Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
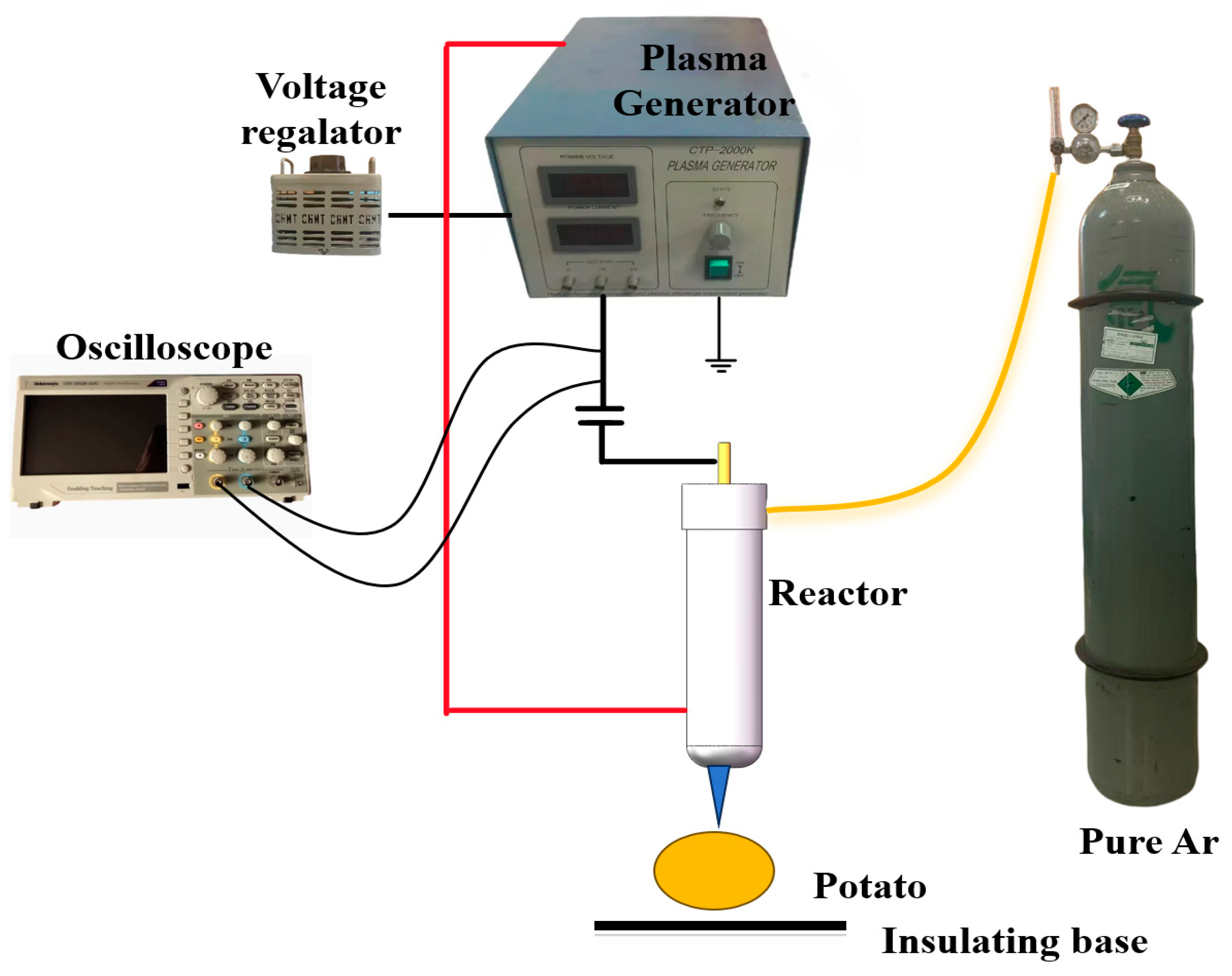
2.2. Experimental Setup
2.3. A One-Factor Experiment
2.4. Optimization of Parameters for Inhibiting Potato Sprouting
2.5. Germination Rate
2.6. Weight Loss Rate
2.7. Control Experiment Design
2.8. The Determination of Potato Texture
2.9. Reducing Sugar Content
2.10. Dry Matter Determination
2.11. Activities of Catalase (CAT) and Superoxide Dismutase (SOD) Enzymes
2.12. Peroxidase (POD) Activity
2.13. Polyphenol Oxidase (PPO) Activity
2.14. Statistical Analysis
3. Results and Discussion
3.1. One-Factor Experiment
3.1.1. Impact of Varying Treatment Voltage on the Germination Process of Potatoes
3.1.2. The Impact of Treatment Time on the Sprouting of Potatoes
3.1.3. The Impact of Gas Flow Rate on the Sprouting of Potatoes
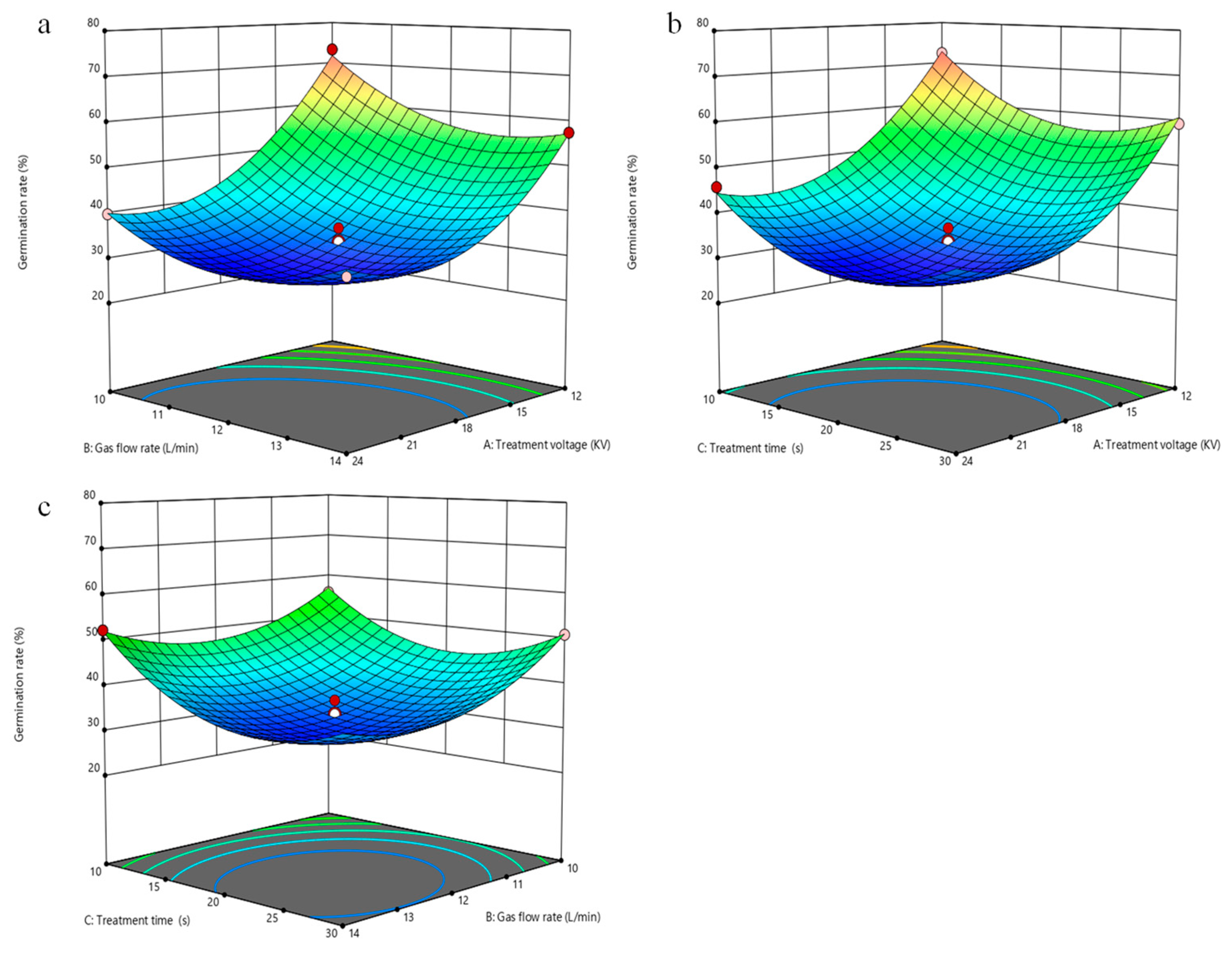
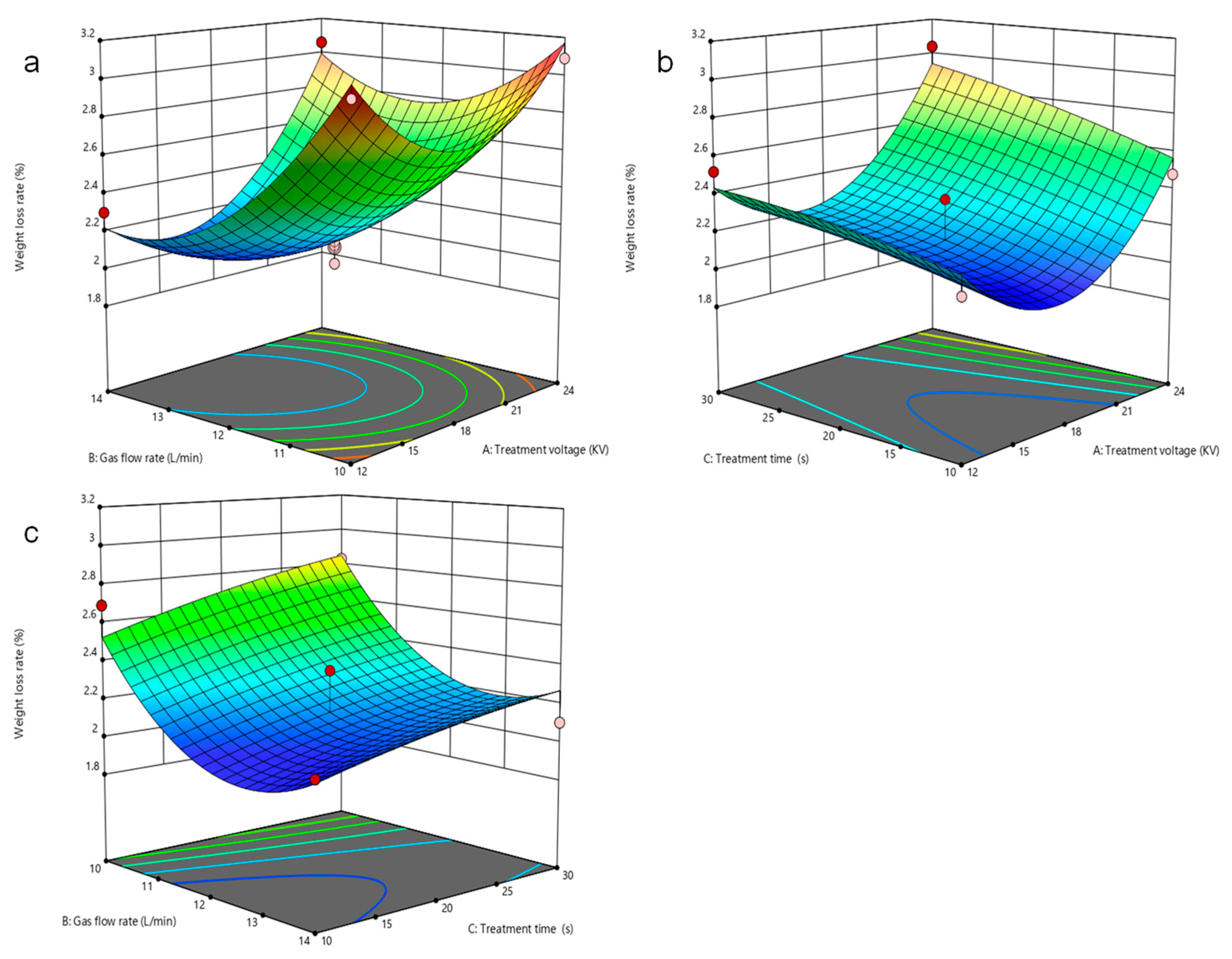
3.2. Response Surface Experimental Design and Result Analysis
3.3. Analysis of Model Interactions
3.4. Parameter Optimization and Verification Experiment
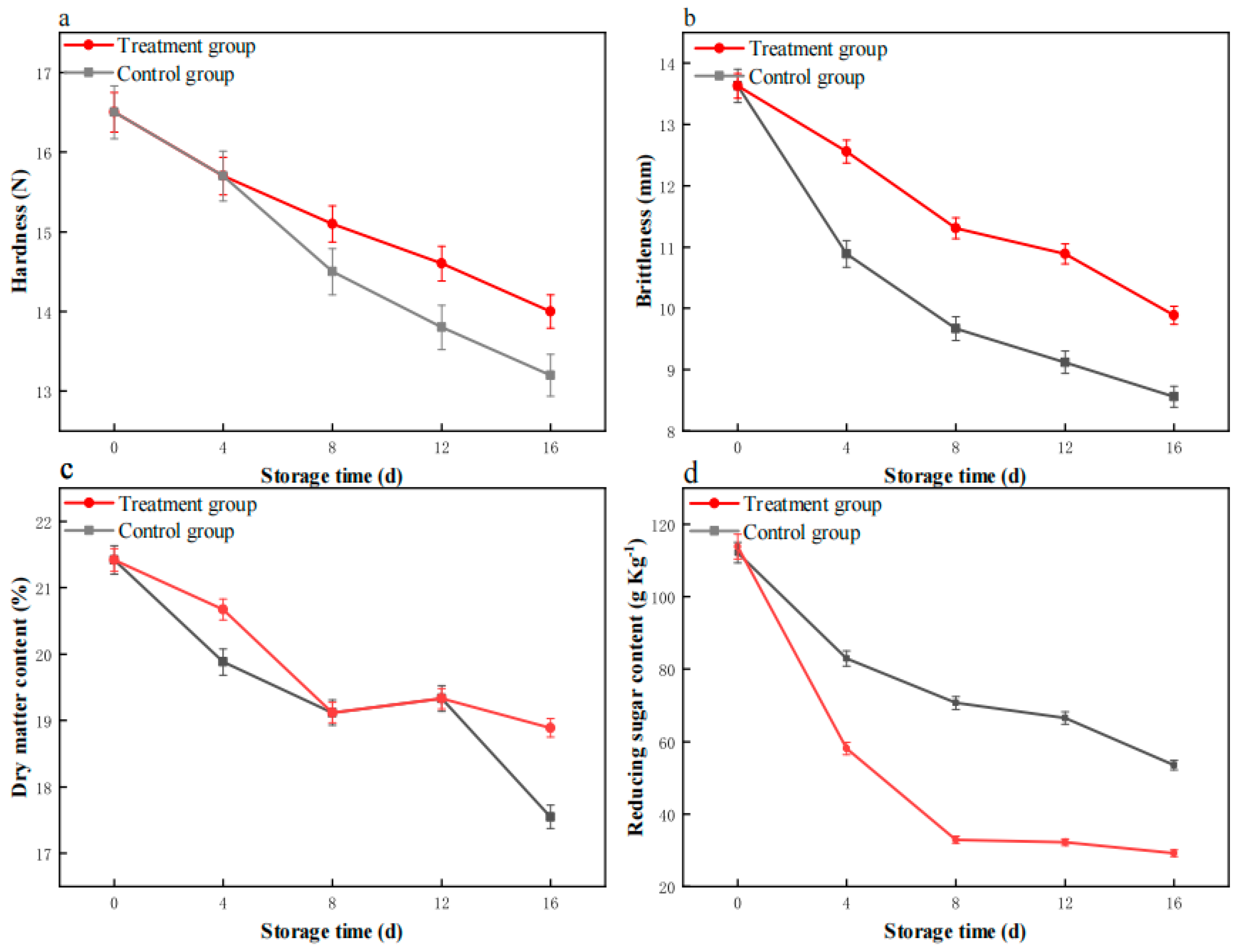
3.5. The Impact of Plasma Treatment on the Hardness and Brittleness
3.6. The Effect of Plasma Treatment on the Dry Matter Content of the Potato
3.7. The Impact of Plasma Treatment on Reducing Sugars
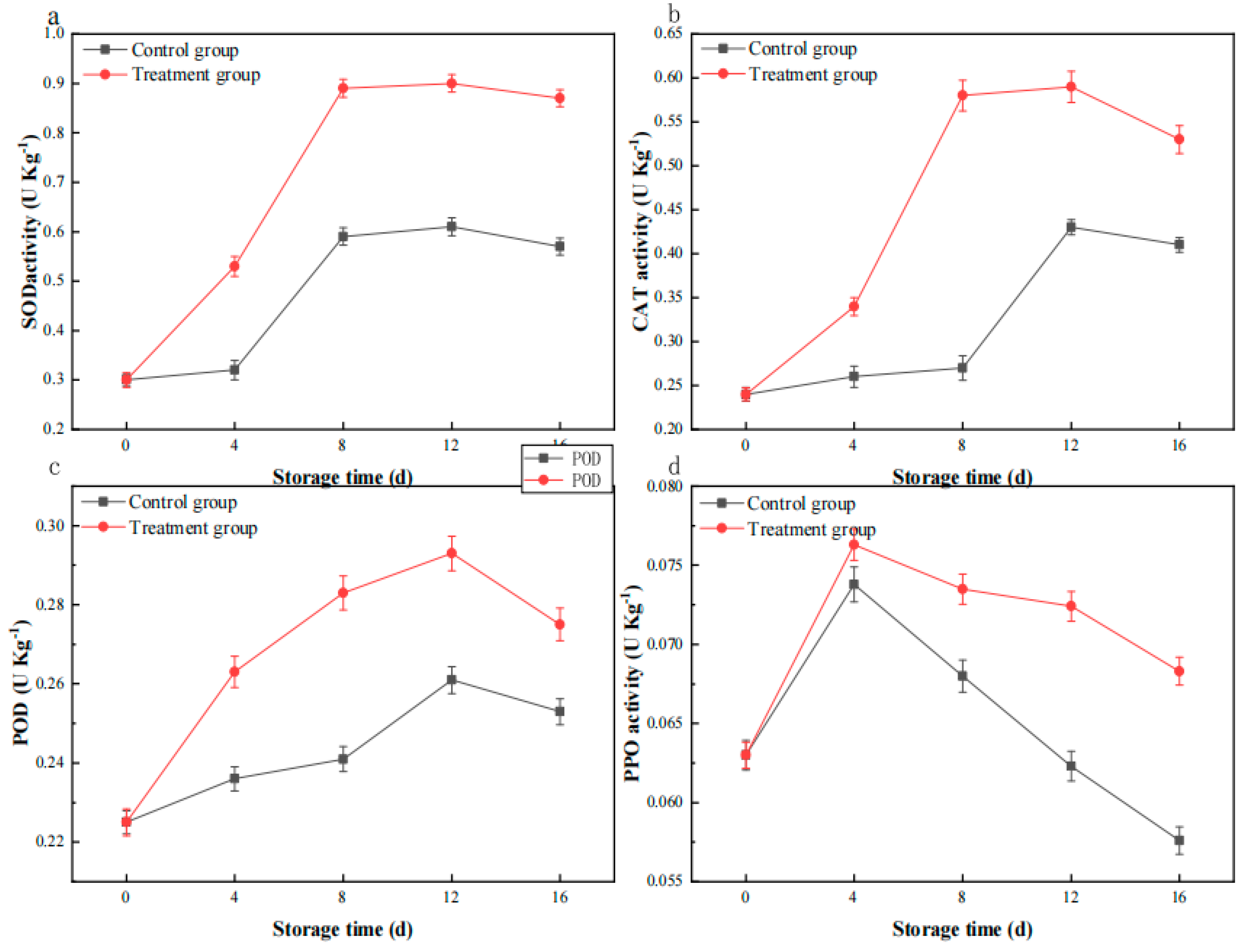
3.8. The Impact of Plasma Treatment on the Activity of SOD and CAT
3.9. Effect of Plasma Treatment on POD Activity
3.10. The Impact of Plasma Treatment on PPO Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
References
- Kirmaci, B.; Singh, R.K. Process Severity Affects Texture and Color of Potato Strips Baked in Pilot-Scale Infrared Radiant Wall Oven. LWT 2018, 97, 261–268. [Google Scholar] [CrossRef]
- Baur, S.; Frank, O.; Hausladen, H.; Hückelhoven, R.; Hofmann, T.; Eisenreich, W.; Dawid, C. Biosynthesis of α-Solanine and α-Chaconine in Potato Leaves (Solanum tuberosum L.)—A 13CO2 Study. Food Chem. 2021, 365, 130461. [Google Scholar] [CrossRef]
- Decker, E.A.; Ferruzzi, M.G. Innovations in Food Chemistry and Processing to Enhance the Nutrient Profile of the White Potato in All Forms. Adv. Nutr. 2013, 4, 345S–350S. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Stratakos, A.C.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusi, R.; Manfreda, G.; Trevisani, M. Atmospheric Cold Plasma Process for Vegetable Leaf Decontamination: A Feasibility Study on Radicchio (Red Chicory, Cichorium intybus L.). Food Control 2016, 60, 552–559. [Google Scholar] [CrossRef]
- Blenkinsop, R.W.; Copp, L.J.; Yada, R.Y.; Marangoni, A.G. Effect of Chlorpropham (CIPC) on Carbohydrate Metabolism of Potato Tubers during Storage. Food Res. Int. 2002, 35, 651–655. [Google Scholar] [CrossRef]
- Vijay, P.; Ezekiel, R.; Pandey, R. Use of CIPC as a Potato Sprout Suppressant: Health and Environmental Concerns and Future Options. Qual. Assur. Saf. Crops Foods 2018, 10, 17–24. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; Chen, G.; Li, Y.; Yang, H. Molecular Mechanisms through Which Short-Term Cold Storage Improves the Nutritional Quality and Sensory Characteristics of Postharvest Sweet Potato Tuberous Roots: A Transcriptomic Study. Foods 2021, 10, 2079. [Google Scholar] [CrossRef]
- Mutlu, S.; Palabiyik, I.; Kopuk, B.; Gunes, R.; Boluk, E.; Bagcı, U.; Özmen, D.; Toker, O.S.; Konar, N. Modification of Chia (Salvia hispanica L.) Seed Mucilage (A Heteropolysaccharide) by Atmospheric Pressure Cold Plasma Jet Treatment. Food Biosci. 2023, 56, 103178. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold Plasma Seed Priming Modulates Growth, Redox Homeostasis and Stress Response by Inducing Reactive Species in Tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef]
- de Morais, J.S.; Cabral, L.; Fonteles, T.V.; Silva, F.A.; Sant’Ana, A.S.; Lima, M.D.S.; Rodrigues, S.; Fernandes, F.A.N.; Magnani, M. Effects of Different Cold Plasma Treatments on Chemical Composition, Phenolics Bioaccessibility and Microbiota of Edible Red Mini-Roses. Food Chem. 2024, 460, 140522. [Google Scholar] [CrossRef]
- Yang, X.; An, J.; Wang, X.; Wang, L.; Song, P.; Huang, J. Ar Plasma Jet Treatment Delay Sprouting and Maintains Quality of Potato Tubers (Solanum tuberosum L.) by Enhancing Antioxidant Capacity. Food Biosci. 2023, 51, 102145. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of Meristem Activity and Sprout Growth in Potato Tubers Require Both Cytokinin and Gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Moens, L.G.; Van Wambeke, J.; De Laet, E.; Van Ceunebroeck, J.-C.; Goos, P.; Van Loey, A.M.; Hendrickx, M.E.G. Effect of Postharvest Storage on Potato (Solanum tuberosum L.) Texture after Pulsed Electric Field and Thermal Treatments. Innov. Food Sci. Emerg. Technol. 2021, 74, 102826. [Google Scholar] [CrossRef]
- Cao, R.; Yan, L.; Xiao, S.; Hou, B.; Zhou, X.; Wang, W.; Bai, T.; Zhu, K.; Cheng, J.; Zhang, J. Effects of Different Low-Temperature Storage Methods on the Quality and Processing Characteristics of Fresh Beef. Foods 2023, 12, 782. [Google Scholar] [CrossRef]
- Singh, A.; Raigond, P.; Lal, M.K.; Singh, B.; Thakur, N.; Changan, S.S.; Kumar, D.; Dutt, S. Effect of Cooking Methods on Glycemic Index and In Vitro Bioaccessibility of Potato (Solanum tuberosum L.) Carbohydrates. LWT 2020, 127, 109363. [Google Scholar] [CrossRef]
- Li, B.; Huang, A.; Wang, L.; Wu, S.; Xu, Z.; Chen, X.; Zhang, Z.; Peng, X. Increased Sugar Content Impairs Pollen Fertility and Reduces Seed-Setting in High-Photosynthetic-Efficiency Rice. Crop J. 2024, 12, 1547–1558. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity and Physiological Changes in Wheat (Triticum aestivum L.) Seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Wang, X.; Chang, F.; Dong, Q.; Jia, P.; Luan, H.; Wang, X.; Zhang, J.; Yuan, X.; Zhang, X.; Yang, S.; et al. Selenium Application during Fruit Development Can Effectively Inhibit Browning of Fresh-Cut Apples by Enhancing Antioxidant Capacity and Suppressing Polyphenol Oxidase Activity. J. Plant Physiol. 2023, 287, 154050. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xie, W.; Li, S.; Wang, Y.; Wang, Q.; Li, Q. Prohibitin StPHB3 Affects the Browning of Fresh-Cut Potatoes via Influencing Antioxidant Capacity and Polyphenol Oxidase Activation. Postharvest Biol. Technol. 2024, 207, 112598. [Google Scholar] [CrossRef]
- Xing, Y.; Liao, X.; Wu, H.; Qiu, J.; Wan, R.; Wang, X.; Yi, R.; Xu, Q.; Liu, X. Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage. Foods 2022, 11, 2788. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of Reactive Species Using Various Gas Plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Baek, E.J.; Joh, H.M.; Kim, S.J.; Chung, T.H. Effects of the Electrical Parameters and Gas Flow Rate on the Generation of Reactive Species in Liquids Exposed to Atmospheric Pressure Plasma Jets. Phys. Plasmas 2016, 23, 073515. [Google Scholar] [CrossRef]
- Norberg, S.A.; Johnsen, E.; Tian, W.; Kushner, M.J. Atmospheric Pressure Plasma Jets Interacting with Liquid Covered Tissue: Touching and Not-Touching the Liquid. J. Phys. D Appl. Phys. 2014, 47, 475203. [Google Scholar] [CrossRef]
- Almady, S.S.; Al-Hamed, S.A.; Marey, S.A.; Al-Sager, S.M.; Aboukarima, A.M. An Assessment of Some Mechanical Properties of Harvested Potato Tubers cv. Spunta. Agronomy 2024, 14, 1116. [Google Scholar] [CrossRef]
- Di, X.; Wang, Q.; Zhang, F.; Feng, H.; Wang, X.; Cai, C. Advances in the Modulation of Potato Tuber Dormancy and Sprouting. Int. J. Mol. Sci. 2024, 25, 5078. [Google Scholar] [CrossRef]
- Islam, M.M.; Naznin, S.; Naznin, A.; Uddin, M.N.; Amin, M.N.; Rahman, M.M.; Tipu, M.M.H.; Alsuhaibani, A.M.; Gaber, A.; Ahmed, S. Dry Matter, Starch Content, Reducing Sugar, Color and Crispiness Are Key Parameters of Potatoes Required for Chip Processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Wen, L.; Meng, M.; Liu, K.; Zhang, Q.; Zhang, T.; Chen, Y.; Liang, H. Effect of Photoperiod on Dry Matter Accumulation and Partitioning in Potato. Agriculture 2024, 14, 1156. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, M.M.; Akter, S. Potato Processing Quality Evaluation of Different Varieties in Bangladesh. Horticulturae 2020, 6, 54. [Google Scholar]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Tian, M.; Chen, J.; Chen, C.; Luo, Z.; Guo, T.; Huo, X.; Xiao, W. OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content. Int. J. Mol. Sci. 2024, 25, 11291. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.A.; Khah, M.A. Chapter 10—Recent Progress in Enzymatic Antioxidant Defense System in Plants Against Different Environmental Stresses. In Improving Stress Resilience in Plants; Ahanger, M.A., Bhat, J.A., Ahmad, P., John, R., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 203–224. [Google Scholar]
- Seol, Y.B.; Kim, J.; Park, S.H.; Chang, H.Y. Atmospheric Pressure Pulsed Plasma Induces Cell Death in Photosynthetic Organs via Intracellularly Generated ROS. Sci. Rep. 2017, 7, 589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.X.; Zeng, L.; Suo, H.; Li, C.C.; Shan, J.; Liu, J.T.; Luo, H.; Li, X.B.; Xiong, X. Characteristics and Differences of Polyphenol Oxidase, Peroxidase Activities and Polyphenol Content in Different Potato (Solanum tuberosum) Tubers. Appl. Ecol. Environ. Res. 2020, 18, 8171–8187. [Google Scholar] [CrossRef]
- Hu, W.; Guan, Y.; Ji, Y.; Yang, X. Effect of Cutting Styles on Quality, Antioxidant Activity, Membrane Lipid Peroxidation, and Browning in Fresh-Cut Potatoes. Food Biosci. 2021, 44 Pt B, 101435. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Tahsiri, Z.; Hedayati, S.; Niakousari, M. Improving the techno-functionality of wild almond protein isolate-based films by its hydrolysates and cold plasma treatment. Food Hydrocoll. Health 2025, 7, 100199. [Google Scholar] [CrossRef]
- Sreelakshmi, V.P.; Vendan, S.E.; Negi, P.S. The effect of cold plasma treatment on quality attributes and shelf life of apples. Postharvest Biol. Technol. 2024, 214, 112975. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; He, L.; Zhang, M.; Yang, T.; Yu, X.; Jiang, H. Application of antioxidants in cold plasma treatment of fish oil. Innov. Food Sci. Emerg. Technol. 2024, 97, 103799. [Google Scholar] [CrossRef]
- Du, Y.; Gao, F.; Yuan, S.; Yu, H.; Guo, Y.; Cheng, Y.; Yang, L.; Yao, W. Metabolomic, transcriptomic and physiological analysis reveal the effects and potential mechanisms of cold plasma treatment on resistance of wolfberry during storage. Postharvest Biol. Technol. 2024, 218, 113128. [Google Scholar] [CrossRef]
- Singh, S.P.; Thakur, R. Postharvest applications of cold plasma treatment for improving food safety and sustainability outcomes for fresh horticultural produce. Postharvest Biol. Technol. 2024, 209, 112694. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, M.; Shi, W.; Bao, Y.; Lei, Y.; Jiang, H. Evaluation of cold plasma activated water composite reagent on kiwifruit plants with bacterial canker and the external qualities of kiwifruit after spray treatment. Future Foods 2024, 9, 100348. [Google Scholar] [CrossRef]
- Chavan, P.; Prendeville, J.; Hamid; Jaiswal, S.; Jaiswal, A.K. Cold plasma treatment in food packaging: Effects on material properties, sterilization, and safety considerations. In Food Packaging and Preservation; Jaiswal, A.K., Shankar, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 215–234. ISBN 9780323900447. [Google Scholar]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Impacts of Cold Plasma Treatment on Physicochemical, Functional, Bioactive, Textural, and Sensory Attributes of Food: A Comprehensive Review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef] [PubMed]

| Treatment Voltage (kV) | Treatment Time (s) | Gas Flow Rate (L/min) | |
|---|---|---|---|
| Treatment voltage (kV) | 9, 12, 15, 18, 21, 24, 27 | 20 | 10 |
| Treatment time (s) | 15 | 10, 15, 20, 25, 30, 35, 40 | 10 |
| Gas flow rate (L/min) | 20 | 20 | 9, 10, 11, 12, 13, 14 |
| Experiment Number | Treatment Voltage (kV) | Gas Flow Rate (L/min) | Treatment Time (s) | Germination Rate (%) | Weight Loss Rate (%) |
|---|---|---|---|---|---|
| 1 | 12 | 10 | 20 | 73.4 | 3.05 |
| 2 | 24 | 10 | 20 | 39.8 | 3.09 |
| 3 | 12 | 14 | 20 | 57.5 | 2.3 |
| 4 | 24 | 14 | 20 | 34.8 | 3.06 |
| 5 | 12 | 12 | 10 | 72.5 | 2.14 |
| 6 | 24 | 12 | 10 | 45.8 | 2.48 |
| 7 | 12 | 12 | 30 | 59.45 | 2.52 |
| 8 | 24 | 12 | 30 | 36.8 | 3.04 |
| 9 | 18 | 10 | 10 | 55.9 | 2.69 |
| 10 | 18 | 14 | 10 | 52.2 | 2.05 |
| 11 | 18 | 10 | 30 | 50.9 | 2.83 |
| 12 | 18 | 14 | 30 | 38.9 | 2.1 |
| 13 | 18 | 12 | 20 | 36.5 | 2.36 |
| 14 | 18 | 12 | 20 | 33.4 | 2.1 |
| 15 | 18 | 12 | 20 | 30.9 | 2.11 |
| 16 | 18 | 12 | 20 | 32.4 | 2.13 |
| 17 | 18 | 12 | 20 | 30.01 | 2.01 |
| Source | Sum of Squares | Degree of Freedom | F-Value | p-Value |
|---|---|---|---|---|
| Model | 3106.56 | 9 | 65.92 | <0.0001 |
| A- Treatment Voltage | 1395.24 | 1 | 266.45 | <0.0001 |
| B- Gas Flow Rate | 167.45 | 1 | 31.98 | 0.0008 |
| C- Treatment Time | 203.52 | 1 | 38.87 | 0.0004 |
| AB | 29.70 | 1 | 5.67 | 0.0488 |
| AC | 4.10 | 1 | 0.7831 | 0.4056 |
| BC | 17.22 | 1 | 3.29 | 0.1126 |
| A2 | 551.79 | 1 | 105.38 | <0.0001 |
| B2 | 223.47 | 1 | 42.68 | 0.0003 |
| C2 | 383.83 | 1 | 73.30 | <0.0001 |
| Residual | 36.65 | 7 | ||
| Lack of Fit | 11.18 | 3 | 0.5848 | 0.6561 |
| Pure Error | 25.48 | 4 | ||
| Cor total | 3143.21 | 16 |
| Source | Sum of Squares | Degree of Freedom | F-Value | p-Value |
|---|---|---|---|---|
| Model | 2.45 | 9 | 10.46 | 0.0027 |
| A- Treatment Voltage | 0.3445 | 1 | 13.24 | 0.0083 |
| B- Gas Flow Rate | 0.5778 | 1 | 22.20 | 0.0022 |
| C- Treatment Time | 0.1596 | 1 | 6.13 | 0.0424 |
| AB | 0.1296 | 1 | 4.98 | 0.0608 |
| AC | 0.0081 | 1 | 0.3113 | 0.5943 |
| BC | 0.0020 | 1 | 0.0778 | 0.7883 |
| A2 | 0.7794 | 1 | 29.95 | 0.0009 |
| B2 | 0.3859 | 1 | 14.83 | 0.0063 |
| C2 | 0.0031 | 1 | 0.1202 | 0.7391 |
| Residual | 0.1822 | 7 | ||
| Lack of Fit | 0.1143 | 3 | 2.24 | 0.2254 |
| Pure Error | 0.0679 | 4 | ||
| Cor total | 2.63 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, X.; Lou, J. Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology. Appl. Sci. 2025, 15, 3233. https://doi.org/10.3390/app15063233
Chen S, Wang X, Lou J. Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology. Applied Sciences. 2025; 15(6):3233. https://doi.org/10.3390/app15063233
Chicago/Turabian StyleChen, Shengfa, Xiangyou Wang, and Jing Lou. 2025. "Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology" Applied Sciences 15, no. 6: 3233. https://doi.org/10.3390/app15063233
APA StyleChen, S., Wang, X., & Lou, J. (2025). Optimization of Low-Temperature Plasma Inhibition of Potato Germination Using Response Surface Methodology. Applied Sciences, 15(6), 3233. https://doi.org/10.3390/app15063233






