Pb removal was found to be significantly dependent on the adsorbent dose, with all pH levels displaying a positive correlation in terms of performance at higher doses. This enhancement is due to the higher dosages resulting in a greater surface area and more active adsorption sites. However, for adsorbent dosages in the range of 40–50 g/L, the removal efficiency becomes relatively constant, indicating the saturation of active sites on the DAC surface. This plateau is consistent with the Langmuir isotherm formulation, which explains the limited adsorption capacity of adsorbents. Therefore, optimizing the adsorbent dose is crucial to maximizing the efficiency of the adsorbent [75].
3.4. Effect of Disinfectants
Disinfectants are a significant element of water and wastewater treatment. To investigate the impact of disinfectant presence during heavy metal adsorption, a chlorine dose of 2 and 4 ppm and a monochloramine dose of 4 ppm were used during the adsorption of lead with DAC. The metal removal rates for these tests are shown in Figure 13. The results indicate that increasing the chlorine concentration generally led to lower lead removal efficiency for DAC across different doses. Specifically, at elevated chlorine concentrations, both adsorbents exhibited substantial deterioration, achieving lower levels of lead removal efficiency. This highlights the negative correlation between disinfectant concentration and the efficiency levels of DAC in removing lead from water. It was also observed that the addition of monochloramine resulted in 99% lead removal by DAC. The paired t-test analysis revealed a significant variation in Pb removal rates across different disinfectants (p = 4.80 × 10−6 to 0.002 < 0.05).
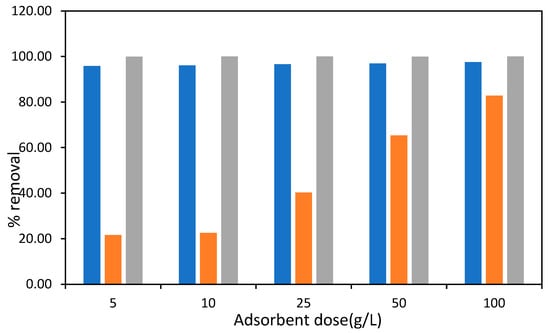
Figure 13.
Lead removal efficiency (%) at 2 and 4 ppm of chlorine and 4 ppm monochloramine dose by DAC (blue bar: DAC+ 2 ppm Cl2; orange bar: DAC+ 4 ppm Cl2; grey bar: DAC + NH2C).
At 2 ppm Cl2, DAC shows a consistent and high lead removal efficiency, exceeding 90% across all adsorbent doses. The intense oxidative environment generated by chlorine is responsible for the superior performance, as it facilitates the breakdown of lead-containing complexes and increases the interaction of Pb2⁺ ions with the active sites on the DAC surface. Additionally, a previous study corroborated the enhanced removal of lead from water through the use of activated carbon in oxidative environments [76]. At 4 ppm Cl2, a slight decrease in removal efficiency can be observed in comparison to 2 ppm Cl2, mainly in the case of lower adsorbent dosages. The explanation for this decline in efficiency may come from the changes brought about in surface chemistry as a result of chlorination. For instance, chlorination can change the functional groups existing on the DAC surface, such as -OH and -COOH groups [77], which is expected to decrease the number of active sites available for lead binding. Previous studies have indicated that chlorination enhances the surface acidity and changes the amount of surface oxide present [77], which means that the adsorption process can be modified. In addition, the attachment of Cl− to the carbon surface may result in altered structure of the adsorbent surface, which could affect the surface interaction of the adsorbent with lead ions. These changes are known to reduce adsorption efficiency, particularly at higher levels of chlorine than were reported in previous studies [76].
At a concentration of 4 ppm NH2Cl, the efficiency of lead removal was maintained at a level that was either comparable to or exceeded the outcomes achieved with 2 ppm Cl2. Monochloramine appeared to operate as a milder oxidant compared to chlorine, preserving the integrity of the functional groups on the surface of DAC while still facilitating the adsorption of lead. The stability of NH2Cl in aqueous environments ensures that it does not undergo excessive oxidation, thereby preserving its adsorption efficiency even at elevated concentrations [78].
3.5. Effect of DOM
The relationship between heavy metals and dissolved organic matter (DOM) is complex and multifaceted [79,80]. The interaction between soluble organic molecules and metals occurs through processes such as anion exchange, ligand exchange, cationic overlapping, Van der Waals forces, and adsorption [81]. Numerous heavy metals exhibit a pronounced attraction for dissolved organic matter (DOM) and form metal complexes, potentially disrupting the adsorption process. The mobility of metals, their elimination rate, and their ability to adhere to adsorption sites are directly affected by the concentration of dissolved organic matter (DOM) [80]. In order to examine the influence of DOM on the process of lead removal, humic acid doses of 1, 3 and 6 ppm, were employed during the adsorption of lead using DAC. The metal removal rates for these experiments are depicted in Figure 14.
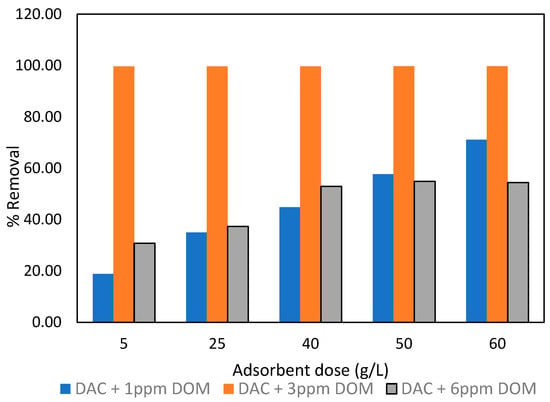
Figure 14.
Lead removal efficiency (%) at 1, 3, and 6 ppm of dissolved organic matter by DAC.
The examination of dissolved organic matter (DOM) concentration on lead removal efficiency by DAC reveals noteworthy patterns. Generally, as the DOM concentration increases, there is a corresponding rise in lead removal efficiency, especially evident at higher adsorbent doses. Strikingly, the 3 ppm level of DOM consistently yields the highest lead removal efficiency across various doses of DAC. Ninety percent removal was observed across all adsorbent doses considered. Moderate DOM levels enhance the adsorption of lead by forming stable complexes with the lead ions [82], which are subsequently adsorbed onto the surface of the DAC. The DOM levels within this limit could also help remove interfering hydroxylated lead species; thus, more Pb2⁺ adsorption sites are available. The paired t-test analysis revealed no significant variation in Pb removal rates between 1 and 6 ppm of DOM (p = 0.96 > 0.05). However, the removal rate at 3 ppm of DOM was significantly different from that at 1 and 3 ppm DOM (p = 5.23 × 10−6 to 0.0003 < 0.05).
At a concentration of 1 ppm DOM, removal efficiency was below average and less competitive. It was also anticipated that the DOM concentration at this level would not be sufficient to create stronger complexes with Pb2⁺ ions, as there seems to be reduced occupancy in the active sites on the DAC. This is the same phenomenon exhibited at 6 ppm DOM, where the % removal was lower than that of the 3 ppm adsorbent dose. This decline may be attributed to DOM oversaturation, which hinders adsorption and competes for the active adsorbent surfaces, thereby limiting the adsorption of Pb ions. Increasing the adsorbent dose from the range of 50–60 g/L proved to be effective at all concentrations of DOM, indicating an increased availability of active sites and an enhanced surface area. This observation underscores the significance of the 3 ppm DOM concentration in optimizing the effectiveness of these activated carbons for lead removal.
4. Conclusions
Cost-effective and innovative adsorbents derived from date seeds (DAC) were effectively employed for the removal of heavy metals from aqueous solutions. This investigation explored the impact of factors such as adsorbent dose, pH, the presence and type of disinfectant, as well as the influence of dissolved organic matter (DOM), on the adsorption process. Scanning electron microscopy (SEM) images highlighted substantial changes in surface morphology pre- and post-adsorption. Key findings include the superior performance of date seed activated carbon (DAC) in removing heavy metals, notably Pb(II) and Cu(II). This study revealed a positive correlation between metal removal and increased adsorbent dosage for all three materials. Noteworthy trends emerged, such as higher copper ion removal at neutral pH (7) and lower removal under acidic conditions, while lead ions exhibited higher removal at acidic pH and reduced efficiency at pH 7. In the presence of monochloramine, both DACs exhibited exceptional Pb removal efficiency, nearing 100%. However, increasing the chlorine concentration from 2 to 4 ppm resulted in decreased Pb removal efficiency for DAC. Maximum lead removal was observed in the presence of 3 ppm of DOM by DAC with lower removal rates at 1 and 6 ppm DOM. SEM images further illustrated distinct morphological changes in the activated carbons before and after the adsorption process, providing visual insights into their structural transformations. Therefore, the findings of this study will be utilized in a wide variety of applications, including the purification of drinking water, the treatment of ground and municipal water, the reduction of gas emissions from power plants and landfills, and the recovery of precious metals. Even though the use of waste materials resolves concerns related to disposal, there may be concerns about the cost benefit of such a technology. The performance of date seed-derived activated carbon as an adsorbent is far superior to that of date seed in general. For this reason, the additional cost is justified by the benefits. The use of HCl and NaOH was minimal, indicating insignificant cost implications. In addition, there were no requirements for the removal of residual chloride or sodium. Previous studies suggested that AC can be regenerated and can be reused in a cost-effective way [83,84]. Overall, even with the constraint of additional requirements, it still appears to be a cost-effective option. Future studies should also focus on optimizing the different factors affecting the activated carbon production from date seeds.
Author Contributions
Conceptualization, M.S.R. and M.M.; methodology, M.S.R. and N.B.; validation, M.S.R., M.H.R. and M.M.; formal analysis, M.S.R., M.H.R. and N.B.; investigation, M.S.R. and N.B.; writing—original draft preparation, M.S.R., M.H.R. and N.B.; writing—review and editing, M.S.R., M.H.R. and M.M.; visualization, M.S.R. and N.B.; supervision, M.S.R. and M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sharjah National Oil Corporation grants for the American University of Sharjah, grant number 223215. The work in this paper was supported, in part, by the Open Access Program from the American University of Sharjah.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The author wishes to express gratitude to Pat Scallion from the Clean Technologies Research Institute at Dalhousie University for her technical assistance throughout the execution of SEM studies. The authors would like to recognize Daniel Chevalier from the Mineral Resource Engineering Department at Dalhousie University for his contribution in preparing the activated carbon. This paper represents the opinions of the author(s) and does not mean to represent the position or opinions of the American University of Sharjah.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zada, A.; Qu, Y.; Ali, S.; Sun, N.; Lu, H.; Yan, R.; Zhang, X.; Jing, L. Improved Visible-Light Activities for Degrading Pollutants on TiO2/g-C3N4 Nanocomposites by Decorating SPR Au Nanoparticles and 2,4-Dichlorophenol Decomposition Path. J. Hazard. Mater. 2018, 342, 715–723. [Google Scholar] [CrossRef]
- World Health Organization. Lead in Drinking-Water: Health Risks, Monitoring and Corrective Actions: Technical Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- González-Fernández, L.A.; Medellín-Castillo, N.A.; Navarro-Frómeta, A.E.; Carranza-Álvarez, C.; Castillo-Ramos, V.; Sánchez-Polo, M.; Vilasó-Cadre, J.E.; Díaz-Flores, P.E.; Morales-Oyervides, L.; Pérez-Aguilar, N.V.; et al. Heavy Metal Pollution in Water: Cause and Remediation Strategies. In Current Status of Marine Water Microbiology; Springer: Singapore, 2023; pp. 221–262. [Google Scholar]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Jarvis, P.; Fawell, J. Lead in Drinking Water—An Ongoing Public Health Concern? Curr. Opin. Environ. Sci. Health 2021, 20, 100239. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Huq, A.O.; binti Yahya, R. The Removal of Heavy Metal Ions from Wastewater/Aqueous Solution Using Polypyrrole-Based Adsorbents: A Review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- El-Fahla, N.A.; Mohallal, M.E.; Gad El-Hak, H.N.; Dessouki, A.A.; Abdelrazek, H.M.A. Effect of Lead Toxicity on The Kidney of Nile Tilapia: Amelioration by Beta-MOS®. Egypt. Acad. J. Biol. Sci. Histol. Histochem. 2022, 14, 31–45. [Google Scholar] [CrossRef]
- Fabolude, G.; Knoble, C.; Vu, A.; Yu, D. Comprehensive Lead Exposure Vulnerability for New Jersey: Insights from a Multi-Criteria Risk Assessment and Community Impact Analysis Framework. Ecol. Indic. 2024, 167, 112585. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global Health Burden and Cost of Lead Exposure in Children and Adults: A Health Impact and Economic Modelling Analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef]
- Hiwatari, M.; Yamada, D.; Narita, D.; Hangoma, P.; Chitah, B. Toxic Pollution and Poverty: Economic Impacts of Lead (Pb) Exposure on Household Welfare in Zambia. Ecol. Econ. 2024, 221, 108209. [Google Scholar] [CrossRef]
- Li, B.; Trueman, B.F.; Rahman, M.S.; Gagnon, G.A. Controlling Lead Release Due to Uniform and Galvanic Corrosion—An Evaluation of Silicate-Based Inhibitors. J. Hazard. Mater. 2021, 407, 124707. [Google Scholar] [CrossRef]
- Health Canada. Water Talk. Minimizing Exposure to Lead from Drinking Water Distribution Systems; Health Canada: Ottawa, ON, Canada, 2016.
- US Environmental Protection Agency. Use of Lead Free Pipes, Fittings, Fixtures, Solder and Flux for Drinking Water; US Environmental Protection Agency: Washington, DC, USA, 2024.
- Santucci, R.J.; Scully, J.R. The Pervasive Threat of Lead (Pb) in Drinking Water: Unmasking and Pursuing Scientific Factors That Govern Lead Release. Proc. Natl. Acad. Sci. USA 2020, 117, 23211–23218. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological Relevance and Mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Ghosh, S.; Sadhu, A.; Mandal, A.H.; Biswas, J.K.; Sarkar, D.; Saha, S. Copper Oxide Nanoparticles as an Emergent Threat to Aquatic Invertebrates and Photosynthetic Organisms: A Synthesis of the Known and Exploration of the Unknown. Curr. Pollut. Rep. 2025, 11, 6. [Google Scholar] [CrossRef]
- El-Araby, H.A.; Ibrahim, A.M.M.A.; Mangood, A.H.; Abdel-Rahman, A.A.-H.; El-Araby, H.A.; Ibrahim, A.M.M.A.; Mangood, A.H.; Abdel-Rahman, A.A.-H. Sesame Husk as Adsorbent for Copper(II) Ions Removal from Aqueous Solution. J. Geosci. Environ. Prot. 2017, 5, 109–152. [Google Scholar] [CrossRef]
- Rahman, M.S. Experimental Investigation on the Effect of Physio-Chemical Properties of Water on Galvanic Corrosion and Turbidity Due to Iron Oxidation. Environ. Process. 2023, 10, 37. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hachisuga, N.; Serpone, N. Recycling of E-Waste Power Cables Using Microwave-Induced Pyrolysis—Process Characteristics and Facile Recovery of Copper Metal. RSC Adv. 2024, 14, 29955–29964. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef]
- Manne, R.; Kumaradoss, M.M.R.M.; Iska, R.S.R.; Devarajan, A.; Mekala, N. Water Quality and Risk Assessment of Copper Content in Drinking Water Stored in Copper Container. Appl. Water Sci. 2022, 12, 27. [Google Scholar] [CrossRef]
- Schwartz, H.; Marushka, L.; Chan, H.M.; Batal, M.; Sadik, T.; Ing, A.; Fediuk, K.; Tikhonov, C. Metals in the Drinking Water of First Nations across Canada. Can. J. Public Health 2021, 112, 113–132. [Google Scholar] [CrossRef]
- Althomali, R.H.; Abbood, M.A.; Saleh, E.A.M.; Djuraeva, L.; Abdullaeva, B.S.; Habash, R.T.; Alhassan, M.S.; Alawady, A.H.R.; Alsaalamy, A.H.; Najafi, M.L. Exposure to Heavy Metals and Neurocognitive Function in Adults: A Systematic Review. Environ. Sci. Eur. 2024, 36, 18. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Fadila, C.R.; Othman, M.H.D.; Adam, M.R.; Takagi, R.; Yoshioka, T.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Adsorptive Membrane for Heavy Metal Removal: Material, Fabrication, and Performance. Mater. Today Proc. 2022, 65, 3037–3045. [Google Scholar] [CrossRef]
- Ren, D.; Zhang, M.; Han, G.; Li, Z.; Yang, L.; Li, X.; Gong, Y.; Shang, Z.; Yang, F. Preparation of CO2/N2-Switchable Hydrogel Beads and Adsorption, Desorption, Regeneration for Cu(II). Colloids Surf. Physicochem. Eng. Asp. 2023, 674, 131882. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Wu, Y.; Geng, L.; Zhang, X.; Zhang, D.; Hu, H.; Zhang, Y.; Li, X.; Liu, W.; et al. Synthesis of Uniform-Sized and Microporous MIL-125(Ti) to Boost Arsenic Removal by Chemical Adsorption. Polyhedron 2021, 196, 114980. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent Advances on Sustainable Adsorbents for the Remediation of Noxious Pollutants from Water and Wastewater: A Critical Review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Rahman, M.H.; Wasiuddin, N.M.; Islam, M.R. Experimental and Numerical Modeling Studies of Arsenic Removal with Wood Ash from Aqueous Streams. Can. J. Chem. Eng. 2004, 82, 968–977. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Mtei, K.M.; Choi, J.W. Adsorptive Removal of Heavy Metals from Wastewater Using Low-Cost Adsorbents Derived from Agro-Based Materials. In Heavy Metal Remediation: Sustainable Nexus Approach; Springer: Cham, Switzerland, 2024; pp. 237–271. [Google Scholar]
- You, Z.; Yu, Z.; Hu, Y.; Dong, J.; Yue, W.; Li, J.; Ma, X. Effect of Modified Ca-Al Adsorbents on Heavy Metal Fixation during Co-Combustion of Coal and Coconut Shells: Experiments and Simulations. Environ. Pollut. 2024, 361, 124856. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Mohan, B.C.; Tai, M.H.; Yao, Z.; Su, W.; Lay-Ming Teo, S.; Wang, C.-H. Green, Non-Toxic and Efficient Adsorbent from Hazardous Ash Waste for the Recovery of Valuable Metals and Heavy Metal Removal from Waste Streams. Chemosphere 2023, 329, 138524. [Google Scholar] [CrossRef]
- Singh, J.; Jadeja, R. Recent Advances in Agricultural Waste Derived Magnetic Biochar for Removal of Heavy Metal Ions: Mechanistic Insights and Technological Innovation. J. Mol. Struct. 2025, 1325, 141005. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Wu, J.; Yu, X. Eggshell-Enhanced Biochar with in-Situ Formed CaO/Ca(OH)2 for Efficient Removal of Pb2+ and Cd2+ from Wastewater: Performance and Mechanistic Insights. Sep. Purif. Technol. 2025, 354, 129352. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, K.; Wang, L.; Wang, W.; Du, M.; Li, R.; Fang, J.; Jiang, Z. Utilization of Rice Husk Ash as a Potential Catalyst for Diatom Growth and Adsorbent for Heavy Metals in Aquaculture Systems. Aquaculture 2025, 595, 741533. [Google Scholar] [CrossRef]
- Gama de Almeida, M.B.; Dantas de Jesus, A.M.; Pereira, A.S.; Fiore, F.A. Evaluating Centrifuged Water Treatment Plant Sludge as an Adsorbent for Nutrients, Microorganisms, and Heavy Metals Removal from Wastewater. J. Clean. Prod. 2024, 468, 142975. [Google Scholar] [CrossRef]
- Yazid, H.; Amour, L.; Terkmani, A.; Maachi, R. Biosorption of Lead from Aqueous Solution by Biologically Activated Date Pedicels: Batch and Column Study. Desalin. Water Treat. 2013, 51, 1690–1699. [Google Scholar] [CrossRef]
- Tariq, A.; Yahaya, N.; Sajid, M. Low Cost Adsorbents Derived from Vegetables and Fruits: Synthesis, Properties, and Applications in Removal of Heavy Metals from Water. Desalin. Water Treat. 2024, 320, 100626. [Google Scholar] [CrossRef]
- Naseer, A. Role of Nanocomposites and Nano Adsorbents for Heavy Metals Removal and Dyes. An Overview. Desalina. Water Treat. 2024, 320, 100662. [Google Scholar] [CrossRef]
- Boughrara, L.; Sebba, F.Z.; Sebti, H.; Choukchou-Braham, E.; Bounaceur, B.; Kada, S.O.; Zaoui, F. Removal of Zn(II) and Ni(II) Heavy Metal Ions by New Alginic Acid-Ester Derivatives Materials. Carbohydr. Polym. 2021, 272, 118439. [Google Scholar] [CrossRef] [PubMed]
- Gomravi, Y.; Karimi, A.; Azimi, H. Adsorption of Heavy Metal Ions via Apple Waste Low-Cost Adsorbent: Characterization and Performance. Korean J. Chem. Eng. 2021, 38, 1843–1858. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Palm Kernel Shell as an Effective Adsorbent for the Treatment of Heavy Metal Contaminated Water. Sci. Rep. 2019, 9, 18955. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R. Oak (Quercus Robur) Acorn Peel as a Low-Cost Adsorbent for Hexavalent Chromium Removal from Aquatic Ecosystems and Industrial Effluents. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Madzin, Z.; Zahidi, I.; Talei, A.; Raghunandan, M.E.; Hermawan, A.A.; Karam, D.S. Optimising Spent Mushroom Compost Biochar for Heavy Metal Removal: Mechanisms and Kinetics in Mine Water Treatment. J. Water Process Eng. 2025, 69, 106829. [Google Scholar] [CrossRef]
- Rahman, M.S. Adsorption of Methyl Blue onto Activated Carbon Derived from Red Oak (Quercus rubra) Acorns: A 26 Factorial Design and Analysis. Water. Air. Soil Pollut. 2021, 232, 1. [Google Scholar] [CrossRef]
- Lebel Desrosiers, S.; Collin, A.; Bélanger, N. Factors Affecting Early Red Oak (Quercus rubra L.) Regeneration near Its Northern Distribution Limit in Quebec. Front. For. Glob. Change 2024, 7, 1451161. [Google Scholar] [CrossRef]
- FAO Statistics. World Food and Agriculture—Statistical Yearbook 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Nasser, R.A.; Salem, M.Z.M.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical Analysis of Different Parts of Date Palm (Phoenix dactylifera L.) Using Ultimate, Proximate and Thermo-Gravimetric Techniques for Energy Production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- Alsulaili, A.D.; Refaie, A.A.; Garcia, H.A. Adsorption Capacity of Activated Carbon Derived from Date Seeds: Characterization, Optimization, Kinetic and Equilibrium Studies. Chemosphere 2023, 313, 137554. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of Lead(II) from Aqueous Solution Using Date Seed-Derived Biochar: Batch and Column Studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Peaches and Nectarines: Global Growth in Production Slowing While Exports Plateau. 2023. Available online: https://fas.usda.gov/data/peaches-and-nectarines-global-growth-production-slowing-while-exports-plateau (accessed on 5 February 2025).
- Eisnor, J.D.; Gagnon, G.A. Impact of Secondary Disinfection on Corrosion in a Model Water Distribution System. J. Water Supply Res. Technol. Aqua 2004, 53, 441–452. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G. A Comparative Study on the Removal Efficiency of Metal Ions (Cu2+, Ni2+, and Pb2+) Using Sugarcane Bagasse-Derived ZnCl2-Activated Carbon by the Response Surface Methodology. Adsorpt. Sci. Technol. 2016, 35, 72–85. [Google Scholar] [CrossRef]
- Vunain, E.; Kenneth, D.; Biswick, T. Synthesis and Characterization of Low-Cost Activated Carbon Prepared from Malawian Baobab Fruit Shells by H3PO4 Activation for Removal of Cu(II) Ions: Equilibrium and Kinetics Studies. Appl. Water Sci. 2017, 7, 4301–4319. [Google Scholar] [CrossRef]
- Nasirian, A. Synthesis and Characterization of Cu Nanoparticles and Studying of Their Catalytic Properties. Int. J. Nano Dim. 2012, 2, 159–164. [Google Scholar]
- Theivasanthi, T.; Alagar, M. X-Ray Diffraction Studies of Copper Nanopowder. arXiv 2010, arXiv:1003.6068. [Google Scholar]
- Roushdy, N.; Elnouby, M.S.; Farag, A.A.M.; Ramadan, M.; El-Shazly, O.; El-Wahidy, E.F. Structural and Electrical Characterization of Nickel Sulfide Nanoparticles. Opt. Quantum Electron. 2024, 56, 1794. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The Comparative Analyses of Reduced Graphene Oxide (RGO) Prepared via Green, Mild and Chemical Approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Allahkarami, E.; Dehghan Monfared, A.; Silva, L.F.O.; Dotto, G.L. Toward a Mechanistic Understanding of Adsorption Behavior of Phenol onto a Novel Activated Carbon Composite. Sci. Rep. 2023, 13, 167. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Sreematha, B.; Arundhathi, N.; Ravinder, D. Influence of Cerium Substitution on Structural, Optical, and Electrical Transport Properties of Ni-Nano Ferrites Prepared by Citrate Gel Auto Combustion Method. Results Chem. 2023, 5, 100929. [Google Scholar] [CrossRef]
- Benjamine, M.; Rahman, A. Structural, Morphological and Optical Properties of CuO Nanoparticles Prepared through Chemical Method. J. Emerg. Technol. Innov. Res. 2018, 5, 273–278. [Google Scholar]
- Kamalabadi, M.; Madrakian, T.; Afkhami, A.; Ghoorchian, A. Crystal Violet-Modified HKUST-1 Framework with Improved Hydrostability as an Efficient Adsorbent for Direct Solid-Phase Microextraction. Microchim. Acta 2021, 188, 305. [Google Scholar] [CrossRef]
- Nasir, H.; Rahman, N.; Zulfiqar; Khan, T.; Ali, S.; Khan, R.; Hayat, K. Variations in Structural, Optical, and Dielectric Properties of CuO Nanostructures with Thermal Decomposition. J. Mater. Sci. Mater. Electron. 2020, 31, 10649–10656. [Google Scholar] [CrossRef]
- Koseoglu, E.; Akmil-Başar, C. Preparation, Structural Evaluation and Adsorptive Properties of Activated Carbon from Agricultural Waste Biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Devi, B.; Goswami, M.; Rabha, S.; Kalita, S.; Sarma, H.P.; Devi, A. Efficacious Sorption Capacities for Pb(II) from Contaminated Water: A Comparative Study Using Biowaste and Its Activated Carbon as Potential Adsorbents. ACS Omega 2023, 8, 15141–15151. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Farag, S.; Fikry, M.; Aly, A.A.; Amr, A. Evaluation of the Adsorption Capacity of Surfactant-Modified Biomass in an Aqueous Acid Blue 193 System. Waste Manag. Bull. 2024, 2, 172–183. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Sellaoui, L.; Dhaouadi, F.; Taamalli, S.; Louis, F.; Bakali, A.E.; Badawi, M.; Bonilla-Petriciolet, A.; Silva, L.; da Boit Martinello, K.; Dotto, G.L. Understanding the Cu2+ Adsorption Mechanism on Activated Carbon Using Advanced Statistical Physics Modelling. Environ. Sci. Pollut. Res. 2022, 29, 54882–54889. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Cheng, S.; Xia, H.; Zhang, L.; Peng, J.; Li, C.; Zhang, S. Copper Loaded on Activated Carbon as an Efficient Adsorbent for Removal of Methylene Blue. RSC Adv. 2017, 7, 14395–14405. [Google Scholar] [CrossRef]
- Djezzar, Z.; Aidi, A.; Rehali, H.; Ziad, S.; Othmane, T. Characterization of Activated Carbon Produced from the Green Algae Spirogyra Used as a Cost-Effective Adsorbent for Enhanced Removal of Copper(Ii): Application in Industrial Wastewater Treatment. RSC Adv. 2024, 14, 5276–5289. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.; García-Díaz, I.; Alguacil, F.J.; López Gómez, F.A. Removal of Copper Ions from Wastewater by Adsorption onto a Green Adsorbent from Winemaking Wastes. BioResources 2020, 15, 1112–1133. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Removal of Lead (II) Ions from Aqueous Solutions Using Manganese Oxide-Coated Adsorbents: Characterization and Kinetic Study. Adsorpt. Sci. Technol. 2009, 27, 177–191. [Google Scholar] [CrossRef]
- Amrutha; Jeppu, G.; Girish, C.R.; Prabhu, B.; Mayer, K. Multi-Component Adsorption Isotherms: Review and Modeling Studies. Environ. Process. 2023, 10, 38. [Google Scholar] [CrossRef]
- Aguilar, K.M.M.; Kose, Y.; Amano, Y.; Machida, M.; Imazeki, F. Influence of Oxidation Conditions of Activated Carbon on Adsorption of Pb (II) from Aqueous Solution. J. Environ. Chem. 2016, 26, 109–114. [Google Scholar] [CrossRef]
- Evans, M.J.B.; Halliop, E.; Liang, S.; MacDonald, J.A.F. The Effect of Chlorination on Surface Properties of Activated Carbon. Carbon 1998, 36, 1677–1682. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans World Health Organization International Agency for Research on Cancer. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans World Health Organization International Agency for Research on Cancer: Lyon, France, 2004; Volume 84. [Google Scholar]
- Minkina, T.M.; Pinskii, D.L.; Zamulina, I.V.; Nevidomskaya, D.G.; Gülser, C.; Mandzhieva, S.S.; Bauer, T.V.; Morozov, I.V.; Sushkova, S.N.; Kizilkaya, R. Chemical Contamination in Upper Horizon of Haplic Chernozem as a Transformation Factor of Its Physicochemical Properties. J. Soils Sediments 2018, 18, 2418–2430. [Google Scholar] [CrossRef]
- Zamulina, I.V.; Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Bauer, T.V.; Burachevskaya, M.V. The Influence of Long-Term Zn and Cu Contamination in Spolic Technosols on Water-Soluble Organic Matter and Soil Biological Activity. Ecotoxicol. Environ. Saf. 2021, 208, 111471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H. Co-Transport of Dissolved Organic Matter and Heavy Metals in Soils Induced by Excessive Phosphorus Applications. J. Environ. Sci. 2010, 22, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Katoh, M. Feature of Lead Complexed with Dissolved Organic Matter on Lead Immobilization by Hydroxyapatite in Aqueous Solutions and Soils. Chemosphere 2020, 249, 126122. [Google Scholar] [CrossRef]
- Qiu, L.; Suo, C.; Zhang, N.; Yuan, R.; Chen, H.; Zhou, B. Adsorption of Heavy Metals by Activated Carbon: Effect of Natural Organic Matter and Regeneration Methods of the Adsorbent. Desalin. Water Treat. 2022, 252, 148–166. [Google Scholar] [CrossRef]
- Da’na, E.; Awad, A. Regeneration of Spent Activated Carbon Obtained from Home Filtration System and Applying It for Heavy Metals Adsorption. J. Environ. Chem. Eng. 2017, 5, 3091–3099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).