Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Aqueous Herbal Infusions
2.3. HPLC Analysis of Phenolic Compounds
2.4. In Vitro Antioxidant Activity
2.4.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Scavenging Activity)
2.4.2. FRAP (Ferric Reducing/Antioxidant Power)
2.4.3. Rancimat Assay (Oxidative Stability Test)
2.5. Antibacterial Activity
2.6. In Vitro Antidabetic Activity
2.6.1. Glucosidase Inhibitory Activity
2.6.2. Amylase Inhibitory Activity
2.7. Cell Culture
2.8. Cell Proliferation Assay (MTT)
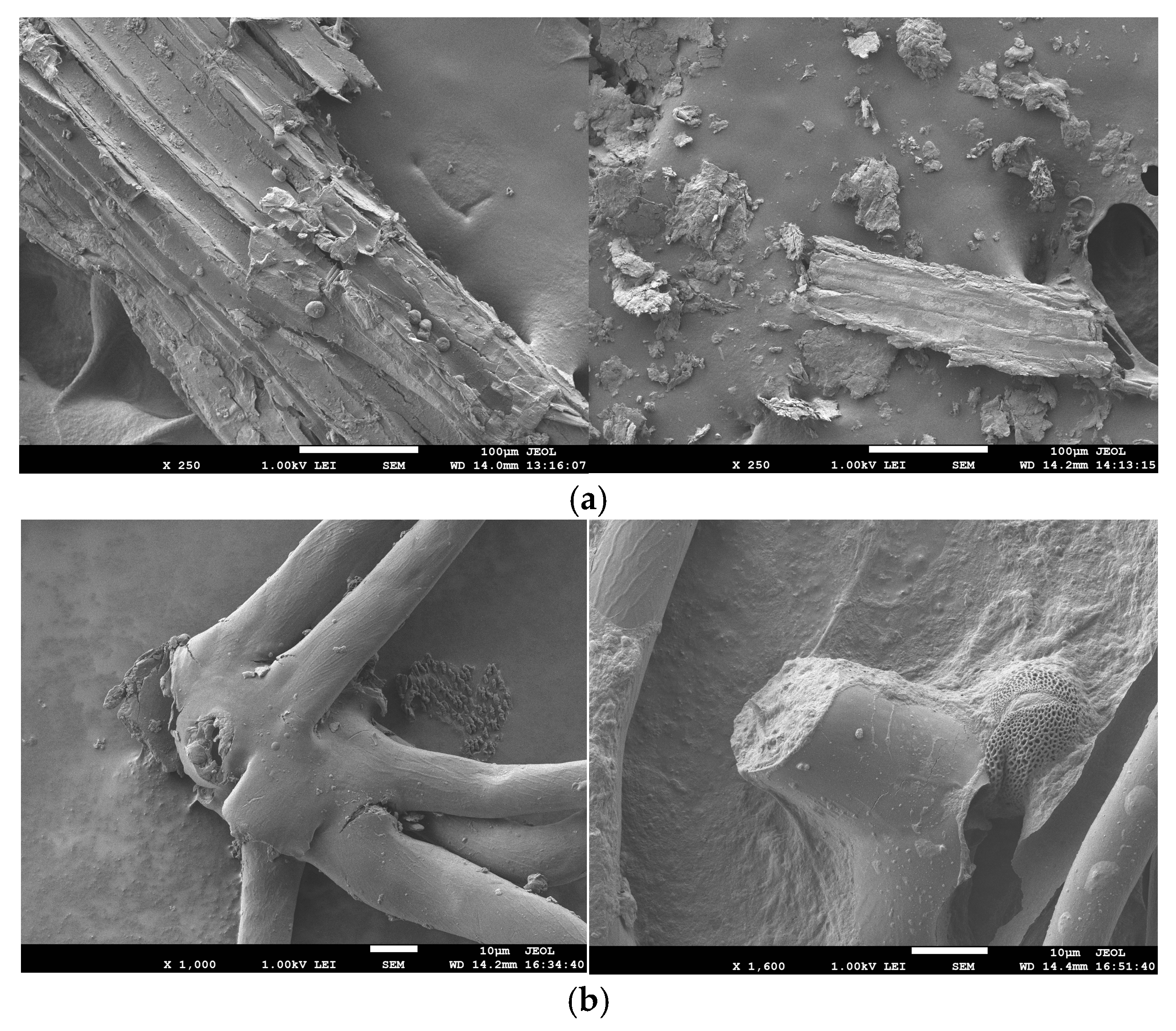
2.9. Microstructure Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis of Aqueous Herbal Infusions
3.2. Antioxidant Activity of Aqueous Herbal Infusions
3.3. Antibacterial and Antidabetic Activities of Aqueous Herbal Infusions
3.4. Antiproliferative Activity of Aqueous Herbal Infusions
3.5. Microstructure Analysis
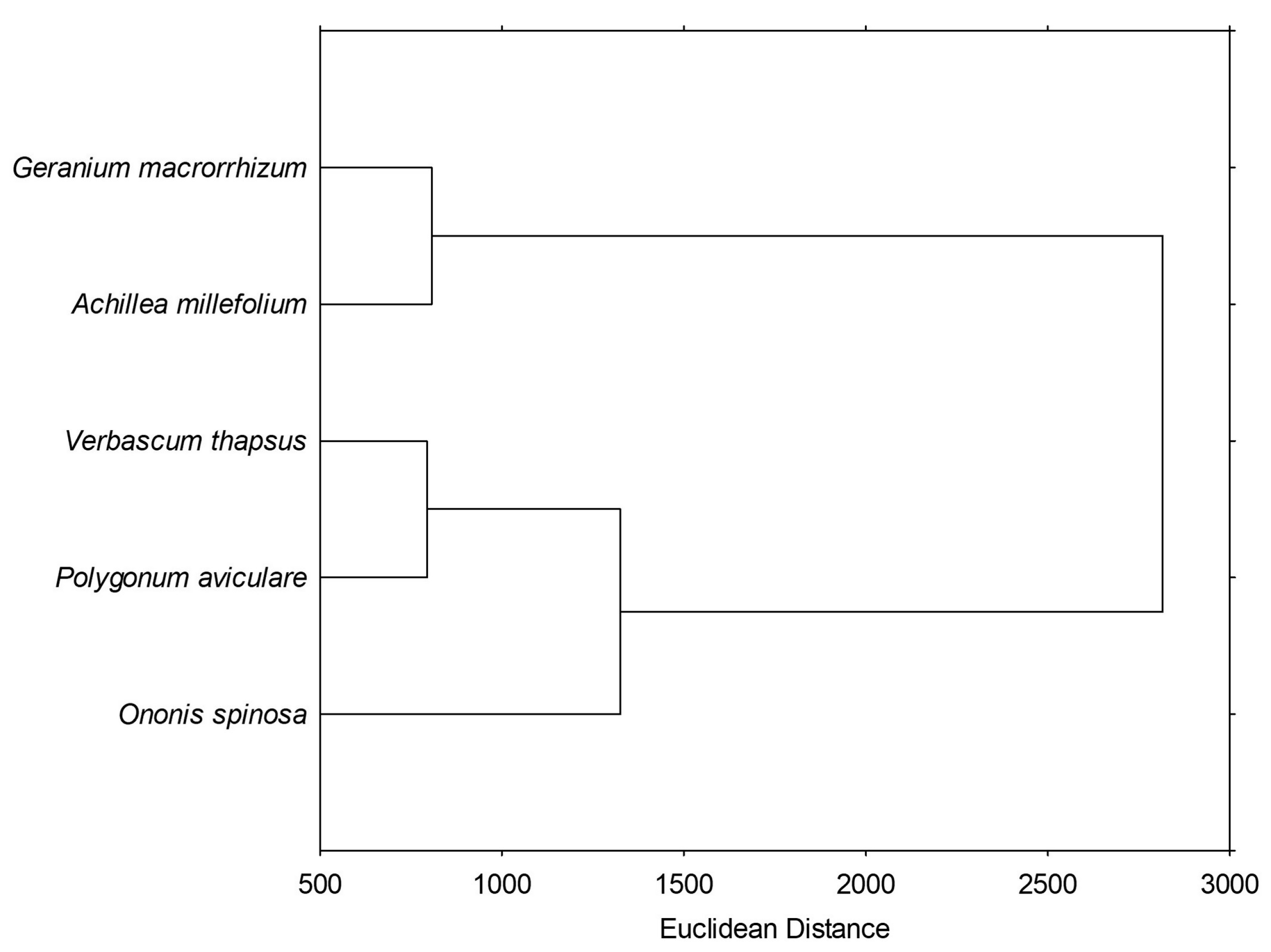
3.6. Statistical Analysis
Principal Component Analysis and Hierarchical Clustering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Global Industry Analysts Inc. A $178.4 Billion Global Opportunity for Herbal Medicines by 2026—New Research from Strategy. 2021. Available online: https://www.prnewswire.com/news-releases/a-178-4-billion-global-opportunity-for-herbal-medicines-by-2026---new-research-from-strategyr-301400269.html (accessed on 12 March 2025).
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241515436. Available online: https://www.who.int/publications/i/item/978924151536 (accessed on 22 July 2017).
- Tomou, E.M.; Peppa, E.; Trichopoulou, A. Consumption of herbal infusions/decoctions and tea in Greece: A Planeterranean perspective on the results of Hydria survey. J. Transl. Med. 2023, 21, 899. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Pádua, A.; Gonçalves, V.M.F.; Ribeiro, C.; Leal, S. Exploring tea and herbal infusions consumption patterns and behaviours: The case of Portuguese consumers. Heliyon 2024, 10, e28779. [Google Scholar] [CrossRef] [PubMed]
- Vasić, D.; Katanić Stanković, J.S.; Urošević, T.; Kozarski, M.; Naumovski, N.; Khan, H.; Popović-Djordjević, J. Insight into bioactive compounds, antioxidant and anti-diabetic properties of rosehip (Rosa canina L.)-based tisanes with addition of hibiscus flowers (Hibiscus sabdariffa L.) and saffron (Crocus sativus L.). Beverages 2024, 10, 1. [Google Scholar] [CrossRef]
- Studzínska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Klaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef] [PubMed]
- Soares Mateus, A.R.; Crisafulli, C.; Vilhena, M.; Cruz Barros, S.; Pena, A.; Sanches Silva, A. The Bright and Dark Side of Herbal Infusions: Assessment of Antioxidant Capacity and Determination of Tropane Alkaloids. Toxins 2023, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Stojković, M.B.; Mitić, S.S.; Randjelović, P.J.; Ilić, I.R.; Stojanović, N.M.; Stojanović-Radić, Z.Z. Exploitation of the Antioxidant Potential of Geranium macrorrhizum (Geraniaceae): Hepatoprotective and Antimicrobial Activities. Nat. Prod. Commun. 2012, 7, 1934578X1200701218. [Google Scholar] [CrossRef]
- Światek, L.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Ozmen Guler, G.; Sadeer, N.B.; Fawzi Mahomoodally, M.; et al. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm.f: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 11, 7621. [Google Scholar] [CrossRef]
- Ghasemi, F.; Rezaei, F.; Araghi, A.; Abouhosseini Tabari, M. Antimicrobial Activity of Aqueous-Alchocolic Extracts and the Essential Oil of Verbascum thapsus L. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e23004. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-UI-Hay, M.; Jaafar, H.Z. Common Mullein, Pharmacological and Chemical Aspects. Rev. Bras. Farmacogn. 2013, 23, 948–959. [Google Scholar] [CrossRef]
- Tuker, A.U.; Ekrem, G. Common Mullein (Verbascum thapsus L.): Recent Advances in Research. Phytother. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef]
- Saeidnia, S.; Sarkhail, P.; Moradi-Afrapoli, F.; Gohari, A.R.; Nikan, M.; Mokhber-Dezfuli, N.; Amin, G.R.; Hadjiakhoondi, A. GC/MS Analysis of the Volatile Compounds of P. hyrcanicum Diethyl Ether Extract and GC Profiling of Some Iranian Polygonum Species. Res. J. Pharmacogn. 2014, 1, 3–7. [Google Scholar]
- Dong, X.; Fu, J.; Yin, X.; Li, X.; Wang, B.; Cao, S.; Zhang, H.; Zhao, Y.; Ni, J. Pharmacological and Other Bioactivities of the Genus Polygonum—A Review. Trop. J. Pharm. Res. 2014, 13, 1749–1759. [Google Scholar]
- Al-Snafi, A. The Traditional Uses, Constituents and Pharmacological Effects of Ononis spinosa. IOSR J. Pharm. 2020, 10, 53–59. [Google Scholar]
- Stojković, D.; Drakulić, D.; Gašić, U.; Zengin, G.; Stevanović, M.; Rajčević, N.; Soković, M. Ononis spinosa L., an Edible and Medicinal Plant: UHPLC-LTQ-Orbitrap/MS Chemical Profiling and Biological Activities of the Herbal Extract. Food Funct. 2020, 11, 7138. [Google Scholar] [CrossRef] [PubMed]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef] [PubMed]
- Uçar, E. Polygonum aviculare L.’s biological activities: Investigating its anti-proliferative, antioxidant, chemical properties supported by molecular docking study. Inorg. Chem. Commun. 2024, 162, 112228. [Google Scholar] [CrossRef]
- Aleksieiev, A.; Maslowski, M.; Efenberger-Szmechtyk, M.; Strzelec, K. The influence of freeze-dried alcohol-water extracts from common yarrow (Achillea millefolium L.) and german chamomile (Matricaria chamolilla L.) on the properties of elastomer vulcanizates. Int. J. Mol. Sci. 2022, 23, 15048. [Google Scholar] [CrossRef]
- Bilušić, T.; Kalušević, A.; Marijanović, Z.; Jerković, I.; Mužek, M.N.; Bratanić, A.; Skroza, D.; Zorić, Z.; Pedišić, S.; Nedović, V.; et al. Influences of Freeze- and Spray-Drying vs. Encapsulation with Soy and Whey Proteins on Gastrointestinal Stability and Antioxidant Activity of Mediterranean Aromatic Herbs. Int. J. Food Sci. Technol. 2021, 56, 1582–1596. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tavares Silva, E.; Spacino, K.R.; Chanan Silva, L.R.; Signori Romagnoli, E.; Gomes Angilelli, K.; Borsato, D. Modelling of Relative Protection Factor of Antioxidants TBHQ, BHT and BHA in Mixture with Biodiesel. Acta Scient Technol. 2018, 40, e35108. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 22 July 2017).
- Rončević, T.; Gerdol, M.; Mardirossian, M.; Maleš, M.; Cvjetan, S.; Benincasa, M.; Maravić, A.; Gajski, G.; Krce, L.; Aviani, I.; et al. Anisaxins, Helical Antimicrobial Peptides from Marine Parasites, Kill Resistant Bacteria by Lipid Extraction and Membrane Disruption. Acta Biomater. 2022, 146, 131–144. [Google Scholar] [CrossRef]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Saravia de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant and α-glucosidase-inhibiting activity of aqueous and hydroethanolic extracts of traditional antidiabetics from Croatian ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- Marijan, M.; Jakupović, L.; Vujić, L.; Jurić, M.; Zovko Končić, M. Urban parks biowaste as a sustainable source of new antidiabetics. Acta Pharm. 2024, 74, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Bilušić, T.; Šola, I.; Čikeš Čulić, V. Identification of flavonoids, antioxidant and antiproliferative activity of aqueous infusions of Calendula officinalis L., Chelidonium majus L., Teucrium chamaedrys L. and Alchemilla vulgaris L. Food Technol. Biotechnol. 2024, 62, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueňas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveria, B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Composition of Wild and Commerical Achillea millefolium L. and Bioactivity of the Methanolic Extract, Infusion and Decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Aljaafreh, I.; Tarawneh, A.; Al Shbailat, Y.; Alzagameem, A.; Al-Marafi, H.; Albadaineh, H.; Rababah, T.M.; Al-u-Datt, M. Antioxidant and α-Amylase Inhibition Activities of Medicinal Plants are Traditionally Used for the Treatment of Digestive Disorders in the near East Region. J. Microbiol. Bioctechnol Food Sci. 2023, 13, e9072. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, G.; Piao, M.; Lang, M.; Wang, Y.; Jin, M.; Li, G.; Zheng, M. Chemical Constituents of Polygonum aviculare L. and their Chemotaxonomic Significance. Biochem. Systemat Ecol. 2022, 105, 104529. [Google Scholar] [CrossRef]
- Idoudi, S.; Tourrette, A.; Bouajila, J.; Romdhane, M.; Elfalleh, W. The Genus Polygonum: An Updated Comprehensive Review of its Ethnomedicinal, Phytochemical, Pharmacological Activities, Toxicology, and Phytopharmaceutical Formulation. Heliyon 2024, 10, e28947. [Google Scholar] [CrossRef]
- Renda, G.; Sari, S.; Barut, B.; Šoral, M.; Liptaj, T.; Korkmaz, B.; Özel, A.; Erik, I.; Şöhretoğlu, D. α-Glucosidase Inhibitory Effects of Polyphenols from Geranium asphodeloides: Inhibition Kinetics and Mechanistic Insights Through In Vitro and In Silico Studies. Bioorganic Chem. 2018, 81, 545–552. [Google Scholar] [CrossRef]
- Alshehri, B. The Geranium Species: A Comprehensive Study on Ethnomedicinal Uses, Phytochemical Compounds, and Pharmacologhical Importance. Saudi J. Biol. Sci. 2024, 31, 103940. [Google Scholar] [CrossRef] [PubMed]
- Ivancheva, S.; Petrova, A. A Chemosystematic Study of Eleven Geranium Species. Biochem. Systemat Ecol. 2000, 28, 255–260. [Google Scholar] [CrossRef]
- Miliauskas, G.; Beek, T.A.; Venskutonis, P.R.; de Ward, P. Antioxidant Activity of Geranium macrorrhizum. Eur. Food Res. Technol. 2004, 218, 253–261. [Google Scholar] [CrossRef]
- Gupta, A.; Atkinson, A.N.; Kumar Pandey, A.; Bishayee, A. Health-Promoting and Disease-Mitigating Potential of Verbascum thapsus L. (Common Mullein): A Review. Phyotherapy Res. 2022, 36, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Amiradalat, M.; Babashpour, M.; Sheikhlooei, H.; Miransari, M. The Antioxidant, Anticancerogenic and Antimicrobial Properties of Verbascum thapsus L. Medicinal Chem. 2020, 16, 991–995. [Google Scholar] [CrossRef]
- Selseleh, M.; Ebrahimi, S.N.; Alihamadi, A.; Sonboli, A.; Mirjalili, M.H. Metabolic Profiling, Antioxidant, and Antibacterial Activity of some Iranian Verbascum L. Species. Ind. Crops Prod. 2020, 153, 112609. [Google Scholar] [CrossRef]
- Ilić, M.; Samardžić, S.; Kotur-Stevuljević, J.; Ušjak, D.; Milenković, M.; Kovačević, N.; Drobac, M. Polyphenol Rich Extracts of Geranium L. Species as Potential Natural Antioxidant and Antimicrobial Agents. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6283–6294. [Google Scholar] [CrossRef]
- Navaie, B.A.; Kavoosian, S.; Fattahi, S.; Hajian-Tilaki, K.; Asouri, M.; Bishekolaie, R.; Akhavan-Niaki, H. Antioxidant and Cytotoxic Effect of Aqueous and Hydroalcoholic Extracts of the Achillea Millefolium L. on MCF-7 Breast Cancer Cell Line. Int. Biol. Biomed. J. 2015, 1, 119–125. [Google Scholar]
- Ivancheva, S.; Manolova, N.; Serkedjieva, J.; Dimov, V.; Ivanovska, N. Polyphenols from Bulgarian Medicinal Plants with Anti-Infectious Activity. Basic. Life Sci. 1992, 59, 717–728. [Google Scholar] [CrossRef]
- Turker, A.U.; Camper, N.D. Biological Activity of Common Mullein, A Medicinal Plant. J. Ethnopharmacol. 2002, 82, 117–125. [Google Scholar] [CrossRef]
- Shahbaz, F.; Akhter, N.; Shahid, M.; Riaz, M.; Anjum, F.; Hussain, F. Ultrasound Assisted Extraction and Characterization of Biaoctives from Verbascum thapsus Roots to Evaluate Their Antioxidant and Medicinal Potential. Dose Response 2022, 3, 15593258221097665. [Google Scholar] [CrossRef]
- Grigore, A.; Colceru-Mihul, S.; Bazdoaca, C.; Yuksel, R.; Ionita, C.; Glava, L. Antimicrobial Activity of an Achillea millefolium L. Proceedings 2020, 57, 34. [Google Scholar] [CrossRef]
- Noda, K.; Kato, E.; Kawabata, J. Intestinal α-Glucosidase Inhibitors in Achillea millefolium. Nat. Prod. Commun. 2017, 12, 1259–1261. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, L.; Lin, X.; Hu, X.; Wang, L. Phenolic Profiles and Screening of Potential α-Glucosidase Inhibitors from Polygonum aviculare L. Leaves Using Ultra-Filtration Combined with HPLC-ESI-qTOF-MS/MS and Molecular Docking Analysis. Ind. Crops Prod. 2020, 154, 112673. [Google Scholar] [CrossRef]
- Zhang, N.; Baran, A.; Valioglu, F.; Teng, L.; Atalar, M.N.; Keskin, C.; Wang, X.X.; Hatipoğlu, A.; Baran, M.F.; Abdelsalam, A.H.; et al. Antioxidant, AChE Inhibitory, and Anticancer Effects of Verbascum thapsus Extract. Cell. Mol. Biol. 2024, 69, 211–216. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding Phenolic Acids Inhibition of α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Deipenbrock, M.; Sendker, J.; Hensel, A. Aqueous Root Extract from Ononis spinosa Exerts Anti-Adhesive Activity against Uropathogenic Escherichia coli. Plant Med. 2020, 86, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wiśniewska, A.; Halas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and Antimetastatic Action of Quercetin on A549 Non-Small Lung Cancer Cells through its Effects on the Cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef]

| Phenolic Compounds | Mass Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| Achillea Millefolium L. | Polygonum Aviculare L. | Ononis Spinosa L. | Geranium Macrorrhizum L. | Verbascum Thapsus L. | |
| Gallic acid | 12.25 ± 0.65 | n.d. | 21.36 ± 0.79 | 23.79 ± 1.01 | 66.72 ± 2.40 |
| Protocatechuic acid | 6.30 ± 0.05 | 9.97 ± 0.15 | n.d. | n.d. | 16.34 ± 0.49 |
| Chlorogenic acid and its derivatives | 337.38 ± 4.72 | 4.34 ± 0.07 | 3.29 ± 0.10 | n.d. | 39.32 ± 0.97 |
| Caffeic acid and its derivatives | 13.76 ± 0.17 | n.d. | 5.13 ± 0.24 | 2.39 ± 0.04 | 16.34 ± 0.49 |
| p-coumaric acid and its derivatives | 2.79 ± 0.02 | 3.27 ± 0.06 | 6.44 ± 0.14 | n.d. | n.d. |
| Ferulic acid and its derivatives s | 189.66 ± 4.23 | n.d. | n.d. | n.d. | n.d. |
| Syringic acid and its derivatives | n.d. | n.d. | n.d. | 71.30 ± 2.05 | 16.39 ± 0.21 |
| Rosmarinic acid | n.d. | n.d. | n.d. | n.d. | 23.01 ± 0.58 |
| Hydroxycinnamic acid (derivatives) | 85.98 ± 0.78 | n.d. | 82.58 ± 0.48 | 67.55 ± 1.77 | 25.47 ± 0.60 |
| Total phenolic acids | 648.13 ± 5.47 a** | 17.59 ± 0.17 e | 118.79 ± 0.90 d | 165.03 ± 2.29 c | 190.91 ± 3.78 b |
| Flavan-3-ols | n.d. | 520.69 ± 7.43 | 161.33 ± 4.88 | n.d. | n.d. |
| Quercetin and its derivatives | 45.89 ± 0.69 | 274.88 ± 2.93 | n.d. | 300.44 ± 1.57 | 39.43 ± 0.94 |
| Kaempferol and its derivatives | n.d. | 223.26 ± 3.34 | n.d. | 192.55 ± 3.14 | 44.15 ± 0.98 |
| Luteolin and its derivatives | 56.02 ± 0.48 | 4.71 ± 0.29 | n.d. | 128.96 ± 3.45 | 3.76 ± 0.14 |
| Apigenin and its derivatives | 72.84 ± 0.95 | 1.22 ± 0.06 | n.d. | n.d. | n.d. |
| Catechin and its derivatives | n.d. | 36.23 ± 0.28 | n.d. | n.d. | n.d. |
| Total flavonoids | 174.74 ± 1.97 c | 1060.99 ± 6.96 a | 161.33 ± 4.88 d | 621.94 ± 2.00 b | 87.34 ± 1.83 e |
| Procyanidins | n.d. | 176.13 ± 3.85 | n.d. | 744.48 ± 9.86 | 94.46 ± 0.87 |
| Total phenolic compounds | 822.87 ± 6.18 c | 1254.72 ± 8.73 b | 280.12 ± 5.64 e | 1531.45 ± 11.58 a | 372.71 ± 1.94 d |
| Sample | DPPH Assay IC50 (mg/mL) | FRAP Assay (mg FeCl2/L) | Rancimat (PF **) |
|---|---|---|---|
| Geranium macrorrhizum L. | 3.75 ± 0.02 d | 3584.61 ± 18.05 a | 1.14 ± 0.02 a |
| Verbascum thapsus L. | 23.78 ± 0.04 b | 265.24 ± 11.70 e | n.d. |
| Achillea millefolium L. | 3.75 ± 0.02 d | 3308.24 ± 18.33 b | 1.10 ± 0.01 a |
| Polygonum aviculare L. | 11.90 ± 0.01 c | 832.91 ± 10.04 c | 1.11 ± 0.02 a |
| Ononis spinosa L. | 30.00 ± 0.01 a | 495.81 ± 24.17 d | n.d. |
| BHT | 0.018 ± 0.002 | / | 3.90 ± 0.34 |
| BHA | 0.054 ± 0.002 | / | 7.2 ± 0.08 |
| Ascorbic acid | n.d. | (2.60 ± 0.22) × 10−4 | n.d. |
| Sample | Inhibition of α-Glucosidase IC50 (mg/mL) | Inhibition of α-Amylase IC50 (mg/mL) |
|---|---|---|
| Geranium macrorrhizum L. | 1.22 ± 0.01 c** | 5.03 ± 0.03 b |
| Verbascum thapsus L. | 43.61 ± 1.09 d | 136.39 ± 16.65 a |
| Achillea millefolium L. | n.d. | n.d. |
| Polygonum aviculare L. | 55.18 ± 5.67 a | n.d |
| Ononis spinosa L. | 4.09 ± 0.09 c | 14.45 ± 0.18 b |
| acarbose | 1.44 ± 0.01 c | 1.84 ± 0.01 b |
| Sample (g/L) | Inhibition of Cell Proliferation (%) | |||
|---|---|---|---|---|
| t/h | ||||
| 1.00 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 15.82 ± 1.06 a** | 17.03 ± 2.92 b | 40.48 ± 0.46 a | 54.99 ± 2.80 a |
| Verbascum thapsus | 12.33 ± 3.00 ab | 12.64 ± 2.24 c | 16.60 ± 1.20 d | 26.79 ± 0.70 d |
| Ononis spinosa | 13.61 ± 3.10 ab | 19.02 ± 0.96 b | 30.11 ± 0.85 b | 49.05 ± 1.47 b |
| Achillea millefolium | 11.12 ± 2.59 ab | 19.61 ± 1.94 b | 38.09 ± 1.89 a | 48.53 ± 3.73 b |
| Polygonum aviculare | 7.95 ± 2.45 b | 25.43 ± 3.18 a | 27.26 ± 0.72 c | 36.30 ± 3.03 c |
| 0.50 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 13.41 ± 2.49 a | 16.41 ± 1.43 ab | 29.77 ± 1.21 a | 46.07 ± 1.38 a |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 10.56 ± 1.12 ab | 15.41 ± 2.39 ab | 30.54 ± 2.13 a | 38.05 ± 1.17 b |
| Achillea millefolium | 9.71 ± 0.43 ab | 13.48 ± 2.90 b | 31.70 ± 1.78 a | 36.78 ± 3.00 b |
| Polygonum aviculare | 8.62 ± 2.93 b | 19.07 ± 1.27 a | 20.52 ± 1.21 b | 28.83 ± 1.25 c |
| 0.25 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 7.35 ± 0.88 a | 11.34 ± 1.39 a | 27.93 ± 1.14 a | 43.98 ± 1.36 a |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 8.75 ± 1.05 a | 11.69 ± 1.04 a | 22.06 ± 1.47 b | 37.08 ± 2.88 b |
| Achillea millefolium | 10.95 ± 3.48 a | 12.74 ± 2.45 a | 31.16 ± 1.44 a | 40.20 ± 3.31 ab |
| Polygonum aviculare | 2.20 ± 0.85 b | 12.30 ± 1.82 a | 15.60 ± 3.31 c | 20.33 ± 1.11 c |
| 0.10 g/L | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 2.96 ± 0.14 b | 10.57 ± 2.87 a | 16.31 ± 1.44 b | 22.81 ± 2.50 b |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 5.75 ± 0.45 a | 13.28 ± 3.14 a | 19.56 ± 0.94 b | 21.14 ± 3.84 b |
| Achillea millefolium | 4.07 ± 0.96 b | 9.11 ± 1.08 ab | 23.68 ± 2.11 a | 30.05 ± 1.74 a |
| Polygonum aviculare | 0.41 ± 0.58 c | 5.07 ± 1.11 b | 9.92 ± 2.51 c | 13.47 ± 0.70 c |
| Positive control (cisplatin 50 µg/mL) | 13.62 ± 2.27 | 20.74 ± 1.76 | 50.22 ± 0.95 | 79.45 ± 1.54 |
| Sample (g/L) | Inhibition of Cell Proliferation (%) | |||
|---|---|---|---|---|
| t/h | ||||
| 1.00 | 4 | 24 | 48 | |
| Geranium macrorrhizum | 10.17 ± 0.98 b** | 74.02 ± 2.70 a | 81.30 ± 3.37 a | 96.15 ± 0.16 a |
| Verbascum thapsus | 0.00 ± 0.00 | 3.37 ± 2.19 d | 19.77 ± 0.90 d | 29.88 ± 3.47 c |
| Ononis spinosa | 11.24 ± 0.75 b | 22.37 ± 1.80 c | 70.77 ± 1.56 b | 96.45 ± 1.24 a |
| Achillea millefolium | 5.17 ± 1.33 c | 23.12 ± 0.38 c | 39.84 ± 1.04 c | 88.84 ± 1.44 b |
| Polygonum aviculare | 17.82 ± 2.65 a | 37.54 ± 0.43 b | 78.50 ± 1.75 a | 94.40 ± 3.18 a |
| 0.50 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 9.91 ± 1.08 a | 23.75 ± 2.65 b | 29.36 ± 0.60 c | 34.71 ± 2.67 d |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 7.95 ± 1.49 a | 15.30 ± 2.26 c | 21.26 ± 0.95 d | 41.25 ± 0.89 c |
| Achillea millefolium | 4.23 ± 1.05 b | 18.99 ± 1.70 bc | 34.24 ± 3.00 b | 74.98 ± 0.88 b |
| Polygonum aviculare | 8.70 ± 0.45 a | 32.40 ± 3.27 a | 74.80 ± 3.39 a | 87.93 ± 1.60 a |
| 0.25 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 7.51 ± 0.25 a | 16.11 ± 0.84 b | 25.34 ± 0.58 c | 26.68 ± 2.68 c |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 5.42 ± 2.14 a | 10.65 ± 0.67 c | 17.17 ± 2.27 d | 20.92 ± 0.80 d |
| Achillea millefolium | 4.69 ± 0.83 a | 20.92 ± 1.93 a | 30.31 ± 0.62 b | 56.41 ± 1.91 b |
| Polygonum aviculare | 4.33 ± 1.94 a | 15.33 ± 1.61 b | 49.16 ± 1.45 a | 67.96 ± 1.73 a |
| 0.10 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 8.80 ± 1.18 a | 6.66 ± 0.41 b | 8.44 ± 1.60 c | 15.52 ± 1.24 c |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 7.41 ± 1.58 a | 8.33 ± 0.77 b | 10.16 ± 0.53 c | 16.00 ± 0.18 c |
| Achillea millefolium | 2.16 ± 0.81 bc | 11.10 ± 2.28 a | 30.09 ± 3.05 b | 38.56 ± 1.75 a |
| Polygonum aviculare | 3.53 ± 0.73 b | 12.46 ± 1.26 a | 18.15 ± 1.65 a | 28.47 ± 1.93 b |
| Positive control (50 µg/mL cisplatin) | 0.00 ± 0.00 | 7.96 ± 1.22 | 17.74 ± 1.78 | 15.3 ± 0.10 |
| Sample (g/L) | Inhibition of Cell Proliferation (%) | |||
|---|---|---|---|---|
| t/h | ||||
| 1.00 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 3.85 ± 1.58 bc ** | 22.13 ± 1.54 a | 51.17 ± 0.82 a | 96.20 ± 2.52 a |
| Verbascum thapsus | 3.48 ± 1.47 bc | 6.28 ± 0.69 c | 10.79 ± 2.38 d | 18.05 ± 2.71 d |
| Ononis spinosa | 10.94 ± 1.83 a | 16.26 ± 2.99 b | 37.64 ± 2.67 b | 58.96 ± 1.55 b |
| Achillea millefolium | 5.74 ± 1.85 b | 15.09 ± 0.82 b | 18.69 ± 3.11 c | 32.44 ± 2.55 c |
| Polygonum aviculare | 1.39 ± 0.44 c | 17.62 ± 1.38 b | 19.51 ± 0.60 c | 10.06 ± 1.44 e |
| 0.50 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 4.33 ± 1.98 a | 18.17 ± 1.48 a | 38.48 ± 1.74 a | 92.28 ± 2.06 a |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 7.94 ± 1.56 a | 20.69 ± 3.44 a | 35.58 ± 1.03 a | 45.83 ± 3.35 b |
| Achillea millefolium | / | / | / | / |
| Polygonum aviculare | / | / | / | / |
| 0.25 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 7.23 ± 1.02 a | 15.46 ± 0.70 a | 32.75 ± 1.92 a | 39.51 ± 1.70 a |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 6.66 ± 0.41 a | 10.43 ± 0.47 b | 20.28 ± 2.52 b | 25.61 ± 3.35 b |
| Achillea millefolium | / | / | / | / |
| Polygonum aviculare | / | / | / | / |
| 0.10 | 4 | 24 | 48 | 72 |
| Geranium macrorrhizum | 2.35 ± 0.55 a | 11.46 ± 2.50 a | 26.76 ± 0.45 a | 32.70 ± 1.42 a |
| Verbascum thapsus | / | / | / | / |
| Ononis spinosa | 2.50 ± 0.68 a | 11.82 ± 1.12 a | 15.60 ± 0.45 b | 18.25 ± 2.31 b |
| Achillea millefolium | / | / | / | / |
| Polygonum aviculare | / | / | / | / |
| Positive control (cisplatin 50 µg/mL) | 8.44 ± 1.22 | 13.67 ± 2.19 | 43.83 ± 1.55 | 47.69 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilušić, T.; Čulić, V.Č.; Zorić, Z.; Čošić, Z.; Vujić, L.; Šola, I. Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions. Appl. Sci. 2025, 15, 3254. https://doi.org/10.3390/app15063254
Bilušić T, Čulić VČ, Zorić Z, Čošić Z, Vujić L, Šola I. Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions. Applied Sciences. 2025; 15(6):3254. https://doi.org/10.3390/app15063254
Chicago/Turabian StyleBilušić, Tea, Vedrana Čikeš Čulić, Zoran Zorić, Zrinka Čošić, Lovorka Vujić, and Ivana Šola. 2025. "Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions" Applied Sciences 15, no. 6: 3254. https://doi.org/10.3390/app15063254
APA StyleBilušić, T., Čulić, V. Č., Zorić, Z., Čošić, Z., Vujić, L., & Šola, I. (2025). Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions. Applied Sciences, 15(6), 3254. https://doi.org/10.3390/app15063254








